Abstract
Objective: Chemical and mechanical injury in myelomeningocele (MMC) during the fetal life results in functional disorders of multiple organs. Prenatal MMC repair reduces sequelae of spinal cord injury.
Design: Histopathological evaluation of dura mater and skin specimens to assess the severity of inflammatory changes.
Setting: Histopathological laboratory and operated patients.
Participants: 45 cases (Group I)-intrauterine surgery due to MMC and 42 cases (Group II)-postnatal surgery.
Outcome measures: Specimens of the skin and of the dura mater adjacent directly to the uncovered section of the spinal cord were collected for assessment. The specimens were histopathologically evaluated to assess the severity of inflammatory changes.
Results: The analysis of the severity of inflammatory changes in the skin and the dura mater showed only small lymphocytic infiltration in 5 fetuses (Group I). Medium and large infiltration in the skin and the dura mater was found in all children who underwent postnatal surgery (Group II). Lymphocytic and granulocytic infiltration in the skin and the dura mater were statistically significantly more prevalent in children who underwent postnatal surgery compared to the group of children who underwent prenatal surgery (P < 0.000003).
Conclusions: By reducing the time of exposure to damaging factors, prenatal MMC repair statistically reduces the risk of inflammatory changes in the exposed spinal cord and spinal nerves. Prenatal closure of spina bifida before 24 week of gestation does not reduce the severity of inflammatory changes in the exposed spinal cord.
Keywords: Myelomeningocele, Prenatal surgery, Inflammatory changes
Introduction
Myelomeningocele (MMC), or spina bifida, is the most prevalent malformation of the nervous system and the second most frequent congenital failure after heart defects. The worldwide prevalence ranges between 0.3 and 5 per 1000 of live births. This differentiation in the number of deliveries of children with MMC is most probably associated with diverse economic level, acceptance of termination of pregnancy and availability of medical services.1 The malformed spine impairs spinal function, thus leading to disorders in innervation of locomotor, urinary and gastrointestinal systems. Impaired development of the spinal cord, vertebrae and dorsal integuments results in malformation of the telencephalon and impaired drainage of the cerebrospinal fluid (CSF) due to a tethered spinal cord. In most cases, abnormal circulation of the CSF leads to hydrocephalus.2 According to the two-hit theory by Heffez, spinal cord injury occurs in two stages. The first stage is associated with abnormal neurulation of the spinal cord, whereas the second stage results from mechanical injury to uncovered spinal cord due to fetal movements in the uterus and the toxic effect of the amniotic fluid on the spinal cord.
The theory has been supported by studies on animal models.3 The aim of prenatal closure of MMC is to reduce the second hit effects. The first repair of spina bifida with an open uterine approach was performed in 1997 in Nashville by J.P. Bruner.4 It was followed by MMC repair in the human fetus in the open uterus performed at the Children’s Hospital of Philadelphia (CHOP) in 1998. Currently, such surgical procedures are performed in many centers in Europe and in both Americas.5,6
The assessment of the results showed that prenatal MMC repair reduced statistically the incidence of hydrocephalus, improved motor functions of the lower limbs and increased the chance for social dryness.7,8
The aim of the study was to establish whether inflammatory changes in the skin and in the dura mater adjacent to the uncovered spinal cord in fetuses with spina bifida exacerbated during pregnancy.
Materials and methods
The material was obtained during spina bifida repair in the lumbosacral section. Spina bifida extended from L1–L2 to S1–S2 (not higher than L1 and not lower than S2). The procedure was performed intrauterinally (45 cases – Group I) and postnatally (42 cases – Group II).
Sample collection for this study was performed in a prospective manner. However, indication for therapy and decision which particular therapy was applied was purely based on medical reasons and was not effected by the study.
The approval of the Bioethics Committee was not required.
All patients who met the Management of Myelomeningocele Study (MOMS) criteria were eligible for prenatal MMC surgery. Postnatal surgery was conducted in those children who did not meet the MOMS criteria for intrauterine procedure, or in children whose parents did not give consent for prenatal surgery despite the fact that the criteria had been met. Patients in whom the features of intrauterine infection were found or in whom the analysis of the amniotic fluid showed the features of infection were excluded from the study.
Prenatal and postnatal surgical procedures with sample collection were performed between 2016 and 2018 by the same team of surgeons and gynaecologists.
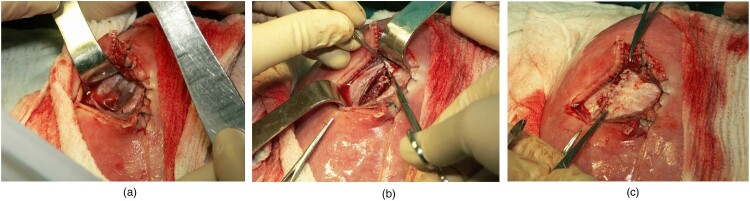
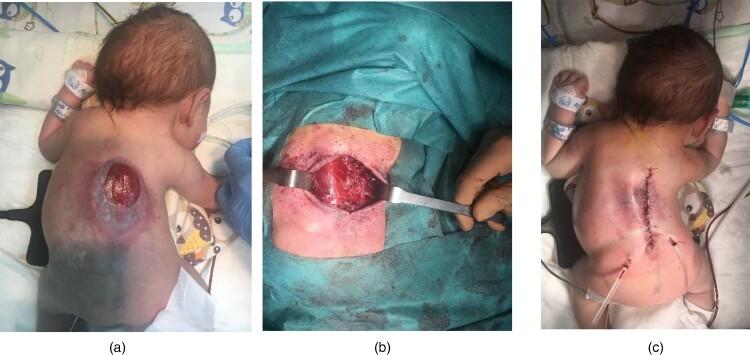
MMC repair involves dissection of hernial sac and then releasing the vertebral neural plate placed at the entry of spina bifida to ensure free movement of the neural plate in the spinal canal. The spinal cord is then covered with the dura. The next stage of the procedure involves mobilization of dorsal muscles symmetrically on both sides. Such a procedure allows repositioning of the relaxed muscles to the posterior midline. The translocated muscles are sutured over the dura, thus forming one of the most important protective layers. As a result, the spinal cord is well protected against another injury in the future. Symmetrical translocation of dorsal muscles reduces the risk of developing scoliosis in the future. Skin plasty is the final stage of the procedure (Fig. 1A–C). The subsequent stages of surgery performed during intrauterine MMC repair are the same as those performed during surgery after delivery (Fig. 2A–C).
Figure 1.
In Utero MMC repair. A – Dissection of hernial sac; B – Mobilization of dorsal muscles; C – Skin plasty.
Figure 2.
Postnatal MMC repair. A – Hernial sac; B – Mobilization of dorsal muscles; C – Skin plasty.
Intrauterine closure of MMC was performed by open fetal surgery (OFS) between 22 and 25 weeks of gestation. All the subjects who underwent postnatal and prenatal surgery were delivered by Cesarean section. The fetal age of the subjects was between 36 and 38 weeks of gestation. Closure of MMC in all subjects from this group was performed within 12 hours following delivery. During the prenatal and postnatal spina bifida repair, specimens of the skin and of the dura mater adjacent directly to the uncovered section of the spinal cord were collected for assessment. The samples obtained were not required for closure or plasty of MMC. Routinely, during the prenatal and postnatal spina bifida repair, excessive fragments of the skin and of the dura mater that are not needed for the reconstruction of consecutive layers covering the spinal cord are destroyed (utilized). In our study, excessive fragments were preserved for histological examination.
The resected tissues were fixed in buffered formalin immediately upon collection. The specimens were embedded in paraffin blocks, cut into 4 µm sections, mounted on slides and deparaffinized at 60°C for 5 minutes. The specimens were routinely stained with hematoxylin and eosin. The material was finally mounted in DPX medium.
The specimens of the skin and the dura mater were histologically evaluated to assess the stage of inflammatory lesions. Lymphocytes and granulocytes were counted for each section in three large high-power fields at 400× magnification.
Patients in whom the features of intrauterine infection were found or in whom the analysis of the amniotic fluid showed the features of infection were not assessed for the intensity of inflammatory changes in the skin and the dura mater.
The following study groups were distinguished:
Group I – specimens were taken during intrauterine surgery
Depending on the time of the procedure two subgroups were distinguished:
Subgroup IA – surgery was performed between 22 and 23 weeks of gestation
Subgroup IB – surgery was performed between 24 and 25 weeks of gestation
Group II – specimens were taken during the surgical procedure on day 1 after delivery
The aim of the division patients from Group I into 2 subgroups was to assess whether the time of prenatal MMC repair affected the exacerbation of inflammatory lesions of the skin and the dura mater.
Intensity of inflammatory lesions in all specimens of the skin and the dura mater was assessed with the use of a three-stage scheme:
Stage I:
− small lymphocytic infiltration (0–15 lymphocytes/high power field – HPF)
− small granulocytic infiltration (0–15 granulocytes/HPF)
Stage II:
− medium lymphocytic infiltration (16–30 lymphocytes/HPF)
− medium granulocytic infiltration (16–30 granulocytes/HPF)
Stage III:
− large lymphocytic infiltration (>30 lymphocytes/HPF)
− large granulocytic infiltration (>30 granulocytes/ HPF)
The obtained results were statistically analyzed and the chi square test was used to assess the relations between individual variables and Z-Test for proportions was used to compare proportion values in particular groups.
Results
The assessment of the severity of inflammatory changes in the skin showed the presence of small lymphocytic infiltration (0–15 lymphocytes) in two fetuses in subgroup IA and in three fetuses in subgroup IB. No small lymphocytic infiltration was observed in any subject from Group II.
Medium lymphocytic infiltration (16–30 lymphocytes) was found in one fetus in subgroup IA, in two fetuses in subgroup IB and in seven patients in Group II. No large lymphocytic infiltration was noted in the skin in any subgroup (IA or IB) of fetuses. However, they were found in 35 children from Group II.
No statistical significance related to small and medium lymphocytic infiltration was found between IA and IB subgroups. Nevertheless, it showed a statistically higher prevalence of lymphocytic infiltration (medium and large) in children who underwent postnatal surgery (P < 0.005).
The comparison of the prevalence of medium and large skin infiltration in children operated postnatally showed that the occurrence of large lymphocytic infiltration was statistically more prevalent compared to middle lymphocytic infiltration (P < 0.0006) (Table 1).
Table 1. Lymphocytic infiltration in the skin.
| Inflammatory changes – skin | Number of patients in groups | Z-test | |||
|---|---|---|---|---|---|
| Lymphocytes | Group IA | Group IB | Group IA + IB | Group II | P-values: IA + IB/II |
| Small (0–15) | 2 | 3 | 5 | 0 | P < 0.005 |
| Medium (16–30) | 1 | 2 | 3 | 7 | P < 0.005 |
| Large >30 | 0 | 0 | 0 | 35 | P < 0.000006 |
| Total | 3 | 5 | 8 | 42 | |
The assessment of the severity of inflammatory changes in the skin, comprising the presence of granulocytes, showed medium infiltration (16–30 granulocytes) in 10 patients and large infiltration in 32 patients from Group II.
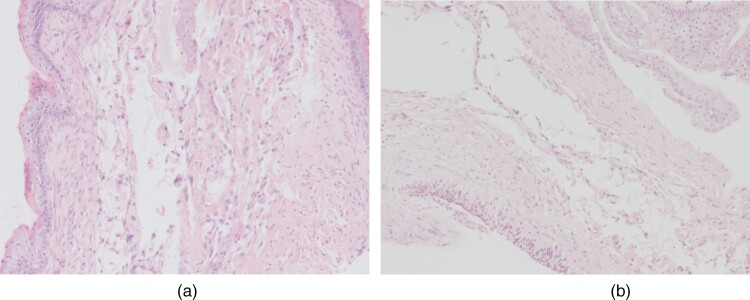
No granulocytic skin infiltration was observed in any operated fetus. (Fig. 3).
Figure 3.
Absence of inflammatory infiltration in the skin (A) and the dura mater (B).
The comparison of the severity of the granulocytic infiltration in the skin in children who underwent postnatal surgery showed that large infiltration was statistically more frequent compared to medium granulocytic infiltration (P < 0.0006) (Table 2).
Table 2. Granulocytic infiltration in the skin.
| Inflammatory changes – skin | Number of patients in groups | Z-test | |||
|---|---|---|---|---|---|
| Granulocytes | Group IA | Group IB | Group IA + IB | Group II | P-values: IA + IB/II |
| Small (0–15) | 0 | 0 | 0 | 0 | |
| Medium (16–30) | 0 | 0 | 0 | 10 | P < 0.000005 |
| Large >30 | 0 | 0 | 0 | 32 | P < 0.000003 |
| Total | 0 | 0 | 0 | 42 | |
The assessment of the severity of inflammatory changes in the dura mater showed small lymphocytic infiltration in two fetuses in subgroup IA and in three fetuses in subgroup IB. The embryos were the same fetuses in which small skin infiltration was observed.
Small lymphocytic infiltration was not observed in the dura mater in any patient who underwent postnatal surgery (Group II). Middle lymphocytic infiltration (16–30 lymphocytes) was observed in 11 patients from Group II, whereas no such infiltration was found in any operated fetus.
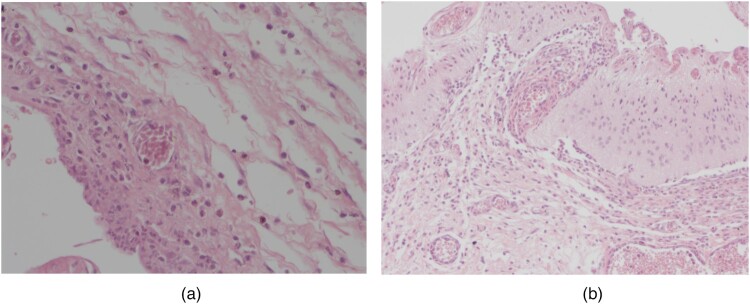
Also, large infiltration of the dura mater was absent in all operated fetuses, whereas such infiltration was observed in 31 patients in the postnatal group (Fig. 4).
Figure 4.
Lymphocytic–granulocytic infiltration in the skin (A) and the dura mater (B).
No statistical significance related to small and medium lymphocytic infiltration in the dura mater was found between IA and IB subgroups. However, it indicated statistically higher prevalence of medium and large lymphocytic infiltration in the dura mater in children who underwent postnatal repair (Group II) (P < 0.000003).
The analysis of the prevalence of medium and large lymphocytic infiltration in the dura mater in children who underwent postnatal surgery showed that large lymphocytic infiltration was statistically more prevalent compared to middle lymphocytic infiltration (P < 0.0006) (Table 3).
Table 3. Lymphocytic infiltration in the dura mater.
| Inflammatory changes – dura | Number of patients in groups | Z-test | |||
|---|---|---|---|---|---|
| Lymphocytes | Group IA | Group IB | Group IA + IB | Group II | P-values: IA + IB/II |
| Small (0-15) | 2 | 3 | 5 | 0 | P < 0.005 |
| Medium (16-30) | 0 | 0 | 0 | 11 | P < 0.005 |
| Large >30 | 0 | 0 | 0 | 31 | P < 0.000003 |
| Total | 2 | 3 | 5 | 42 | |
The assessment of the severity of inflammatory changes in the dura mater, comprising the presence of granulocytes, showed no granulocytic infiltration in any fetus (Fig. 3).
Granulocytic infiltration in the dura mater was present only in children who underwent postnatal surgery. Medium infiltration (16–30 granulocytes) was observed in 18 patients and large infiltration was found in the remaining 24 patients in Group II (Fig. 4).
The comparison of the severity of granulocytic infiltration of the dura mater in children who underwent postnatal surgery showed that large infiltration was statistically more prevalent compared to medium granulocytic infiltration (P < 0.0006) (Table 4).
Table 4. Granulocytic infiltration in the dura mater.
| Inflammatory changes – dura | Number of patients in groups | Z-test | |||
|---|---|---|---|---|---|
| Granulocytes | Group IA | Group IB | Group IA + IB | Group II | P-values: IA + IB/II |
| Small (0–15) | 0 | 0 | 0 | 0 | P < 0.005 |
| Medium (16–30) | 0 | 0 | 0 | 18 | P < 0.00005 |
| Large >30 | 0 | 0 | 0 | 24 | P < 0.000003 |
| Total | 0 | 0 | 0 | 42 | |
Discussion
During fetal life, the uncovered spinal cord is exposed to chemical and mechanical injury. Histopathological evaluation of the spinal cord in human embryos with MMC subjected to therapeutic abortions after prenatal diagnosis showed some degenerative changes of the nervous tissue and focal hemorrhage in the uncovered section of the spinal cord. Degenerative changes were due to permanent chemical injury to the exposed spinal cord by the amniotic fluid, whereas focal hemorrhage resulted most probably from mechanical injury to the spinal cord during fetal life and the passage through the birth canal.3
No nervous tissue specimens could not be taken for histopathological evaluation due to medical reasons. Some excessive fragments of the skin and the dura mater directly adjacent to the uncovered spinal cord were collected for the analysis.
Histopathological assessment of the collected tissues indicated the presence of large lymphocytic and granulocytic infiltration in all children who underwent postnatal surgery. The subjects were delivered by Cesarean section, hence inflammatory changes could not be associated with delivery.
The key elements of MMC repair are the same regardless of surgery time (prenatal or postnatal procedure). In both groups, the method of tissue collection for histopathological examination was the same. The difference in the severity of inflammatory changes was due to the length of pregnancy. Fragments of the mislocated dura mater and skin that were adjacent to the uncovered neural plate in patients who underwent MMC repair in utero, i.e. between 22 and 25 weeks of gestation were exposed to the toxic effect of amniotic fluid and mechanical injury for a significantly shorter period of time. The shorter time of permanent damage to abnormally located tissues resulted in reduction of the inflammatory process. Mislocated perispinal tissues in patients who underwent surgery after delivery, i.e. between 36 and 38 weeks of gestation were exposed to chemical and mechanical damage for several weeks longer, which exacerbated inflammatory changes.
Studies on rats, where MMC was induced prenatally and then diluted human meconium was added to the amniotic fluid, showed some inflammatory, destructive and necrotic changes in all cases, comprising the uncovered spinal cord and the surrounding tissues.9
In mice, experimentally induced MMC showed that the spinal cord developed normally only during the very early stage of fetal life which was followed by progressive degenerative and inflammatory changes.10
Studies on the amniotic fluid of healthy embryos indicate the presence of intestinal enzymes which are most likely the effect of physiological fetal defecation.11
The amniotic fluid contains maternal and fetal body fluids. Its composition is substantially different from the cerebrospinal fluid, whereas its chemical and physical properties change during pregnancy. The amniotic fluid concentration of intestinal enzymes increases during pregnancy, which may contribute to the elevated toxic effect on the uncovered spinal cord and the spinal nerves during subsequent gestational weeks, resulting in progressive inflammatory and degenerative changes in the uncovered section of the spinal cord and the spinal nerves.12
Our study is in line with the above observations. Skin and dura mater specimens showed no presence of granulocytic infiltration in any patient who underwent prenatal surgery and only in five fetuses small lymphocytic infiltration was found in the skin and the dura mater, whereas only three fetuses showed medium skin infiltration.
Post-traumatic spinal cord injury is a two-hit phenomenon. First, the structures are damaged due to hypoxia and trauma-related hemorrhage. Then, progressive inflammatory and degenerative changes occur and are associated with multiple injury. This complex post-traumatic process may lead to manifestations of spinal cord injury comprising the regions above the fractured vertebrae.13 Similar phenomena are sometimes observed in children born with MMC where neurological disorders comprise the sections of the spinal cord above the entry of the spina bifida.
The obtained results prove that early separation of the embryonic spinal cord from the toxic effect of the amniotic fluid components and protection against mechanical injury during fetal movements in the uterus can reduce the severity of inflammatory and degenerative changes in the spinal cord, thus improving its proper function.
Lesser spinal cord injury allows to preserve better functions of locomotor, alimentary and urinary systems.
Conclusions
By reducing the time of exposure to damaging factors, prenatal MMC repair statistically reduces the risk of inflammatory changes in the exposed spinal cord and spinal nerves.
Prenatal closure of spina bifida before 24 weeks of gestation does not reduce the severity of inflammatory changes in the exposed spinal cord.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest None.
References
- 1.Cope H, McMahon K, Heise E, Eubanks S, Garrett M, Gregory S, et al. Outcome and life satisfaction of adults with myelomeningocele. Disabil Health J. 2013;3:236–43. doi: 10.1016/j.dhjo.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osaka K, Tanimura T, Hirayama A, Matsumato S.. Myelomeningocele before birth. J Neurosurg. 1978;49:711–24. doi: 10.3171/jns.1978.49.5.0711 [DOI] [PubMed] [Google Scholar]
- 3.Meuli M, Meuli-Simmen C, Hutchins GM, Seller MJ, Harrison MR, Adzick NS. The spinal cord lesion in human fetus with myelomeningocele: implications for fetal surgery. J Pediatr Surg. 1997;32:448–52. doi: 10.1016/S0022-3468(97)90603-5 [DOI] [PubMed] [Google Scholar]
- 4.Tulipan N, Bruner JP.. Myelomeningolele repair in utero: a rapport of three cases. Pediatr Neurosurg. 1998;28(4):177–80. doi: 10.1159/000028645 [DOI] [PubMed] [Google Scholar]
- 5.Adzick NS, Sutton LN, Crombleholme TM, Flake AW.. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675–6. doi: 10.1016/S0140-6736(98)00070-1 [DOI] [PubMed] [Google Scholar]
- 6.Zamłyński J, Olejek A, Koszutski T, Ziomek G, Horzelska E, Gajewska-Kucharek A, et al. Compresion of prenatal and postnatal treatments of spina bifida in Poland – a non- randomized, single center study. J Matern Fetal Neonatal Med. 2014;27(14):1409–17. doi: 10.3109/14767058.2013.858689 [DOI] [PubMed] [Google Scholar]
- 7.Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, Johnson MP, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastuszka A, Bohosiewicz J, Koszutski T.. Prenatal myelomeningocele repair improves urinary continence and reduces the risk of constipation. Neurourol Urodyn. 2018;37:2792–8. doi: 10.1002/nau.23771 [DOI] [PubMed] [Google Scholar]
- 9.Correia-Pinto J, Reis JL, Hutchins GH, Baptista MJ, Estevão-Costa J, Flake AW, et al. In utero meconium exposure increases spinal cord Necrosis in Rat model of myelomeningocele. J Pediatr Surg. 2002;37(3):488–92. doi: 10.1053/jpsu.2002.30872 [DOI] [PubMed] [Google Scholar]
- 10.Stiefel D, Meuli M.. Scanning electron microscopy of fetal murine melomeningocele reveals growth and development of the spinal cord in esrly gestation and neural tissue destruction around birth. J Pediatr Surg. 2007;42:1561–5. doi: 10.1016/j.jpedsurg.2007.04.019 [DOI] [PubMed] [Google Scholar]
- 11.Ciftci AO, Tanyel FC, Bingöl-Koloğlu M, Sahin S, Büyükpamukçu N.. Fetal distress does not affect in utero defecation but does impair the clearance of amniotic fluid. J Pediatr Surg. 1999;34:246–50. doi: 10.1016/S0022-3468(99)90183-5 [DOI] [PubMed] [Google Scholar]
- 12.McLone DG, Dias MS, Goossens W, Knepper PA.. Pathological changes in exposed neural tissue of fetal delayed splotch (Spd) mice. Childs Nerv Syst. 1997;13:1–7. doi: 10.1007/s003810050028 [DOI] [PubMed] [Google Scholar]
- 13.Ning Z, Yin Ying, Sheng-Jie X, Yong-Ping W, Wei-Shan C.. Inflammation and apoptosis in spinal cord injury. Indian J Med Res. 2012;135(3):287–96. [PMC free article] [PubMed] [Google Scholar]