Abstract
Adipose tissue is a critical organ for nutrient sensing, energy storage and maintaining metabolic health. The failure of adipose tissue homeostasis leads to metabolic disease that is seen during obesity or aging. Local metabolic processes are coordinated by interacting microenvironments that make up the complexity and heterogeneity of the adipose tissue. Catecholamine-induced lipolysis, a critical pathway in adipocytes that drives the release of stored triglyceride as free fatty acid after stimulation, is impaired during aging. The impairment of this pathway is associated with a failure to maintain a healthy body weight, body-temperature or mount an immune response. Along with impairments in aged adipocytes, aging is associated with an accumulation of inflammation, immune cell activation, and increased dysfunction in the nervous and lymphatic systems within the adipose tissue. Together these microenvironments support the initiation of stimulated lipolysis and the transport of free fatty acid under conditions of metabolic homeostasis. However, during aging, the defects in these cellular systems result in a reduction in ability to stimulate lipolysis. This review will focus on how the immune, nervous and lymphatic systems interact during tissue homeostasis, review areas that are impaired with aging and discuss areas of research that are currently unclear.
Keywords: Adipose tissue lipolysis, aging, immune cells, lymphatic vessels, inflammation, sympathetic nervous system, inflammation
1.1. Introduction:
Adipose tissue, a heterogenous organ with critical functions in endocrine signaling and immune homeostasis, plays multiple roles in chronic and infectious diseases (1-4). Aging is associated with increased visceral adiposity and increased inflammation (5). Aging also shows increased risk for metabolic disease and various metabolic functions. Lipolysis, a critical metabolic pathway, is tightly regulated in adipocytes by inhibitory factors (eg. insulin and adenosine) or activating factors (catecholamines and cytokines) and the subsequent intracellular signaling. At basal levels, reduced sensitivity to insulin is thought to be a major contributing factor of ectopic fat deposition, via elevated levels of circulating free fatty acids that are stored as triglyceride in other tissues (6, 7). We will focus on situations of stimulated lipolysis, that is largely regulated by sympathetic nervous system, the release of catecholamines and the adipocyte lipase, hormone sensitive lipase (HSL) (8-10). This pathway is activated during energetic need, such as calorie deficiency, exercise, cold stress, and immune responses (11-15). It results in the hydrolysis of stored adipocyte triglycerides into free fatty acids (FFA) that are subsequently released into the blood to be taken up by other tissues and utilized for β-oxidation or UCP1-mediated uncoupling of the mitochondrial respiration to generate heat through thermogenesis (16, 17). Catecholamine-stimulated lipolysis is impaired in elderly and obese individuals, where the reduction in their ability to induce lipolysis is associated with increased visceral adiposity, reduced core body-temperature and reduced ability to fight viral infection (18-25). This is consistent findings that show genetic knockouts of proteins in the lipolysis pathway or in humans with mutations in those proteins leads to adiposity, dyslipidemia and metabolic syndrome (26-28). Given the heterogeneity of the adipose tissue, there are several interacting systems within the adipose tissue that will alter the induction of the stimulated lipolytic pathway. With age there is also accumulating chronic inflammation that alters the homeostasis of adipose (29). This review will focus on the interacting immune, lymphatic, and sympathetic nervous system that are altered with age and how those changes may affect catecholamine-stimulated lipolysis. We will also mainly focus on results from rodent research, with highlights of important findings from human biology.
2.1. White adipose tissue and cell types within
Adipose tissue can be classified into white and brown tissue, based on their tissue color which reflects the unilocular lipid droplet in white adipocytes and more concentrated mitochondria in brown adipocytes (2). Brown and white adipocytes are generally found separated into distinct depots. Brown adipocytes are primarily found in the supraclavicular and thoracic depot, while the white adipocytes are primarily found in visceral locations surrounding the vital organs and are contained within the gonadal, perirenal, mesenteric, retroperitoneal, omental and pericardial depots. Subcutaneous depots, including the inguinal, interscapular and at the anterior flank, which are primarily composed of white adipocytes, but can accumulate brown adipocytes in some conditions (30, 31). There are large, but imperfect overlaps between rodent and human adipose depots that need to be taken into account when interpreting data (18). The visceral white adipose tissue (primarily the omentum in human and the perigonadal in rodent) is associated with the greatest amount of risk for metabolic disease (2, 18). The primary role of the white adipose is to store excess energy in the form of triglyceride, and, during times when additional energetic substrates are needed, the stored triglyceride is hydrolyzed to produced FFA (32). Brown adipocytes also store low amounts of lipids, but their primary purpose is to utilize the FFA as energy substrates during the process of thermogenesis, which generates heat from fatty acids through the uncoupling of mitochondria gradient (16, 33, 34). Visceral adipose tissue from obese human individuals shows increased capacity for inducing lipolysis, as compared to the subcutaneous adipose tissue (30, 35).
Adipocytes are a predominant cell type in the adipose tissue. They are large, terminally differentiated cells primarily composed of lipid droplets. Adipocytes differentiate from precursor cells, also termed pre-adipocytes, and require that they are identified by multiple markers (Lin− CD29+ CD34+ Sca1+) (36). Adipose tissue expansion occurs via hyperplastic proliferation of precursors or hypertrophy of the differentiated adipocytes. The white adipose tissue from older humans and obese individuals are almost exclusively hypertrophic (37, 38). Excess hypertrophy that is associated with obesity and hyperinsulinemia, results in a premature senescent transcriptome and secretory profile the activation of necrotic pathways and recruitment of macrophages, forming a crown-like structures (38, 39). Weight loss is accompanied by a decrease in adipocyte size, indicating the loss of the stored triglyceride, improvements in insulin sensitivity and other metabolic parameters (40, 41). In addition to the adipocytes, stromal cells, precursors and immune cells make up the stroma vascular fraction of adipose tissue (42). In both humans and rodents, adipose tissue in younger individuals is highly vascular, requiring coordination of nutrient supply and maintenance of fluid homeostasis by draining lymph from extracellular space and returning it to the blood stream (43). Lymphatic vessels are also important for transport of antigen to lymph nodes, absorption of dietary fat, management of immune cell trafficking and inflammation and regulation of blood pressure (43, 44). White adipose tissue shows reduced vascularity in obese and older individuals, which would support an overall reduction of nutrient supply, blood and lymph flow (45).
3.1. White adipose tissue sympathetic innervation initiates lipolytic signaling
White and brown adipose tissues are highly innervated by sympathetic nerves. Brown adipose tissue innervation was recently reviewed (46, 47). Locally released catecholamines from sympathetic nerves are sufficient and necessary for the initiation of lipolysis in the white adipose tissue (8). Sympathetic innervations occur as postganglionic nerves originating from the celiac ganglia and they release catecholamines from varicosities, which permits immediate and widespread release of catecholamines throughout the tissue, as opposed to localized and contained release from a nerve terminal (48). Parasympathetic innervation is not found in rodent white adipose tissue (49); however sensory nerves recognize the lipid products from lipolysis and trigger thermogenesis in brown adipose tissue (50). Innervation is present in both visceral and subcutaneous depot, although whether nerves are present to the same density is unclear (51). Within the subcutaneous white adipose tissue, sympathetic innervation shows regional variations with the highest density localized in the inguinal region, medial to the lymph node (51), which supports a direct, local action of SNS on immune cells and their activation (52-54). The density of innervation, and the structure or organization of innervation are likely altered by conditions such as obesity, infection, or age, but precise quantification is still needed.
Sympathetic nerves express the tyrosine hydroxylase, which catalyzes the synthesis of norepinephrine (8, 55). Tyrosine hydroxylase levels are increased by cold stress and fasting, which increases catecholamines quantities (51). Catecholamine binds and activate β-adrenergic receptors (β-ARs) on adipocytes (56). β-ARs are coupled with G-proteins that signal to adenylyl cyclase to generate cyclic AMP. cAMP binds protein kinase A, which phosphorylates hormone sensitive lipase (HSL) to drive triglyceride hydrolysis into free fatty acid and glycerol (57). HSL, along with adipose triglyceride lipase (ATGL) are responsible for most of the lipase activity in adipose tissue (14). Aged adipocytes have reduced phosphorylated HSL following β-ARs stimulation (23, 58). Changes in the aged adipocytes, are affected by a reduction in adipocyte precursors, limiting ability to generate new adipocytes (59). Precursors may also become senescent, contributing to the inflammatory milieu of the tissue (21, 60). In vitro research suggesting that β-ARs are altered on aged adipocytes is controversial, but alterations in these receptors would have direct impact on lipolytic signaling (23, 61). (See figure 1A and 3)
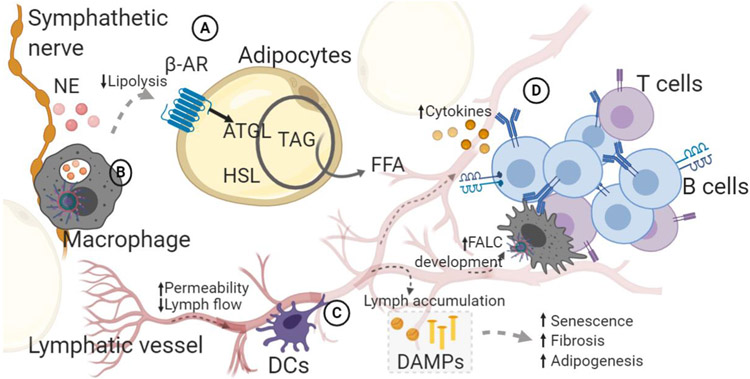
Figure 1. Interactions within aged adipose tissue.
Schematic shows major cell types and interactions within aged adipose tissue that drive catecholamine-impaired lipolysis. Letters indicate interacting microenvironments within adipose tissue. A. Adipocytes induce lipase activation and hydrolysis of triglycerides in response to catecholamines (norepinephrine: NE) that signal through β-adrenergic (β-AR) receptors. Details of β-AR signaling described in the text (2.2). This pathway is reduced during aging, in part due to changes within adipocytes, but also due to altered interactions in the aged tissue. B. Macrophages are found throughout the tissue, including lining sympathetic nerves. Macrophages drive inflammaging in adipose tissue, contributing to tissue dysfunction. Macrophages may also degrade catecholamines or take-up lipid which directly impairs the lipolysis pathway. C. Lymphatic vessels maintain fluid homeostasis. Lymph flow and contractions are reduced, while permeability is increased, indicating that lymph accumulation can occur throughout adipose tissue. Lymph contains damage associated molecular products (DAMPs), cholesterol, fatty acid, antigen that induce adipogenesis, inflammation and senescence. Dendritic cells interaction with lymphatic vessels control their permeability. D. Fat-associated lymphoid clusters, containing B and T cells, accumulate in aged adipose tissue on lymphatic vessels, potentially a result of accumulating DAMPs in lymph. The direct connection is unknown, but aged adipose B cells inhibit lipolysis, possibly because of their close contact with adipocytes and lymphatic vessels. ATGL: adipocyte triglyceride lipase; HSL: hormone sensitive lipase; TAG: triacylglyceride; FFA: free fatty acid; FALC; fat-associated lymphoid cluster.
Figure 3. Age-induced changes in systems within white adipose tissue (WAT) coordinately drive impaired stimulated lipolysis.
Schematic to describe the known factors and unknown factors in the various systems that may contribute to impaired white adipose tissue lipolysis. Tyrosine hydroxylase: TH; Norephinephrine: NE; Fat-associated lymphoid cluster: FALC; β-Adrenergic Receptor: β-AR; Hormone sensitive lipase: HSL; Stimulated: Stim.; Free fatty acid: FFA; Antibodies: Abs; White adipose tissue (WAT).
4.1. Lymphatic system transports lipid, protein and excess fluid
The lymphatic system is an essential component of the adipose tissue. It is responsible for draining interstitial fluid, for removal of cholesterol from peripheral tissues, and for transport of dietary vitamins or triglycerides packaged in chylomicrons, lipids, immune cell and antigen (43, 44). This fluid uptake and transport is carefully regulated and occurs against a pressure gradient. Blind ended microvessels, lacking smooth muscle, prevent the backflow of fluid after entering the network. The microvessels merge into larger collecting vessels composed of individually contracting units known as lymphangions. The lymphangion is lined with smooth muscle that allows contraction (62, 63). Lymphangion are separated by valve leaflets that, with the contractions, help promote unidirectional flow of lymph (64). Visceral depots have extensive lymphatic networks, whereas subcutaneous depots lymphatic vessels have reduced vessel densities that appear mainly at tissue edges (65). Lymphatic vessels density in the visceral adipose may be reflective of and correlated with its elevated inflammatory environment. The presence of triglyceride, free fatty acid and antigen makes lymph a critical immunometabolic-regulating fluid.
Lymphatic vessels respond to multiple stimuli of inflammatory or dietary origin, allowing them to alter their contractions and flow rate and thus the removal of lymph components. Lipopolysaccharide treatment reduces contractile responses, impairs the lymphatic pump function and flow in lymphatic vessels (66). Similar immunosuppressive activities are seen in response to nitric oxide and histamine (67). A single high fat meal is also sufficient to reduce contraction frequency and amplitude in lymphatic vessels, where reduced flow, reduced diameter, increased permeability, and reduced responsiveness to calcium are seen with diet-induced obesity (68, 69). In a model of intestinal inflammation in guinea pigs, 2,4,6-trinitrobenzenesulfonic acid impaired lymphatic diameter, constriction frequency and lymph flow rate (70). These findings are consistent with impaired function of the lymphatic vessel that leads to accumulating lymph and secreted inflammatory factors in local tissue, but inconsistent with heightened immune trafficking that is usually seen in inflammatory situations. Additional research is needed to clarify these findings.
Lymph contents including free fatty acid, chylomicrons and protein, can act as damage-associated molecular patterns (DAMPs), and both drive adipogenesis and inflammation, following accumulation in the tissues (43, 71). Evidence supporting this arises from analysis of mice that are haploinsufficient in the transcription factor driving the differentiation of lymphatic endothelial cells, prospero homeobox protein (PROX)-1 (72). Prox1+/− mice fail to show normal lymphatic vessel development and show overt obesity due to lymph accumulation (71, 73). In a mouse model, secondary lymphedema can result in lipid accumulation that persists even after lymphatic drainage was restored (74). Moreover, imaging reveals that lymph contents are dispersed throughout the adipose tissue and can induce inflammation during obesity (75). Although the mechanistic link has not been identified, worsened lymphatic drainage is associated with reduced lipolytic responsiveness of adipose tissue to adrenergic stimuli (76). The action of the lymphatic vessels and the lymph contents are closely tied to adipocyte ability to store or release triglyceride. (See figure 1C)
4.2. Aged lymphatic system
Aging alone results in an impairment in lymphatic vessel function (77, 78). Aged lymphatic vessels show high permeability that is caused by the loss of glycocalyx and the dysfunction of junctional proteins (78). The main role of the glycocalyx is to aid lymphocyte rolling by generating a chemokine gradient to prevent immune cells from adhering to endothelium (79). The increased permeability of the aged lymphatic vessel drives the reduced contractility and impaired pathogen transport (78). Aged lymphatic vessels also have reduced responses to inflammatory agents, such as lipopolysaccharide (LPS) (80). Basal activation of mast cells in aged animals is responsible for the impaired LPS-induced lymphatic vessel contractile response, suggesting that both intrinsic and extrinsic age-related defects may occur (80). The aged tissue surrounding vessels may also have altered responses to unexpected exposure to lymph. Young pre-adipocytes normally respond to lymph fluid and oleic acid present in lymph by inducing adipogenesis, however the aged adipocytes induce a p53-dependent apoptotic pathway following exposure to oleic acid (71, 81). Therefore, aged pre-adipocytes fail to appropriately store lipid, and the induction of the p53/p21 pathway is directly linked to cellular senescence, suggesting that accumulating lymph may drive senescence, which further increases inflammation and tissue function. The reduced contractility and impaired lymph transport, fluid homeostasis and pathogen clearance could lead to localized accumulation of inflammatory triggers within adipose tissue that cannot be cleared.
5.1. Adipose tissue immune cells
Immune cells are a contributing cellular component of adipose tissue stroma vascular fraction, with the CD45+ hematopoietic fraction making up 20-30% of non-adipocytes. Most subsets of immune cells are found in the adipose tissue, including αβ T cells, B cells and innate cells including macrophages, eosinophils, neutrophils, γδ T cells, and type 2 innate lymphoid cells. Lean healthy adipose contains a large number of anti-inflammatory, growth-supportive macrophages (82). Adipose tissue macrophages have a diverse transcriptional profile that represents a diverse spectrum of in vitro activated macrophages, suggesting that they are individually responsive to the immediate environment (24). Tissue resident macrophages are supported by other immune cells, including innate lymphoid cells type 2, eosinophils and CD4+ T regulatory cells secreting anti-inflammatory cytokines IL-4, IL-13 and IL-10 (83, 84). Macrophages originate and are recruited by several different chemokines from the bone marrow, or they can maintain themselves through proliferation (41, 85-89). Macrophages in lean, healthy tissue are also maintained through adrenergic receptor stimulation (48, 90, 91).
Adipose tissue immune cells are critical to regulate local metabolism. Fasting-induced infiltrating macrophages take up lipid. Deletion of those macrophages, increases free fatty acid release, suggesting that they act as a buffer to regulate excess lipid availability during fasting. This occurs in an ATGL-dependent manner, indicating that adipocyte lipolysis is required (90). In addition, anorexia and white adipose tissue decrease occurs independent of food intake or energy expenditure during viral infection. Instead, CD8+ T cell secreting IFNγ are required for adipose tissue loss (92). In contrast, CD4+ T cells are required for fat mass increase in a weight gain-loss-regain model (93). These results indicate that the lipolysis pathway may be modulated by other cellular targets beyond the adipocyte.
5.2. Aging related changes in adipose tissue immune cells
Aging drives alterations in the frequency and number of immune cells that may be an attempt to control tissue homeostasis and triglyceride accumulation. Over time, accumulating inflammation results in tissue dysfunction including elevated levels of fibrosis, insulin resistance and impaired catecholamine-stimulated lipolysis (94-96). Macrophages, eosinophils, innate lymphoid cells and lymphocytes have been linked to metabolic regulation of adipose tissue (24, 84, 97-100). Recent research supports a role for immune cell activation in the subcutaneous white adipose tissue from healthy, aged, non-obese human individuals (101, 102). Pathways that are significantly increased include those associated with innate and acquired immune processes and the inflammatory processes (101).
Aging and obesity show some similar mechanisms of adipose tissue inflammation (94). Surprisingly there is no clear accumulation of macrophages in white adipose tissue from human or mice (24, 102, 103). However, in mouse models of aging, adipose tissue macrophages do show a heightened inflammatory profile (24, 103, 104). There may also be sex-specific differences as aged female mice show a consistent reduction in adipose tissue macrophages, that occurs quite early, at 8 months of age (24, 98). This suggests that alterations in adipose tissue macrophages may be an initial sign of inflammation in the aging adipose tissue, also revealed in proteomics and transcriptomics analysis across multiple tissues (105, 106). One major pathway of macrophage activation is through the NOD-, LRR- and pryin domain containing protein (NLRP)-3 inflammasome, a pattern-like-recognition-receptor that recognizes a wide range of DAMPs (107, 108). Elevated levels of DAMPs during aging drive increased activation of the NLRP3 inflammasome in aged adipose tissue (109). Consistent with a role for the NLRP3 inflammasome in age-related inflammation, aged Nlrp3−/− mice have increased macrophage frequency, reduced adipose tissue inflammation, improved insulin sensitivity and increased fasting-induced lipolysis (24, 109, 110). Lymphatic fluid or necrotic adipocytes are a potential rich source of DAMPs, suggesting a direct connection between local environments like lymphatic vessels, the NLRP3 inflammasome activation, adipose tissue immune cells and age-related metabolic impairments.
Aging is also associated with an expansion in multiple lymphocyte populations within the adipose tissue from both rodents and humans (97, 98, 103). The importance of adipose tissue lymphocytes has been recently reviewed (94), so we will focus on B lymphocytes. The expansion in B cells has been described by multiple publications (Figure 2) (98, 99, 105). B cell accumulation is localized to fat-associated lymphoid clusters (FALCs) within adipose tissue and occurs after decreases in macrophages or expansion in T cells (98). Although, it is unclear whether FALCs accumulate due to age or diet alone in humans, lymphoid clusters develop in patients with Chron’s disease (111) (discussed later). The expansion of FALCs is dependent on inflammation, as aged Nlrp3−/− mice have restored frequencies of B cells, and T cells and a reduction in FALC number (98). A portion of the adipose B cells are Ki67+, a marker for proliferation. Their proliferation is partly dependent on the cytokine IL-1β, which is secreted by the NLRP3 inflammasome-activated macrophages (24). B cells accumulate via recruitment as Cxcl13-knockout mice, a chemotactic factor for CXCR5+ B cells, fail to accumulate B cells in the FALCs (112), indicating that both proliferation and recruitment likely contribute to the B cell expansion, but whether these make up separate populations or whether a subset of B cells are first recruited and then undergo proliferation is unknown.
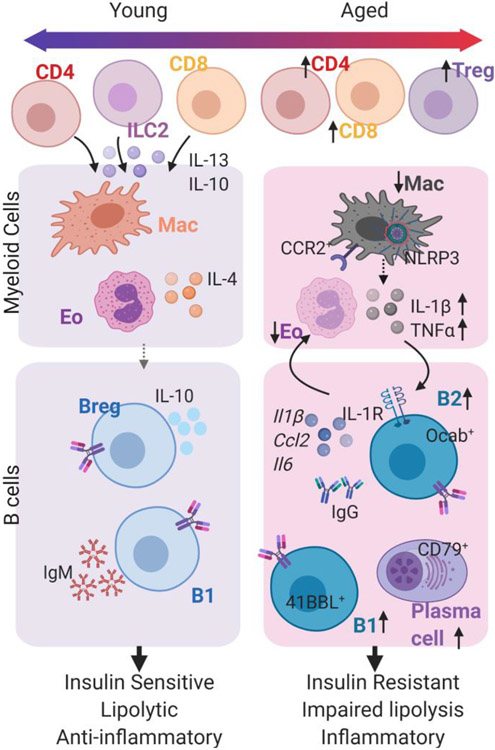
Figure 2. Adipose tissue immune cells are altered with age.
Schematic to describe the changes in adipose tissue immune cells with age. CD4 T cells and CD8 T cells accumulate in aged tissue, but less is known about their function. The changes in myeloid cells, including eosinophils and macrophages, and B cells are well-described and illustrated here. Macrophages (Mac) in young mice are anti-inflammatory and they become more inflammatory with age. Eosinophils (Eo) are abundant in young healthy tissue, secreting IL-4 to maintain anti-inflammatory macrophages and B regulatory B cells. B1 cells secreting IgM are also present in adipose tissue. With age, there is a reduction in Eo, and expansion in T cells (top) and B cells (bottom). Multiple subsets of B cells are expanded including B2, B1 and plasma cells.
Aged adipose B cells have a unique phenotype as compared to B cells from other tissues. Adipose B cells are a subset of age-associated B cells (ABCs), which lack CD93, CD21 and CD23 (113). The adipose environment and obesity drive the inflammatory ABC-like phenotype (98, 114-116). Aged Adipose B cells do not express CD11c and Tbet, which are expressed by splenic ABCs, and express high levels of cytokines (98). The B cell-specific transcription factor Oct coactivator (Ocab), that is important for transcription of the variable part of the kappa light chain, is required for B cell numbers in aged adipose tissue, as hematopoietic deletion of OcaB reduced IgG2c antibody levels and improved insulin sensitivity (99). There is also an expansion of B1 cells and plasma cells that express the J chain and CD79 (98, 105). Germinal center B cells accumulate in FALCs that develop during acute inflammation and are in close proximity with follicular dendritic cells and T cells (112). Finally, peritoneal B cells, which may circulate into the adipose tissue, and express 4-1BBL and TNFα, accumulate in a manner dependent on CCR2+ monocytes accumulation during aging (117). It is currently unclear how these subsets interact and whether they independently support or inhibit inflammation or impaired lipolysis.
Initial studies to understand adipose tissue B cells were performed in mouse models of diet-induced obesity. Obesity results in alterations of adipose tissue B cell subsets, including increased inflammatory B2 B cells expressing IgG2c and decreased tissue protective B regulatory cells expressing IL-10 (118-122). More importantly these were the first to show that B cells regulate adipose tissue metabolism. Acute systemic depletion of B cells in diet obese mice or analysis of B cell null obese mice revealed an improvement in insulin sensitivity as compared to their lean, chow-fed counterpart (119). Transfer of IgG2c antibodies from obese mice to lean mice, also transfers insulin resistance (119). Consistently, acute depletion of B cells in aged mice improves insulin sensitivity (98). B cell depletion in aged mice also results in improved fasting-induced lipolysis and improved responses to cold stress. There was increased phosphorylated HSL in visceral adipose tissue after fasting and increased core body-temperature during cold stress (98). Transfer of the aged adipose B cells to young hosts impairs glycerol release after fasting, suggesting there may be cell-intrinsic properties of aged B cells that lead to the reduction in stimulated-adipocyte lipolysis.
6.1. Fat-associated lymphoid clusters with lymphatic vessels accumulate during inflammation
Aged adipose tissue lymphocytes are relatively sparse throughout the parenchyma, and instead accumulate in FALCs (98, 112, 123). FALCs are a non-traditional tertiary lymphoid organ (TLO) that are closely associated with adipocytes. TLOs can form during chronic inflammation, including metabolic disease, aging, cancer and autoimmune disease (124). FALCs may also form in response to acute peritoneal inflammation (125). The most common trigger appears to be toll-like receptor (TLR) signaling. This can be direct, through peritoneal injection of zymosan, the TLR-2 ligand or modeled in mouse models of infection, obesity or aging (112, 126). Infection with the bacteria, Yersinia pseudotuberculosis, results in leaky lymphatic vessels exposing the adipose tissue directly to lymph contents and the intracellular gram-negative bacteria that activates TLR signaling and causes the formation of FALCs (126, 127). Age-related microbiota changes are at least partially responsible for the expansion in FALCs, as germ free mice had reduced numbers of FALCs per adipose tissue (112). It’s unclear whether increased permeability of the intestines, or even lymphatic vessels, are the mechanistic link for FALC formation.
FALC formation is a multi-step process requiring the development of a vascular network to support the immune cells, recruitment of those immune cells and formation of a cluster. Although TLOs require lymphotoxin signaling, and specialized cells called lymphoid tissue inducer cell (LTi) to permit the formation of the TLO, FALCs develop in a LTi, lymphotoxin receptor-independent manner (112, 128). Instead, research indicates that immune cells, including invariant natural killer cells, innate lymphoid cells type 2 and macrophages, drive the formation of the stromal network, and secrete cytokines to drive FALC development (112, 123). Critical cytokines that are involved in the FALC development during acute inflammation are macrophage-derived TNFα and stromal cell-derived CXCL13 (112). iNKT cells may play multiple roles by secreting Th2-type cytokines, IL-4 and IL-13, which may help limit excess expansion and may also be involved in the resolution of acute inflammation. The production of antibodies from B cells in FALCs is highly dependent on the environmental cues present during the inflammatory process, but they can contributes to pathogen clearance and autoimmunity (125). Neither the process to clear FALCs from adipose tissue, nor the differences between FALCs that develop during acute inflammation versus chronic inflammation, are fully understood.
FALCs that develop during age-related chronic inflammation, have lymphatic vessels and high endothelial venules, specialized cells that permit lymphocyte exit from vessel and into the microenvironment (98, 129). It is currently unclear whether FALCs develop around already existing lymphatic vessels, or whether new vessels form as a result of the FALC development. The identification of these Prox1+ lymphatic vessels in FALCs, indicates a direct link between lymph fluid and the abundant immune cells. Immune cells, especially macrophages and dendritic cells may line lymphatic vessels throughout tissues as a mechanism of sampling lymph fluid (44). CCR7+ dendritic cells, which accumulate during obesity, control lymphatic collecting vessel permeability through their interactions with lymphatic vessels. This interaction increases tissue fibrosis, ultimately increasing permeability and enhancing adipose tissue inflammation during obesity. In humans, Crohn’s Disease is characterized by inflammatory creeping fat, where TLOs are positioned along collecting lymphatic vessels. Lymph flow may be affected by TLOs that are filled with accumulating B cells suggesting that crosstalk exists between these two environments (111). Inflammatory cytokines are sufficient to initiate and drive the process, as constitutive activation of TNFα or adipocyte-released cytokines results in TLO development, altered lymphatic transport, and lymphangiogenesis (130, 131). Depletion of B cells does not eliminate the FALC but does improve metabolic responses and reduce age-expanded T cell subsets (98). The interaction of lymphocytes within the FALC with the lymphatic and stromal network on catecholamine-induced lipolysis requires additional studies. (See figure 1d)
7.1. Interaction of nerves and immune cells
Sympathetic nerve growth and development are supported by locally acting neurotropic factors. Initial work identified adipocytes as a major producer of nerve growth factor which plays a key role in the growth and maintenance of sympathetic neurons (132). Leptin signaling induces brain derived neurotrophic factor (BDNF) to regulate adipose tissue innervation (133). Work has also revealed that adipose macrophage express BDNF that is required for maintaining subcutaneous adipose tissue innervation, energy expenditure and preventing fat mass gain. (134). These results suggest that the cellular source of neurotrophic factors may be more widespread than was initially thought. The activation of the nerves directly contributes to macrophage phenotype and activation status. Surgical denervation and β-adrenergic antagonist treatment increases macrophage-specific TNFα expression (91). Macrophage-expression of the β-AR2 and HDAC4 play a role on mediating this regulation (91, 135). Macrophages, including BDNF+ subsets, line the sympathetic nerve, thus giving them localized access to directing sympathetic nerve growth. Initial results that have not held out, suggested that macrophages themselves may make catecholamines (136), but more recent work shows that macrophages do not express TH and therefore cannot enzymatically produce catecholamines (51, 137). Instead, macrophages lining the nerve-associated macrophages are found to express a high level of inflammatory cytokines such as IL-1β, IL-6 and express catecholamine uptake receptor (SLC6A2), as well as the catecholamine degradation enzymes (monoamine oxidase; MAOA). MAOA is also upregulated in total F4/80+ CD11b+ adipose tissue macrophages from aged or obese mice (24, 138). Therefore, catecholamine levels in obese and aged adipose tissue may be reduced by inflammatory macrophage subsets which can oxidize these amines, removing them from activating lipolysis in adipocytes. Human adipocytes, but not human adipose tissue macrophages, express MAOA, suggesting that similar mechanisms may be present in adipose tissue from obese or elderly humans (139). Moreover, aged macrophage inability to engulf excess lipids or dying adipocytes within aged tissue may lead to heightened levels of FFAs and contribute to chronic inflammation activation.
8.1. Conclusions
Stimulated lipolysis in the white adipose tissue is a critical metabolic pathway that drives the release of stored triglyceride as free fatty acid, which can then be used as energetic substrates (57). This pathway is precisely controlled in the adipocyte through integration of multiple diverse signals or interactions from the nervous system, the lymphatic system and the immune system. Many interesting advances have been made to understand the importance of the microenvironments containing the lymphatic system and interacting adipose tissue immune cells that help enforce stimulated-adipocyte lipolysis. The importance of these interactions is highlighted in aged adipose tissue, where catecholamine-induced lipolysis is impaired (Summarized in figure 3). Much remains to be elucidated regarding the in vivo interactions of these systems within the complexity of the heterogenous adipose tissue. These advances show promise of identifying new candidates to target to improve tissue function and lipolysis in the dysfunctional adipose tissue.
Acknowledgements
This work was supported by NIH grants R01 AG069819 (CDC), R00 AG058800 (CDC), and the Medical Discovery Team on the Biology of Aging (CDC). CDC also is supported by the Fesler-Lampert Chair in Aging Studies and the Glenn Foundation for Medical Research/AFAR Grants for Junior Faculty. These figures were created with BioRender.com. Thank you to Stephanie Cholensky for comments and edits on the final draft.
Abbreviations:
- HSL
Hormone sensitive lipase
- FFA
Free fatty acid
- β-ARs
β-adrenergic receptors
- ATGL
adipose triglyceride lipase
- DAMPs
damage-associated molecular patterns
- LPS
lipopolysaccharide
- NLRP-3
NOD-, LRR- and pryin domain containing protein- 3
- FALC
fat-associated lymphoid cluster
- ABCs
Aged B cells
- TLO
tertiary lymphoid organ
- TLR
Toll-like receptor
- BDNF
brain derived neurotrophic factor
- MAOA
monoamine oxidase-A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Christina Camell: Conceptualization, writing and editing
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hotamisligil GS, Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 47, 406–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cinti S, The adipose organ at a glance. Disease models & mechanisms 5, 588–594 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer PE, Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55, 1537–1545 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Rosen ED, Spiegelman BM, What we talk about when we talk about fat. Cell 156, 20–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huffman DM, Barzilai N, Role of visceral adipose tissue in aging. Biochimica et biophysica acta 1790, 1117–1123 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shetty SS, Kumari S, Fatty acids and their role in type-2 diabetes (Review). Experimental and therapeutic medicine 22, 706 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ertunc ME, Hotamisligil GS, Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. Journal of lipid research 57, 2099–2114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartness TJ, Liu Y, Shrestha YB, Ryu V, Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 35, 473–493 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arner P, Langin D, The role of neutral lipases in human adipose tissue lipolysis. Current opinion in lipidology 18, 246–250 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Langin D et al. , Adipocyte Lipases and Defect of Lipolysis in Human Obesity. Diabetes 54, 3190–3197 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Haemmerle G et al. , Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Han SJ et al. , White Adipose Tissue Is a Reservoir for Memory T Cells and Promotes Protective Memory Responses to Infection. Immunity 47, 1154–1168 e1156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayari A et al. , Influenza infection rewires energy metabolism and induces browning features in adipose cells and tissues. Commun Biol 3, 237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zechner R, FAT FLUX: enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO molecular medicine 7, 359–362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanford KI, Goodyear LJ, Exercise regulation of adipose tissue. Adipocyte 5, 153–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend KL, Tseng Y-H, Brown fat fuel utilization and thermogenesis. Trends in Endocrinology & Metabolism 25, 168–177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan B, Zierath JR, Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell metabolism 17, 162–184 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Chusyd DE, Wang D, Huffman DM, Nagy TR, Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front Nutr 3, 10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolander J, Effect of cold exposure on older humans. Int J Sports Med 23, 86–92 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Milner JJ, Beck MA, The impact of obesity on the immune response to infection. Proc Nutr Soc 71, 298–306 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry DC et al. , Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell metabolism 25, 166–181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pontzer H et al. , Daily energy expenditure through the human life course. Science 373, 808–812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lonnqvist F, Nyberg B, Wahrenberg H, Arner P, Catecholamine-induced lipolysis in adipose tissue of the elderly. The Journal of clinical investigation 85, 1614–1621 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camell CD et al. , Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Large V et al. , Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. Journal of lipid research 40, 2059–2066 (1999). [PubMed] [Google Scholar]
- 26.Albert JS et al. , Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. The New England journal of medicine 370, 2307–2315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G, Triglyceride deposit cardiomyovasculopathy. The New England journal of medicine 359, 2396–2398 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Zechner R et al. , FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell metabolism 15, 279–291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A, Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14, 576–590 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Tchkonia T et al. , Mechanisms and metabolic implications of regional differences among fat depots. Cell metabolism 17, 644–656 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jong J. M. A. d., Larsson O, Cannon B, Nedergaard J, A stringent validation of mouse adipose tissue identity markers. American Journal of Physiology-Endocrinology and Metabolism 308, E1085–E1105 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Richard AJ, White U, Elks CM, Stephens JM, in Endotext, Feingold KR et al. , Eds. (South Dartmouth (MA), 2000). [Google Scholar]
- 33.CANNON B, NEDERGAARD J, Brown Adipose Tissue: Function and Physiological Significance. Physiological reviews 84, 277–359 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Matthias A et al. , Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. The Journal of biological chemistry 275, 25073–25081 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Arner P, Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med 27, 435–438 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Church CD, Berry R, Rodeheffer MS, Isolation and study of adipocyte precursors. Methods in enzymology 537, 31–46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spalding KL et al. , Dynamics of fat cell turnover in humans. Nature 453, 783–787 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Li Q et al. , Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nature medicine, (2021). [DOI] [PubMed] [Google Scholar]
- 39.Cinti S et al. , Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research 46, 2347–2355 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Jung DY et al. , Short-term weight loss attenuates local tissue inflammation and improves insulin sensitivity without affecting adipose inflammation in obese mice. American journal of physiology. Endocrinology and metabolism 304, E964–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamarron BF et al. , Macrophage Proliferation Sustains Adipose Tissue Inflammation in Formerly Obese Mice. Diabetes 66, 392–406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoettl T, Fischer IP, Ussar S, Heterogeneity of adipose tissue in development and metabolic function. The Journal of experimental biology 221, (2018). [DOI] [PubMed] [Google Scholar]
- 43.Escobedo N, Oliver G, The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell metabolism 26, 598–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP, The Lymphatic System: Integral Roles in Immunity. Annual review of immunology 35, 31–52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye J, Adipose tissue vascularization: its role in chronic inflammation. Current diabetes reports 11, 203–210 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.François M et al. , Sympathetic innervation of the interscapular brown adipose tissue in mouse. Annals of the New York Academy of Sciences 1454, 3–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartness TJ, Vaughan CH, Song CK, Sympathetic and sensory innervation of brown adipose tissue. International journal of obesity 34 Suppl 1, S36–42 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang H, Ding X, Cao Y, Wang H, Zeng W, Dense Intra-adipose Sympathetic Arborizations Are Essential for Cold-Induced Beiging of Mouse White Adipose Tissue. Cell metabolism 26, 686–692 e683 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK, Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and cellular endocrinology 318, 34–43 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garretson JT et al. , Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Molecular metabolism 5, 626–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chi J et al. , Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell metabolism 27, 226–236 e223 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Lorton D, Bellinger DL, Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci 16, 5635–5665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma D, Farrar JD, Adrenergic regulation of immune cell function and inflammation. Seminars in immunopathology 42, 709–717 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madden KS, Sanders VM, Felten DL, Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annual review of pharmacology and toxicology 35, 417–448 (1995). [DOI] [PubMed] [Google Scholar]
- 55.Zeng W et al. , Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins S, Cao W, Robidoux J, Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Molecular endocrinology 18, 2123–2131 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS, Regulation of lipolysis in adipocytes. Annual review of nutrition 27, 79–101 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mancuso P, Bouchard B, The Impact of Aging on Adipose Function and Adipokine Synthesis. Frontiers in endocrinology 10, 137 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkland JL, Hollenberg CH, Gillon WS, Ageing, differentiation, and gene expression in rat epididymal preadipocytes. Biochem Cell Biol 71, 556–561 (1993). [DOI] [PubMed] [Google Scholar]
- 60.Baker DJ et al. , Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gettys TW et al. , Age-dependent changes in beta-adrenergic receptor subtypes and adenylyl cyclase activation in adipocytes from Fischer 344 rats. Endocrinology 136, 2022–2032 (1995). [DOI] [PubMed] [Google Scholar]
- 62.Dixon JB, Lymphatic lipid transport: sewer or subway? Trends in endocrinology and metabolism: TEM 21, 480–487 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D, Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 17, 920–922 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Dixon JB et al. , Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13, 597–610 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Redondo PAG et al. , Lymphatic vessels in human adipose tissue. Cell Tissue Res 379, 511–520 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Chakraborty S et al. , Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. American journal of physiology. Heart and circulatory physiology 309, H2042–2057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao S et al. , Impaired lymphatic contraction associated with immunosuppression. Proceedings of the National Academy of Sciences of the United States of America 108, 18784–18789 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kassis T et al. , Postprandial lymphatic pump function after a high-fat meal: a characterization of contractility, flow, and viscosity. Am J Physiol Gastrointest Liver Physiol 310, G776–789 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zawieja SD et al. , Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. American journal of physiology. Heart and circulatory physiology 302, H643–653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY, Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol 291, G566–574 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Escobedo N et al. , Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong YK et al. , Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225, 351–357 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Harvey NL et al. , Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nature genetics 37, 1072–1081 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA, Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 72, 161–171 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuan EL et al. , Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. Journal of immunology 194, 5200–5210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varaliova Z et al. , Lymphatic drainage affects lipolytic activity of femoral adipose tissue in women. International journal of obesity 44, 1974–1978 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Shang T, Liang J, Kapron CM, Liu J, Pathophysiology of aged lymphatic vessels. Aging 11, 6602–6613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zolla V et al. , Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging cell 14, 582–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG, The endothelial glycocalyx: composition, functions, and visualization. Pflugers Archiv : European journal of physiology 454, 345–359 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nizamutdinova IT et al. , Mast cells and histamine are triggering the NF-kappaB-mediated reactions of adult and aged perilymphatic mesenteric tissues to acute inflammation. Aging 8, 3065–3090 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo W et al. , Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. American journal of physiology. Endocrinology and metabolism 292, E1041–1051 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Boutens L, Stienstra R, Adipose tissue macrophages: going off track during obesity. Diabetologia 59, 879–894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feuerer M et al. , Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature medicine 15, 930–939 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brigger D et al. , Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat Metab 2, 688–702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu L, Kitade H, Ni Y, Ota T, Roles of Chemokines and Chemokine Receptors in Obesity-Associated Insulin Resistance and Nonalcoholic Fatty Liver Disease. Biomolecules 5, 1563–1579 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weisberg SP et al. , Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation 112, 1796–1808 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weisberg SP et al. , CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation 116, 115–124 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amano SU et al. , Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell metabolism 19, 162–171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng C et al. , Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell death & disease 7, e2167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kosteli A et al. , Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of clinical investigation 120, 3466–3479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang L et al. , Sympathetic Nerve Activity Maintains an Anti-Inflammatory State in Adipose Tissue in Male Mice by Inhibiting TNF-alpha Gene Expression in Macrophages. Endocrinology 156, 3680–3694 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Baazim H et al. , CD8(+) T cells induce cachexia during chronic viral infection. Nature immunology 20, 701–710 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zou J et al. , CD4+ T cells memorize obesity and promote weight regain. Cell Mol Immunol 15, 630–639 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan S, Chan YT, Revelo XS, Winer DA, The Immune Landscape of Visceral Adipose Tissue During Obesity and Aging. Frontiers in endocrinology 11, 267 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frasca D, Blomberg BB, Paganelli R, Aging, Obesity, and Inflammatory Age-Related Diseases. Frontiers in immunology 8, 1745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee AH, Dixit VD, Dietary Regulation of Immunity. Immunity 53, 510–523 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bapat SP et al. , Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528, 137–141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Camell CD et al. , Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell metabolism 30, 1024–1039 e1026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carter S et al. , Loss of OcaB Prevents Age-Induced Fat Accretion and Insulin Resistance by Altering B-Lymphocyte Transition and Promoting Energy Expenditure. Diabetes 67, 1285–1296 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Goldberg EL et al. , IL-33 causes thermogenic failure in aging by expanding dysfunctional adipose ILC2. Cell metabolism, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trim WV et al. , Divergent immunometabolic changes in adipose tissue and skeletal muscle with ageing in healthy humans. The Journal of physiology, (2021). [DOI] [PubMed] [Google Scholar]
- 102.Ortega Martinez de Victoria E et al. , Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes 58, 385–393 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lumeng CN et al. , Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. Journal of immunology 187, 6208–6216 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu D et al. , Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. Journal of immunology 179, 4829–4839 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Schaum N et al. , Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu Q et al. , Sample multiplexing for targeted pathway proteomics in aging mice. Proceedings of the National Academy of Sciences of the United States of America 117, 9723–9732 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Camell C, Goldberg E, Dixit VD, Regulation of Nlrp3 inflammasome by dietary metabolites. Seminars in immunology 27, 334–342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swanson KV, Deng M, Ting JP, The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature reviews. Immunology 19, 477–489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Youm YH et al. , Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell metabolism 18, 519–532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Youm YH et al. , The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell reports 1, 56–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Randolph GJ et al. , Lymphoid Aggregates Remodel Lymphatic Collecting Vessels that Serve Mesenteric Lymph Nodes in Crohn Disease. The American journal of pathology 186, 3066–3073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benezech C et al. , Inflammation-induced formation of fat-associated lymphoid clusters. Nature immunology 16, 819–828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rubtsova K, Rubtsov AV, Cancro MP, Marrack P, Age-Associated B Cells: A T-bet-Dependent Effector with Roles in Protective and Pathogenic Immunity. Journal of immunology 195, 1933–1937 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frasca D, Blomberg BB, Adipose Tissue Inflammation Induces B Cell Inflammation and Decreases B Cell Function in Aging. Frontiers in immunology 8, 1003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB, Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mechanisms of ageing and development 162, 91–99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frasca D et al. , Obesity decreases B cell responses in young and elderly individuals. Obesity 24, 615–625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bodogai M et al. , Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Science translational medicine 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winer DA, Winer S, Chng MH, Shen L, Engleman EG, B Lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cellular and molecular life sciences : CMLS 71, 1033–1043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winer DA et al. , B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature medicine 17, 610–617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu L, Parekh VV, Hsiao J, Kitamura D, Van Kaer L, Spleen supports a pool of innate-like B cells in white adipose tissue that protects against obesity-associated insulin resistance. Proceedings of the National Academy of Sciences of the United States of America 111, E4638–4647 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nishimura S et al. , Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell metabolism, (2013). [DOI] [PubMed] [Google Scholar]
- 122.DeFuria J et al. , B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences of the United States of America 110, 5133–5138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moro K et al. , Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 (2010). [DOI] [PubMed] [Google Scholar]
- 124.Ruddle NH, Lymphatic vessels and tertiary lymphoid organs. The Journal of clinical investigation 124, 953–959 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cruz-Migoni S, Caamano J, Fat-Associated Lymphoid Clusters in Inflammation and Immunity. Frontiers in immunology 7, 612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Misumi I et al. , Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell reports 27, 514–524 e515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fonseca DM et al. , Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity. Cell 163, 354–366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rangel-Moreno J et al. , Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 30, 731–743 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruddle NH, High Endothelial Venules and Lymphatic Vessels in Tertiary Lymphoid Organs: Characteristics, Functions, and Regulation. Frontiers in immunology 7, 491 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rehal S, von der Weid PY, TNFDeltaARE Mice Display Abnormal Lymphatics and Develop Tertiary Lymphoid Organs in the Mesentery. The American journal of pathology 187, 798–807 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guedj K et al. , Adipocytes orchestrate the formation of tertiary lymphoid organs in the creeping fat of Crohn's disease affected mesentery. J Autoimmun 103, 102281 (2019). [DOI] [PubMed] [Google Scholar]
- 132.Peeraully MR, Jenkins JR, Trayhurn P, NGF gene expression and secretion in white adipose tissue: regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. American journal of physiology. Endocrinology and metabolism 287, E331–339 (2004). [DOI] [PubMed] [Google Scholar]
- 133.Wang P et al. , A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 583, 839–844 (2020). [DOI] [PubMed] [Google Scholar]
- 134.Blaszkiewicz M et al. , The involvement of neuroimmune cells in adipose innervation. Molecular medicine 26, 126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luan B et al. , Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell metabolism 19, 1058–1065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nguyen KD et al. , Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fischer K et al. , Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nature medicine 23, 623–630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pirzgalska RM et al. , Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nature medicine 23, 1309–1318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao H et al. , Age-Induced Reduction in Human Lipolysis: A Potential Role for Adipocyte Noradrenaline Degradation. Cell metabolism 32, 1–3 (2020). [DOI] [PubMed] [Google Scholar]