Abstract
Background:
It is unclear whether race and type 2 diabetes (T2D) modulate the effects of omega-3 supplementation on the incidence of heart failure (HF). Our primary aim was to evaluate whether prevalent T2D modifies the effects of omega-3 supplementation on HF hospitalization. Our secondary aim was to examine if race modifies the effects of omega-3 supplements on HF risk.
Methods:
In this ancillary study of the parent VITAL – a completed randomized trial testing the efficacy of vitamin D and omega-3 fatty acids on cardiovascular diseases and cancer, we assessed the role of T2D and race on the effects of omega-3 supplements on incidence of HF hospitalization (adjudicated by review of medical records and supplemented with query of Centers for Medicare and Medicaid Services data).
Results:
Comparing omega-3 supplements with placebo, hazard ratio (95% CI) for first HF hospitalization was 0.69 (0.50- 0.95) in participants with prevalent T2D and 1.09 (0.88-1.34) in those without T2D, p for interaction 0.019. Furthermore, prevalent T2D modified the effects of omega-3 fatty acids on incidence of recurrent HF hospitalization [HR=0.53 (0.41-0.69) in participants with prevalent T2D vs. 1.07 (0.89-1.28) in those free of T2D, p interaction <0.0001]. In our secondary analysis, omega-3 supplementation reduced recurrent HF hospitalization only in Black participants (p interaction race x omega-3: 0.0497).
Conclusions:
Our data showed beneficial effects of omega-3 fatty acid supplements on incidence of HF hospitalization in participants with T2D but not in those without T2D and such benefit appeared to be stronger in Blacks with T2D.
Keywords: Marine omega-3 fatty acids, heart failure, race, type 2 diabetes
Introduction
About 6.2 million Americans live with heart failure (HF) and it is estimated that HF prevalence will reach 8 million by 20301. Among older adults, HF is one of the leading causes of hospitalization in the US2,3 and is associated with high costs and high mortality1. Although data from the Olmsted County showed that among HF patients, 83% will be re-hospitalized at least once and 67% at least twice, most prior clinical trials have focused on initial HF hospitalization4. Emerging trial data on the effects of marine omega-3 fatty acids on the incidence of HF remain scarce and inconsistent. In particular, few trials have enrolled adequate number of Black participants to allow assessment of efficacy of omega-3 supplements by race/ethnicity. While the Risk and Prevention Study5 reported a 33% reduction (95% CI: 13%-48%) in first HF hospitalization comparing 1g/d of marine omega-3 fatty acids with olive oil placebo among Italians, other trials reported no benefits of marine omega-3 fatty acid supplements on HF incidence6-10. Our group has previously reported a reduction in recurrent but not initial HF hospitalization with marine omega-3 fatty acid supplements among participants of the VITamin D and OmegA-3 TriaL (VITAL)11.
Underlying reasons for heterogeneity across clinical trials assessing the effects of marine omega-3 fatty acids on HF hospitalization have not been fully elucidated. Although type 2 diabetes mellitus (T2D) is a major risk factor for HF12-14 and might modify the effects of marine omega-3 fatty acids on HF incidence, no previous study has considered the role of race/ethnicity in assessing the interaction of T2D with omega-3 supplements on initial and recurrent HF hospitalization. With the exception of VITAL that enrolled 5,087 Black participants, most other trials enrolled a small percentage of Black participants [i.e., n=346 Blacks in the STRENGTH trial9 and <10% Blacks in REDUCE-IT6]. Lastly, the parent VITAL trial reported greater reduction in myocardial infarction with omega-3 fatty acid supplements among Blacks and participants with T2D15. Hence, the current analysis of VITAL-HF ancillary study sought to test the hypothesis that supplementation with 1g/d of marine omega-3 fatty acids versus placebo has a greater effect on reducing initial and recurrent HF hospitalization among people with T2D than those without T2D among VITAL participants. In a secondary analysis, we examined whether race (Black vs. White) modified the effects of marine omega-3 supplements on both initial and recurrent HF hospitalization.
Methods
VITAL-HF is an ancillary study of the parent VITAL trial, a completed randomized, double-blind, placebo-controlled trial with a two-by-two factorial design16. The main trial’s objectives were to examine the efficacy and risks of 2000 IU per day of vitamin D3 (cholecalciferol) and 1 gram per day of marine omega-3 fatty acids [capsule containing 840 mg of n-3 fatty acids including 460 mg of EPA and 380 mg of docosahexaenoic acid (DHA)] for the prevention of cardiovascular disease and cancer from 2011 to 2017. Detailed description of the VITAL design and main results have been published previously15,16. Of the 25,871 persons randomized into parent VITAL, 36 participants with prevalent HF were excluded from our primary outcome analyses. Each participant signed informed consent and the study protocol was approved by the Institutional Review Board of Brigham and Women’s Hospital. The primary aim of the VITAL-HF ancillary study was to assess the effects of vitamin D and omega-3 supplements on the incidence of HF hospitalization and a secondary aim sought to examine potential effect modification by HF risk factors including T2D.
Ascertainment of HF
We considered the first hospitalization for HF after randomization as the primary outcome in 25,835 participants free of HF at randomization. Recurrent hospitalization for HF was considered a secondary outcome (n=25,871). A detailed description of HF ascertainment in VITAL-HF has been published11.
Other important variables
Information on demographics, comorbidity, lifestyle factors, and medication was initially obtained at baseline.
Statistical analysis
We computed person-time of follow up from randomization until the first occurrence of HF hospitalization, death, or end of the trial on December 31, 2017. We used Cox proportional hazards models to calculate hazard ratios and 95% CI for the primary outcome , stratified by prevalent T2D, controlling for stratification factors age, and sex, and randomization to vitamin D using the intention-to-treat approach. Cumulative incidences were plotted and tested using log rank test. We used the product term between T2D and omega-3 supplement in the Cox regression to evaluate interaction. For the secondary outcome, we used the Andersen-Gill model17, which allows for varying numbers of events per person with different time between events. In secondary analysis we evaluated effect modification by race (Black vs. White). All analyses were performed using SAS 9.4. Alpha level of 0.05 and 2-tailed test.
Results
Overall, the mean age at randomization was 67.1 (SD=7.1) years; 50.6% were women; 71.3% were non-Hispanic White, 20.2% were Black. The overall prevalence of T2D was 14% (24% in Blacks and 10% in Whites), and mean body mass index was 28.1 (SD=5.7) kg/m2 (Table 1).
Table 1.
Baseline characteristics of the 25,871 participants by randomized assignment and prevalent type 2 diabetes (T2D)*
| Prevalence of T2D at baseline | P comparing people with and without T2D |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | YES | NO | ||||||
| Omega-3 (n=1,791) |
Placebo (n=1,746) |
P | Omega-3 (n= 11,117) |
Placebo (n=11,170) |
P | |||
| Female sex (%) | 52.7 | 50.5 | 0.20 | 50.3 | 50.6 | 0.74 | 0.20 | |
| Age ( y) | 67.1±7.0 | 67.0±7.0 | 0.65 | 67.2 ±7.1 | 67.1±7.1 | 0.92 | 0.65 | |
| Race/ ethnic group (%) | 0.25 | 0.46 | <0.001 | |||||
| Non-Hispanic White | 54.0 | 53.5 | 74.3 | 73.9 | ||||
| Black | 35.0 | 34.7 | 17.7 | 17.9 | ||||
| Non-Black Hispanic | 6.0 | 5.8 | 3.5 | 3.9 | ||||
| Asian or Pacific Islander | 2.5 | 2.1 | 1.4 | 1.4 | ||||
| Native American or Alaskan native | 0.7 | 1.3 | 1.0 | 0.8 | ||||
| Other or unknown | 2.2 | 2.6 | 2.0 | 2.1 | ||||
| Body mass index (kg/m2) | 31.8±6.7 | 32.0±7.1 | 0.27 | 27.6±5.3 | 27.4±5.2 | 0.14 | <0.001 | |
| Smoking status | 0.55 | 0.91 | <0.001 | |||||
| Never smoker | 47.1 | 48.6 | 52.3 | 52.5 | ||||
| Past smoker | 43.3 | 42.6 | 40.9 | 40.6 | ||||
| Current smoker | 9.7 | 8.8 | 6.8 | 6.9 | ||||
| Current alcohol use (Yes) | 52.6 | 52.2 | 0.79 | 71.3 | 70.8 | 0.42 | <0.001 | |
| Treated hypertension | 79.7 | 79.8 | 0.98 | 44.4 | 45.7 | 0.07 | <0.001 | |
| Use of lipid-lowering drug | 63.9 | 64.7 | 0.62 | 33.5 | 33.0 | 0.39 | <0.001 | |
| Statin use | 59.9 | 61.0 | 0.52 | 31.2 | 30.6 | 0.34 | <0.001 | |
| Aspirin use | 59.5 | 58.3 | 0.46 | 43.0 | 43.5 | 0.44 | <0.001 | |
| Fish intake (serv/week)median (Q1-Q3) | 1.47 (0.93-2.47) | 1.47 (0.93-2.87) | 0.84 | 1.47 (0.93-2.47) | 1.47 (0.93-2.47) | 0.73 | 0.46 | |
| Fish intake ≥ median (1.5 servings/week) | 46.5 | 47.8 | 0.45 | 46.7 | 46.9 | 0.74 | 0.72 | |
| Prevalent cases of HF at randomization* | 7 | 8 | 0.76 | 14 | 7 | 0.12 | <0.001 | |
36 subjects with prevalent heart failure at randomization were excluded from analyses of incident heart failure but retained for recurrent heart failure
Race and ethnic group were reported by study participants
Data are reported as percent or mean± standard deviation, unless specified otherwise
Prevalent T2D, omega-3 fatty acid supplements, and HF hospitalization
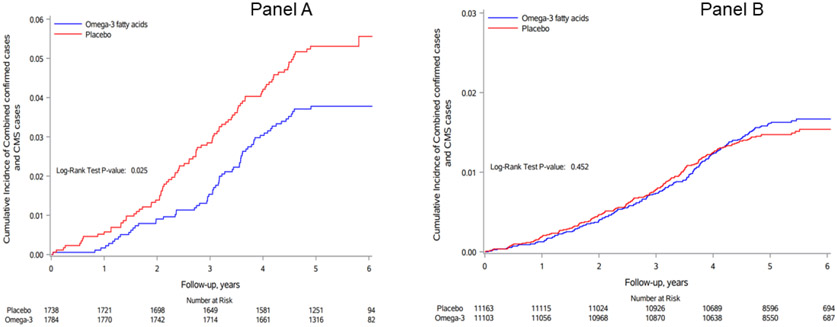
During a median person-time of follow-up of 5.3 years (range: 0 to 6.1 years), the primary endpoint of first HF hospitalization occurred in 65 out of 1784 participants with T2D assigned to omega-3 (3.6%) and 90 out of 1738 participants with T2D assigned to placebo (5.2%) [hazard ratio 0.69 (95% CI: 0.50-0.95)], adjusting for age, sex, and vitamin D assignment (Table 2 and Central Illustration). Corresponding hazard ratio (95% CI) for participants free of T2D at baseline was 1.09 (0.88-1.34), Table 2 and Central Illustration. The p value for interaction between T2D and omega-3 fatty acid supplementation was 0.019. For recurrent HF hospitalization, we also observed evidence for effect modification by T2D with stronger benefits of omega-3 fatty acids observed in T2D participants but no effect of omega-3 supplements on recurrent HF hospitalization in people without prevalent T2D (p for interaction <0.0001, Table 2).
Table 2.
Hazard ratios (95% confidence interval) for initial and recurrent heart failure hospitalization according to randomized groups and type 2 diabetes (T2D) status*
| Sequence of heart failure hospitalization |
Prevalent T2D at baseline |
No prevalent T2D at baseline |
P interaction T2D x omega-3 fatty acids |
||||
|---|---|---|---|---|---|---|---|
| Active Omega-3 Events/Total |
Omega-3 placebo Events/Tota1 |
Hazard Ratio (95% CI) p-value |
Active Omega-3 Events/Total |
Omega-3 placebo Events/Tota1 |
Hazard Ratio (95% CI) p-value |
||
| Initial | 65/1,784 | 90/1,738 | 0.69 (0.50-0.95) | 177/11,053 | 164/11,163 | 1.09 (0.88-1.34) | 0.019 |
| Recurrent ƚ | 88/1,879 | 158/1,904 | 0.53 (0.41-0.69) | 240/11,357 | 226/11,395 | 1.07 (0.89-1.28) | <0.0001 |
All models adjusted for age, sex, and vitamin D assignment versus placebo
Hnclusive of 36 participants with prevalent heart failure at randomization
Central Illustration: Cumulative Incidence rate of heart failure.
Cumulative incidence rates of first heart failure hospitalization (combined confirmed cases and cases identified via CMS), according to year of follow-up and randomization to n-3 fatty acids (blue) versus placebo (red) in people with T2D (panel A) or without T2D (panel B)
Secondary analyses: Race and HF hospitalization
Supplementation with omega-3 fatty acids was not associated with initial HF hospitalization among Blacks [54 out of 2,538 in omega-3 vs. 62 out of 2,549 in placebo; HR: 0.87 (95% CI: 0.60-1.25)] or Whites [HR: 0.95 (95% CI: 0.77-1.18)], p interaction 0.60; in contrast, there was evidence for race x omega-3 interaction for recurrent HF hospitalization with benefits in Blacks [72 out of 2,621 in omega-3 vs. 109 out of 2,666 in placebo; HR: 0.65 (95% CI: 0.49-0.88)] but not Whites [HR: 0.90 (95% CI: .75-1.08)], p interaction 0.0497, Table 3. Among participants with prevalent T2D, the benefits of omega-3 fatty acid supplements on recurrent (but not initial) HF hospitalization were observed in Blacks [HR: 0.46 (95% CI: 0.30-0.70)] as well as Whites [HR: 0.65 (95% CI: 0.45-0.92)], supplemental Table 1. In exploratory analyses, there was no evidence for 3-way interaction across T2D, fish consumption, and omega-3 supplements or race, fish consumption, and omega-3 supplements for initial or recurrent HF hospitalization (all p >0.05).
Table 3.
Hazard ratios (95% confidence interval) for initial and recurrent heart failure hospitalization according to randomized groups and race*
| Black participants |
White participants |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sequence of heart failure hospitalization |
Active Omega-3 Events/Total |
Omega-3 placebo Events/Total |
Hazard Ratio (95% CI) p-value |
Active Omega-3 Events/Total |
Omega-3 placebo Events/Total |
Hazard Ratio (95% CI) p-value |
P interaction race x omega-3 fatty acids |
|
| Initial | 54/2538 | 62/2549 | 0.87 (0.60-1.25) | 162/9035 | 168/8997 | 0.95 (0.77-1.18) | 0.60 | |
| Recurrent ƚ | 72/2621 | 109/2666 | 0.65 (0.49-0.88) | 221/9265 | 243/9244 | 0.90 (0.75-1.08) | 0.0497 | |
All models adjusted for age, sex, and vitamin D assignment versus placebo
Inclusive of 36 participants with prevalent heart failure at randomization
Discussion
Main findings
In this post-hoc analysis of a large randomized clinical trial, we found evidence for a statistically significant interaction between prevalent T2D and supplementation with 1g/d of omga-3 fatty acid on the incidence of HF hospitalization. Among participants with T2D, supplementation with omega-3 fatty acids led to a 31% [95% CI: 5-50%] reduction of initial HF hospitalization and 47% [95% CI: 31-59%] reduction of recurrent HF hospitalization compared to placebo. In contrast, there were no benefits of omega-3 fatty acid supplements on incidence of HF hospitalization among participants without T2D. While omega-3 supplements reduced the incidence of recurrent HF hospitalization only in Blacks in the entire cohort, the reduction in recurrent HF hospitalization with omega-3 supplements was observed in both Black and White participants with T2D.
T2D as effect modifier of marine omega-3 supplementation on incident HF
Our findings of reduced incidence of initial HF hospitalization with omega-3 supplements in participants with T2D are (i) consistent with greater reduction in MI (a major risk factor for HF) with n-3 supplements among VITAL participants with T2D [HR=0.40 (95% CI: 0.22-0.74)] compared to those without T2D [HR=0.80 (95% CI: 0.63-1.00)]15 and (ii) in line with data from the Risk and Prevention Study5 where intervention with 1g/d of omega-3 fatty acids led to a 33% reduction [95% CI: 13-48%] in HF hospitalization compared to olive oil placebo among 12,513 Italian adults, 60% of whom had T2D at baseline. In contrast, the ASCEND trial reported no benefit of 840 mg/d of EPA/DHA supplements on non-fatal HF [HR: 0.81 (95% CI: 0.60-1.10)] among 15,480 participants with diabetes (94% with T2D)7. Furthermore, the ORIGIN trial that enrolled 12,536 participants with T2D, impaired glucose tolerance or impaired fasting glucose showed no benefit of EPA/DHA (1g/d) on incidence of HF hospitalization (HR: 1.02 (95% CI: 0.88-1.19)]8. In the STRENGTH trial9 with 70% of 13,078 participants diagnosed with diabetes, intervention with 4g/d of omega-3 fatty acids had no effects on initial HF hospitalization [HR: 1.12 (95% CI: 0.88-1.42)]. It is also important to note that the STRENGTH trial9 included both urgent outpatient visit for HF and HF hospitalization in their outcome. Lastly, the REDUCE IT trial6 (58% T2D participants) and the OMEMI trial10 (21% T2D participants) showed no benefits of omega-3 fatty acid intervention on HF hospitalization compared to placebo. The inconsistency of findings on the effects of EPA/DHA on HF in people with T2D merits additional investigation in future clinical trials. As to underlying biologic mechanisms that could explain observed benefits of omega-3 supplements on HF in people with T2D, our working hypothesis is that omega-3 supplements reduce serum advanced glycation end-products18 that accelerate the development and worsening of HF and improved insulin sensitivity19 in people with T2D. However, our working hypothesis merits further evaluation in future mechanistic studies.
Race as potential modifier of the effects of marine omega-3 supplements on incident HF
The reduction in rates of recurrent HF hospitalization in Blacks but not Whites in the current study is consistent with reported greater reduction in MI with n-3 supplements among Blacks [HR=0.23 (95% CI: 0.11-0.47)] but not Whites [HR: 0.93 (95% CI: 0.73-1.18)] in VITAL15. Our study is the first large study to focus on the role of race as potential effect modifier of omega-3 supplements on HF incidence. A lack of enrollment of an adequate number of Black participants in previous large clinical trials prevents us from comparing our results to any previous data. For example, the STRENGTH trial9 enrolled only 2.6% (n=346) Black participants while other large randomized trials including REDUCE-IT6, OMEMI10, and ASCEND7 enrolled 90% or more White participants. The paucity of data on the efficacy of marine omega-3 supplements on incidence of HF as well as cardiovascular disease in Blacks underscores the need for future trials to assess the efficacy of omega-3 supplements among Blacks, who are at risk of developing HF due to high prevalence of Hf risk factors (i.e., obesity, hypertension, and T2D)1.
Limitations and strengths
Limitations of the current study include insufficient statistical power to evaluate the interaction of T2D with omega-3 fatty acids on HF with preserved vs. reduced ejection fraction. Of the 106 confirmed HF cases via review of medical records, only 101 had echocardiographic data on ejection fraction in their medical records to subclassify HF with preserved (n=48) or reduced (n=53) ejection fraction. It is possible that we missed some hospitalizations due to HF, especially fatal ones; however, the use of the CMS database to capture unreported HF on annual follow up questionnaires helped to mitigate this limitation. It is possible that some of the HF events identified via CMS were misclassified; however, such misclassification is likely to be non-differential because of randomization and would bias the results towards the null (no effect of either intervention on HF rate). Given the paucity of data on effect modifiers of omega-3 supplements on HF incidence in the literature, our findings should be considered as hypothesis generating for future randomized trials. Lastly, although the use of sodium glucose co-transporter-2 inhibitors (SGLT2i) has been shown to reduce HF incidence20,21 among T2D, it is less likely that SGLT2i played any role in the observed interaction by T2D given the balanced distribution of medications including hypoglycemic agents between omega-3 and placebo groups in VITAL22. Nonetheless, our study has numerous strengths including novel investigation of potential effect modification of the effects of omega-3 fatty acids on both initial and recurrent HF hospitalization by T2D in a large and multi-ethnic cohort; standardized methods to adjudicate HF hospitalization; and randomization with double-blinding to eliminate confounding by known and unknown factors.
Conclusions
Our data provide evidence in support of beneficial effects of marine omega-3 supplements on incidence rate of both initial and recurrent HF hospitalization among participants with T2D. Our secondary findings of benefits of marine omega-3 supplements on HF risk among Blacks merit further investigation.
Supplementary Material
Clinical perspectives:
While omega-3 fatty acid supplements might reduce the risk of HF risk factors and HF hospitalization in general, it is less clear whether patients with T2D may benefit more that non-diabetic patients from omega-3 fatty acid supplements. Furthermore, the paucity of trial data among Blacks is an important gap to address. In this study, we found that supplementation with omega-3 fatty acids reduced the incidence of HF hospitalization, especially among patients with T2D and Blacks. If confirmed by future trials, these findings could help clinicians improve management and prevention of HF in subgroups of high-risk patients.
Translational outlook:
While promising, our findings do not address the question of adequate dose of omega-3 fatty acid supplements, nor whether EPA is equally effective as DHA. Furthermore, no previous study has examined the efficacy of omega-3 fatty acid supplements on HF with preserved versus reduced ejection fraction. Future clinical trials should address these important gaps that are critical for informed and appropriate use of omega-3 supplementation for effective management of HF.
Acknowledgements:
We are indebted to study participants and staff for their commitment to the trial.
Funding sources:
The current ancillary study was supported by grant R01HL131687 (L Djousse) from the National Heart, Lung, and Blood Institute, Bethesda MD. The parent VITAL was supported by grants U01 CA138962 and R01 CA138962 from the National Institutes of Health and the Office of Dietary Supplements. Dr. Mora was supported by K24HL136852, R01HL134811, and R01DK112940.
List of abbreviations:
- CI
Confidence interval
- CMS
Centers for Medicare and Medicaid Services
- EPA
Eicosapentaenoic acid
- DHA
Docosahexaenoic acid
- HF
Heart failure
- HR
Hazard ratio
- SD
Standard deviation
- SGLT2i
Sodium glucose co-transporter-2 inhibitor
- T2D
Type 2 diabetes
- VITAL
Vitamin D and Omega-3 Trial
Footnotes
Disclosures: Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Quest Diagnostics (San Juan Capistrano, CA) measured serum 25-hydroxyvitamin D and plasma phospholipid omega-3 fatty acids at no cost to the study.
In the past years, Dr. Djousse received investigator-initiated grants from Amarin Inc and Merck; he is currently serving as Co-PI on a project funded by Novartis. Dr. Mora has served as a consultant to Quest Diagnostics and Pfizer.
Other co-authors have no disclosures.
Trial registration: NCT01169259 (parent VITAL) and NCT02271230 (current study).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137(2):352–360. [DOI] [PubMed] [Google Scholar]
- 3.Koelling TM, Chen RS, Lubwama RN, L'Italien GJ, Eagle KA. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004;147(1):74–78. [DOI] [PubMed] [Google Scholar]
- 4.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54(18):1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risk, Prevention Study Collaborative G, Roncaglioni MC, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–1808. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. [DOI] [PubMed] [Google Scholar]
- 7.Group ASC, Bowman L, Mafham M, et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379(16):1540–1550. [DOI] [PubMed] [Google Scholar]
- 8.Investigators OT, Bosch J, Gerstein HC, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–318. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA. 2020;324(22):2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalstad AA, Myhre PL, Laake K, et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients after Myocardial Infarction: A Randomized Controlled Trial. Circulation. 2021;143:528–539. [DOI] [PubMed] [Google Scholar]
- 11.Djousse L, Cook NR, Kim E, et al. Supplementation With Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure. Circulation. 2020;141(9):784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister DA, Read SH, Kerssens J, et al. Incidence of Hospitalization for Heart Failure and Case-Fatality Among 3.25 Million People With and Without Diabetes Mellitus. Circulation. 2018;138(24):2774–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronow WS, Ahn C. Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest. 1999;115(3):867–868. [DOI] [PubMed] [Google Scholar]
- 14.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879–1884. [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Cook NR, Lee IM, et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N Engl J Med. 2019;380(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen PKG, R.D. Cox’s regression model for counting processes: a large sample study. . Ann Stat. 1982;10(4):1100–1120. [Google Scholar]
- 18.Mirhashemi SM, Rahimi F, Soleimani A, Asemi Z. Effects of Omega-3 Fatty Acid Supplementation on Inflammatory Cytokines and Advanced Glycation End Products in Patients With Diabetic Nephropathy: a Randomized Controlled Trial. Iran J Kidney Dis. 2016;10(4):197–204. [PubMed] [Google Scholar]
- 19.Soleimani A, Taghizadeh M, Bahmani F, Badroj N, Asemi Z. Metabolic response to omega-3 fatty acid supplementation in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36(1):79–84. [DOI] [PubMed] [Google Scholar]
- 20.Lam CSP, Chandramouli C, Ahooja V, Verma S. SGLT-2 Inhibitors in Heart Failure: Current Management, Unmet Needs, and Therapeutic Prospects. J Am Heart Assoc. 2019;8(20):e013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. [DOI] [PubMed] [Google Scholar]
- 22.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.