A third mRNA-based booster vaccination is the currently favoured strategy to maintain protection against SARS-CoV-2 infection. Yet, significant waning of specific immunity within 6 months after two doses,1 along with a higher incidence of breakthrough infections associated with the time elapsed since the second dose,2, 3 raise concerns regarding the durability of immunity also after the booster vaccination.
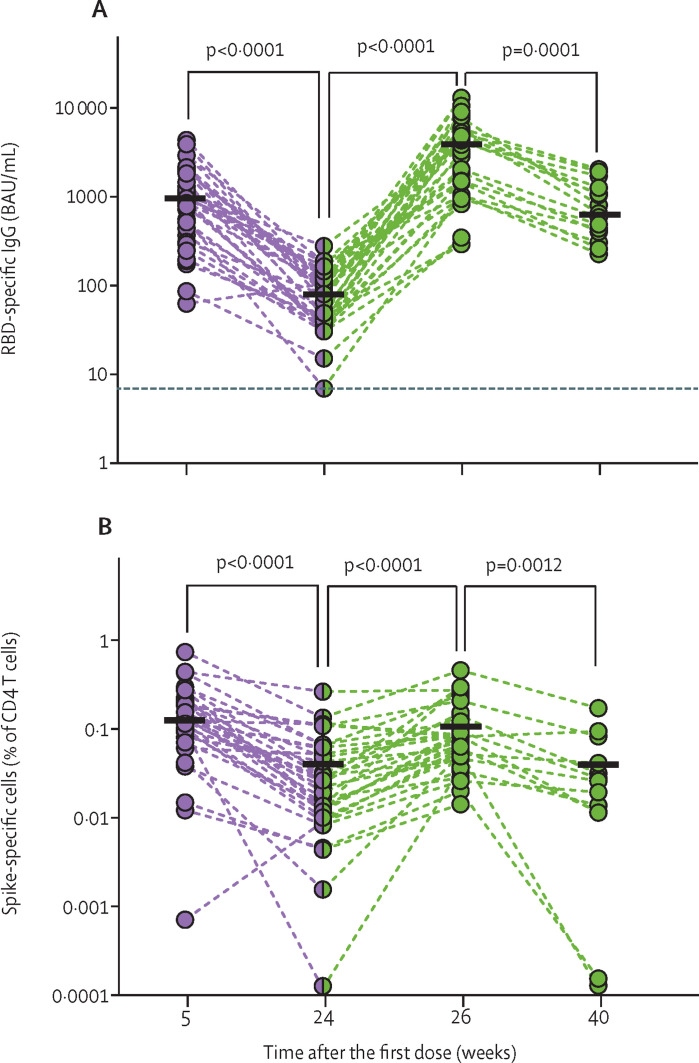
We compared the specific humoral and cellular responses (figure ; appendix pp 10–13) after three versus two BNT162b2 (Pfizer-BioNTech) doses in a cohort of adults older than 80 years (median age 83 years [IQR 81–86]; appendix pp 3–4) at risk for severe COVID-19 and immune senescence. Our data demonstrate the induction of marginally higher spike S1-specific blood IgG concentrations 2 weeks after three than after two doses (appendix p 5). By contrast, functionally relevant receptor binding domain-specific IgG (figure A) and SARS-CoV-2-neutralising antibody (appendix p 5) titres were substantially increased after three compared with two doses, reflecting enhanced antibody production or affinity maturation.
Figure.
Humoral and cellular SARS-CoV-2 immunity in donors older than 80 years after two and three doses of BNT162b2
Immune response kinetics were followed in older adults in the course of vaccinations with BNT162b2 (second vaccination occurred 3 weeks and third vaccination occurred a median of 24 weeks [IQR 23–25] after first vaccination). Green indicates data related to the third dose of BNT162b2. Each symbol represents data of one donor at one timepoint. Horizontal lines indicate median values of datapoints in each column. p values were determined by two-tailed Wilcoxon matched-pairs signed rank test. (A) SARS-CoV-2 RBD-specific serum IgG levels; for weeks 5, 24, 26, and 40, number of participants was 35, 36, 34, and 15, respectively. The dotted horizontal line indicates the cutoff for antibody positivity at 7·1 BAU/mL. (B) Frequencies of SARS-CoV-2 spike-specific CD4 T cells identified as CD40 ligand-positive, interferon γ-positive CD4 T cells after overnight stimulation of peripheral blood mononuclear cells with SARS-CoV-2 spike peptides; for weeks 5, 24, 26, and 40, number of participants was 34, 35, 33, and 13, respectively. RBD=receptor-binding domain. BAU=binding antibody units.
By contrast, spike-specific CD4 T-cell frequencies reached similar levels after two and three doses (figure B; appendix p 5). After the respective acute response, frequencies returned to approximately pre-third vaccination levels, with no significant differences in the rate of decline after the second and third vaccinations (figure B; appendix p 6). Quantified cytoplasmic expression of the effector cytokine interferon γ (IFNγ) indicated functional enhancement of spike-specific T cells upon second but not further upon third vaccination, while more cytoplasmic IFNγ was found in spike-specific CD4 T cells from adults older than 80 years who had recovered from COVID-19 (appendix p 5). Thus, even a third BNT162b2 dose failed to induce durably enhanced quantities of spike-specific T cells and a functional quality reached after natural infection. Neither age nor comorbidities were significantly correlated with the observed immune response, perhaps due to the limited size of our cohort (appendix pp 7–9).
Concentrations of S1-specific IgG and neutralising antibodies also declined from the acute responses at weeks 5 and weeks 26, but at a lower rate and with an extended half-life after the third (week 40) compared with the second (week 24) dose (figure A; appendix pp 5–6), yielding more persistent, enhanced IgG quantity or quality after the third than after the second vaccination.
We conclude that a third dose of BNT162b2 in older adults, while establishing immunity in primary non-responders,4 induces a durably escalated humoral response in the bulk of vaccinees for at least 3 months, indicating longer lasting humoral immunity. In a younger cohort, this boost also led to a strong increase of neutralising antibodies against the omicron (B.1.1.529) variant and protection from infection with the omicron variant.5, 6 Although neutralising antibody data for omicron are not yet available for our cohort, the strong rise in titres of neutralising antibodies against the BavPat1/2020 isolate used in our neutralisation assay (appendix p 5) suggests better neutralisation against omicron by the booster dose than for the second dose, as also demonstrated by others,7 at least in the short term. The level of T-cell immunity to SARS-CoV-2 in peripheral blood required for protection is still not established, although peripheral T cells induced by BNT162b2 apparently react well against the omicron variant.8 As for our cohort, our data show two important aspects of a third compared with a second dose—namely, peak virus-specific T-cell frequencies were not further increased by a third dose, and average per-cell production of IFNγ remained unaltered and was still remarkably lower than in recovered donors of a similar age. Thus, at least in older adults, the durability and quality of vaccine-induced immunity should be considered in the recommendation of booster vaccinations, in addition to the severity of breakthrough SARS-CoV-2 infections caused by current and future viral mutants.
We declare no competing interests. This research was supported in part by grants from the Government of Hesse (Pandemie Netzwerk), Germany, by the Else-Kroener-Fresenius-Stiftung, Germany, by the Senate of Berlin, by the Deutsche Forschungsgemeinschaft (grant LO 396/8-1 to ML), and by the German Center for Infection Research, Section Emergency Vaccines (FKZ:8033801809 to VK). AJR-O and ARS contributed equally. HEM, CK, and ML contributed equally as senior authors.
Supplementary Material
References
- 1.Tober-Lau P, Schwarz T, Vanshylla K, et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir Med. 2021;9:e104–e105. doi: 10.1016/S2213-2600(21)00456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Olmedo AJ, Schulz AR, Hochstatter S, et al. Induction of robust cellular and humoral immunity against SARS-CoV-2 after a third dose of BNT162b2 vaccine in previously unresponsive older adults. Nat Microbiol. 2022;7:195–199. doi: 10.1038/s41564-021-01046-z. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Collier AY, Rowe M, et al. Comparable neutralization of the SARS-CoV-2 omicron BA.1 and BA.2 variants. medRxiv. 2022 doi: 10.1101/2022.02.06.22270533. published online Feb 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai CG, Maragakis LL, Connolly S, et al. Booster protection against omicron infection in a highly vaccinated cohort. medRxiv. 2022 doi: 10.1101/2022.02.24.22271347. published online Feb 26. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanshylla K, Tober-Lau P, Gruell H, et al. Durability of omicron-neutralising serum activity after mRNA booster immunisation in older adults. Lancet Infect Dis. 2022;22:445–446. doi: 10.1016/S1473-3099(22)00135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Cai C, Grifoni A, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the omicron variant. Nat Med. 2022;28:472–476. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.