Abstract
Background
Coronavirus disease 19 (COVID-19) vaccination has been established as preventing severe and mortal COVID-19. Vaccination is critical strategy in controlling the COVID-19 pandemic, to restrict infections and reduce disease severity. Vaccination coverage will be more extensive if we can better identify vaccination barriers in the population, especially among vulnerable groups, of which one is pregnant women. The aim of this study was to determine the level of acceptance of COVID-19 vaccination and detect the factors that influence vaccine acceptance among pregnant women in Saudi Arabia.
Methods
This was a cross-sectional, web-based study conducted in Western, Eastern, North, South, and Central Regions in Saudi Arabia between July and September 2021 among pregnant women, using multi-stage sampling. All pregnant women above 18 years were invited to participate in the study. Pregnant under 18 years of age and those with a contraindication to receiving COVID-19 vaccination were excluded. Binomial logistic regression (univariate and multivariate) was used to identify the influencing factors on vaccination acceptance.
Results
Among the 5307 pregnant women, the acceptance level of COVID-19 vaccine was 68%. In the multivariate regression model analysis, the most common predictors of acceptance were living in North Region (P = 0.001, OR = 1.9), living in South Region (P = 0.000, OR = 3.06), and living in Central Region (P = 0.035, OR = 1.42) in comparison to living in Western Region. Gestational week (P = 0.018, OR=0.98), income more than 8000 SR (P = 0.000, OR = 0.51), education level (primary, secondary, and university; P = 0.002, 0.008, and 0.010, respectively), having had gestational diabetes mellitus (P = 0.013, OR = 1.86), being vaccinated with influenza vaccine during present pregnancy (P = 0.000, OR = 4.55, OR = 1.81), being vaccinated with tetanus vaccine during present pregnancy (P = 0.039), and believing that the COVİD-19 vaccine could harm their baby (P = 0.000, OR = 0.12).
Conclusion
Our study reported high acceptance of COVID-19 vaccination. The major two reasons for refusal were concerns about a lack of data on COVID-19 vaccination safety and the possibility of harming the fetus. Continued public health efforts, such as educational television programs and awareness campaigns about the safety of the COVID-19 vaccine for pregnant women, are required to raise awareness. More studies of COVID-19 vaccine safety in pregnant women would assist in overcoming these obstacles and encourage pregnant women to be vaccinated.
Keywords: COVID-19, vaccine, pregnancy, Saudi Arabia, pregnant women, acceptance
Introduction
Worldwide, coronavirus disease 19 (COVID-19) has imposed large burdens of morbidity and mortality among the general population.1 Up until September 2021, SARS-CoV-2 virus had caused 226,236,577 confirmed cases of COVID-19, resulting in 4,654,548 deaths. More than 500,000 cases were recorded in Saudi Arabia, as reported to the World Health Organization (WHO).2
Vaccination is a critical strategy in controlling the COVID-19 pandemic, to restrict infections and reduce disease severity.3 As in other viral epidemic diseases in the past, vaccination has undoubtedly made a significant contribution to human and animal health, notably in developing countries.4 Vaccination coverage will be more extensive if we can better identify vaccination barriers in the population, especially among vulnerable groups, of which one is pregnant women.
Recommendations from the Society for Maternal-Fetal Medicine and American College of Obstetricians and Gynecologists (ACOG) have maintained that the vaccine should be offered to pregnant and lactating women based on their risk, and that mRNA-based vaccines are thought to pose a low risk to the fetus as the mRNA is expected to degrade in the circulation.5,6
According to the data, pregnant women are at high risk for severe COVID-19 disease.7 Pregnant women were three times more likely than non-pregnant women to be hospitalized to the ICU or require intubation, and one and half times more likely to die from COVID-19, according to a report from the Centers for Disease Control and Prevention (CDC).8
Acceptance of vaccination depends on society’s awareness of disease risk, vaccine attitudes, and demand, and is crucial for immunization programs to achieve high vaccination coverage rates, particularly for newly developing contagious diseases.9–11 Even before the current COVID-19 pandemic, the WHO identified vaccine hesitancy, as the delay in accepting vaccines or refusal of them, as one of the top ten threats to global health.12
The general population’s acceptance of the COVID-19 vaccine was high, and is recorded as at 90% in China,13 70% in the United States,14,15 and 75% in France.16,17 This is in contrast to a previous study conducted in the United States in 2020, in which less than half of pregnant women stated they were likely to obtain the COVID-19 vaccination.18 Another study reported that pregnant respondents had a lower rate of vaccine acceptance (44.3%) than non-pregnant women (76.2%).19
Understanding the causes of COVID-19 vaccine hesitancy in pregnant women is critical for devising specific approaches to manage hesitancy and increase vaccination awareness. No studies have been conducted to evaluate the acceptance of COVID-19 vaccination among pregnant women in Saudi Arabia. Therefore, the purpose of this study was to determine the level of acceptance of COVID-19 vaccination and detect the factors that influence vaccine acceptance among pregnant women in Saudi Arabia.
Materials and Methods
Study Design, Setting, and Sampling
This was a cross-sectional, web-based study conducted in the Western, Eastern, North, South and Central regions in Saudi Arabia between July and September 2021 among pregnant women. Sample size was calculated using Raosoft online sample size calculator. The calculation was based on 95% confidence interval and 5% margin of error. The calculated sample size was 306 participants. We further inflated that number to ensure accurate and generalizable results. Multistage sampling technique was used. First, we divided Saudi Arabia into North, South, East, West and Central Regions. Second, we assigned data collectors in each region to distribute the questionnaire link to the target population. The COVID-19 vaccine was initially approved for adults above 18 years old. Therefore, all pregnant women above 18 years were invited to participate in the study. Pregnant under 18 years of age and those with a contraindication to receiving COVID-19 vaccination (ie, allergy to vaccine components) were excluded. The patient’s pregnancy stage was determined based on their latest menstrual cycle or first-trimester crown-rump length.
Data Collection Instruments
The data were collected online using a standardized, anonymous questionnaire that had previously been used in an epidemiological study (in both English and Arabic) via various social media platforms using Google Forms.20 We obtained written permission from the original publisher to use the questionnaire. The online method was used to avoid physical contact during the ongoing pandemic. The original English-language questionnaire was forward-translated into Arabic and then back-translated to English to ensure the accuracy of the translated version. Participants were informed that all responses would be anonymous and confidential. The informed consent form had two options: “yes” for those who agreed to participate in the study, and “no” for those who did not want to participate. Only those who selected “yes” were directed to the questionnaire page, where they could complete the survey.
All the questions were obligatory and exhibited in running order on the participant’s screen. Participants were prompted to complete the outstanding questions before submitting the form and they were unable to save responses or change their answers after submission.
Every submitted questionnaire was included in the study analysis. Vaccine acceptance was defined as a response of having received or being willing to receive a COVID-19 vaccination, and non-acceptance was defined as a response of not having had and being unwilling to receive a COVID-19 vaccination. The questionnaire was divided into four sections: demographic information, the impact of the COVID-19 pandemic on perceptions of risk, vaccination history, including acceptance of and attitude toward COVID-19 vaccination, and reasons for refusal.
The first section of the study focused on demographic data and general health status such as age, gravidity, parity, and gestational week; number of household members and school-aged children; comorbidities such as diabetes, hypertension, and heart disease; number of people over 65 living in the same household; monthly income in Saudi Riyal; and high-risk pregnancy, which included preterm labor, gestational hypertension, gestational diabetes, fetal structural anomalies, multifetal gestation, epilepsy, and placenta previa. The second section evaluated risk perception about the COVID-19 pandemic, as well as the pandemic’s effects, and it included four questions: 1) Are you at a high risk of COVID-19 transmission at work or have you had close contact with a COVID-19 positive individual? 2) Are you concerned about hand hygiene during the pandemic? 3) Do you pay attention to social distancing? and 4) Did you have COVID-19 during your pregnancy?
The third section discussed the participants’ history of vaccination and acceptance and attitude toward the COVID-19 vaccine. The participants were asked if they had had a vaccination in the last 5 years or during their current pregnancy, whether they had been recommended to have a flu vaccine during this pregnancy, and whether they planned to have their baby vaccinated after birth. The fourth section asked about the reasons for their refusal, and they were invited to choose from the following statements: “vaccines are harmful to a pregnant woman’s health”; “COVID-19 infection is a result of the vaccine”; “the vaccine is harmful to infants”; “COVID-19 is not a life-threatening illness”; “COVID-19 infection is unlikely to affect me, even if I get sick”; “I believe my infant and I will not have any terrible experiences”; “I believe that the vaccine will not work against the virus”; “some or all of my family members are wary of the COVID-19 vaccine”; and “there is a doubt about the safety of the COVID-19 immunization in pregnant women.”
Ethical approval was obtained from the Institutional Review Board/Ethics Committee at King Abdulaziz University Hospital (Ref. 348-21). Our study also complies with the Declaration of Helsinki.
Statistical Analysis
The data were collected, coded, entered, and analyzed using SPSS version 20 (IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was used to determine the distribution of normality; for categorical data, Chi square test was used to test and describe the relationship between two categorized variables. Continuous variables were presented as mean and standard deviations using t-test. Binomial logistic regression (univariate and multivariate) was used to test the predictors of the binary outcome variable. A type-1 error below 0.05 was considered statistically significant.
Results
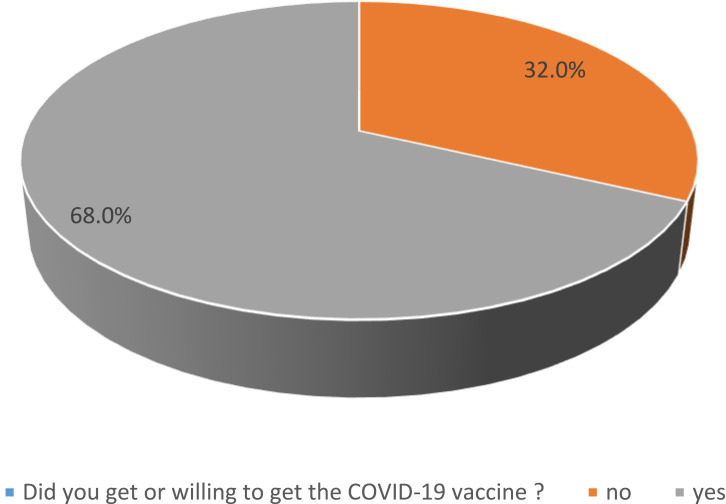
A total of 5307 pregnant women responded. Of these 68% answered that they had received or were willing to receive a COVID-19 vaccination, with the remaining 32% unvaccinated and not wishing to receive it (Figure 1). A total of 53.4% received the COVID-19 vaccine, and 14.4% were willing to receive the vaccine. Most of participants were from Western Region (28.8%) and Eastern Region (27.6%), followed by Central Region (21%), South Region (11.6%), and North Region (10.7%); however, 0.3% were from other regions. Approximately half of the sample (54.1%) reported their income was over 8000 SR per month; almost one-third of the sample (34.4%) had an income ranging between 3000 and 8000 SR per month, and 11.5% had an income less than 3000 SR per month. Table 1 shows the relationship between sociodemographic characteristics and COVID vaccination willingness. The average age of those vaccinated or willing to be vaccinated was 31.57 years vs 30.08 years for those unwilling. Other sociodemographic features and their relationship with vaccine willingness are demonstrated in Table 1.
Figure 1.
The prevalence of acceptance of COVID-19 vaccine among the participant group.
Table 1.
Relationship Between Acceptance of COVID-19 Vaccination and Sociodemographic Characteristics
| Variable | Acceptance of COVID-19 Vaccine | |
|---|---|---|
| Did Not Receive or Not Willing to Receive COVID-19 Vaccine | Received or Willing to Receive COVID-19 Vaccine | |
| n=1685 | n=3548 | |
| Age (mean±S.D) | (30.08±5.99) | (31.57±7.79) |
| Gravidity n (%) | ||
| <5 | 1438 (34.7%) | 2702 (65.3%) |
| ≥ 5 | 243 (23.3%) | 799 (76.7%) |
| Parity n (%) | ||
| <5 | 1567 (33.8%) | 3073 (66.2%) |
| ≥ 5 | 116 (20%) | 464 (80%) |
| Regions n (%) | ||
| Western Region | 525 (34.4%) | 1003 (65.6%) |
| Eastern Region | 516 (35.2%) | 949 (64.8%) |
| North Region | 147 (26.0%) | 419 (74.0%) |
| South Region | 112 (18.2%) | 502 (81.8%) |
| Central Region | 392 (35.1%) | 725 (64.9%) |
| Other | 5 (29.4%) | 12 (70.6%) |
| Gestational week (mean±S.D) | (25.98±9.82) | (24.21±12.48) |
| Number of household members (mean±S.D) | (3.97±2.44) | (4.57±2.40) |
| Number of school-age children n (%) | ||
| <5 | 1641 (32.9%) | 3350 (67.1%) |
| ≥ 5 | 44 (18.2%) | 198 (81.8%) |
| Number of household members > 65 y (mean±S.D) | (0.14±0.44) | (0.18±0.57) |
| Nationality n (%) | ||
| Saudi | 1626 (31.8%) | 3494 (68.2%) |
| Non-Saudi | 71 (38%) | 116 (62%) |
| Education status n (%) | ||
| None | 12(41.1%) | 17 (58.6%) |
| Primary school | 30 (21.4%) | 110 (78.6%) |
| Secondary school | 284 (31.3%) | 623 (68.7%) |
| University | 1371(32.4%) | 2860 (67.6%) |
| Wife job n (%) | ||
| Housewife | 1106 (35.9%) | 1976 (64.1%) |
| Private sector | 230 (29.6%) | 546 (70.4%) |
| Government sector | 361 (24.9%) | 1088 (75.1%) |
| Husband job n (%) | ||
| Not working | 91 (31.2%) | 201(68.8%) |
| Private sector | 614 (36.4%) | 1073 (63.6%) |
| Government sector | 926 (29.9%) | 2173 (70.1%) |
| Merchant | 66 (28.8%) | 163 (71.2%) |
| Do you have any medical conditions? n (%) | ||
| None | 1544 (32.9%) | 3150 (67.1%) |
| Diabetes | 36 (19.7%) | 147 (80.3%) |
| Hypertension | 23 (16.8%) | 114 (83.2%) |
| Heart disease | 5 (13.9%) | 31 (86.1%) |
| Other | 89 (34.6%) | 168 (65.4%) |
| Do you have a high-risk pregnancy? n (%) | ||
| No | 1451 (31.8%) | 3117 (68.2%) |
| Preterm labor | 61 (33%) | 124 (67%) |
| Gestational hypertension | 33 (35.5%) | 60 (64.5%) |
| Fetal structural abnormality | 12 (41.4%) | 17 (58.6%) |
| Multifetal pregnancy | 34 (41.5%) | 48 (58.5%) |
| Gestational diabetes mellitus | 78 (29.7%) | 185 (70.3%) |
| Epilepsy | 0 (0.0%) | 11 (100%) |
| Placenta previa | 28 (36.8%) | 48 (63.2%) |
The associations between acceptance and attitude toward the COVID-19 vaccine and sociodemographic data, general health status, and history of vaccination are shown in Table 2 (univariate regression analysis). We observed that the following variables were significantly associated with being vaccinated or willingness to be COVİD-19 vaccinated: age (P=0.000); residence in North Region (P=0.000) and residence in Region (P=0.000); gravidity (P=0.001) and parity (P=0.000); gestational week (P=0.000); number of household members (P=0.000), number of school-age children (P=0.000), and number of household members >65 years of age (P=0.002); income more than 8000 SR (P=0.030), primary school education (P=0.017), and job (private sector and government official, P=0.001 and P=0.000, respectively); history of diabetes mellitus (P=0.000), hypertension (P=0.000), or heart disease (P=0.025); mask-wearing during the pandemic period (P=0.009); having had COVID-19 in this pregnancy (P=0.000); having had influenza vaccination during present pregnancy (P=0.000); having been vaccinated for tetanus during present pregnancy (P=0.000); planning to have their baby vaccinated after birth (P=0.022); and believing that COVİD-19 vaccination can possibly harm their babies (P=0.000). Other variables did not show significant association (Table 2).
Table 2.
The Association Between Acceptance and Attitude Toward the COVID-19 Vaccine and Sociodemographic Data, General Health Status, and History of Vaccination, Univariate Logistic Regression Analysis
| Variables* | P-value | Odds Ratio (OR) | 95% C.I. for OR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age (year) | 0.000 | 1.027 | 1.018 | 1.036 | |
| Nationality | Saudi | Ref | |||
| Non Saudi | 0.68 | 0.755 | 0.558 | 1.021 | |
| Regions | Western Region | Ref | |||
| Eastern Region | 0.592 | 0.959 | 0.825 | 1.116 | |
| North Region | 0.000 | 1.510 | 1.217 | 1.874 | |
| South Region | 0.000 | 2.288 | 1.816 | 2.883 | |
| Central Region | 0.602 | 0.958 | 0.814 | 1.127 | |
| Other | 0.926 | 1.052 | 0.358 | 3.095 | |
| Gravidity (the number of times that a woman has been pregnant) ≥5 | 0.001 | 1.750 | 1.495 | 2.048 | |
| Parity (as the number of times that woman has given birth) ≥5 | 0.000 | 2.040 | 1.649 | 2.522 | |
| Gestational week: | 0.000 | 0.986 | 0.981 | 0.991 | |
| Number of householders: | 0.000 | 1.117 | 1.088 | 1.147 | |
| Number of school age children ≥5 | 0.000 | 1.175 | 1.128 | 1.223 | |
| Number of household members > 65 y | 0.002 | 1.221 | 1.076 | 1.385 | |
| Income (month): | Less than 3000 SR | Ref | |||
| 3000–8000 SR | 0.594 | 0.948 | 0.781 | 1.152 | |
| More than 8000 SR | 0.030 | 1.230 | 1.020 | 1.483 | |
| Education status: | None | Ref | |||
| Primary school | 0.017 | 2.853 | 1.207 | 6.745 | |
| Secondary school | 0.159 | 1.741 | 0.804 | 3.769 | |
| University | 0.196 | 1.653 | 0.772 | 3.542 | |
| Wife job | Housewife | Ref | |||
| Private sector | 0.001 | 1.328 | 1.118 | 1.577 | |
| Government official | 0.000 | 1.685 | 1.464 | 1.939 | |
| Husband job | Not Working | Ref | |||
| Private sector | 0.115 | 0.805 | 0.615 | 1.054 | |
| Government sector | 0.575 | 1.078 | 0.830 | 1.400 | |
| Merchant | 0.545 | 1.125 | 0.769 | 1.645 | |
| Do you have any medical condition? | None | Ref | |||
| Diabetes | 0.000 | 1.947 | 1.344 | 2.822 | |
| Hypertension | 0.000 | 2.340 | 1.486 | 3.684 | |
| Heart disease | 0.025 | 2.962 | 1.147 | 7.650 | |
| Other | 0.483 | 0.909 | 0.696 | 1.187 | |
| Do you have high risk pregnancy? | No | Ref | |||
| Preterm labor | 0.737 | 0.947 | 0.691 | 1.299 | |
| Gestational hypertension | 0.427 | 0.840 | 0.546 | 1.292 | |
| Fetal structural anomalies | 0.171 | 0.587 | 0.274 | 1.258 | |
| Multifetal pregnancy | 0.070 | 0.663 | 0.425 | 1.034 | |
| Gestational diabetes mellitus. | 0.402 | 1.125 | 0.854 | 1.481 | |
| Epilepsy | 0.999 | - | - | - | |
| Placenta previa | 0.155 | 0.705 | 0.435 | 1.141 | |
| Do you have high risk of COVID-19 transmission at work? Yes | 0.959 | 0.995 | 0.816 | 1.213 | |
| Did you have close contact with COVID-19 positive person? Yes | 0.436 | 0.928 | 0.768 | 1.121 | |
| Did you care about hand hygiene during pandemic period? Yes | 0.184 | 1.359 | 0.864 | 2.137 | |
| Did you care about social distancing during pandemic period? Yes | 0.541 | 1.107 | 0.799 | 1.534 | |
| Did you care about using mask during pandemic period? Yes | 0.009 | 1.508 | 1.106 | 2.057 | |
| Did you have COVID-19 in this pregnancy? Yes | 0.000 | 1.721 | 1.332 | 2.223 | |
| Have you ever been vaccinated in last 5 years? Yes | 0.569 | 1.058 | 0.872 | 1.283 | |
| Was the Influenza vaccine recommended in the present pregnancy? Yes | 0.000 | 1.560 | 1.233 | 1.972 | |
| If the Influenza vaccine recommended would you have vaccinated in the present pregnancy? Yes | 0.000 | 6.499 | 5.343 | 7.904 | |
| Have you been vaccinated for Influenza in the present pregnancy? Yes | 0.000 | 2.639 | 1.847 | 3.771 | |
| Was the tetanus vaccine recommended in the present pregnancy ? yes | 0.001 | 1.515 | 1.174 | 1.955 | |
| Have you been vaccinated for tetanus in the present pregnancy ? yes | 0.000 | 2.094 | 1.524 | 2.877 | |
| Are you going to have vaccinated your baby after birth? Yes | 0.022 | 1.910 | 1.099 | 3.318 | |
| Did you heard about COVID-19 vaccine before? Yes | 0.637 | 1.068 | 0.811 | 1.407 | |
| Do you think that you have enough information about COVID-19 vaccine? Yes | 0.053 | 1.194 | 0.998 | 1.429 | |
| Do you think COVİD-19 vaccine carry out possibility of harm for your baby? Yes | 0.000 | 0.104 | 0.085 | 0.128 | |
Notes: *Variables adapted with permission from John Wiley and Sons: Ayhan SG, Oluklu D, Atalay A, et al. COVID‐19 vaccine acceptance in pregnant women. Int J Gynecol Obstet. 2021;154:291–296. DOI:10.1002/ijgo.13713.21 © 2021 International Federation of Gynecology and Obstetrics.
The multivariate logistic regression models showed the most common predictors of acceptance and positive attitude toward the COVID-19 vaccine among pregnant women were living in North Region (P=0.001, OR=1.9), living in South Region (P=0.000, OR=3.06), and living in Central Region (P=0.035, OR=1.42) in comparison to living in Western Region. Gestational week (P=0.018), income more than 8000 SR (P=0.000), education level (primary, secondary, and university; P=0.002, 0.008, and 0.010, respectively), having had gestational diabetes mellitus (P=0.013), being vaccinated with influenza vaccine during present pregnancy (P=0.000), being vaccinated with tetanus vaccine during present pregnancy (P=0.039), and believing that the COVİD-19 vaccine could harm their baby (P=0.000) were significantly associated with having or being willing to have COVİD-19 vaccination. Other variables did not show significant association (Table 3).
Table 3.
Predictors of the Acceptance and Attitude Toward COVID-19 Vaccination Among Pregnant Women, Multivariate Logistic Regression Model
| Variables* in the Equation | p-value | Odds Ratio (OR) | 95% C.I. for OR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age (year) | 0.653 | 1.005 | 0.983 | 1.028 | |
| Nationality | Saudi | Ref | |||
| Non Saudi | 0.734 | 1.096 | 0.646 | 1.859 | |
| Regions | Western Region | Ref | |||
| Eastern Region | 0.536 | 1.099 | 0.815 | 1.483 | |
| North Region | 0.001 | 1.914 | 1.293 | 2.834 | |
| South Region | 0.000 | 3.065 | 2.059 | 4.562 | |
| Central Region | 0.024 | 1.424 | 1.047 | 1.938 | |
| Other | 0.146 | 2.987 | 0.684 | 13.037 | |
| Gravidity (the number of times that a woman has been pregnant) ≥5 | 0.185 | 1.324 | 0.874 | 2.004 | |
| Parity (as the number of times that woman has given birth)≥5 | 0.907 | 0.967 | 0.554 | 1.689 | |
| Gestational week: | 0.018 | 0.987 | 0.977 | 0.998 | |
| Number of householders: | 0.750 | 1.009 | 0.955 | 1.066 | |
| Number of school age children≥5 | 0.901 | 0.957 | 0.483 | 1.897 | |
| Number of household members > 65 y | 0.262 | 1.149 | 0.901 | 1.466 | |
| Income (month): | Less than 3000 SR | Ref | |||
| 3000–8000 SR | 0.058 | 0.722 | 0.516 | 1.011 | |
| More than 8000 SR | 0.000 | 0.516 | 0.363 | 0.734 | |
| Education status: | None | Ref | |||
| Primary school | 0.002 | 12.469 | 2.566 | 60.601 | |
| Secondary school | 0.008 | 7.659 | 1.706 | 34.385 | |
| University | 0.010 | 7.196 | 1.615 | 32.068 | |
| Wife job: | Housewife | Ref | |||
| Private sector | 0.666 | 0.926 | 0.654 | 1.312 | |
| Government official | 0.567 | 1.091 | 0.810 | 1.471 | |
| Do you have any medical condition? | None | Ref | |||
| Diabetes | 0.265 | 1.456 | 0.752 | 2.820 | |
| Hypertension | 0.167 | 0.433 | 0.132 | 1.419 | |
| Heart disease | 0.964 | 0.966 | 0.213 | 4.384 | |
| Other | 0.223 | 0.712 | 0.413 | 1.228 | |
| Do you have high risk pregnancy? | No | Ref | |||
| Preterm labor | 0.303 | 0.721 | 0.387 | 1.343 | |
| Gestational hypertension | 0.419 | 0.695 | 0.287 | 1.682 | |
| Fetal structural anomalies | 0.999 | 0.000 | 0.000 | ||
| Multifetal pregnancy | 0.488 | 0.755 | 0.341 | 1.671 | |
| Gestational diabetes mellitus. | 0.013 | 1.867 | 1.142 | 3.054 | |
| Epilepsy | 1.000 | - | - | - | |
| Placenta previa | 0.392 | 0.688 | 0.291 | 1.623 | |
| Did you care about using mask during pandemic period? Yes | 0.206 | 1.281 | 0.872 | 1.883 | |
| Did you have COVID-19 in this pregnancy? Yes | 0.192 | 1.245 | 0.896 | 1.731 | |
| Was the Influenza vaccine recommended in the present pregnancy? Yes | 0.324 | 1.186 | 0.845 | 1.665 | |
| If the Influenza vaccine recommended would you have vaccinated in the present pregnancy? Yes | 0.000 | 4.557 | 3.583 | 5.795 | |
| Have you been vaccinated for Influenza in the present pregnancy? Yes | 0.197 | 0.706 | 0.417 | 1.197 | |
| Was the tetanus vaccine recommended in the present pregnancy? Yes | 0.511 | 0.858 | 0.545 | 1.353 | |
| Have you been vaccinated for tetanus in the present pregnancy? Yes | 0.039 | 1.816 | 1.031 | 3.200 | |
| Are you going to have vaccinated your baby after birth? Yes | 0.411 | 1.339 | 0.667 | 2.685 | |
| Do you think that you have enough information about COVID-19 vaccine? Yes | 0.186 | 1.163 | 0.930 | 1.456 | |
| Do you think COVİD-19 vaccine carry out possibility of harm for your baby? Yes | 0.000 | 0.125 | 0.099 | 0.158 | |
Notes: *Variables adapted with permission from John Wiley and Sons: Ayhan SG, Oluklu D, Atalay A, et al. COVID‐19 vaccine acceptance in pregnant women. Int J Gynecol Obstet. 2021;154:291–296. DOI:10.1002/ijgo.13713.21 © 2021 International Federation of Gynecology and Obstetrics.
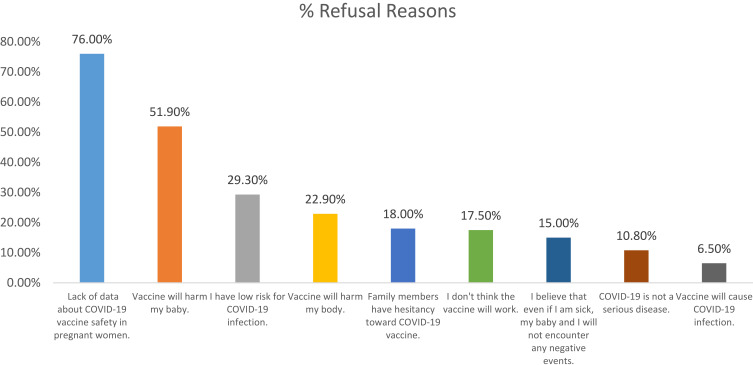
The two major reasons for refusing COVID-19 vaccination were lack of data about COVID-19 vaccination safety (76%) and the possibility of harm to their baby (51.9%) (Figure 2).
Figure 2.
Reasons of refusal of getting a COVID-19 vaccine among the participant group.
Discussion
Vaccination programs can only be considered effective if they have a high level of acceptance and coverage. Assessing COVID-19 risk perception is critical to achieving this, as is assessing the acceptability of COVID-19 vaccination and trust in the health system and media sources, particularly those used to learn about the COVID-19 pandemic. The acceptance level of COVID-19 vaccination among our sample of pregnant women was high, with 68% having received or willing to receive COVID-19 vaccination. This is in contrast to a previous study conducted in the United States in 2020, in which less than half of pregnant women stated they were likely to obtain the COVID-19 vaccination.19 Another study reported that pregnant respondents had a lower rate of vaccine acceptance (44.3%) than non-pregnant women (76.2%).18 A Turkish study, also found that acceptance of the COVID-19 vaccination was low in a sample of pregnant women.21 The acceptance rate in our study, however, was lower than that found in a study conducted in China (91.3%).22 The possible explanations for these differences are differences in access to healthcare services, differing awareness of the severity of COVID- 19, and study population differences.
In the univariate regression analysis, older women were more likely to receive the vaccine in comparison to younger women. This finding is consistent with previous studies conducted in the United Kingdom and Turkey.20,23 Those with an income of more than 8000 SR were more likely to receive the vaccine. Higher income, and older age were the strongest predictor of COVID-19 vaccine acceptance in a study conducted in 16 countries.24 Moreover, working in a governmental sector among participants was found to be associated with higher acceptance of COVID-19 vaccine when compared to working in a private sector or being a housewife. This might be due to the Saudi government’s efforts to raise societal awareness and knowledge about COVID-19 vaccination through various communication channels.
Women with 5 or more school-age children were more likely to receive COVID-19 vaccination. This finding is consistent with a previous study conducted in Turkey.20 This might be due to higher anxiety about the risk of transmission by these children to other members of the household. We also found that the women with higher number of household members above 65 years were more likely to accept the vaccine. This is probably due to higher concern about the risk of disease transmission to elderly. While multiparity was associated with greater acceptance of COVID-19 in our study, another study performed in six European countries found the opposite.25
In the multivariate regression analysis, we discovered significant geographic variation in pregnant women’s acceptance of COVID-19 vaccination. Women in the South, North, and Central Regions were three times, twice, and one and a half times more likely to receive the vaccine compared to Western Region, respectively. This may be because of more comprehensively distributed primary healthcare centers in Southern Region. Better health education to pregnant women from healthcare workers may have led to an increase in their knowledge about the disease, through, for example, dissemination of brochures about COVID-19 preventive measures.
According to education status, primary school-, secondary school-, and university-educated women were 12, 8, and 7 times more likely to receive the COVID-19 vaccine than uneducated women. These results are consistent with a previous study that showed that as people’s education level rises, they are more willing to receive a COVID-19 vaccination.26 These findings suggest that if a pregnant woman has an educational degree, she would be more likely to receive COVID-19 vaccination.
Women with high-risk pregnancy due to gestational diabetes mellitus were nearly twice as likely to be vaccinated compared with women with a low-risk pregnancy. Additionally, we found that increase in the gestational week was associated with reduced COVID-19 vaccination acceptance. This finding is consistent with a previous study in Turkey that showed that pregnant women in the second and third trimester expressed lower vaccine acceptance than those in the first trimester.20 Other studies have found that anxiety and depression are prevalent symptoms in the first trimester of pregnancy and we suggest that women in their first trimester of pregnancy during the COVID-19 pandemic may be more distressed than during prior pregnancies.21,27,28 This degree of anxiety may lead to greater acceptance of COVID-19 vaccination in the first trimester of pregnancy and in high-risk pregnant women.
Influenza vaccination is recommended during pregnancy, as pregnancy is considered a low immune state and influenza can cause more severe illness in pregnant women when compared with non-pregnant women. A retrospective study conducted between 2010 and 2016 reported that influenza vaccines provided moderate protection against laboratory-confirmed influenza-associated hospitalizations during pregnancy; this adds to the evidence that maternal influenza immunization programs are beneficial.29 We asked our participants if they would be willing to receive an influenza vaccine if it was recommended and if they had been vaccinated against influenza in the present pregnancy, to assess their acceptance of a new vaccine such as the COVID-19 vaccine. Surprisingly, our study found that pregnant women were almost four times more likely to accept COVID-19 vaccination, similar to findings in a Turkish study.5
Pregnant women in our study who thought that the vaccine has the possibility of harm to their babies were less likely to accept vaccination. The CDC has stated that the safety and efficacy of COVID-19 vaccination for pregnant women are proven.30 The ACOG has reported no evidence of maternal or fetal complications from vaccinating pregnant women with COVID-19 vaccines.31 The WHO recommends using the Pfizer-BioNTech COVID-19 vaccine in pregnant women where the benefits of immunization exceed the risks. To aid pregnant women in making this decision, information about the risks of COVID-19 during pregnancy, the potential advantages of vaccination, and the existing limits of safety evidence should be provided.32
The primary reason for vaccine rejection in our study was the absence of evidence on COVID-19 vaccine safety in pregnant women, which is consistent with a previous study published in March 2021;13 the second-most cited reason was a belief that the vaccine might harm the baby, and the third was the belief that they have a low risk of becoming infected with COVID-19. These findings are similar to those found in a study conducted by Ayhan et al.21 This can be easily explained by the natural fear of a mother toward her pregnancy. As more data become available, there will be more opportunity to build trust in the scientific approval of these vaccines. A rapid systematic review published in June 2021 did not find any safety concerns in any of the studies they reviewed.33
Strengths and Limitations
Our study had some key strengths. First, our sample size was large, with a large number of study parameters. Second, the sample included participants from all regions of Saudi Arabia which is likely to provide generalizable results that represent pregnant Saudi population. However, the study was subject to some limitations. The survey was conducted online, and so study participants were restricted to those who had access to the required technology and resources to complete it. Another limitation of this study was its cross-sectional design, meaning the results cannot show the relationship between cause and effect.
Conclusion and Recommendations
Pregnant women in Saudi Arabia are highly acceptant of COVID-19 vaccination. The Saudi government and ministry of health have been encouraging pregnant women to be vaccinated, as recommended by the WHO. A high level of awareness about COVID-19 vaccination in the study population was noticeable; however, lack of data and fear of harming the fetus are two major concerns among pregnant women when considering COVID-19 vaccination. Considering the reasons some pregnant women refuse to receive the COVID-19 vaccine, continued public health efforts, such as educational television programs and awareness campaigns about the safety of the COVID-19 vaccine for pregnant women, are required to raise awareness. More studies of COVID-19 vaccine safety in pregnant women would assist in overcoming these obstacles and encourage pregnant women to be vaccinated.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Nersesjan V, Amiri M, Christensen HK, Benros ME, Kondziella D. Thirty-day mortality and morbidity in COVID-19 positive vs. COVID-19 negative individuals and vs. individuals tested for influenza A/B: a population-based study. Front Med. 2020;7:1–10. doi: 10.3389/fmed.2020.598272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-19) dashboard. Geneva: World Health Organization; 2021. Available from: https://covid19.who.int/. Accessed March 9, 2022. [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans Roy Soc B Biol Sci. 2014;369:20130433. doi: 10.1098/rstb.2013.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen SA, Jamieson DJ. Pregnancy, postpartum care, and COVID-19 vaccination in 2021. JAMA. 2021;325:1099–1100. doi: 10.1001/jama.2021.1683 [DOI] [PubMed] [Google Scholar]
- 6.The American College of Obstetrics and Gynaecology. Vaccinating pregnant and lactating patients against COVID-19 – practice advisory; 2020. Available from: www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19. Accessed March 9, 2022.
- 7.Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. Morb Mortal Wkly Rep. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. Morb Mortal Wkly Rep. 2020;69:1641. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen T, Henningsen KH, Brehaut JC, Hoe E, Wilson K. Acceptance of a pandemic influenza vaccine: a systematic review of surveys of the general public. Infect Drug Resist. 2011;4:197. doi: 10.2147/IDR.S23174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: a critical review. Soc Sci Med. 2014;112:1–11. doi: 10.1016/j.socscimed.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 11.Dubé E, MacDonald NE. Vaccine acceptance: barriers, perceived risks, benefits, and irrational beliefs. In: Bloom BR, Lambert PH, editors. The Vaccine Book. Elsevier; 2016:507. [Google Scholar]
- 12.World Health Organization. Ten threats to global health in 2019; 2019. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed March 9, 2022.
- 13.Wang J, Jing R, Lai X, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. 2020;8:482. doi: 10.3390/vaccines8030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann Intern Med. 2020;173:964–973. doi: 10.7326/M20-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogue K, Jensen JL, Stancil CK, et al. Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines. 2020;8:582. doi: 10.3390/vaccines8040582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38:7002–7006. doi: 10.1016/j.vaccine.2020.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peretti-Watel P, Seror V, Cortaredona S, et al. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect Dis. 2020;20:769–770. doi: 10.1016/S1473-3099(20)30426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton D, D’Alton M, Zhang Y, et al. COVID-19 Vaccine acceptance among pregnant, breastfeeding and non-pregnant reproductive aged women. Am J Obstet Gynecol MFM. 2021;3:100403. doi: 10.1016/j.ajogmf.2021.100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battarbee A, Stockwell M, Varner M, et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August–December 2020. medRxiv. 2021. doi: 10.1101/2021.03.26.21254402 [DOI] [PubMed] [Google Scholar]
- 20.Goncu Ayhan S, Oluklu D, Atalay A, et al. COVID‐19 vaccine acceptance in pregnant women. Inter J Gynecol Obstet. 2021;154:291–296. doi: 10.1002/ijgo.13713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayhan SG, Oluklu D, Atalay A, et al. COVID‐19 vaccine acceptance in pregnant women. Int J Gynecol Obstet. 2021;154:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao L, Wang R, Han N, et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum Vaccin Immunother. 2021;17:2378–2388. doi: 10.1080/21645515.2021.1892432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236–e1. doi: 10.1016/j.ajog.2021.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceulemans M, Foulon V, Panchaud A, et al. Vaccine willingness and impact of the COVID-19 pandemic on women’s perinatal experiences and practices—a multinational, cross-sectional study covering the first wave of the pandemic. Int J Environ Res Public Health. 2021;18(7):3367. doi: 10.3390/ijerph18073367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S, Eto M. Screening for depressive and anxiety symptoms during pregnancy and postpartum at a Japanese perinatal center. J Clin Med Res. 2017;9:512. doi: 10.14740/jocmr3035w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki S. Psychological status during the first trimester of pregnancy under the COVID-19 epidemic in Japan. J Matern Fetal Neonatal Med. 2020;1–2. doi: 10.1080/14767058.2020.1793319 [DOI] [PubMed] [Google Scholar]
- 29.Thompson MG, Kwong JC, Regan AK, et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: a multi-country retrospective test negative design study, 2010–2016. Clin Infect Dis. 2019;68:1444–1453. doi: 10.1093/cid/ciy737 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control. New CDC data: COVID-19 vaccination safe for pregnant people; 2021. Available from: https://www.cdc.gov/media/releases/2021/s0811-vaccine-safe-pregnant.html. Accessed March 9, 2022.
- 31.American College of Obstetricians and Gynecologists. COVID-19 vaccines and pregnancy: conversation guide for clinicians; 2021. Available from: https://www.acog.org/covid-19/covid-19-vaccines-and-pregnancy-conversation-guide-for-clinicians. Accessed March 9, 2022.
- 32.World Health Organization. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under emergency use listing: interim guidance; 2021. Available from: https://apps.who.int/iris/handle/10665/338484. Accessed March 9, 2022.
- 33.Ciapponi A, Bardach A, Mazzoni A, et al. Safety of COVID-19 vaccines, their components or their platforms for pregnant women: a rapid review. medRxiv. 2021. doi: 10.1101/2021.06.03.21258283 [DOI] [Google Scholar]