Abstract
In 2015, the American Heart Association (AHA) awarded four-year funding for a Strategically Focused Research Network focused on hypertension (HTN) composed of 4 Centers - Cincinnati Children’s Hospital (CCH), Medical College of Wisconsin (MCW), University of Alabama at Birmingham (UAB), and University of Iowa (UI). Each Center proposed 3 integrated (basic, clinical, and population science) projects around a single area of focus relevant to HTN. Along with scientific progress, AHA put a significant emphasis on training of next generation HTN researchers by sponsoring 3 post-doctoral fellows per Center over 4 years. With the Center projects being spread across the continuum of basic, clinical, and population sciences, post-doctoral fellows were expected to garner experience in various types of research methodologies. AHA also provided a number of leadership development opportunities for fellows and investigators in these Centers. In addition, collaboration was highly encouraged among the Centers (both within and outside the network) with AHA providing multiple opportunities for meeting and expanding associations. The area of focus for CCH Center was HTN and target organ damage in children utilizing ambulatory blood pressure (BP) measurements. The MCW Center focused on epigenetic modifications and their role in pathogenesis of HTN using human and animal studies. The UAB Center’s areas of research were diurnal BP patterns and clock genes. The UI Center evaluated copeptin as a possible early biomarker for preeclampsia and vascular endothelial function during pregnancy. In this review, challenges faced and successes achieved by the investigators of each of the Centers are presented.
Keywords: strategically focused research network, team science, collaboration, hypertension, preeclampsia
Introduction
In 2015, the American Heart Association (AHA) awarded four years of funding for a Strategically Focused Research Network (SFRN) focused on hypertension (HTN). It was the second SFRN to be awarded and the network’s no-cost extension funding ended in 2020. This network was composed of 4 centers - Cincinnati Children’s Hospital (CCH), Medical College of Wisconsin (MCW), University of Alabama at Birmingham (UAB), and University of Iowa (UI) and oversight advisory board. Each Center had proposed 3 integrated/interlinked projects (basic, clinical, and population science projects) around a single area of focus relevant to HTN. All centers were required to have a Center Director along with Principal Investigators (PI) for each of the projects; Center Directors could also function as PIs for one of the projects.
Along with scientific progress, AHA put a significant emphasis on training of next generation HTN researchers. Therefore, each Center had a fellowship Training Director and one post-doctoral fellow (MD or PhD) per year for the first 3 years with 2-year training period for each fellow. Each of the fellows were embedded into one or more research projects of the Center. With the Center projects being spread across the continuum of basic, clinical, and population sciences, post-doctoral fellows were expected to garner experience in methodologies from bench to bedside to community-based research. The AHA also provided a number of leadership development opportunities for fellows and investigators in these Centers. In addition, collaboration was highly encouraged among the Centers (both within and outside network) with AHA providing multiple avenues for meeting and expanding associations. Each Center’s investigators and fellows were also required to present work-in-progress data to investigators from other centers as well as to the oversight committee at least annually throughout the award period, receive feedback, and consider suggestions received.
The area of focus for CCH Center was HTN and target organ damage in children and adolescents utilizing ambulatory blood pressure measurements (ABPM) as a tool to measure blood pressure (BP). The MCW Center focused on epigenetic modifications (DNA methylation changes) and their role in pathogenesis of HTN using human and animal studies. The UAB Center’s areas of research were diurnal BP patterns and clock genes. The UI Center evaluated copeptin as a possible early biomarker for preeclampsia and vascular endothelial function during pregnancy. This review was commissioned to describe the science from research projects proposed by each Center as well as successes and challenges experienced in implementation of proposed projects, fellowship training, and development of collaborations.
Our Experience
Cincinnati Children’s Hospital (CCH) Center
Overall hypotheses and goals:
To examine the concept that hypertensive cardiovascular injury emerges during early stages of primary HTN, 3 projects were designed by the CCH Center (SHIP AHOY study). Nearly 400 adolescents were enrolled at 3 BP thresholds - normal (SBP<75th% percentile), mid-risk (SBP>80th% but <90th%) and high-risk (systolic BP >90th%) categories (Figure 1). Investigators hypothesized that the prevalence of target organ damage (left ventricular mass (LVM), pulse wave velocity, urinary albumin excretion and cognitive function) would be greater at higher levels of BP (population science project). In addition, it was hypothesized that (sustained) 24-hour ambulatory HTN and multiple metabolic syndrome risk factors would predict presence of target organ damage (clinical science project). The third part of the study (basic science) investigated epigenetic changes that influence the development of target organ damage in youth with HTN.
Figure 1:
Schematic describing Cincinnati Children’s Hospital’s hypertension SFRN Center’s SHIP-AHOY Study Investigators hypothesized that the prevalence of target organ damage would be greater at higher levels of BP (left ventricular mass (LVM), pulse wave velocity, urinary albumin excretion and cognitive function) in children. In addition, it was hypothesized that (sustained) 24-hour ambulatory HTN and multiple metabolic syndrome risk factors would predict presence of target organ damage. The third part of the study investigated epigenetic changes that influence the development of target organ damage in youth with HTN.
Successes and Challenges:
Success of the CCH center was rooted in its ability to develop multiple collaborating centers to ensure adequate enrollment since pediatric HTN is not common. This multicenter collaboration was effective due to previous partnerships among the PIs as part of the International Pediatric HTN association (IPHA). Weekly web-based calls ensured close communication between various centers. Center PI’s coordinator had weekly contacts with other site coordinators facilitating recruitment and timely data transfer. Fellowship recruitment was enhanced by a strong pre-existing pediatric cardiology fellowship program resulting in a diverse group of fellows including a pediatric cardiologist, a pediatric nephrologist, and an investigator with expertise in laboratory science (Table 1). A multi-disciplinary group of topic experts (cardiology, nephrology, genetics, psychology, and statistics) provided didactic lectures in the population, clinical, and basic science areas to CCH and other SFRN fellows. SHIP AHOY study included cognitive assessments of teens using face-to-face interviews and questionnaires. These assessments required training of non-psychologists, development of a standardized process for certification, regular quality assurance checks, and follow-ups to specific site examiners when questions arose. The Center was able to ensure quality of cognitive data through these rigorous processes. These methods will form a blueprint for upcoming studies that include cognitive assessments in multicenter studies.
Table 1:
Highlights of trainees in HTN SFRN
| Name | AHA SFRN Fellow Project | Position after AHA SFRN Fellowship |
|---|---|---|
| Cincinnati Children’s Hospital Center | ||
| Brenda Mendizábal, MD | Population: Threshold BP predicting LVH | Assistant Professor, Pediatric Cardiology Children’s Hospital of Pittsburgh University |
| Gilad Hamdani, MD | Clinical: ABPM cut points predicting LVH | Faculty Physician, Pediatric Nephrology, Schneider Children’s Medical Center of Israel |
| Mohamed Arif, PhD | Basic: Epigenetic predictors of LVH | Post-doctoral fellow University of Cincinnati |
| Medical College of Wisconsin Center | ||
| Yingchuan Li, M.D., Ph.D. | Clinical and Population: Methylation sequencing | Faculty, Shanghai Jiao Tong University affiliated the Sixth People’s Hospital, Shanghai, China |
| Louise C. Evans | All projects | Assistant Professor, Department of Surgery, University of Minnesota |
| John Henry Dasinger, Ph.D. | Basic: Performed animal experiments | Post-doctoral fellow Medical College of Georgia |
| Xiaoqing Pan, Ph.D. | All projects: Methylation analyses | Faculty, Shanghai Normal University, Shanghai, China |
| University of Alabama at Birmingham Center | ||
| S. Justin Thomas, PhD | Clinical: Mechanisms of Nocturnal HTN and Non-Dipping BP | Assistant Professor Sleep and Circadian Research Core, Co-Director Department of Psychiatry, University of Alabama at Birmingham |
| John Booth III, PhD | Population: Race and sex differences in nocturnal hypertension. | Epidemiology research consultant |
| Daien Chen, PhD | Basic: Dysregulation of diurnal sodium handling and BP | Biomedical industry |
| University of Iowa Center | ||
| Anand R. Nair, PhD | Basic and Population: Effects of in utero exposure to vasopressin to offspring | Project Scientist Cedars-Sinai Los Angeles, CA |
| Alexandria (Andie) Betz, DO | Population and Clinical: Early pregnancy plasma endothelin-1, arterial stiffness, and mean arterial pressure are predictive of preeclampsia. | Maternal Fetal Medicine Subspecialist Maine Medical Center |
| Guorui (Gary) Deng, PhD | Basic and Population: Hypothalamic mechanisms controlling autonomic and neurohormonal control in preeclampsia. | Postdoctoral Fellow University of Iowa |
CCH was also successful in initiating several new collaborations banking on pre-existing connections with HTN investigators in other Centers – (1) UI Center’s project on pre-eclampsia was the impetus to gather data on maternal pre-eclampsia from subjects enrolled in SHIP AHOY study. Additional blood was collected to assess copeptin levels from the participants. Analyses were performed to determine if maternal pre-eclampsia and/or copeptin levels in teens influenced BP levels or target organ damage. No relationship was discovered between maternal pre-eclampsia and/or copeptin levels in teens with teen BP levels or HTN; (2) CCH also developed collaboration with MCW Center and collected and isolated T-cells using the MCW’s protocol on 45 subjects. However, neither site had funding for analysis to explore the epigenetics of salt sensitivity in youth; and (3) SFRN renewal grant was submitted with investigators from Disparities in Cardiovascular Disease (CVD) SFRN Center at Northwestern University in Chicago to examine the relationship between fibroblast growth factor 23 and LVM in youth. This was not funded but future applications for NIH funding are in preparation.
Main challenges were focused around recruitment. Nearly 50% of new subjects deemed hypertensive at pediatrician’s office and referred for enrollment were normotensive when BP was measured appropriately in the research setting. For this reason, investigators had to add additional recruitment sites. Additional difficulties were faced by CCH Center in developing new and ongoing collaborations. Although collaboration with UI Center proceeded smoothly, the results were negative, and the collaboration did not continue. A collaboration with the UAB Center to develop ambulatory BP monitoring arena further was contemplated but this did not proceed due to time constraints placed on the fellows. Due to funding issues, collaboration with MCW Center could not be pursued further. CCH Center also faced challenges with the basic science project because it had to be delayed until sufficient samples were collected for processing. Rapid changes in the fields of genetics, bioinformatics, and systems-based molecular biology necessitated changes in alteration to original candidate gene-based approach to genome wide gene expression products.
Future Directions for the Center:
Preliminary findings from the epigenetic studies will be used to apply for funding for translational studies including collecting new samples from established cohort, culturing cardiomyocytes from participant inducible pluripotent stem cells, and testing hypotheses in animal studies. CCH also plans to pursue funding to evaluate the relationship between FGF23 and target organ damage.
Medical College of Wisconsin (MCW) Center
Overall hypotheses and goals:
The objective of the MCW Center program was to carry out a systematic investigation of the relevance of genome-wide DNA methylation patterns to HTN. HTN has long been considered a result of interactions between one’s genetic background and environmental factors, including diet and other lifestyle choices1. One way the environment may interact with the genome and influence organismal physiology and disease is through epigenetic modifications, which are molecular changes to the DNA (unrelated to its sequence), that lead to changes in gene expression2, 3. Overall hypothesis was that dietary salt intake, maternal dietary exposures, and other lifestyle factors cause genome-wide changes in DNA methylation (a type of epigenetic modification), which contribute to the development of HTN and can be used as predictive or diagnostic markers of HTN and related diseases (Figure 2). All three projects analyzed DNA methylation at near-genome-wide scale using the technology of reduced representation bisulfite sequencing as described previously4, 5. Basic and clinical science projects used immune cells for methylation analyses.
Figure 2:
Schematic describing Medical College of Wisconsin’s hypertension SFRN Center’s investigators hypothesized that dietary salt intake, maternal dietary exposures, and other lifestyle interactions cause genome-wide changes in DNA methylation (type of epigenetic modification), which contribute to the development of HTN and can be used as predictive or diagnostic markers of HTN and related diseases
Successes and Challenges:
MCW’s SFRN catalyzed the expansion of Team Science by promoting the interaction among basic scientists, clinical investigators, epidemiologists, bioinformatics experts and genomic scientists to design studies and obtain and analyze data. Several published manuscripts were supported by this SFRN with several additional abstracts containing data that we plan to publish in the future6–13. Investigators were able to adhere to the hypotheses proposed with no major changes due to innovative ideas in implementation of the projects. In the basic science project, changes were observed in DNA methylation and gene expression in T lymphocytes (T-cells) of Dahl salt-sensitive (SS) rats fed different diets6, 7, providing new insight into the mechanisms by which immune mechanisms amplify SS HTN and renal damage. The Clinical project started with de novo recruitment of 150 pairs of identical twins in collaboration with Michigan State University Twin Registry (MSUTR). They were phenotyped and sequenced for differential methylation in T-cells among BP concordant and discordant twins (publication pending). In addition, salt-sensitivity was assessed in 50 human subjects using a 2-week 1200 mg low-sodium diet and completed methylation analyses of T-cells (pending publication). As part of population study, a 10-year follow-up database of 1000 African Americans was created and is the basis of multiple ongoing observations. Whole blood DNA methylation sequencing of ~500 subjects was completed. It was observed that stored DNA samples (as long as 20 years at 4° C) are suitable for comparative studies of DNA methylation8. In addition, it was observed that in African Americans, 24-hour BP monitoring provides limited added value as a predictor of cardiovascular/renal disease events10. SFRN enabled post-doctoral fellows to participate in multiple types of research and learn how basic and clinical studies are conducted and strengths and limitations of each (Table 1). In addition to specific successes in each of the projects, MCW Center also made critical methodological advances in the realm of methylation sequencing and analyses14.
MCW Center faced challenges in the arena of subject recruitment as expected with any clinical study with complex inclusion criteria and cost overruns. It was realized that despite having access to a database of twins (from Michigan State University), recruitment was still difficult and costly. The award duration was short (4 years), given the scope of the projects and the delays in start of de novo projects after acquisition of funding. Data analyses took longer than expected as methylation data analysis is new and required innovative approaches, however, MCW Center’s strength was a strong bioinformatics and statistics team which allowed robust data analyses. It was challenging to recruit clinical fellows to the post-doctoral fellowship, however, in the end MCW Center was able to be successful in training very qualified fellows that provided much needed complimentary expertise.
Future Directions for the Center:
Of equal or perhaps greater value than scientific advances, the SFRN facilitated continued collaboration between basic and clinical scientists and opened up translational opportunities that will be fruitful in future basic science projects led to an important new area to pursue, the microbiome. Initial work in that field has been presented in abstract form (FASEB Journal 2019;33, Supplement 1, abstract 866.9) and has presently been submitted for publication. Clinical Science project’s success in assessing salt-sensitivity established a track record and will be basis of new project that will be submitted for future funding. Investigators believe that some of the best work from the SFRN is still to come as the work from the clinical and population projects come to completion. It is hoped that correlates can be found between the data in the animal models and the humans and interrogate those genes to understand the mechanistic basis of human disease.
University of Alabama at Birmingham (UAB) Center
Overall hypotheses and goals:
The University of Alabama at Birmingham (UAB) SFRN HTN Center was designed to generate new evidence on the role of diurnal BP on CVD risk. Investigators proposed that an abnormal diurnal BP pattern (i.e., nocturnal HTN and a non-dipping BP pattern) is an under-detected “silent” CVD risk factor, its underlying mechanisms can be identified through basic and clinical research, and these patterns can be diagnosed and treated cost-effectively at the population-level. Therefore, the exploration of the etiologies and complications of abnormal diurnal BP patterns was the central theme of UAB Center (Figure 3). Their integrated program of research included 1) a population science project that generated data on the burden of nocturnal HTN and non-dipping BP, 2) a clinical science project of a randomized crossover study designed to determine if dietary sodium restriction reduces nocturnal BP and restores normal BP dipping through improvements in sleep disordered breathing, and 3) a series of basic science studies that further examined novel salt-dependent mechanisms promoting renal microvascular dysfunction, which in turn leads to dysregulation of diurnal sodium handling and BP. Three outstanding early-stage investigators were mentored who gained trans-disciplinary population, clinical and basic science training (Table 1).
Figure 3:

Schematic describing the University of Alabama at Birmingham’s hypertension SFRN Center investigators research on racial differences in diurnal blood pressure, mechanisms underlying abnormal diurnal blood pressure and salt-sensitivity and circadian clock gene expression.
Successes and Challenges:
The successes of the UAB SFRN resulted from the long-standing collaborations between basic, clinical and population HTN research at UAB. When the SFRN was initiated in 2015, there was a strong foundation including a T32 that is currently in its 40th year of continuous funding and the UAB Vascular Biology and HTN symposium, which has been held annually since 1989. In 2019 and 2020, this symposium received funding from NHLBI through an R13 grant. The population science project published findings on (1) the prevalence of out-of-office BP phenotypes according to the 2017 American College of Cardiology/American Heart Association guideline15, (2) the association between nocturnal BP and CVD risk among African Americans16, (3) the association of health behaviors with nocturnal HTN17 and (4) the estimated prevalence of nocturnal HTN among US adults18. The clinical science project had 53 participants successfully complete all phases of their dietary intervention crossover study by the end of 2019. Preliminary analyses of 24-hour urinary sodium excretion demonstrated that participants were adherent to their assigned diets (6 g versus 1.5 g of sodium per day) without evidence of any carryover effects. Given these positive results, analyses are being conducted to determine the effect of dietary sodium intake on severity of sleep apnea and diurnal BP levels. The basic science project has focused on delineating novel high salt-dependent pathways (histone deacetylases and the E3 ubiquitin protein ligase, Rfwd2) that are regulated by clock genes. The clinical and basic science teams collaborated to publish a study defining a novel method to integrate circadian measurements with peripheral clock genes in humans and a review titled “Circadian Regulation of BP: of Mice and Men”19. Furthermore, the clinical and population science teams collaborated to publish a manuscript on the association of sleep characteristics with nocturnal HTN and non-dipping BP20. In addition, the UAB SFRN collaborated with the MCW SFRN to publish data on clinic and ambulatory BP among African Americans in the Jackson Heart Study21.
Despite the successes of the UAB SFRN, many challenges were experienced over the funding period. Only 835 participants completed ambulatory BP monitoring as part of the population science project. However, investigators were able to supplement these data with ambulatory BP monitoring recordings from other studies. The clinical science project was delayed due to staffing issues. However, during no-cost extension period, they were able to successfully complete the study and results are currently being prepared for publication. The basic science project also had staffing issues that resolved with collaborations within the clinical and population teams to assist in data analyses. Finally, personnel turn-over including an investigator who retired and another who took a leadership position at another institution resulted in minor delays. Fortunately, these investigators have remained engaged with the Center and the mentoring of the fellows.
Future Directions for the Center:
The UAB center was re-funded by the AHA for a study focused on whether reduced sodium intake is associated with higher levels of short-chain fatty acids (an endogenous histone deacetylase inhibitor), and lower levels of oxidative stress. Additionally, the Center has received funding as a University-Wide Interdisciplinary Research Center and the HTN Research Center was designated as a center by the University of Alabama board of trustees. Dr. S. Justin Thomas, the first UAB SFRN fellow and now a faculty member is a founding co-director of the UAB Sleep and Circadian Research Core. This core supports research focused on time-restricted feeding, timing of sodium intake and sleep patterns as they relate to BP and other cardiometabolic risk factors. Ongoing NIH and AHA funded studies for the UAB SFRN investigators is focused on time-restricted feeding, timing of sodium intake, testing home BP measurement devices to assess BP during sleep, an ambulatory BP monitoring pooling project, home BP monitoring in older adults, and central and peripheral mechanisms underlying non-dipping BP in black adults.
University of Iowa (UI) Center
Overall hypotheses and goals:
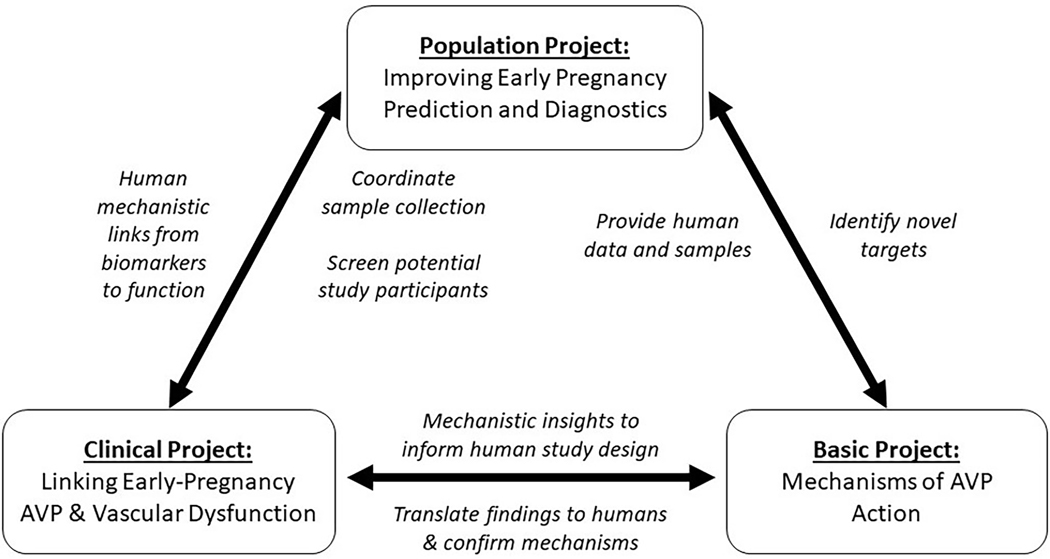
The University of Iowa SFRN HTN Center was designed to investigate the involvement of the neurohypophyseal hormone, arginine vasopressin (AVP) and its metabolic products in the prediction, diagnosis, modeling, and pathogenesis of the prevalent pregnancy-related hypertensive disorder, preeclampsia which is associated with immediate and long-term maternal-fetal morbidity and mortality (Figure 4).22–27 It was previously demonstrated that AVP release is robustly predictive of the development of human preeclampsia and successfully models the disease in mice.28, 29 These early findings initiated the center’s future studies in both mice and humans.
Figure 4:
Schematic describing the University of Iowa’s Hypertension Strategically Focused Research Network (SFRN) Center’s synergy over all projects and hypothesis on the early pregnancy predictive power and etiologic mechanisms of arginine vasopressin (AVP) in the development of preeclampsia.
Successes and Challenges:
The successes of the Iowa Center predominantly arose from existing, organic, and close collaborations of the center director and the individual study PIs. Goal-oriented project focus and group structure, multiple partially overlapping regularly occurring (i.e., weekly) cross-project group meetings, preexisting shared resources (i.e., pathology & phenotyping cores, biobanks, and clinical research support teams), and complementary cross-disciplinary areas of expertise helped accelerate the projects. A key resource that propelled the translational nature of the basic, clinical, and population studies was the Iowa Maternal Fetal Tissue Bank (MFTB) as the central machinery in which 1) pregnant women and their children are enrolled, 2) biomaterials are collected, and 3) and clinical data is curated to improve our knowledge of pregnancy and its effects on maternal and child health30. This machinery has developed a national cohort of perinatal clinics and practices which are a rich resource of clinical data and biological samples. Early mechanistic and biomarker work in plasma and urine from this cohort has resulted in multiple patents that can be easily be translated to clinical practice31, 32. This work resulted in many new mechanistic insights in the pleotropic vascular and immune role of AVP in the development of preeclampsia28, 33, 34. Additional unanticipated extensions of this work have implicated Regulator of G-protein Signaling-2 (RGS2; a regulator of common immunovascular hormones such as angiotensin, endothelin-1, and AVP) in preeclampsia and its vascular dysfunction33–36. In addition, a potential novel hemodynamic biomarker, short-term beat-to-beat BP variability, that is prospectively associated with the development of preeclampsia and elevated arterial stiffness in the first trimester was discovered. Further, this study also highlighted that vascular dysfunction can easily be detected as early as the first trimester long before the development of the other clinical signs and symptoms.37
While the UI Center has established many successful collaborations, a major challenge to the progression of this translational work at the national level is the delay inherent in building collaborative sites with no research architecture. Using the lessons learned from building the system associated within this SFRN, the team has been successful in obtaining NIH funding to expand this cohort to build research architecture using tele-research technologies in research-void environments.
Future Directions for the Center:
The Iowa center’s current focus is upon exploring the mechanisms of preeclampsia that lead to future CVD, cognitive impairment in the mother and offspring, and further immunologic mechanisms underpinning this disease. AHA has funded an ongoing collaboration between elements of the now-former centers at UI and Magee Women’s Research Institute to evaluate novel RGS2-related preeclampsia immunovascular mechanisms leading to future HTN, including masked HTN identified with home BP monitoring, and CVD. Ongoing NIH R01 and AHA Innovative Project and Established Investigator awards to individual members of the team support these continued efforts in identifying novel diagnostic and therapeutic tools for preeclampsia and its downstream cardiovascular morbidity and mortality.
The Strengths and Future Opportunities for Strategically Focused Research Networks
SFRNs are an unprecedented effort by AHA to bring team science to the forefront to solve big challenging healthcare problems that require multiple disciplines to work together. By mandating that each center proposes projects spanning basic, clinical, and population sciences, it facilitated interdisciplinary and interprofessional investigator teams to collaborate. Based on experiences of the PIs, intra-Center collaborations were highly successful among the PIs and Center Directors. However, inter-Center collaborations were faced with difficulties due to lack of specific funding to develop and continue projects initiated. While there are examples of AHA-funded collaborative efforts in the SFRN-HTN like the Iowa (HTN) and Magee (Go Red For Women) AHA Strategic Collaborative Grant, this mechanism was meant for all existing SFRNs (Table 2). SFRN related projects have been productive as seen by the addition of significant publications to the literature that cover a wide variety of study types including epidemiologic, clinical, translational, and basic science studies (Table 2). In addition, the duration of the award was just four years and was felt to be too short for many of the projects to be completed and analyzed. It was difficult to continue many promising projects due to lack of opportunities to renew the funding.
Table 2:
Intra-SFRN/Inter-SFRN Productivity
| HTN SFRN Center | Collaborating Institution/Center | Project | Funding |
|---|---|---|---|
| Iowa HTN SFRN | Magee Go Red For Women SFRN | Mechanisms for Early and Late Postpartum Hypertension in Human Preeclampsia | AHA Strategic Collaborative Grant 18SCG34350001 |
| Iowa HTN SFRN | Vasopressin and Preeclampsia: Early Mechanisms for Prevention | NICHD R01 HD089940-01 | |
| Iowa HTN SFRN | Treatment of Preeclampsia Using Cellular Immunotherapy | AHA Innovative Project Award 19IPL134760288 | |
| Iowa HTN SFRN | Iowa ICTS + PREDICTV Study Cohort Institutions | iELEVATE (Improving womEn’s and chiLdren’s hEalth Via biobanking and elecTronic rEgistry) | NCATS 3 UL1 TR002537-02SI |
| UAB HTN SFRN | Duke University | Improving the Detection of Masked Hypertension: Analysis of Pooled Population- and Community-Based Studies | NHLBI R01HL144773-01 |
| UAB HTN SFRN | Columbia University | Evaluating novel approaches for estimating awake and sleep blood pressure | NHLBI R01HL139716 |
| UAB HTN SFRN | Central and Peripheral Circadian Mechanisms Underlying Non-Dipping Blood Pressure in Blacks | AHA 19CDA34660139 | |
| Publications | |||
| Muntner P, Becker RC, Calhoun D, Chen D, Cowley AW, Flynn JT, Grobe JL, Kidambi S, Kotchen TA, Lackland D, Leslie KK, Li Y, Liang M, Lloyd A, Mattson DL, Mendizabal B, Mitsnefes M, Nair A, Pierce GL, Pollock JS, Safford MM, Santillan MK, Sigmund CD, Thomas SJ, Urbina EM. Introduction to the AHA hypertension strategically focused research network. Hypertension 2016;67:674-80. | |||
| Mendizábal B, Urbina EM, Becker R, Daniels S, Falkner BE, Hamdani G, Hanevold C, Hooper SR, Ingelfinger J, Lande M, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels J, Flynn JT. Rationale, Design and Methods for the SHIP AHOY Study. Hypertension 2019;72:625-631. | |||
| Hamdani G, Flynn JT, Becker RC, Daniels SR, Falkner B, Hanevold CD, Ingelfinger JR, Lande MB, Martin LJ, Meyers KE, Mitsnefes M, Rosner B, Samuels JA, Urbina EM. Prediction of Ambulatory Hypertension Based on Clinic Blood Pressure percentile in adolescents. Hypertension 2018;72:955-961. | |||
| Hamdani G, Flynn JT, Daniels S, Falkner B, Hanevold C, Inglefinger J, Lande MB, Martin LJ, Meyers KE, Mitsnefes M, Rosner B, Samuels J, Urbina EM. Ambulatory Blood Pressure Monitoring Tolerability and Blood Pressure Status in Adolescents. BP Monitoring 2019;24:12-17 | |||
| Arif M, Sadayappan S, Becker RC, Martin LJ, Urbina EM. Epigenetic Modification: A Regulatory Mechanism in Essential Hypertension. Hypertension Res 2019;42:1099-1113. | |||
| Urbina EM, Mendizábal B, Becker RC, Daniels S, Falkner BE, Hamdani G, Hanevold C, Hooper SR, Ingelfinger JR, Lande M, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels J, Flynn JT. Association of Blood Pressure Level With Left Ventricular Mass in Adolescents. Hypertension 2019;74:590-596. | |||
| Tran AH, Flynn JT, Becker RC, Daniels SR, Falkner BE, Ferguson M, Hanevold CD, Hooper SR, Ingelfinger JR, Lande MC, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels JA, Urbina EM, Subclinical systolic and diastolic dysfunction is evident in youth with elevated blood pressure. Hypertension 2020;75:1551-1556. | |||
| Poudel B, Booth JN 3rd, Sakhuja S, Moran AE, Schwartz JE, Lloyd-Jones DM, Lewis CE, Shikany JM, Shimbo D, Muntner P. Prevalence of ambulatory blood pressure phenotypes using the 2017 American College of Cardiology/ American Heart Association blood pressure guideline thresholds: data from the Coronary Artery Risk Development in Young Adults study. J Hypertens. 2019 Jul;37(7):1401-1410. | |||
| Booth JN 3rd, Hubbard D, Sakhuja S, Yano Y, Whelton PK, Wright JT Jr, Shimbo D, Muntner P. Proportion of US Adults Recommended Out-of-Clinic Blood Pressure Monitoring According to the 2017 Hypertension Clinical Practice Guidelines. Hypertension. 2019 Aug;74(2):399-406. Epub 2019 Jun 24. | |||
| Pugliese DN, Booth JN 3rd, Deng L, Anstey DE, Bello NA, Jaeger BC, Shikany JM, Lloyd-Jones D, Lewis CE, Schwartz JE, Muntner P, Shimbo D. Sex differences in masked hypertension: the Coronary Artery Risk Development in Young Adults study. J Hypertens. 2019 Dec;37(12):2380-2388. doi: 10.1097/HJH.0000000000002175. | |||
| Bello NA, Jaeger BC, Booth JN 3rd, Abdalla M, Anstey DE, Pugliese DN, Lewis CE, Gidding SS, Lloyd-Jones D, Shah SJ, Schwartz JE, Shikany JM, Muntner P, Shimbo D. Associations of awake and asleep blood presssure and blood pressure dipping with abnormalities of cardiac structure: the Coronary Artery Risk Development in Young Adults study. J Hypertens. 2020 Jan;38(1):102-110. doi: 10.1097/HJH.0000000000002221. | |||
| Jaeger BC, Booth JN 3rd, Butler M, Edwards LJ, Lewis CE, Lloyd-Jones DM, Sakhuja S, Schwartz JE, Shikany JM, Shimbo D, Yano Y, Muntner P. Development of Predictive Equations for Nocturnal Hypertension and Nondipping Systolic Blood Pressure. J Am Heart Assoc. 2020 Jan 21;9(2):e013696. doi: 10.1161/JAHA.119.013696. Epub 2020 Jan 9. | |||
| Thomas SJ, Booth JN 3rd, Jaeger BC, Hubbard D, Sakhuja S, Abdalla M, Lloyd-Jones DM, Buysse DJ, Lewis CE, Shikany JM, Schwartz JE, Shimbo D, Calhoun D, Muntner P, Carnethon MR. Association of Sleep Characteristics With Nocturnal Hypertension and Nondipping Blood Pressure in the CARDIA Study. J Am Heart Assoc. 2020 Apr 7;9(7):e015062. doi: 10.1161/JAHA.119.015062. | |||
| Sakhuja S, Booth JN III, Antsey DE, Jaeger BC, Lewis CE, Lloyd-Jones DM, Schwartz JE, Shimbo D, Shikany JM, Sims M, Muntner P. Using predicted atherosclerotic cardiovascular disease risk for discrimination of awake or nocturnal hypertension. American Journal of Hypertension. 2020 Nov 3;33(11):10111020 | |||
| Li S, Schwartz JE, Shimbo D, Muntner P, Shikany JM, Booth JN III, Allen NB, Jaeger BC, Bress AP, King JB, Clark D, Butler KR, Correa A, Moran AE, Bellows BK, Zhang Y, Estimated prevalence of asleep and masked hypertension in US adults. Submitted: JAMA Cardiology 2020 Oct 28: e205212. doi: 10.1001/jamacardio.2020.5212. | |||
| Nuckols VR, Holwerda SW, Luehrs RE, DuBose LE, Stroud AK, Brandt D, Betz AM, Fiedorowicz JG, Scroggins SM, Santillan DA, Grobe JL, Sigmund CD, Santillan MK, Pierce GL. Beat-to-Beat Blood Pressure Variability in the First Trimester Is Associated With the Development of Preeclampsia in a Prospective Cohort: Relation With Aortic Stiffness. Hypertension. 2020 Dec;76(6):1800-1807. doi: 10.1161/HYPERTENSIONAHA.120.15019. Epub 2020 Sep 21. PMID: 32951467 | |||
| Gumusoglu SB, Chilukuri ASS, Santillan DA, Santillan MK, Stevens HE. Neurodevelopmental Outcomes of Prenatal Preeclampsia Exposure. Trends Neurosci. 2020 Apr;43(4):253-268. doi:10.1016/j.tins.2020.02.003. Epub 2020 Mar 6. PMID: 32209456 | |||
| Gumusoglu SB, Chilukuri ASS, Hing BWQ, Scroggins SM, Kundu S, Sandgren JA, Santillan MK, Santillan DA, Grobe JL, Stevens HE. Altered offspring neurodevelopment in an arginine vasopressin preeclampsia model. Transl Psychiatry. 2021 Jan 28;11(1):79. doi: 10.1038/s41398-021-01205-0. PMID: 33510137 | |||
| Perschbacher KJ, Deng G, Sandgren JA, Walsh JW, Witcher PC, Sapouckey SA, Owens CE, Zhang SY, Scroggins SM, Pearson NA, Devor EJ, Sebag JA, Pierce GL, Fisher RA, Kwitek AE, Santillan DA, Gibson-Corley KN, Sigmund CD, Santillan MK, Grobe JL. Reduced mRNA Expression of RGS2 (Regulator of G Protein Signaling-2) in the Placenta Is Associated With Human Preeclampsia and Sufficient to Cause Features of the Disorder in Mice. Hypertension. 2020 Feb;75(2):569-579. doi: 10.1161/HYPERTENSIONAHA.119.14056. | |||
| Nair AR, Silva SD Jr, Agbor LN, Wu J, Nakagawa P, Mukohda M, Lu KT, Sandgren JA, Pierce GL, Santillan MK, Grobe JL, Sigmund CD. Endothelial PPARγ (Peroxisome Proliferator-Activated Receptor-γ) Protects From Angiotensin II-Induced Endothelial Dysfunction in Adult Offspring Born From Pregnancies Complicated by Hypertension. Hypertension. 2019 Jul;74(1):173-183. doi: 10.1161/HYPERTENSIONAHA.119.13101. | |||
| Maric-Bilkan C, Abrahams VM, Arteaga SS, Bourjeily G, Conrad KP, Catov JM, Costantine MM, Cox B, Garovic V, George EM, Gernand AD, Jeyabalan A, Karumanchi SA, Laposky AD, Miodovnik M, Mitchell M, Pemberton VL, Reddy UM, Santillan MK, Tsigas E, Thornburg KLR, Ward K, Myatt L, Roberts JM. Research Recommendations From the National Institutes of Health Workshop on Predicting, Preventing, and Treating Preeclampsia. Hypertension. 2019 Apr;73(4):757-766. doi: 10.1161/HYPERTENSIONAHA.118.11644. PMID: 30686084 | |||
| Sandgren JA, Deng G, Linggonegoro DW, Scroggins SM, Perschbacher KJ, Nair AR, Nishimura TE, Zhang SY, Agbor LN, Wu J, Keen HL, Naber MC, Pearson NA, Zimmerman KA, Weiss RM, Bowdler NC, Usachev YM, Santillan DA, Potthoff MJ, Pierce GL, Gibson-Corley KN, Sigmund CD, Santillan MK, Grobe JL. Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight. 2018 Oct 4;3(19):e99403. doi: 10.1172/jci.insight.99403. PMID: 30282823 | |||
| Perschbacher KJ, Deng G, Fisher RA, Gibson-Corley KN, Santillan MK, Grobe JL.Regulators of G protein signaling in cardiovascular function during pregnancy. Physiol Genomics. 2018 Aug 1;50(8):590-604. doi: 10.1152/physiolgenomics.00037.2018. Epub 2018 Apr 27. PMID: 29702036 | |||
| Scroggins SM, Santillan DA, Lund JM, Sandgren JA, Krotz LK, Hamilton WS, Devor EJ, Davis HA, Pierce GL, Gibson-Corley KN, Sigmund CD, Grobe JL, Santillan MK. Elevated vasopressin in pregnant mice induces T-helper subset alterations consistent with human preeclampsia. Clin Sci (Lond). 2018 Feb 14;132(3):419-436. doi: 10.1042/CS20171059. Print 2018 Feb 14. PMID: 29371289 | |||
| Sandgren JA, Linggonegoro DW, Zhang SY, Sapouckey SA, Claflin KE, Pearson NA, Leidinger MR, Pierce GL, Santillan MK, Gibson-Corley KN, Sigmund CD, Grobe JL. Angiotensin AT1A receptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to osmotic control of vasopressin. Am J Physiol Regul Integr Comp Physiol. 2018 Jun 1;314(6): R770-R780. doi: 10.1152/ajpregu.00435.2017. Epub 2018 Jan 24. PMID: 29364700 | |||
| Sandgren JA, Santillan MK, Grobe JL. Breaking a Mother’s Heart: Circulating Antiangiogenic Factors and Hypertension During Pregnancy Correlate with Specific Cardiac Dysfunctions. Hypertension. 2016 Jun;67(6):1119-20. doi: 10.1161/HYPERTENSIONAHA.116.07380. Epub 2016 Apr 25. PMID: 27113050. | |||
| Abais-Battad JM, Dasinger JH, Fehrenbach DJ, Mattson DL. Novel Adaptive and Innate Immunity Targets in Hypertension. Pharmacol Res 120:109-115, 2017. | |||
| Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null Dahl SS rats reveal adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Physiol 2018 Jul 1;315(1): R28-R35. | |||
| Abais-Battad JM and Mattson DL. The influence of dietary protein on Dahl Salt-Sensitive hypertension: a potential role for gut microbiota. Am J Physiol 315:R907-R914, 2018. | |||
| Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Alsheikh A, and Mattson DL. Parental dietary protein source and the role of CMKLR1 in determining the severity of Dahl SS hypertension. Hypertension 73:440-448, 2019. | |||
| Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, Roberts M, Cowley AW Jr, Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Dietary Effects on Dahl Salt-Sensitive Hypertension, Renal Damage, and the T Lymphocyte Transcriptome. Hypertension 2019; 74:854-863. | |||
| Dasinger JH, Alsheikh AJ, Abais-Battad JM, Pan X, Fehrenbach DJ, Lund H, Roberts M, Cowley AW Jr, Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Epigenetic Modifications in T Cells The Role of DNA Methylation in Salt-Sensitive Hypertension 2020; 75:372-382 | |||
| Kidambi S, Wang T, Chelius T, Nunuk I, Agarwal P, Laud P, Mattson D, Cowley A, Liang M, Kotchen T. Twenty-four-hour versus clinic blood pressure levels as predictors of long-term cardiovascular and renal disease outcomes among African Americans. Scientific Reports 2020; 10: Article number: 11685 | |||
| Kotchen TA, Cowley AW Jr, Liang M. Ushering Hypertension into a New Era of Precision Medicine. JAMA 315: 343-4, 2016. | |||
| Li Y, Pan X, Roberts ML, Liu P, Kotchen TA, Cowley AW Jr., Mattson DL, Liu Y, Liang M, Kidambi S. Stability of global methylation profiles of whole blood and extracted DNA under different storage durations and conditions. Epigenomics. 2018; 10 :797-811. | |||
| Liang M. Epigenetic Mechanisms and Hypertension. Hypertension. 2018 Dec;72(6):1244-1254. | |||
| Liu Y, Liang M. Functional role of epigenetic regulation in the development of prenatal programmed hypertension. Kidney Int 2019; 96: 10-12. | |||
| Liu P, Liu Y, Liu H, Pan X, Li Y, Usa K, Mishra MK, Nie J, Liang M. Role of DNA De Novo (De)Methylation in the Kidney in Salt-Induced Hypertension. Hypertension. 2018 Nov;72(5):1160-1171. | |||
| Mattson DL. Immune Mechanisms of Salt-Sensitive Hypertension and Renal End-Organ Damage. Nature Rev Nephrol 15, 290-300, 2019. | |||
| Mattson DL, Liang M. From GWAS to functional genomics-based precision medicine. Nature Rev Nephrol. 2017 Apr;13(4):195-196. | |||
| Sun X, Han Y, Zhou L, Chen E, Lu B, Liu Y, Pan X, Cowley AW Jr, Liang M, Wu Q, Lu Y, Liu P. A comprehensive evaluation of alignment software for reduced representation bisulfite sequencing data. Bioinformatics. 2018; 34:2715-2723 | |||
| Thomas SJ, Booth JN 3rd, Bromfield SG, Seals SR, Spruill TM, Ogedegbe G, Kidambi S, Shimbo D, Calhoun D, Muntner P. Clinic and ambulatory blood pressure in a population-based sample of African Americans: the Jackson Heart Study. J Am Soc Hypertens. 2017; 11:204-212. | |||
| Wade B, JM Abais-Battad, and DL Mattson. Role of immune cells in salt-sensitive hypertension and renal injury. Current Opinions in Nephrology and Hypertension. 25:22-27, 2016. | |||
| Williams AM, Liu Y, Regner KR, Jotterand F, Liu P, Liang M. Artificial Intelligence, Physiological Genomics, and Precision Medicine. Physiol Genomics. 2018 Apr 1;50(4):237-243. | |||
Development of next-generation hypertension researchers was a key priority for AHA. Overall, this objective was successful with training of 12 outstanding physicians and scientists interested in the field of HTN. Plenty of mentorship opportunities were facilitated by the AHA via SFRN-specific programs at annual HTN meetings as well as leadership academy meetings. These programs were important strengths of the program since it brought together the trainees at national meetings and provided special forums to network and present their work. Although trainees were always encouraged to attend the national meetings, they are often lost in a sea of more established investigators and do not have the opportunity to meet and get to know one another. Smaller settings organized by the AHA for the Center investigators and Fellows was of immense use. Some parts of the SFRN program for fellows such as grant and manuscript writing, and ethics workshops were of less importance since there was much redundancy with institutional programs.
To improve future SFRNs, we recommend the following specific steps: 1. More robust opportunities be provided for continuation of the work that was initiated in the SFRNs via additional renewal mechanisms. 2. Separate funding to promote collaborations between SFRN centers might improve successes of inter-center collaborations. As an example, one SFRN, Go Red For Women, had additional research funding available for promoting collaborative projects among the Go Red For Women SFRN centers and perhaps this model could be followed for future SFRNs. 3. Specific future funding mechanisms for SFRN trainees could encourage trainees to remain in science and pursue their own future research, this would be particularly helpful to keep clinical trainees in science.
The SFRN-HTN represents a success for the innovative comprehensive multicenter network approach for themed research by facilitating investigators to maximize collaboration and interdisciplinary work. Further development of the SFRN mechanism with more resources will allow AHA encourage and own the advancement and innovation of cardiovascular disease research through this innovative program.
Acknowledgements:
The authors would like to thank the American Heart Association for this fruitful opportunity to enhance collaborative efforts to diversify and strengthen science. The authors would also like to thank the patients affected with cardiovascular disease such as hypertension and preeclampsia for inspiring the work we do.
Sources of Funding:
All the SFRNs were funded by the American Heart Association (AHA)- University of Cincinnati – 15SFRN23680000; Medical College of Wisconsin - 15SFRN23910002; University of Alabama at Birmingham - 15SFRN2390002; University of Iowa −15SFRN23480000.
Disclosures:
The authors report grants from the NIH (MKS, JLG, CDS) and AHA (MKS, RCB, JTF, JLG, KKL,KEM, EMU, SK) during the conduct of the study. KEM reports from TRAVERE and REATA outside the submitted work. CDS reports personal fees from Ionis Pharmaceuticals outside the submitted work. MKS and JLG hold a patent to United States Patent #9,937,182 issued and a patent to European Patent #2954324 issued.
References
- 1.Johnson RJ, Feig DI, Nakagawa T, Sanchez-Lozada LG, Rodriguez-Iturbe B. Pathogenesis of essential hypertension: Historical paradigms and modern insights. J Hypertens. 2008;26:381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang M. Epigenetic mechanisms and hypertension. Hypertension. 2018;72:1244–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotchen TA, Cowley AW Jr., Liang M. Ushering hypertension into a new era of precision medicine. JAMA. 2016;315:343–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Liu P, Yang C, Cowley AW Jr., Liang M. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in dahl s rats: Effect of salt and genomic sequence. Hypertension. 2014;63:827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P, Liu Y, Liu H, Pan X, Li Y, Usa K, Mishra MK, Nie J, Liang M. Role of DNA de novo (de)methylation in the kidney in salt-induced hypertension. Hypertension. 2018;72:1160–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, Roberts ML, Kriegel AJ, Cowley AW Jr., Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Dietary effects on dahl salt-sensitive hypertension, renal damage, and the t lymphocyte transcriptome. Hypertension. 2019;74:854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasinger JH, Alsheikh AJ, Abais-Battad JM, Pan X, Fehrenbach DJ, Lund H, Roberts ML, Cowley AW Jr., Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Epigenetic modifications in t cells: The role of DNA methylation in salt-sensitive hypertension. Hypertension. 2020;75:372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Pan X, Roberts ML, Liu P, Kotchen TA, Cowley AW Jr., Mattson DL, Liu Y, Liang M, Kidambi S. Stability of global methylation profiles of whole blood and extracted DNA under different storage durations and conditions. Epigenomics. 2018;10:797–811 [DOI] [PubMed] [Google Scholar]
- 9.Muntner P, Becker RC, Calhoun D, Chen D, Cowley AW Jr., Flynn JT, Grobe JL, Kidambi S, Kotchen TA, Lackland DT, Leslie KK, Li Y, Liang M, Lloyd A, Mattson DL, Mendizabal B, Mitsnefes M, Nair A, Pierce GL, Pollock JS, Safford MM, Santillan MK, Sigmund CD, Thomas SJ, Urbina EM. Introduction to the american heart association’s hypertension strategically focused research network. Hypertension. 2016;67:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidambi S, Wang T, Chelius T, Nunuk I, Agarwal P, Laud P, Mattson D, Cowley AW Jr., Liang M, Kotchen T. Twenty-four-hour versus clinic blood pressure levels as predictors of long-term cardiovascular and renal disease outcomes among african americans. Sci Rep. 2020;10:11685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null dahl ss rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol. 2018;315:R28-R35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abais-Battad JM, Mattson DL. Influence of dietary protein on dahl salt-sensitive hypertension: A potential role for gut microbiota. Am J Physiol Regul Integr Comp Physiol. 2018;315:R907-R914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Alsheikh AJ, Mattson DL. Parental dietary protein source and the role of cmklr1 in determining the severity of dahl salt-sensitive hypertension. Hypertension. 2019;73:440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Han Y, Zhou L, Chen E, Lu B, Liu Y, Pan X, Cowley AW Jr., Liang M, Wu Q, Lu Y, Liu P. A comprehensive evaluation of alignment software for reduced representation bisulfite sequencing data. Bioinformatics. 2018;34:2715–2723 [DOI] [PubMed] [Google Scholar]
- 15.Poudel B, Booth JN 3rd, Sakhuja S, Moran AE, Schwartz JE, Lloyd-Jones DM, Lewis CE, Shikany JM, Shimbo D, Muntner P. Prevalence of ambulatory blood pressure phenotypes using the 2017 american college of cardiology/american heart association blood pressure guideline thresholds: Data from the coronary artery risk development in young adults study. J Hypertens. 2019;37:1401–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano Y, Tanner RM, Sakhuja S, Jaeger BC, Booth JN 3rd, Abdalla M, Pugliese D, Seals SR, Ogedegbe G, Jones DW, Muntner P, Shimbo D. Association of daytime and nighttime blood pressure with cardiovascular disease events among african american individuals. JAMA Cardiol. 2019;4:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakhuja S, Booth JN, Lloyd-Jones DM, Lewis CE, Thomas SJ, Schwartz JE, Shimbo D, Shikany JM, Sims M, Yano Y, Muntner P. Health behaviors, nocturnal hypertension, and non-dipping blood pressure: The coronary artery risk development in young adults and jackson heart study. Am J Hypertens. 2019;32:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Schwartz JE, Shimbo D, Muntner P, Shikany JM, Booth JN 3rd, Allen NB, Jaeger BC, Bress AP, King JB, Clark D 3rd, Butler KR, Correa A, Moran AE, Bellows BK, Zhang Y. Estimated prevalence of masked asleep hypertension in us adults. JAMA Cardiol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhoads MK, Balagee V, Thomas SJ. Circadian regulation of blood pressure: Of mice and men. Curr Hypertens Rep. 2020;22:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas SJ, Booth JN 3rd, Jaeger BC, Hubbard D, Sakhuja S, Abdalla M, Lloyd-Jones DM, Buysse DJ, Lewis CE, Shikany JM, Schwartz JE, Shimbo D, Calhoun D, Muntner P, Carnethon MR. Association of sleep characteristics with nocturnal hypertension and nondipping blood pressure in the cardia study. J Am Heart Assoc. 2020;9:e015062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas SJ, Booth JN 3rd, Bromfield SG, Seals SR, Spruill TM, Ogedegbe G, Kidambi S, Shimbo D, Calhoun D, Muntner P. Clinic and ambulatory blood pressure in a population-based sample of african americans: The jackson heart study. J Am Soc Hypertens. 2017;11:204–212 e205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garovic VD, Hayman SR. Hypertension in pregnancy: An emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–622 [DOI] [PubMed] [Google Scholar]
- 23.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951 [DOI] [PubMed] [Google Scholar]
- 24.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstetrics and gynecology. 2009;114:961–970 [DOI] [PubMed] [Google Scholar]
- 25.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The helsinki birth cohort study. Stroke; a journal of cerebral circulation. 2009;40:1176–1180 [DOI] [PubMed] [Google Scholar]
- 26.Wu CS, Sun Y, Vestergaard M, Christensen J, Ness RB, Haggerty CL, Olsen J. Preeclampsia and risk for epilepsy in offspring. Pediatrics. 2008;122:1072–1078 [DOI] [PubMed] [Google Scholar]
- 27.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: A population-based cohort study. American journal of obstetrics and gynecology. 2009;201:269 e261–269 e210 [DOI] [PubMed] [Google Scholar]
- 28.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: A novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64:852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandgren JA, Scroggins SM, Santillan DA, Devor EJ, Gibson-Corley KN, Pierce GL, Sigmund CD, Santillan MK, Grobe JL. Vasopressin: The missing link for preeclampsia? Am J Physiol Regul Integr Comp Physiol. 2015;309:R1062–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santillan MK, Leslie KK, Hamilton WS, Boese BJ, Ahuja M, Hunter SK, Santillan DA. “Collection of a lifetime: A practical approach to developing a longitudinal collection of women’s healthcare biological samples”. Eur J Obstet Gynecol Reprod Biol. 2014;179:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grobe JL, Santillan DA, Santillan MK. Therapeutic strategies for the treatment of preeclampsia, united states patent #9,937,182. (april 10, 2018). 2018 [Google Scholar]
- 32.Grobe JL, Santillan MK. Method using copeptin to predict onset of preeclampsia, european patent #2 954 324 b1 (july 31, 2019). 2019 [Google Scholar]
- 33.Sandgren JA, Deng G, Linggonegoro DW, Scroggins SM, Perschbacher KJ, Nair AR, Nishimura TE, Zhang SY, Agbor LN, Wu J, Keen HL, Naber MC, Pearson NA, Zimmerman KA, Weiss RM, Bowdler NC, Usachev YM, Santillan DA, Potthoff MJ, Pierce GL, Gibson-Corley KN, Sigmund CD, Santillan MK, Grobe JL. Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI Insight. 2018;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scroggins SM, Santillan DA, Lund JM, Sandgren JA, Krotz LK, Hamilton WS, Devor EJ, Davis HA, Pierce GL, Gibson-Corley KN, Sigmund CD, Grobe JL, Santillan MK. Elevated vasopressin in pregnant mice induces t-helper subset alterations consistent with human preeclampsia. Clin Sci (Lond). 2018;132:419–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perschbacher KJ, Deng G, Fisher RA, Gibson-Corley KN, Santillan MK, Grobe JL. Regulators of g protein signaling in cardiovascular function during pregnancy. Physiol Genomics. 2018;50:590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perschbacher KJ, Deng G, Sandgren JA, Walsh JW, Witcher PC, Sapouckey SA, Owens CE, Zhang SY, Scroggins SM, Pearson NA, Devor EJ, Sebag JA, Pierce GL, Fisher RA, Kwitek AE, Santillan DA, Gibson-Corley KN, Sigmund CD, Santillan MK, Grobe JL. Reduced mrna expression of rgs2 (regulator of g protein signaling-2) in the placenta is associated with human preeclampsia and sufficient to cause features of the disorder in mice. Hypertension. 2020;75:569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nuckols VR, Holwerda SW, Luehrs RE, DuBose LE, Stroud AK, Brandt D, Betz AM, Fiedorowicz JG, Scroggins SM, Santillan DA, Grobe JL, Sigmund CD, Santillan MK, Pierce GL. Beat-to-beat blood pressure variability in the first trimester is associated with the development of preeclampsia in a prospective cohort: Relation with aortic stiffness. Hypertension. 2020;76:1800–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]