Abstract
Leptomeningeal carcinomatosis (LC) occurs when tumor cells spread to the cerebrospinal fluid-containing leptomeninges surrounding the brain and spinal cord. LC is an ominous complication of cancer with a dire prognosis. Although any malignancy can spread to the leptomeninges, breast cancer, particularly the HER2+ subtype, is its most common origin. HER2+ breast LC (HER2+ LC) remains incurable, with few treatment options, and the molecular mechanisms underlying proliferation of HER2+ breast cancer cells in the acellular, protein, and cytokine-poor leptomeningeal environment remain elusive. Therefore, we sought to characterize signaling pathways that drive HER2+ LC development as well as those that restrict its growth to leptomeninges. Primary HER2+ LC patient-derived (“Lepto”) cell lines in co-culture with various central nervous system (CNS) cell types revealed that oligodendrocyte progenitor cells (OPC), the largest population of dividing cells in the CNS, inhibited HER2+ LC growth in vitro and in vivo, thereby limiting the spread of HER2+ LC beyond the leptomeninges. Cytokine array-based analyses identified Lepto cell-secreted granulocyte-macrophage colony-stimulating factor (GM-CSF) as an oncogenic autocrine driver of HER2+ LC growth. Liquid chromatography-tandem mass spectrometry-based analyses revealed that the OPC-derived protein TPP1 proteolytically degrades GM-CSF, decreasing GM-CSF signaling and leading to suppression of HER2+ LC growth and limiting its spread. Lastly, intrathecal delivery of neutralizing anti-GM-CSF antibodies and a pan-Aurora kinase inhibitor (CCT137690) synergistically inhibited GM-CSF and suppressed activity of GM-CSF effectors, reducing HER2+ LC growth in vivo. Thus, OPC suppress GM-CSF-driven growth of HER2+ LC in the leptomeningeal environment, providing a potential targetable axis.
Keywords: HER2+ Leptomeningeal carcinomatosis (HER2+ LC), Granulocyte-macrophage colony-stimulating factor (GM-CSF), Tripeptidyl Peptidase 1 (TPP1), Oligodendrocyte progenitor cells (OPCs), Pan-Aurora kinase inhibitor (CCT137690)
Introduction
Among patients with metastatic breast tumors, 10–30% develop central nervous system (CNS) metastases (1,2). Several factors positively correlate with a greater risk of brain metastases, among them: poorly differentiated tumors; HER2-enriched, luminal HER2, basal-like, and triple-negative breast cancer subtypes; and having four or more metastatic lymph-nodes (3,4). HER2+ breast leptomeningeal carcinomatosis (HER2+ LC), which occurs when HER2+ breast tumor cells spread to the cerebrospinal fluid (CSF)-containing leptomeninges surrounding the brain and spinal cord (5–8), is an ominous complication of breast cancer with a dire prognosis (6–8). Once established, HER2+ LC can invade the parenchyma to produce focal neurologic damage (9). Any malignancy can spread to the leptomeninges; however, given the high incidence of breast cancer (and particularly the HER2+ subtype) worldwide, breast cancer is the most common origin (10). Although significant progress has been made in developing breast cancer treatments that target systemic disease, efficacy in the CNS remains a challenge, thus leading to an increase in the incidence of HER2+ LC (11). Indeed, HER2+ LC typically develops while the systemic tumor burden is well-managed (12–15), and 30% of HER2+ LC cases are diagnosed as the first manifestation of cancer after a substantial disease-free interval (14,16–18).
HER2+ LC remains incurable, with few treatment options and response rates often less than 20% (19–25). The current standard-of-care for HER2+ LC management is multidisciplinary, including radiotherapy (RT) and intrathecal chemotherapy (ITC) (26–29). Methotrexate (MTX), a DNA alkylating drug, is frequently used as palliative ITC for HER2+ LC (9,30–33). However, this approach has limited success and causes serious side effects (26–29). Furthermore, patients with HER2+ LC are excluded from clinical trials due to poor prognosis and to minimize results that are not reproducible (6,34–36). Therefore, our goal was to identify novel therapeutic targets to improve the management of this intractable disease.
Little is known about how HER2+ breast cancer cells proliferate in the leptomeninges, which are acellular and poor in protein, glucose, and cytokine content (5–8). Thus, in this study, we used primary HER2+ LC patient-derived (“Lepto”) cell lines (37) to identify the molecular mechanisms that promote HER2+ LC development in this unique context. We found that oligodendrocyte progenitor cells (OPCs), which are abundant in white matter, inhibit HER2+ LC growth in vitro (using Lepto cell lines) and in vivo (in HER2+ LC xenograft models in NOD/SCID mice), limiting the spread of HER2+ LC beyond the leptomeninges. We also conducted cytokine array-based analyses of media conditioned by Lepto cells and various CNS cell types, which revealed that granulocyte-macrophage colony-stimulating factor (GM-CSF) is an oncogenic autocrine driver of HER2+ LC that is significantly overexpressed by HER2+ LC patient-derived tissues and cell lines. In addition, using liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based analyses, we demonstrated that the OPC-derived factor TPP1 proteolytically degrades GM-CSF and can thus suppress HER2+ LC growth. Finally, we showed that combined treatment with anti-GM-CSF neutralizing antibodies plus a pan-Aurora kinase inhibitor (CCT137690) synergistically inactivates GM-CSF signaling and reduces HER2+ LC growth in vitro and in vivo. Collectively, these findings indicate that GM-CSF overexpression confers a survival advantage to HER2+ LC cells, suggesting that GM-CSF inhibition could be an effective therapeutic approach to treat patients with HER2+ LC.
Materials and methods
Ethics statements
Use of human specimens was approved by the City of Hope (COH) Institutional Review Board (IRB; protocols #07047 and #16015) (38–40). Written informed consent was obtained from all patients under protocols #07047 and #16015, and the study was conducted in accordance with the Declaration of Helsinki, institutional guidelines, and all local, state, and federal regulations. All mouse studies were approved by the COH Institutional Animal Care and Use Committee (protocol #10044). We only used female NOD/SCID mice for all the in vivo experiments because the HER2+ Leptomeningeal carcinomatosis (HER2+ LC) occurs predominantly in females.
Reagents
All chemical compounds/drugs, antibodies, culture media, its supplements, and the analyses softwares used in this manuscript are listed and described in the Supplementary Tables 1 and 2, along with their source information and research resource identifier numbers.
Culture and maintenance of HER2+ LC patient derived primary Lepto lines
Derivation of Lepto lines from HER2+ LC patient derived tumors is described in (37). Briefly, nodular HER2+ LC tumors from HER2+ LC patients who underwent surgeries to acquire biopsies for pathological confirmation of HER2+ LC or to decompress localized symptomatic lesions (IRB protocols #07047 and #16015). Each specimen was mechanically dissociated, and CD44+/CD24-/EpCAM+/CD49f- cells (displaying epithelial and cancer stem cell phenotypes) were FACS sorted and maintained at 37°C and 5% CO2. Low-passage Lepto lines were cryobanked using STEM-CELLBANKER Cryopreservation Media (AMSBIO) and banked in liquid nitrogen at −180°C. The Lepto cells were cultured in hCSF-supplemented Advanced DMEM/F-12 (Dulbecco’s Modified Eagle Medium/Ham’s F-12 Nutrient Mixture; Life Technologies) with various supplements (Supplementary Table 2) on collagen-coated T-75 flasks, as previously described (37,39).
Cell lines
Cell lines (HEK293T, MDA-MB-231, BT-474, and T47D) were obtained from ATCC (details listed in Supplementary Table 1). All the lines were biweekly tested for mycoplasma contamination. The cell lines were either grown in RPMI+ 10% FBS + Pen-Strep (1X), IMDM +10% FBS + Penn-Strep (1X) or RPMI +20% FBS + Pen-Strep (1X) according to guidance from ATCC. HEK293T were cultured in DMEM +10% FBS+ Penn-Strep (1X). Cell line authentication was done by short tandem repeat profiling at the IDEXX Bioanalytic Laboratories Inc and tested as Mycoplasma negative by PCR (Agilent Mycosenser Mycoplasma Assay Kit) as recent as 15 days prior to last experiments.
FACS sorting, differentiation, and culture of various CNS cell types
Human iPSC-derived multipotent NPCs were obtained from EMD Millipore (Cat. #SCC035) and propagated using ENStem-A neural expansion medium (Cat. #SCM004). Cells were terminally differentiated to neurons or oligodendrocytes using ENStem-A Neuronal Differentiation Medium (Cat. #SCM017) or human OPC Expansion Media (Cat. # SCM107; basal medium with PDGF-AA, NT3, FGF2, T3, and retinoic acid), respectively, following the supplier’s recommendations. Differentiated microglia, oligodendrocytes, neurons, OPCs, and reactive astrocytes were purified by immunostaining with anti-CD45, anti-GALC, anti-CD90, anti-NG2, and anti-HepaCAM antibodies, respectively, sorted by FACS as described in (41), and propagated in supplier-recommended media. To recapitulate the in vivo microenvironment, cells were grown in hCSF for various durations for the in vitro experiments. Cell morphology and differentiation status were monitored using immunofluorescence staining with anti-CD45, anti-GALC, anti-CD90, anti-NG2, and anti-HepaCAM antibodies.
Cell viability and apoptosis assay
Cell viability was assessed using a Cell Titer Glo Luminescent Cell Viability Assay kit, according to the manufacturer’s protocol. Apoptosis was measured using Annexin V-FITC or staining of phycoerythrin PE-conjugated CD326 (EpCAM-PE). Annexin V-FITC binding was analyzed by flow cytometry using an FITC signal detector, and EpCAM-PE staining was analyzed using a PE emission signal detector. Adherent Lepto cells were trypsinized and washed once with FBS-containing media before incubation with Annexin V-FITC or EpCAM-PE.
Mouse studies and in vivo drug and antibody administration
For HER2+ LC derived Lepto lines based xenografts model development, 4–6 weeks old female NOD/SCID mice were used that were maintained under pathogen-free conditions in accordance with guidelines and therapeutic interventions approved by City Of Hope institutional animal care and use committee (IACUC 10044). As HER2+ leptomeningeal carcinomatosis (HER2+ LC) occurs predominantly in females (Gender), only female NOD/SCID mice were used for all the in vivo experiments. For the overall survival analyses, tumor progression and tumor seeding studies, NOD/SCID 4–6 weeks old female mice were utilized. Animals were housed under standard conditions in the ARCH facility at City of Hope. All animal experiments were carried out under approved IACUC protocols and followed COH’s animal care procedures.
In vivo xenografts and OPC/drug administration
To evaluate the effects of treatment on tumor growth, overall survival and tumor seeding analyses in vivo, control, and variously transduced mCherry and firefly luciferase (mCherry: LUC, Addgene_29783) Lepto lines were injected at 100K density or various other densities in 20 μL PBS buffer via cisterna magna puncture into cohorts of female NOD/SCID mice. At 7- and 14-days post-implantation of Lepto cells, mice were intrathecally injected with either OPCs or OPCs-shGFP or OPCs-shTPP1 (100K or 200K in 20 μL PBS), CCT137690 (50 mg/kg (Fig. 5G–I)), TPP1 (50–150 ng/mL), anti-GM-CSF antibodies in PBS (8 μg/g (Fig. 2H–J) or 4 μg/g (Fig. 5G–I).), or vehicle (PBS alone). Tumor growth was monitored weekly by BLI on a Xenogen Imaging System (Xenogen Corp). Mice were injected with 100 mg/kg D-luciferin, and 2 sets of in vivo BLI images in 1 projection were acquired, resulting in a collection of 8 images. Mice were then euthanized, and their brains collected, fixed in formalin (Thermo Fisher Scientific), and subjected to western blot or H&E or IHC analysis. On each in vivo BLI image, a region of interest (ROI) encompassing the entire mouse except the tail was placed, and the total signal in the ROI was quantified using Living Image software (version 2.50; Xenogen). The total signals of all images obtained in a single imaging session were averaged to determine the whole-body signal intensity, which was used as a marker of whole-body tumor burden. As per IACUC (# 10044) the experimental endpoint of the animals were death and/or reaching biologically humane endpoint based on tumor burden, weight loss, mobility, food/water refusal. Once the tumor bearing NOD/SCID mice reached biological end point the animals were subjected to final BLI for tumor burden before euthanization.
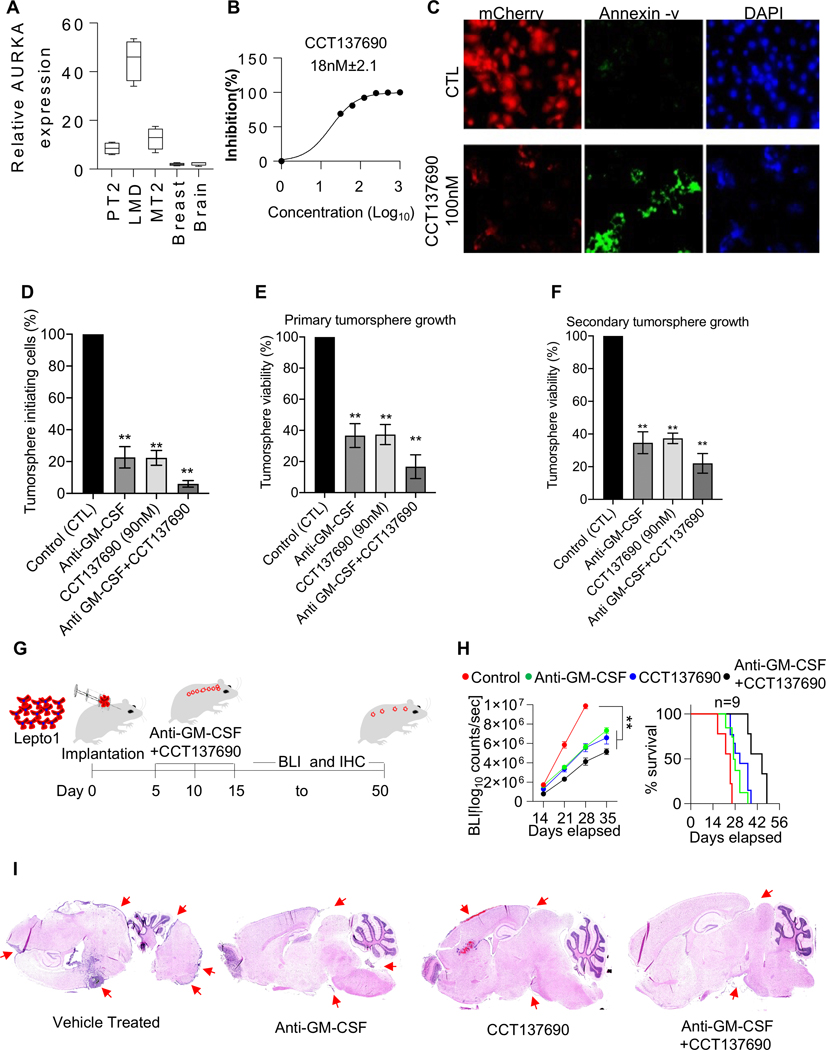
Figure 5. Combination treatment with a pan-Aurora kinase inhibitor and anti-GM-CSF neutralizing antibodies reduces Lepto cell growth in vivo.
(A.) RT-qPCR analysis of Aurora-A transcript levels in nodular HER2+ LC, primary tumor (PT2), metastatic tumor (MT2), normal breast, and normal brain tissues. The HER2+ LC tissues exhibited the highest Aurora-A transcript levels.
(B.) Dose-dependent inhibition of Lepto cell viability by CCT137690, measured by CellTiter-Glo assays. The IC50 value is shown.
(C.) Immunofluorescence images of mCherry: LUC-labeled (red) Lepto cells stained with Annexin V (green) after 24 h of treatment with CCT137690 (100 nM) or 0.1% DMSO (CTL). Nuclei were counterstained with DAPI (blue). Scale bar=50 μm.
(D.) Percentages of tumorsphere-initiating cells after 24-h treatment (as shown in S4A, top row), measured by CCK assays. The number of DMSO-treated cells was set to 100 (n = 3). **p<0.01, compared to DMSO-treated cells.
(E.) Viability of primary tumorspheres after 2-day treatment (as shown in S4A, middle row), measured by CCK assays. The number of DMSO-treated cells was set to 100 (n = 3). **p<0.01, compared to DMSO-treated tumorspheres.
(F.) Viability of secondary tumorspheres 12 days after dissociation of treated primary tumorspheres (as shown in S4A, bottom row), measured by CCK assays. ** p < 0.01, compared to secondary tumorspheres from DMSO-treated primary tumorspheres.
(G.) Schematic showing the protocol used to monitor the effects of the intrathecal administration of anti-GM-CSF neutralizing antibodies and CCT137690 ((8 μg/g and 100 mg/kg, respectively, on days 5, 10, and 15) in mice implanted with mCherry: LUC-labeled Lepto cells (100K). Tumor growth was monitored by BLI from days 15 to 50.
(H.) (Left panel) BLI-based quantification of mCherry: LUC-labeled Lepto tumor growth in mice treated with anti-GM-CSF antibodies alone or with CCT137690 (n=9). Control animals were treated with vehicle (PBS for antibodies and 0.1% DMSO for CCT137690). (Right panel) Survival analysis of the same mice. Combination treatment with anti-GM-CSF antibodies and CCT137690 (anti-GM-CSF+CCT137690) significantly reduced tumor growth and increased survival. Treatment with anti-GM-CSF antibodies alone also significantly reduced tumor growth but to a lesser extent. **p<0.01.
(I.) H&E-stained sagittal brain tissue sections from Lepto bearing NOD/SCID mice treated with Vehicle, Anti-GM-CSF, CCT137690 and CCT137690+Anti-GM-CSF. Red arrows indicate presence of Lepto derived tumor mass.
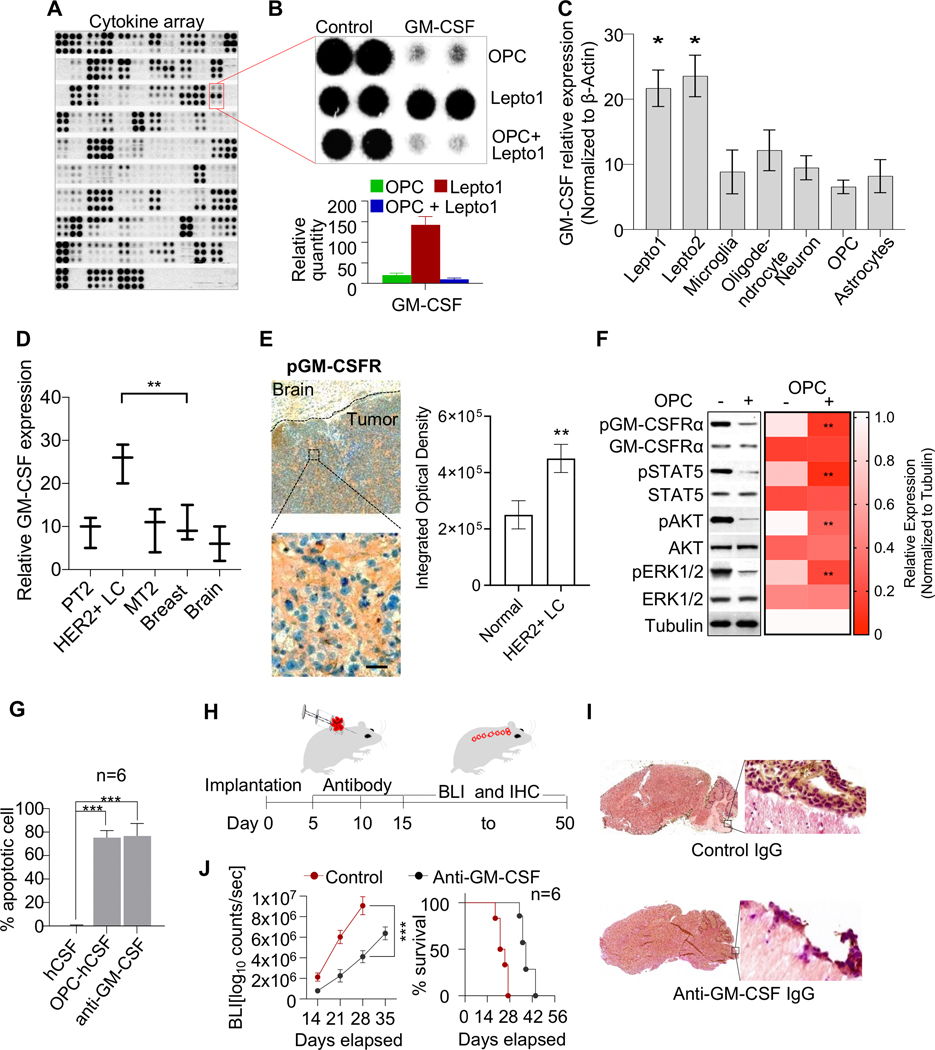
Figure 2. GM-CSF acts as an oncogenic autocrine driver contributing to HER2+ LC cell growth.
(A.) Cytokine XL array-based analyses of conditioned media from OPCs and/or Lepto cells cultured in media supplemented with hCSF. The secreted factors identified in the media of monocultured OPCs and OPCs co-cultured with Lepto cells are listed in Supplementary table 4 and 5.
(B.) (Top panel) Control and GM-CSF-specific blots from the array shown in Fig. 2A. (Bottom panel) Density-based quantification of the GM-CSF blots shown in the top panel.
(C.) RT-qPCR analysis of GM-CSF transcript levels in Lepto1 and Lepto2 cells, as well as in the indicated iPSC-derived CNS cell types. The Lepto lines exhibited the highest GM-CSF mRNA levels among all cell types analyzed (n=3). *p<0.001 relative to OPCs
(D.) RT-qPCR analysis of GM-CSF transcript levels in HER2+ LC tumor, HER2+ breast metastatic tumor (MT2), primary tumor (PT2), normal breast, and normal brain tissues. The HER2+ LC tumor tissues exhibited the highest GM-CSF transcript levels among all tissues analyzed (n=3). **p<0.001.
(E.) (Left panel) Immunohistochemical analysis (IHC) of patient HER2+ LC specimens showing pGM-CSFRα (orange) in tumor cells but not surrounding brain tissue. (Top) Low magnification image showing tumor and surrounding normal brain tissue. (Bottom) High magnification image showing the selected tumor region. Scale bar=100 μm. (Right panel) FIJI based quantification of the IHC image analyses from n=3 patient HER2+ LC specimens showing higher levels of pGM-CSFRα in tumor cells but not in the surrounding brain tissue.
(F.) (Left Panel) Western blot analysis of the indicated signaling proteins in extracts from Lepto cells cultured alone or with OPCs for 48 h. Tubulin was used as the loading control. (Right panel) Heat map showing FIJI based quantification of the western blots. Compared to mono-cultured Lepto cells, Lepto cells co-cultured with OPCs exhibited lower pGM-CSFRα levels and less growth factor phosphorylation/activation (pSTAT5, pAKT, and pERK1/2). Tubulin served as a loading control.
(G.) Quantification of Annexin V-positive Lepto cells grown under the indicated conditions. Cells cultured with OPC-conditioned media or anti-GM-CSF neutralizing antibodies were significantly more apoptotic than control cells grown in hCSF-supplemented media alone (n=6). ***p<0.001.
(H.) Schematic showing the protocol used to monitor the effects of the intrathecal administration of anti-GM-CSF neutralizing vs. control IgG antibodies (8 μg/g on days 5, 10, and 15) in mice implanted with mCherry:LUC-labeled Lepto cells (100K). Tumor growth was monitored by BLI from days 15 to 50.
(I.) IHC analysis of brain sections from Lepto cell-implanted NOD/SCID mice treated with anti-GM-CSF antibodies or control IgG. Anti-GM-CSF antibody treatment suppressed Lepto tumor growth.
(J.) (Left panel) BLI-based quantification of mCherry:LUC-labeled Lepto tumor growth in mice treated with anti-GM-CSF antibodies or control IgG (n=6). Antibody treatment blocked tumor progression. (Right panel) Survival analysis of the same mice. ***p<0.001.
Statistical analyses
Data shown in figures are mean values ± standard error, using data generated from n=3 biological replicates with n=2 technical replicates present in each biological replicate. Statistical significance between groups was determined using one- or two-way analysis of variance (ANOVA), followed by multiple comparisons with Bonferroni multiple comparisons correction. The level of significance used was α:0.05. Other statistical evaluations were performed using the Student t test. The software used for the above-mentioned analyses was GraphPad Prism 8.4.1. Kaplan–Meier curve was used to model overall survival. P value of <0.05 was considered statistically significant. Significance in statistical analyses in the figures is represented by * < 0.05, or ** < 0.05, or *** < 0.05.
All additional methods are described in the Supplemental Material and Methods section
Results
The presence of OPCs reduces HER2+ LC cell viability
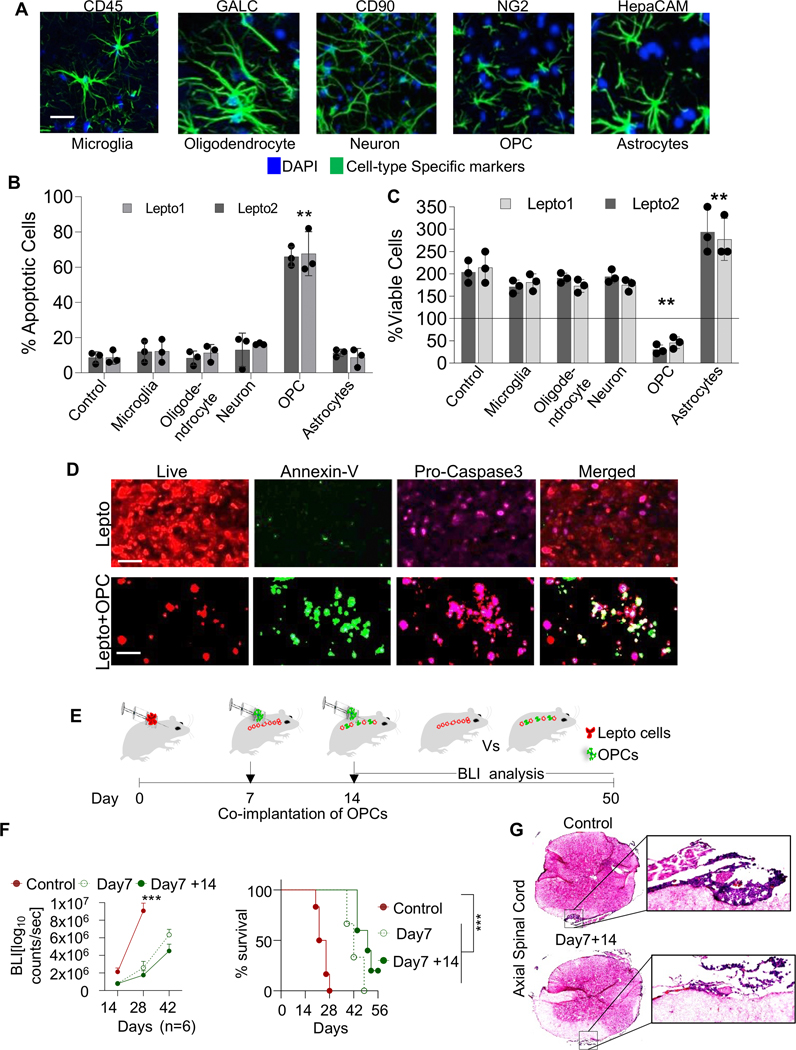
To determine whether host glial cells in the CNS impact HER2+ LC growth and development, we developed HER2+ LC patient-derived lines that we call “Lepto” lines, which demonstrated the unique spinal cord migration functionality in vivo, as do HER2+ LC tumor cells (37). HER2+ LC patient-derived lines differed transcriptomically different from other HER2+/− metastatic breast cancer cell lines (Suppl. Fig. 1A). We then immuno-panned CNS cell types from human induced pluripotent stem cell (iPSC)-derived neural progenitor cells (NPCs). Specifically, we used anti-CD45, anti-GALC, anti-CD90, anti-NG2, and anti-HepaCAM antibodies to sort microglia, oligodendrocytes, neurons, Oligodendrocyte Progenitor Cells (OPCs), and astrocytes, respectively, by fluorescence-activated cell sorting (FACS) (41). Cells were maintained in human CSF (hCSF) for various time periods (4–5 days), during which all cell types maintained typical morphology and marker expression patterns (Fig. 1A). We then co-cultured each cell type in Boyden chambers with primary HER2+ LC patient-derived Lepto1 or Lepto2 cells and assessed their effects on Lepto cell viability (37). Co-culture of both Lepto lines with astrocytes increased their proliferation, whereas co-culture with OPCs induced Lepto cell apoptosis (Annexin-v based FACS staining) (Fig. 1B) and reduced their viability (Cell Titer-Glo Luminescent Cell Viability Assay) (Fig. 1C). Immunofluorescence (IF) imaging of mCherry: LUC-labeled Lepto cells co-cultured 48h with or without OPC-conditioned media indicated more robust Annexin-V (green) and Pro-Caspase 3 (magenta) staining, indicative of increased apoptosis, in cells grown in conditioned media (Fig. 1D and Suppl. Fig. 1B and 2A). To characterize these effects in vivo, we injected mCherry:LUC-labeled Lepto cells (100K) into the cisternae magna of adult NOD/SCID mice (on day 0), followed by OPCs co-implantation (100K) on days 7 and/or 14 (Scheme; Fig. 1E). Then, from days 14 to 50, we monitored tumor growth via bioluminescence imaging (BLI). Mice co-implanted with Lepto cells (on day 0) and OPCs (on day 7 ± day 14) showed significantly decreased tumor growth based on BLI relative to non-OPC injected control animals bearing Lepto tumors and prolonged survival (Fig. 1F left and right panels, respectively, Supplementary Table 3). Furthermore, histopathological analyses of H&E stained axial sections of spinal cord and sagittal sections brain of Lepto bearing NOD/SCID mice injected with no OPCs or OPCs (D7+14) revealed marked reduction in levels of Lepto-derived tumors (Fig. 1G and Suppl. Fig. 2B). These findings confirm the inhibitory effects of OPCs observed in vitro and in vivo.
Figure 1. The presence of OPCs reduces HER2+ LC cell viability.
(A.) Immunofluorescence (IF) images of various CNS cell types immuno-panned from human iPSCs and stained with the indicated antibodies. Nuclei were counterstained with DAPI (blue). Scale bar=100 μm.
(B.) Annexin-V FACS-based analysis of Lepto1 and Lepto2 cells (seeded at 0.5×105 density/well of a 24 well plate, in the bottom chamber) co-cultured with the indicated human CNS cell populations (seeded at 0.5×105 density/well of 24 well plate, in the top inserts). Co-culture with OPCs increased the proportion of apoptotic Lepto cells (n=3). **p<0.001 relative to control Lepto cells exposed to no CNS cell types.
(C.) Viability of Lepto1 and Lepto2 (seeded at 0.1×105 density/well of a 96 well plate, in the bottom chamber) lines co-cultured with various CNS cell types (derived in Fig. 2A) (all seeded at 0.1×105 density/well of a 96 well plate, in the top inserts) for 48 h, measured using CellTiter-Glo assays (n=3). **p<0.001 relative to control Lepto cells exposed to no CNS cell types.
(D.) IF images of mCherry: LUC-labeled (red) Lepto cells stained with Annexin-V (green) and Pro-Caspase 3 (magenta) after 48 h of treatment with or without OPC-conditioned medium. Scale bar=50 μm.
(E.) Schematic showing the protocol used for the in vivo characterization of the effects of OPCs on Lepto cell growth. mCherry: LUC-labeled Lepto cells (100K, red) were injected into the cisternae magna of adult NOD/SCID mice on day 0, and OPCs (100K, green) were injected on days 7 and/or 14. Tumor growth was monitored by BLI from days 14 to 50, with representative images acquired starting on day 28.
(F.) (Left panel) Quantitative analyses showing that the mice that received OPCs exhibited reduced tumor growth (n=6). ***p<0.001 relative to mice with OPCs implanted on days 7 and 14. (Right panel) Kaplan–Meier curves showing the overall survival of mice implanted with Lepto cells on day 0 only (solid red line) or co-implanted with OPCs on day 7 (dashed green line) or on days 7 and 14 (solid green line). ***p<0.001.
(G.) Histopathologic analyses of the H&E stained axial spinal cord sections from control Lepto infused and Lepto+ OPC (OPC infusion on D7 and D14) co-infused mice on the Left and 20x magnified regions showing Lepto deposition
GM-CSF acts as an oncogenic autocrine driver contributing to HER2+ LC cell growth
To identify factors that initiate and drive growth of HER2+ LC tumors, we co-cultured primary patient-derived Lepto cells with OPCs in a Boyden chamber for 72 h, for comparison with OPCs or Lepto cell controls grown as monolayers. When we analyzed growth medium from samples using a Cytokine XL array (Fig. 2A, Suppl. Fig. 3A and Supplementary Table 4 and 5), we observed significantly higher GM-CSF concentrations in media of mono-cultured Lepto cells relative to co-cultured OPC/Lepto cells or mono-cultured OPCs (Fig. 2A and B). In addition, primary Lepto cells expressed higher levels of GM-CSF transcripts than did various iPSC-derived CNS cell types (Fig. 2C). Moreover, nodular patient derived HER2+ LC tissues expressed higher levels of GM-CSF transcripts compared to other primary and metastatic patient derived tumors or normal human breast and brain tissues (Fig. 2D). Immunohistochemical (IHC) analyses of Lepto bearing mouse brain sections revealed significantly higher levels of the phosphoGM-CSF receptor α subunit (GM-CSFRα) in HER2+ LC lepto derivd tumor tissues relative to surrounding brain tissues (Fig. 2E and quantification on right panel). Furthermore, western blot analysis of Lepto cells cultured with or without OPCs revealed decreased phosphorylation of GM-CSFRα and its downstream targets, STAT5, AKT, and ERK1/2 in the presence of OPCs compared to Lepto cells cultured without OPCs (Fig. 2F (Western Blots) and quantification of western blots on right panel), suggesting that OPCs inhibit GM-CSF secretion from Lepto cells
We next assessed disruption of GM-CSF signaling in vitro. To do so first we assessed apoptotic markers in Lepto cells grown in either hCSF-supplemented media (controls), OPC-conditioned hCSF-supplemented media, or hCSF containing anti-GM-CSF neutralizing antibodies, which have been used clinically in various cancer treatments (42,43). Quantification of surface Annexin-V via flow cytometry indicated that Lepto cells grown in OPC-conditioned hCSF or hCSF containing anti-GM-CSF antibodies were significantly more apoptotic (~90%) than control (vehicle treated) Lepto cells (Fig. 2G). Next, we assessed potential anti-tumor effects of anti-GM-CSF neutralizing antibodies on tumor growth in vivo by administering those antibodies intrathecally to xenograft mouse models of HER2+ LC and monitoring tumor formation by BLI (Fig. 2H). Relative to vehicle-treated controls, antibody-treated mice showed reduced tumor progression (based on BLI counts) (Fig. 2J (Left)) and increased overall survival (Fig. 2J (Right) and and Supplementary Table 3). These findings were supported by immunohistochemical analysis of sagittal brain sections from lepto bearing NOD/SCID mice, which demonstrated that the anti-GM-CSF neutralizing antibodies suppressed tumor growth in the brain stem regions (Fig. 2I (Sagittal brain sections stained with HER2 antibody)). Taken together, these results confirm the contributory role of GM-CSF signaling in HER2+ LC tumor progression and suggest that treatment with anti-GM-CSF neutralizing antibodies could serve as a potential strategy to target HER2+ LC.
Modulation of GM-CSF expression alters Lepto cell proliferation in vitro and in vivo
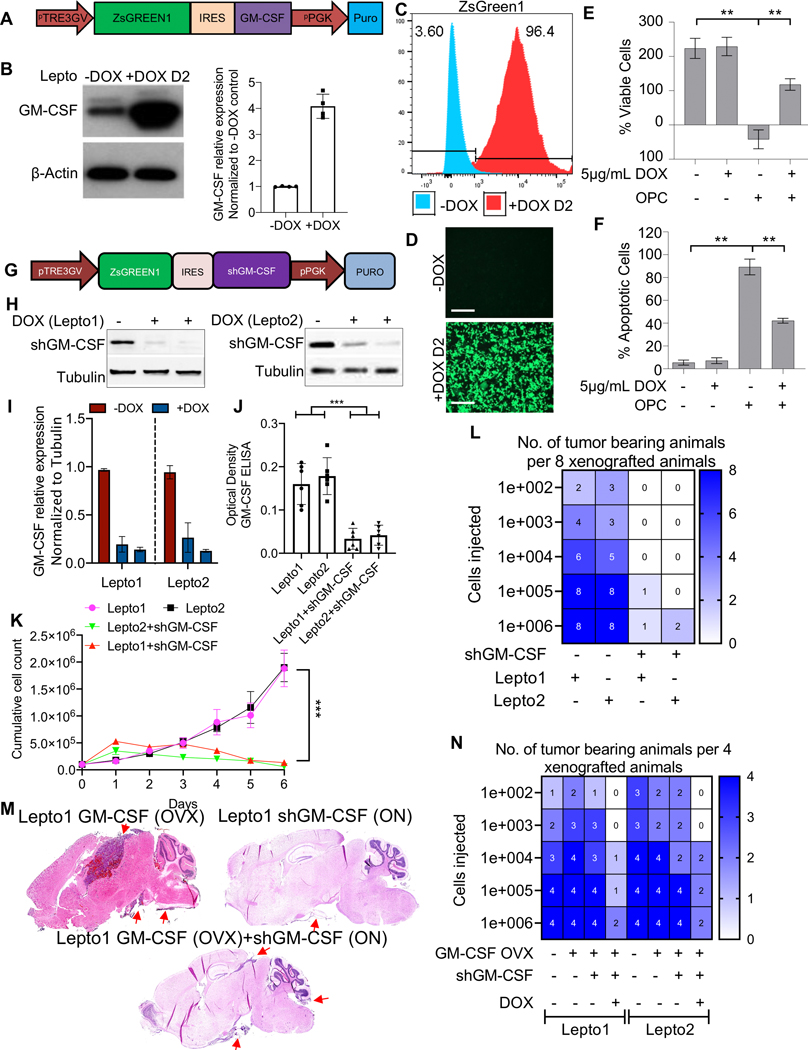
To further assess GM-CSF effects we established Lepto cells conditionally overexpressing GM-CSF by cloning the GM-CSF open reading frame (ORF) downstream of ZsGreen1-IRES in a Tet-On 3G inducible expression system (Vector design; Fig. 3A). Addition of doxycycline (DOX; 5 μg/mL) to the culture media of Lepto cells transduced with this construct significantly increased GM-CSF protein levels (in Lepto cell lysates) (Fig. 3B, Left panel western blot and Right panel Quantification of the western blots), with a concomitant increase in ZsGreen1 levels as detected by FACS and immunofluorescence imaging (Fig. 3C–D). DOX-induced GM-CSF expression in Lepto cells increased their viability in the presence of OPCs relative to that seen in the absence of DOX, based on analysis using Cell Titer-Glo assays (Fig. 3E). Moreover, in the presence of OPCs DOX-exposed GM-CSF overexpressing Lepto cells showed decreased apoptosis as determined by Annexin-V flow cytometry-based analysis than did comparably co-cultured Lepto cells without DOX induction (Fig. 3F).
Figure 3. Modulation of GM-CSF expression alters Lepto cell proliferation in vitro and in vivo.
(A.) Diagram showing the lentiviral Tet-On 3G inducible GM-CSF ORF expression cassette used in this study.
(B.) (Left) Western blot analysis of GM-CSF in Lepto cell lysates collected 48 h after 5 μg/mL DOX administration to induce GM-CSF overexpression. β-Actin served as a loading control. (Right) FIJI based quantification of the western blots shows DOX mediated overexpression of GM-CSF in Lepto cells.
(C.) FACS-based analysis of ZsGreen1expression in Lepto cells cultured for 48 h with (red) or without (blue) 5 μg/mL DOX.
(D.) Fluorescence imaging of Lepto cells 48 h after 5 μg/mL DOX or vehicle (PBS) treatment, showing robust green fluorescence of Lepto cells after GM-CSF induction. Scale bar=50 μm.
(E.) Viability of Lepto cells cultured with or without 5 μg/mL DOX and/or OPCs for 48 h, measured by CellTiter-Glo assays (n=3). **p<0.001.
(F.) Annexin V FACS-based analysis of apoptosis in Lepto cells grown under the conditions shown in Fig. 3E (n=3). **p<0.001.
(G.) Diagram showing the lentiviral Tet-On 3G inducible GM-CSF-shRNA expression cassette used in this study.
(H.) Western blot analysis of GM-CSF in Lepto cell (Lepto1 Left and Lepto2 Right) lysates collected 48 h after 5 μg/mL DOX administration to induce shGM-CSF expression. Tubulin served as a loading control.
(I.) FIJI based quantification of the western blots in Fig. 3H shows DOX mediated overexpression of shGM-CSF in Lepto1 and 2 cells. *p<0.01.
(J.) ELISA-based quantification of GM-CSF concentrations in the media of Lepto1 and 2 cells conditionally expressing shGM-CSF. ***p<0.01.
(K.) Proliferation rates of control Lepto cells and Lepto cells expressing shGM-CSF over 6 days. shGM-CSF-expressing Lepto cells showed prolonged doubling times relative to control Lepto cells.
(L.) Heatmap of the tumor-seeding capacities (per 8 xenografted animals) of control Lepto cells vs. Lepto cells conditionally expressing shGM-CSF.
(M.) H&E-stained sagittal brain tissue sections from NOD/SCID mice implanted with Lepto1 cells (100K) constituitively overexpressing GM-CSF alone, Lepto1 cells (100K) conditionally overexpressing shGM-CSF (DOX; ON) and Lepto1 cells constituitively overexpressing GM-CSF as well as conditionally overexpressing shGM-CSF (DOX; ON). Red arrows indicate presence of Lepto derived tumor mass.
(N.) Heatmap of the tumor-seeding capacities (per 4 xenografted animals) of control Lepto cells vs. constitutive GM-CSF overexpressing Lepto cells vs GM-CSF (constitutive overexpression) combined with conditionally expressing shGM-CSF Lepto cells.
Next, to assess consequences of GM-CSF loss-of-function, we inserted GM-CSF shRNA (shGM-CSF) upstream of ZsGreen1 and employed the same Tet-On 3G inducible expression system to conditionally knockdown GM-CSF in Lepto cells (Fig. 3G). DOX (5 μg/mL) treatment of transduced Lepto cells significantly reduced GM-CSF protein levels relative to “No DOX” controls (Fig. 3H (left (Lepto1) and right (Lepto2) and I (quantification of western blots)). ELISA analysis confirmed decreased GM-CSF secretion from DOX-treated Lepto cells transduced with shGM-CSF (Fig. 3J). DOX-treated, shGM-CSF-transduced Lepto cells also showed decreased proliferation in vitro relative to “No DOX” Lepto cell controls (Fig. 3K).
We next compared the ability of control and GM-CSF knockdown Lepto cells to seed tumors in mice. Briefly 8 NOD/SCID mice per group, were implanted with varying density of Lepto cells with or without shGM-CSF transduction and found that the number of injected cells required to generate tumors in at least 1 of 8 mice was 103-fold less for control Lepto cells (100) than for GM-CSF knockdown cells (105 to 106) (Fig. 3L). To further confirm that GM-CSF drives Lepto cell growth in vivo, we first established constitutive GM-CSF-overexpressing Lepto cells and transduced them with the inducible GM-CSF-shRNA vector (Suppl. Fig. 4A–B). ELISA analysis confirmed abrogation of GM-CSF secretion following DOX treatment (Suppl. Fig. 4C). Then using the DOX induction system, we compared the ability of GM-CSF-overexpressing versus GM-CSF-depleted Lepto cells to seed tumors in NOD/SCID mice. The number of injected cells required to generate tumors in at least 1 of 4 mice was 103-fold less for GM-CSF-overexpressing (100) as compared to GM-CSF-depleted (105 to 106) Lepto cells (Fig. 3N). Histopathological analyses of H&E-stained sagittal brain sections demonstrated that GM-CSF overexpressing Lepto cells were able to form tumor in the various regions of the brain including the brain stem, while shGM-CSF overexpression or coexpression with GM-CSF (ORF) led to significantly decreased tumor growth (Fig. 3M).
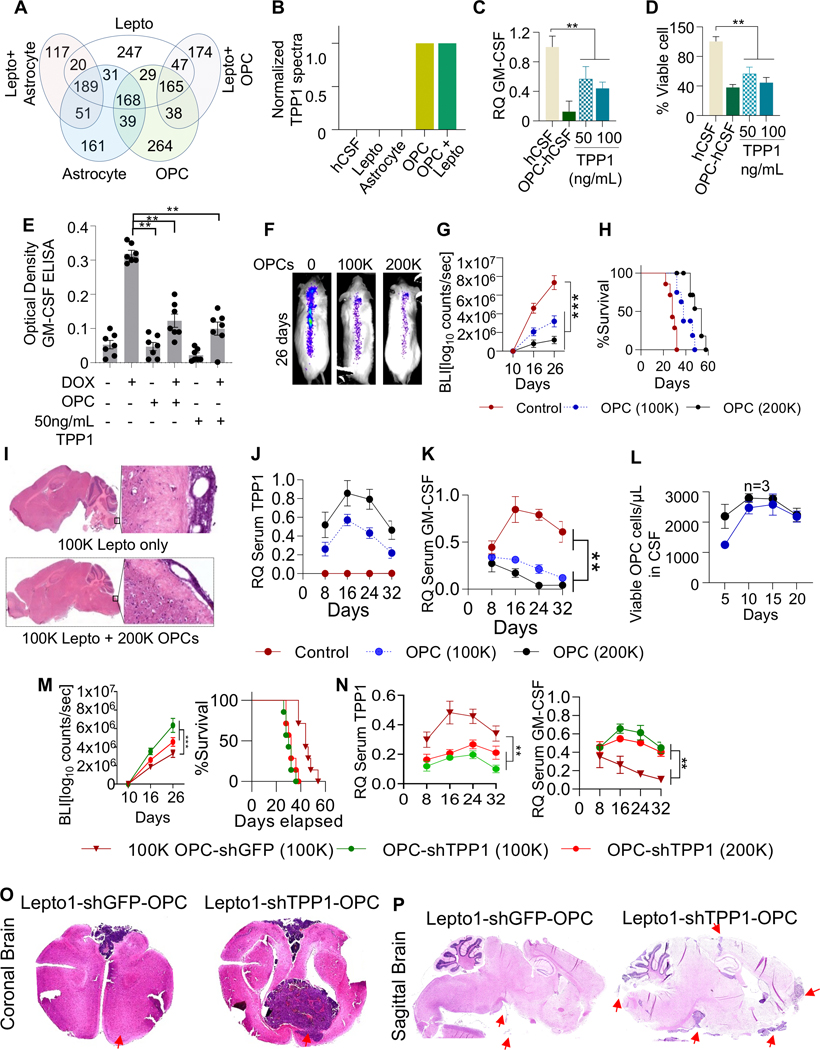
OPC-derived TPP1 is a candidate regulator of GM-CSF
We next asked if OPCs secrete factor(s) that inhibit GM-CSF signaling and potentially induce Lepto cell apoptosis. To do so, we analyzed the secretomes of human astrocytes and OPCs, cultured alone or with Lepto cells, using liquid chromatography-tandem mass spectrometry (LC-MS/MS). We identified 38 unique proteins present in OPC-conditioned hCSF whose levels remained unchanged in OPC-Lepto co-cultures (Fig. 4A and Supplementary Table 5). Of the 38 candidate proteins, only CGREF1, ENO, PTPRZ1, SPARC, and TPP1 were known secreted proteins located extracellularly (Supplementary Table 5). PROSPER-based predictions of GM-CSF protease cleavage sites (44) revealed multiple sites for various serine proteases (Supplementary Table 6). Among the 5 candidates, only TPP1, a serine protease in the sedolisin family, acts as a non-specific lysosomal peptidase that cleaves N-terminal tripeptides (45). When we examined media conditioned by monocultured OPCs or (?) OPCs co-cultured with Lepto cells, we observed higher levels of TPP1 than seen in media of monocultured Lepto cells or in astrocyte-conditioned media (Fig. 4B). To assess whether OPC-derived TPP1 proteolytically degrades GM-CSF, we cultured Lepto cells 24 h with 50 or 100 ng/mL of recombinant TPP1 protein in media supplemented with hCSF and measured GM-CSF levels by ELISA. Both TPP1 concentrations significantly reduced GM-CSF levels in the media relative to vehicle-treated control Lepto cells (Fig. 4C). Furthermore, TPP1 treatment also suppressed Lepto cell viability via usage of Cell Titer-Glo Luminescent Cell Viability Assay (Fig. 4D). To determine if recombinant TPP1 or OPC-secreted TPP1 can degrade Lepto-secreted GM-CSF, we treated culture media of Lepto cells conditionally overexpressing GM-CSF with 50 ng/mL TPP1 and, separately co-cultured GM-CSF-overexpressing Lepto cells with OPCs. Both treatment with exogenous TPP1 and and co-culture with OPCs decreased concentrations of secreted GM-CSF protein in Lepto cell media, as determined by ELISA (Fig. 4E). Furthermore, both conditions induced Lepto cell apoptosis (based on Flow cytometry based surface Annexin-v staining (Suppl. Fig. 5A).
Figure 4. OPC-derived TPP1 is a candidate regulator of GM-CSF.
(A.) Venn diagram of unique and shared secreted proteins identified in the hCSF of mono- or co-cultured astrocytes, OPCs, and Lepto cells.
(B.) Relative TPP1 protein levels in control hCSF (no cells) and hCSF from the indicated cell cultures.
(C.) Relative quantification (RQ) of GM-CSF levels in media from Lepto cells cultured in OPC-conditioned hCSF or in hCSF with exogenous TPP1 (50 or 100 ng/mL), as measured by ELISA. **p<0.01.
(D.) Lepto cell viability following treatment with OPC-conditioned hCSF or hCSF with exogenous TPP1, as shown in Fig. 4C, measured by CellTiter-Glo assays. **p< 0.01.
(E.) ELISA-based analysis of GM-CSF concentrations in the media of Lepto cells conditionally overexpressing GM-CSF (following treatment with 5 μg/mL DOX) and cultured with OPCs or TPP1 (50 ng/mL). Both conditions reduced GM-CSF levels in culture media. **p<0.01.
(F.) Representative BLI images of NOD/SCID mice on day 26 post-implantations of Lepto cells (100K) alone or with OPCs (100K or 200K).
(G.) BLI-based quantification of tumor growth showing OPC density-dependent suppression of Lepto tumor growth. ***p<0.001.
(H.) Kaplan–Meier curves showing the density-dependent effects of OPC implantation on the survival of mice bearing Lepto tumors. ***p<0.001.
(I.) H&E-stained brain tissue sections from NOD/SCID mice implanted with Lepto cells (100K) alone or with OPCs (200K).
(J.) ELISA-based analysis of TPP1 levels in serum extracted from mice on days 8, 16, 24, and 32 post-implantations of with Lepto cells (100K) alone (Control) or with OPCs (100K or 200K).
(K.) ELISA-based analysis of GM-CSF levels in serum extracted from mice on days 8, 16, 24, and 32 post-implantations with Lepto cells (100K) alone (Control) or with OPCs (100K or 200K).
(L.) Viability of OPCs extracted from CSF samples collected between days 5 and 20 after co-implantation with Lepto cells into NOD/SCID mice, measured by CellTiter-Glo assays.
(M.) (Left panel) BLI-based quantification of tumor growth in Lepto1 bearing NOD/SCID mice co-implanted with OPC-shGFP (100K), OPC-shTPP1 (100K) and OPC-shTPP1 (200K) shows density-dependent elevation of Lepto1 tumor growth. ***p<0.001. (Right panel) Kaplan–Meier curves showing survival of mice bearing Lepto1 derived tumors co-implanted with OPC-shGFP (100K), OPC-shTPP1 (100K) and OPC-shTPP1 (200K). ***p<0.001.
(N.) (Left panel) ELISA-based analysis of TPP1 levels in serum extracted from mice on days 8, 16, 24, and 32 post-implantations with Lepto1 cells (100K) followed by co-implantation with 100K OPCs-shGFP, (100K and 200K) OPCs-shTPP1. (Right Panel) ELISA-based analysis of GM-CSF levels in serum extracted from mice on days 8, 16, 24, and 32 post-implantations with Lepto1 cells (100K) followed by co-implantation with 100K OPCs-shGFP, (100K and 200K) OPCs-shTPP1
(O.) H&E-stained coronal brain tissue sections from NOD/SCID mice co-implanted with Lepto1 cells (100K) and with OPCs-shGFP or with OPCs-shTPP1 (100K). Red arrows indicate presence of Lepto derived tumor mass.
(P.) H&E-stained sagittal brain tissue sections from NOD/SCID mice co-implanted with Lepto1 cells (100K) and with OPCs-shGFP or with OPCs-shTPP1 (100K). Red arrows indicate presence of Lepto derived tumor mass.
To determine if TPP1 loss-of-function in OPCs would alter concentrations of secreted GM-CSF in Lepto line co-cultures, we transiently transfected iPSC-derived OPCs with siTPP1 and the following day co-cultured them (or control OPCs transfected with siGFP or siLUC) with Lepto cells conditionally overexpressing GM-CSF (Suppl. Fig. 5B). One day later, siTPP1-transfected OPCs showed significantly decreased levels of TPP1 transcripts relative to control OPCs, as measured by RT-qPCR (Suppl. Fig. 5C). Interestingly, GM-CSF protein levels were significantly higher in media from Lepto cells co-cultured with TPP1-depleted OPCs than in media from Lepto cells co-cultured with control OPCs (Suppl. Fig. 5D).
To assess effects of OPC-derived TPP1 on GM-CSF signaling in Lepto cells in vivo, we co-implanted Lepto cells (100K) with or without OPCs (100K or 200K) into the cisternae magna of NOD/SCID mice. As anticipated, we observed OPC density-dependent suppression of HER2+ LC tumor progression, as indicated by BLI counts (Fig. 4F, G) and histopathological analyses using hematoxylin and eosin (H&E) staining (Fig. 4I). Moreover, co-implantation of OPCs with Lepto cells increased animal survival relative to mice implanted with Lepto cells only (Fig. 4H and Supplementary Table 3). Analysis of sera from these mice revealed that TPP1 protein levels increased with OPC density (Fig. 4J), an effect that corresponded to decreased GM-CSF levels (Fig. 4K). Interestingly, OPCs co-implanted with Lepto cells did not exhibit substantial changes in viability between days 5 and 20 post-implantation (Fig. 4L).
Next, to determine whether OPC-secreted TPP1 inhibits Lepto cell growth in vitro or in vivo, we transduced iPS derived OPCs with either TPP1 shRNA or control GFP shRNA. Analyses of TPP1 protein levels via western blot demonstrated that the protein levels of TPP1 were significantly reduced in OPCs transduced with shTPP1 relative to OPCs transduced with shGFP (Suppl. Fig. 5E and 5F (quantification of western blots in SF5E)). Then, co-culture of GFP depleted OPCs with Lepto cells significantly reduced GM-CSF secretion from Lepto cells relative to Lepto cells exposed to shTPP1 transduced OPCs (Suppl. Fig. 5G). Next, we co-implanted control or TPP1 knockdwon OPCs (100K or 200K) into the cisternae magna of NOD/SCID mice bearing Lepto tumors. We observed decreased growth of Lepto-derived tumors in mice implanted with control OPCs compared to mice implanted with TPP1 knockdown OPCs, based on BLI quantification from days 10 to 26 (Fig. 4M (Left panel)). Accordingly, animal survival was significantly decreased in mice implanted with OPC-shTPP1 relative to control OPCs (Fig. 4M (Right panel) and Supplementary Table 3). Analysis of sera from these animals revealed higher TPP1protein levels in OPC-shGFP- compared to OPC-shTPP1-implanted mice (Fig. 4N (Left panel)). Also GM-CSF levels in sera were also relatively higher in OPC-shTPP1-implanted mice (Fig. 4N (Right Panel)). Finally, H&E staining of coronal (Fig. 4O) and sagittal (Fig. 4P) brain sections showed reduced tumor growth in shGFP-OPC co-implanted Lepto tumor bearing NOD/SCID mice (n=3) compared to shTPP1-OPC co-impanted Lepto tumor bearing mice (n=3). Analysis of horizontal brain sections revealed comparable effects (Suppl. Fig. 5H). Overall, in vitro (Fig. 4B–E) and in vivo (Fig. 4F–P) analyses suggest that OPC-secreted TPP1 degrades GM-CSF and suppresses GM-CSF signaling, decreasing Lepto cell viability and tumor progression.
Combination treatment with a pan-Aurora kinase inhibitor and anti-GM-CSF neutralizing antibodies reduces Lepto cell growth in vivo
Current treatment of HER2+ LC tumors relies on cytotoxic ITC, which indiscriminately kills rapidly dividing cells. Given our findings that GM-CSF signaling can drive HER2+ LC growth, we searched for drugs that could synergistically target and inhibit this signaling pathway. To do so, we performed a chemical genetics screen using Lepto cells treated with compounds from the LOPAC-1280 library (Scheme; Suppl. Fig. 6A). Specifically, GFP-labeled Lepto cells were seeded in 384-well plates at 1,000 cells per well, and one day later, 0.1% DMSO (control) or one of three concentrations of each LOPAC-1280 compound (100 nM, 200 nM, or 500 nM) was added to cells. After 72 h, we analyzed cell viability using MitoTracker staining. Of all compounds tested, the pan-Aurora kinase inhibitor CCT137690 had the strongest effects, inhibiting Lepto cell viability by ~95% (Suppl. Fig. 6B–C and Supplementary Table 7). Dose–response analysis showed Lepto lines to be sensitive to CCT137690 at all concentrations tested with an IC50 value ~18 nM (Fig. 5B). CCT137690 (100 nM) treatment of cultured Lepto cells also significantly induced cell apoptosis based on surface Annexin-v staining (Fig. 5C and Suppl. Fig. 6D and E).
CCT137690 inhibits Aurora-A, B, and C kinases. Aurora-A participates in crosstalk with GM-CSF signaling to regulate effectors such as STAT5, AKT, and mTOR (Suppl. Fig. 7A), which reportedly promote Lepto cell proliferation and viability (46–48). We observed elevated Aurora-A expression levels (mRNA) in nodular HER2+ LC tissues relative to other primary and metastatic tumors and normal breast and brain tissues (Fig. 5A). Thus, we asked whether combining anti-GM-CSF neutralizing antibodies with CCT137690 (anti-GM-CSF+CCT137690) would antagonize Lepto tumor initiation, growth, and/or relapse. To evaluate effects on Lepto tumor initiation, we treated cultured Lepto cells for 24 h with DMSO (control), anti-GM-CSF neutralizing antibodies, CCT137690, or anti-GM-CSF+CCT137690 and then cultured them 7 days in conditions favoring tumorsphere formation. After 7 days, control cells developed numerous round tumorspheres, whereas cells treated with anti-GM-CSF antibodies, CCT137690, or anti-GM-CSF+CCT137690 formed fewer and smaller tumorspheres (Fig. 5D). CCK assays of the same tumorspheres showed that, relative to DMSO, all treatments reduced the proportion of sphere-initiating cells (Fig. 5E). Notably, combining anti-GM-CSF antibodies with CCT137690 reduced the proportion of live, tumorsphere-initiating cells by ~80%, an effect significantly greater than any single reagent. To assess effects on Lepto tumorsphere growth, we allowed untreated Lepto cells to form tumorspheres for 5 days and then treated them 2 days with DMSO (control), anti-GM-CSF antibodies, CCT137690, or anti-GM-CSF+CCT137690. CCK assays confirmed that, relative to DMSO, all treatments—and most significantly the combination treatment—reduced primary tumorsphere cell viability (Fig. 5F).
To assess treatment effects on relapse, we developed secondary tumorspheres from the primary tumorspheres assessed in Fig. 5F. Briefly, after treatment of primary tumorspheres with DMSO (control), anti-GM-CSF antibodies, CCT137690, or anti-GM-CSF+CCT137690, surviving cells were dissociated and allowed to form secondary tumorspheres for 12 days in standard stem cell medium only (Fig. 5D). We then dissociated the secondary tumorspheres and subjected the cells isolated from secondary tumorspheres to CCK assays and found significantly fewer viable cells in secondary tumorspheres pre-treated with anti-GM-CSF+CCT137690 relative to DMSO-treated controls. Tumorspheres treated with CCT137690, and anti-GM-CSF antibodies also generated fewer viable cells than DMSO-treated controls, but the effect was less robust than that observed for anti-GM-CSF+CCT137690 (Fig. 5D).
Finally, to evaluate these effects on HER2+ LC growth in vivo, we administered anti-GM-CSF+CCT137690 (as well as anti-GM-CSF and CCT137690 alone and vehicle control) to NOD/SCID mice on days 5, 10, and 15 after Lepto cell implantation (Fig. 5G). Compared to vehicle control or individual treatments (anti-GM-CSF and CCT137690), the anti-GM-CSF+CCT137690 combination treatment significantly reduced tumor progression as indicated by BLI analysis (Fig. 5H (Left)) and increased overall survival (Fig. 5H (Right) and Supplementary Table 3). Subsequent H&E staining of the sagittal brain sections from variously treated Lepto bearing NOD/SCID mice demonstrated that relative to vehicle treated Lepto bearing mice, anti-GM-CSF+CCT137690 treated as well as anti-GM-CSF and CCT137690 treated mice demonstrated decreased Lepto tumors in the brain stem as well as in other regions of the brain indicated by red arrows (Fig. 5I).
Discussion
CNS metastases from breast cancer occasionally spread to the parenchymal brain or leptomeninges (49–52). HER2+ breast cancer is the most common solid tumor origin of leptomeningeal metastasis (53–60). Once tumor cells reach leptomeninges, they may spread via the CSF (61). Thus, diagnoses can be made via positive cytology of aspirated CSF samples. However, in some cases, adherent nodular deposits develop on the surface of the brain, spinal cord, and spinal roots, allowing diagnosis based on MRI alone (15,22,61,62). The presence of nodular deposits is associated with the greatest suffering from headaches and intractable pain due to cranial and spinal nerve invasion (22,61,62).
Approximately 84% of breast cancers reportedly contain at least one genomic alteration that could be exploited as a treatment target (63), and genetic screens have identified promising therapeutic targets in breast cancer (64). However, only a few targets, including phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), AKT1, and ERBB2 (4,64) have been validated in clinical studies, and the success rate of these strategies is surprising low. Therefore, there is a need to identify additional targets and therapies that effectively target HER2+ LC tumors that metastasize to the leptomeninges. Furthermore, studies have shown that brain metastasis leads to astrocyte-mediated alterations in brain tissue around the tumor, which limit and can negatively impact intra-tumoral chemotherapeutic delivery (65). Thus, identification of drugs that can penetrate through the brain tissue surrounding the HER2+ LC tumor to effectively target growing HER2+ LC is urgently required, which in turn requires a better understanding of molecular mechanisms that govern migration of HER2+ LC tumor cells from the brain stem to the acellular leptomeningeal environment in the spinal cord and support HER2+ LC growth in such a acellular environment. To identify such therapies, we established and analyzed primary HER2+ LC patient-derived Lepto cells (37), which led to the discovery that OPCs found primarily in white matter inhibit HER2+ LC cell viability in vitro and in vivo. Furthermore, we go on to show that GM-CSF serves as an autocrine driver contributing to Lepto cell growth in vitro and in vivo. It is also important to note that while the effects of anti-GM-CSF neutralizing antibodies are significant, but the inhibition of GM-CSF signaling pathway is incomplete (Fig. 2F and 2H–J), suggesting that GM-CSF is not the sole driver HER2+ LC and there must be other signaling pathways enabling the growth of HER2+ LC growth in vivo in the leptomeninges. As evidence, we report that conditional GM-CSF overexpression partially blocks OPC-induced Lepto cell apoptosis in vitro, and that comparable effects seen in vivo are reversible by DOX-induced GM-CSF knockdown in implanted Lepto cells.
LC-MS-based analyses reported here identified the protease TPP1 as a candidate regulator of GM-CSF-mediated signaling in HER2+ LC. Interestingly, TPP1 reportedly functions as a lysosomal serine protease that serves as a non-specific lysosomal peptidase (66,67). TPP1 deficiency is associated with various fatal neurodegenerative diseases (68–72), such as neuronal ceroid lipofuscinoses; however, its role in inhibiting HER2+ LC tumor development in white matter has not been explored. In this study, we observed that extracellular GM-CSF levels dropped significantly when Lepto cells were either cultured with OPCs or treated with recombinant TPP1. In addition, quantification of GM-CSF levels in Lepto cell culture media and in serum derived from mice bearing xenograft Lepto tumors indicated that TPP1 secreted from OPCs degrades GM-CSF. Co-implantation of TPP1 depleted OPCs in Lepto derived tumor bearing NOD/SCID mice reversed the OPC-mediated inhibition of Lepto cell growth (relative to co-implantation with normal OPCs or shGFP-OPCs), suggesting that TPP1 derived from OPCs may degrade GM-CSF and antagonize growth of HER2+ LC tumors in leptomeningeal regions. We propose that intrathecal administration of recombinant TPP1 and/or inhibition of GM-CSF signaling may be a viable therapeutic option to target HER2+ LC growth. That idea is supported by our finding that administration of anti-GM-CSF neutralizing antibodies suppresses GM-CSF-mediated signaling and significantly impairs HER2+ LC development in vivo.
To identify additional drugs that suppress Lepto cell growth, we performed a chemical genetics screen using the LOPAC-1280 compound library. The strongest inhibitor of Lepto cell viability was CCT137690, a highly selective pan-Aurora kinase inhibitor. Interestingly, Aurora kinases regulate mitotic activities, such as centrosome maturation, spindle assembly, chromosome segregation, and cytokinesis (73–78), and their inhibitors have been extensively studied as novel anti-mitotic drug targets (79,80). Aurora-A kinase overexpression was observed in HER2+ LC patient derived tissues and cell lines. Interestingly, our unbiased chemical screen identified Aurora inhibitor I as well as targeted analyses identified MK5108, which are inhibitors of Aurora A kinase. MK-5108 (VX-689) which has entered clinical trials in the US, in patients with advanced and/or refractory solid tumors. Cell-Titre-Glo based dose titrations and comparison of IC50 values of CCT137690, Aurora inhibitor I and MK5108 (Fig. 5B and Suppl. Fig. 7B) showed that CCT137690 was more effective in Lepto cells. Hence, CCT137690 was further pursued for in vitro and in vivo combinatorial analyses along with anti GM-CSF antibodies in Fig. 5. Analysis presented in Fig. 5D–I indicates that CCT137690 and anti-GM-CSF neutralizing antibodies synergize to inactivate GM-CSF effectors and strongly inhibit primary and secondary Lepto tumorsphere initiation, growth, and relapse in vitro. Moreover, in xenograft mouse models, combination treatment with CCT137690 and anti-GM-CSF neutralizing antibodies antagonized Lepto tumor growth and augmented overall animal survival more potently than single treatment with CCT137690, TPP1, or anti-GM-CSF antibody, suggesting that comparable strategies could be used to target HER2+ LC tumors in patients. Future research is warranted to optimize ITC with TPP1, CCT137690, and/or anti-GM-CSF for application in the clinic.
In summary, we have identified and characterized neural niche-specific crosstalk between HER2+ LC tumors and OPCs residing predominantly in white matter. We showed that GM-CSF acts as an autocrine oncogenic driver of HER2+ LC growth in vivo and report that intrathecal administration of the protease TPP1, the selective pan-Aurora kinase inhibitor CCT137690, and/or anti-GM-CSF antibodies may be an potential strategy to treat HER2+ LC in the clinic.
Supplementary Material
Key points.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an oncogenic autocrine driver of HER2+ LC growth in vivo and in vitro.
The oligodendrocyte progenitor cell (OPC)-derived protease TPP1 degrades GM-CSF, decreasing GM-CSF signaling and suppressing HER2+ LC growth.
Synergistic inhibition of GM-CSF signaling via neutralizing anti-GM-CSF antibodies and pan-Aurora kinase inhibitor (CCT137690) significantly reduces development of HER2+ LC
Statement of significance.
This study characterizes molecular mechanisms that drive HER2+ leptomeningeal carcinomatosis and demonstrates the efficacy of anti-GM-CSF antibodies and pan-Aurora kinase inhibitors against this disease.
Acknowledgments:
The authors express their gratitude to the City of Hope Analytical Cytometry Core Facility. This work was made possible by the generous support of the City of Hope Department of Surgery and a grant from the United States Department of Defense Breast Cancer Research Program (W81XWH-19–1-0310). The RNA-seq data analyses were supported by the UND Genomics core. M.T. was supported by the University of North Dakota Start-up and P20GM104360 from the National Institutes of Health.
Footnotes
Competing interests: The authors declare no competing interests.
References
- 1.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 2012;32:4655–62 [PubMed] [Google Scholar]
- 2.Quigley MR, Fukui O, Chew B, Bhatia S, Karlovits S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg Rev 2013;36:377–82 [DOI] [PubMed] [Google Scholar]
- 3.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–7 [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Hoffmann AD, Liu H, Liu X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. npj Precision Oncology 2018;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol 2015;273:57–68 [DOI] [PubMed] [Google Scholar]
- 6.Assi HI, Mahmoud T, Saadeh FS, El Darsa H. Management of leptomeningeal metastasis in breast cancer. Clin Neurol Neurosurg 2018;172:151–9 [DOI] [PubMed] [Google Scholar]
- 7.Scott BJ, Oberheim-Bush NA, Kesari S. Leptomeningeal metastasis in breast cancer - a systematic review. Oncotarget 2016;7:3740–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niwinska A, Rudnicka H, Murawska M. Breast cancer leptomeningeal metastasis: propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med Oncol 2013;30:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayar G, Ejikeme T, Chongsathidkiet P, Elsamadicy AA, Blackwell KL, Clarke JM, et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget 2017;8:73312–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo JC, Kang MJ, Kim JE, Ahn JH, Jung KH, Gong G, et al. Clinical features and outcome of leptomeningeal metastasis in patients with breast cancer: a single center experience. Cancer Chemother Pharmacol 2013;72:201–7 [DOI] [PubMed] [Google Scholar]
- 11.Kodack DP, Askoxylakis V, Ferraro GB, Fukumura D, Jain RK. Emerging strategies for treating brain metastases from breast cancer. Cancer Cell 2015;27:163–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS Metastases in Breast Cancer: Old Challenge, New Frontiers. Clin Cancer Res 2013;19:6404–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin X, Mu P. Targeting Breast Cancer Metastasis. Breast cancer : basic and clinical research 2015;9:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DW, Lee KH, Kim JW, Keam B. Molecular Targeted Therapies for the Treatment of Leptomeningeal Carcinomatosis: Current Evidence and Future Directions. International journal of molecular sciences 2016;17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leal T, Chang JE, Mehta M, Robins HI. Leptomeningeal Metastasis: Challenges in Diagnosis and Treatment. Current cancer therapy reviews 2011;7:319–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeAngelis LM, Boutros D. Leptomeningeal metastasis. Cancer Invest 2005;23:145–54 [PubMed] [Google Scholar]
- 17.Groves MD. Leptomeningeal Disease. Neurosurg Clin N Am 2011;22:67-+ [DOI] [PubMed] [Google Scholar]
- 18.Waki F, Ando M, Takashima A, Yonemori K, Nokihara H, Miyake M, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. Journal of neuro-oncology 2009;93:205–12 [DOI] [PubMed] [Google Scholar]
- 19.Brower JV, Saha S, Rosenberg SA, Hullett CR, Robins HI. Management of leptomeningeal metastases: Prognostic factors and associated outcomes. J Clin Neurosci 2016;27:130–7 [DOI] [PubMed] [Google Scholar]
- 20.Mack F, Baumert BG, Schafer N, Hattingen E, Scheffler B, Herrlinger U, et al. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev 2016;43:83–91 [DOI] [PubMed] [Google Scholar]
- 21.Chowdhary S, Chamberlain M. Leptomeningeal metastases: current concepts and management guidelines. Journal of the National Comprehensive Cancer Network : JNCCN 2005;3:693–703 [DOI] [PubMed] [Google Scholar]
- 22.Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin 2003;21:25-+ [DOI] [PubMed] [Google Scholar]
- 23.Larionov AA. Current Therapies for Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Patients. Front Oncol 2018;8:89- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeckle KA, Phuphanich S, van den Bent MJ, Aiken R, Batchelor T, Campbell T, et al. Intrathecal treatment of neoplastic meningitis due to breast cancer with a slow-release formulation of cytarabine. Brit J Cancer 2001;84:157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamberlain MC, Kormanik PRN. Carcinomatous meningitis secondary to breast cancer: Predictors of response to combined modality therapy. Journal of neuro-oncology 1997;35:55–64 [DOI] [PubMed] [Google Scholar]
- 26.Park MJ. Durable Response of Leptomeningeal Metastasis of Breast Cancer to Salvage Intrathecal Etoposide After Methotrexate: A Case Report and Literature Review. Am J Case Rep 2015;16:524–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwinska A, Rudnicka H, Murawska M. Breast cancer leptomeningeal metastasis: the results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin Breast Cancer 2015;15:66–72 [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Melisko ME, Magbanua MJ, Kablanian AT, Scott JH, Rugo HS, et al. Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res Treat 2015;154:339–49 [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto S, Iwasaki M, Ito M, Niiya Y, Itosaka H, Mabuchi S, et al. [Radiotherapy for Alleviation of Paraparesis due to Leptomeningeal and Cauda Equina Metastasis of HER2-Positive Breast Cancer: A Case Report]. No Shinkei Geka 2015;43:819–23 [DOI] [PubMed] [Google Scholar]
- 30.Drappatz J, Batchelor TT. Leptomeningeal neoplasms. Curr Treat Options Neurol 2007;9:283–93 [DOI] [PubMed] [Google Scholar]
- 31.Hermann B, Hultenschmidt B, Sautter-Bihl ML. Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlenther Onkol 2001;177:195–9 [DOI] [PubMed] [Google Scholar]
- 32.Pentheroudakis G, Pavlidis N. Management of leptomeningeal malignancy. Expert Opin Pharmacother 2005;6:1115–25 [DOI] [PubMed] [Google Scholar]
- 33.Silvani A, Caroli M, Gaviani P, Fetoni V, Merli R, Riva M, et al. Neoplastic meningitis from solid tumors: a prospective clinical study in lombardia and a literature review on therapeutic approaches. J Drug Deliv 2013;2013:147325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Zhang Y, Ding J, Wang M, Li N, Yang H, et al. Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget 2018;9:2705–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenyon SM, Flieth TL, Algeciras-Schimnich A. Comparing the performance of CA 15–3 CSF to cytology in a cohort of patients with breast cancer leptomeningeal metastasis. Clin Biochem 2018;58:122–4 [DOI] [PubMed] [Google Scholar]
- 36.Morikawa A, Jordan L, Rozner R, Patil S, Boire A, Pentsova E, et al. Characteristics and Outcomes of Patients With Breast Cancer With Leptomeningeal Metastasis. Clin Breast Cancer 2017;17:23–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhan A, Ansari KI, Chen MY, Jandial R. Inhibition of Jumonji histone demethylases selectively suppresses HER2+ breast leptomeningeal carcinomatosis growth via inhibition of GM-CSF expression. Cancer Res 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ansari KI, Bhan A, Liu X, Chen MY, Jandial R. Astrocytic IGFBP2 and CHI3L1 in cerebrospinal fluid drive cortical metastasis of HER2+breast cancer. Clin Exp Metastasis 2020;37:401–12 [DOI] [PubMed] [Google Scholar]
- 39.Choy C, Ansari KI, Neman J, Hsu S, Duenas MJ, Li H, et al. Cooperation of neurotrophin receptor TrkB and Her2 in breast cancer cells facilitates brain metastases. Breast Cancer Res 2017;19:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jandial R, Choy C, Levy DM, Chen MY, Ansari KI. Astrocyte-induced Reelin expression drives proliferation of Her2(+) breast cancer metastases. Clin Exp Metastasis 2017;34:185–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016;89:37–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 2019;133:697–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patnaik MM, Sallman DA, Mangaonkar A, Heuer R, Hirvela J, Zblewski D, et al. Phase 1 study of lenzilumab, a recombinant anti-human GM-CSF antibody, for chronic myelomonocytic leukemia (CMML). Blood 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song J, Tan H, Perry AJ, Akutsu T, Webb GI, Whisstock JC, et al. PROSPER: an integrated feature-based tool for predicting protease substrate cleavage sites. PLoS One 2012;7:e50300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wlodawer A, Durell SR, Li M, Oyama H, Oda K, Dunn BM. A model of tripeptidyl-peptidase I (CLN2), a ubiquitous and highly conserved member of the sedolisin family of serine-carboxyl peptidases. BMC structural biology 2003;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung LY, Tseng JT, Lee YC, Xia W, Wang YN, Wu ML, et al. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res 2008;36:4337–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang Y-s. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Molecular Cancer 2018;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsha A, Belkhiri A, Goff L, El-Rifai W. Aurora kinase A in gastrointestinal cancers: time to target. Molecular Cancer 2015;14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen TW, Jan IS, Chang DY, Lin CH, Chen IC, Chen HM, et al. Systemic treatment of breast cancer with leptomeningeal metastases using bevacizumab, etoposide and cisplatin (BEEP regimen) significantly improves overall survival. Journal of neuro-oncology 2020;148:165–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumthekar P, Tang SC, Brenner AJ, Kesari S, Piccioni DE, Anders C, et al. ANG1005, a Brain-Penetrating Peptide-Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin Cancer Res 2020;26:2789–99 [DOI] [PubMed] [Google Scholar]
- 51.Scott BJ, Kesari S. Leptomeningeal metastases in breast cancer. Am J Cancer Res 2013;3:117–26 [PMC free article] [PubMed] [Google Scholar]
- 52.Shigekawa T, Takeuchi H, Misumi M, Matsuura K, Sano H, Fujiuchi N, et al. Successful treatment of leptomeningeal metastases from breast cancer using the combination of trastuzumab and capecitabine: a case report. Breast Cancer 2009;16:88–92 [DOI] [PubMed] [Google Scholar]
- 53.Angus L, Martens JWM, van den Bent MJ, Sillevis Smitt PAE, Sleijfer S, Jager A. Novel methods to diagnose leptomeningeal metastases in breast cancer. Neuro Oncol 2019;21:428–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuppari L, Reccia P, Burei M, Cervino AR, Evangelista L. 18F-Choline PET/CT in Leptomeningeal Breast Cancer Metastases. Clin Nucl Med 2019;44:e96–e7 [DOI] [PubMed] [Google Scholar]
- 55.Ha B, Chung SY, Kim YJ, Gwak HS, Chang JH, Lee SH, et al. Effects of Postoperative Radiotherapy on Leptomeningeal Carcinomatosis or Dural Metastasis after Resection of Brain Metastases in Breast Cancer Patients. Cancer Res Treat 2017;49:748–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung JM, Kim S, Joo J, Shin KH, Gwak HS, Lee SH. Incidence and risk factors for leptomeningeal carcinomatosis in breast cancer patients with parenchymal brain metastases. J Korean Neurosurg Soc 2012;52:193–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaidar-Person O, Deal AM, Anders CK, Ewend MG, Dees EC, Camporeale J, et al. The incidence and predictive factors for leptomeningeal spread after stereotactic radiation for breast cancer brain metastases. Breast J 2018;24:424–5 [DOI] [PubMed] [Google Scholar]
- 58.Pan Z, Yang G, Yuan T, Wang Y, Pang X, Gao Y, et al. ‘Hot cross bun’ sign with leptomeningeal metastases of breast cancer: a case report and review of the literature. World J Surg Oncol 2015;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricciardi GRR, Russo A, Franchina T, Schifano S, Mastroeni G, Santacaterina A, et al. Efficacy of T-DM1 for leptomeningeal and brain metastases in a HER2 positive metastatic breast cancer patient: new directions for systemic therapy - a case report and literature review. BMC Cancer 2018;18:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tham YL, Hinckley L, Teh BS, Elledge R. Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: a case report. Clin Breast Cancer 2006;7:164–6 [DOI] [PubMed] [Google Scholar]
- 61.Chamberlain MC. Leptomeningeal metastasis. Current opinion in oncology 2010;22:627–35 [DOI] [PubMed] [Google Scholar]
- 62.Lin N, Dunn IF, Glantz M, Allison DL, Jensen R, Johnson MD, et al. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: a retrospective, case-controlled study Clinical article. J Neurosurg 2011;115:730–6 [DOI] [PubMed] [Google Scholar]
- 63.Vasan N, Yelensky R, Wang K, Moulder S, Dzimitrowicz H, Avritscher R, et al. A targeted next-generation sequencing assay detects a high frequency of therapeutically targetable alterations in primary and metastatic breast cancers: implications for clinical practice. Oncologist 2014;19:453–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Onesti CE, Vicier C, André F. What to expect from high throughput genomics in metastatic breast cancers? The Breast 2015;24:S19–S22 [DOI] [PubMed] [Google Scholar]
- 65.Uzunalli G, Dieterly AM, Kemet CM, Weng H-Y, Soepriatna AH, Goergen CJ, et al. Dynamic transition of the blood-brain barrier in the development of non-small cell lung cancer brain metastases. Oncotarget 2019;10:6334–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haney MJ, Klyachko NL, Harrison EB, Zhao Y, Kabanov AV, Batrakova EV. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv Healthc Mater 2019;8:e1801271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiemhoefer A, Stargardt A, van der Linden WA, Renner MC, van Kesteren RE, Stap J, et al. Tripeptidyl Peptidase II Mediates Levels of Nuclear Phosphorylated ERK1 and ERK2. Mol Cell Proteomics 2015;14:2177–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collier AM, Nemtsova Y, Kuber N, Banach-Petrosky W, Modak A, Sleat DE, et al. Lysosomal protein thermal stability does not correlate with cellular half-life: global observations and a case study of tripeptidyl-peptidase 1. Biochem J 2020;477:727–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson TB, Cain JT, White KA, Ramirez-Montealegre D, Pearce DA, Weimer JM. Therapeutic landscape for Batten disease: current treatments and future prospects. Nat Rev Neurol 2019;15:161–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lukacs Z, Nickel M, Murko S, Nieves Cobos P, Schulz A, Santer R, et al. Validity of a rapid and simple fluorometric tripeptidyl peptidase 1 (TPP1) assay using dried blood specimens to diagnose CLN2 disease. Clin Chim Acta 2019;492:69–71 [DOI] [PubMed] [Google Scholar]
- 71.Smith PK, Sen MG, Fisher PR, Annesley SJ. Modelling of Neuronal Ceroid Lipofuscinosis Type 2 in Dictyostelium discoideum Suggests That Cytopathological Outcomes Result from Altered TOR Signalling. Cells 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong LC, Hsu CJ, Lee WT. Perampanel attenuates myoclonus in a patient with neuronal ceroid lipofuscinoses type 2 disease. Brain Dev 2019;41:817–9 [DOI] [PubMed] [Google Scholar]
- 73.Dhanasekaran K, Bose A, Rao VJ, Boopathi R, Shankar SR, Rao VK, et al. Unraveling the role of aurora A beyond centrosomes and spindle assembly: implications in muscle differentiation. FASEB J 2019;33:219–30 [DOI] [PubMed] [Google Scholar]
- 74.Fielding AB, Dobreva I, Dedhar S. Beyond focal adhesions: integrin-linked kinase associates with tubulin and regulates mitotic spindle organization. Cell Cycle 2008;7:1899–906 [DOI] [PubMed] [Google Scholar]
- 75.Kobayashi A, Hashizume C, Dowaki T, Wong RW. Therapeutic potential of mitotic interaction between the nucleoporin Tpr and aurora kinase A. Cell Cycle 2015;14:1447–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Magnaghi-Jaulin L, Eot-Houllier G, Gallaud E, Giet R. Aurora A Protein Kinase: To the Centrosome and Beyond. Biomolecules 2019;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mielgo A, Seguin L, Huang M, Camargo MF, Anand S, Franovic A, et al. A MEK-independent role for CRAF in mitosis and tumor progression. Nat Med 2011;17:1641–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.So C, Seres KB, Steyer AM, Monnich E, Clift D, Pejkovska A, et al. A liquid-like spindle domain promotes acentrosomal spindle assembly in mammalian oocytes. Science 2019;364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faisal A, Vaughan L, Bavetsias V, Sun C, Atrash B, Avery S, et al. The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo. Mol Cancer Ther 2011;10:2115–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cirak Y, Furuncuoglu Y, Yapicier O, Aksu A, Cubukcu E. Aurora A overexpression in breast cancer patients induces taxane resistance and results in worse prognosis. J BUON 2015;20:1414–9 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.