ABSTRACT
The CoronaVac vaccine was found to be effective against symptomatic COVID-19 and protective against severe disease in phase 3 studies. However, there are little data about its effectiveness in real-world conditions. The aim of the current study was to investigate the protective effect of the CoronaVac vaccine in health-care workers (HCWs) in Turkey, a country where CoronaVac is widely used. The questionnaire was sent to all employees in the form of a survey link by using a telephone application. In the survey, HCWs were asked about demographic characteristics; CoronaVac vaccination status, history of a COVID-19 infection, whether COVID-19 infection was before or after the CoronaVac vaccination; the time between being vaccinated and the COVID-19 infection; the clinical pictures of COVID-19 infection. Those who experienced COVID-19 before vaccination were compared with the breakthrough cases in terms of demographic and clinical features. A total of 628 HCW agreed to participate in the study. A total of 536 (85.3%) volunteers had been vaccinated and 92 (14.6%) had not been vaccinated against COVID-19 with CoronaVac. There was a history of COVID-19 infection in 234 (37.2%) subjects and 188 (35%) had been vaccinated and 46 (50%) not vaccinated. The rate experiencing COVID-19 disease was significantly lower in the vaccinated than the unvaccinated volunteers. The rate of breakthrough cases after CoronaVac was found to be 7%. The hospitalization rate was similar in the breakthrough cases and those who had COVID-19 before CoronaVac vaccination. The results of our study indicate that CoronaVac provides protection against COVID-19.
KEYWORDS: CoronaVac, Sinovac, COVID-19, pneumonia, hospitalization, healthcare workers, mortality, survey, vaccine, intensive care unit
Introduction
The SARS-CoV-2 pandemic continues to wreak havoc around the world although 18 months have gone by since it first began. The pandemic has caused a large number of deaths worldwide and adversely affected how we live in many ways, with especially significant effects on the education system, the economy, and social life. The agent is highly contagious and has a wide range of clinical courses from asymptomatic to acute respiratory distress syndrome (ARDS), multi-organ failure, and death. Asymptomatic persons can also be contagious. The method thought to present the best hope to end the pandemic is vaccines and the discovery of vaccines against SARS-CoV-2 has opened a new era in the fight against the disease. The vaccines approved by the World Health Organization (WHO) by August 2021 were the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech), the ChAdOx1 nCoV-19 vaccine (Oxford–AstraZeneca), the mRNA-1273 vaccine (Moderna), the Ad26.COV2.S vaccine (Johnson & Johnson), the inactivated SARS-CoV-2 vaccine (Vero cell) (Sinopharm/BIBP), and the inactivated SARS-CoV-2 vaccine (Vero cell) (Sinovac Life Sciences).1
Following the emergency use approval of the CoronaVac vaccine by the Turkish Ministry of Health on 13 January 2021, vaccination was started in Turkey on 14 January 2021. The first target group was health-care workers (HCW) and the vaccine was administered in two doses at a 28-day interval. Application of the BioNTech vaccine in Turkey started on 1 June 2021. The third dose of the CoronaVac or the BioNTech vaccine is currently being administered to health-care workers at the request of the person, starting at 5 months after the second dose.
The CoronaVac vaccine is an inactivated whole-virion vaccine. The World Health Organization (WHO) has approved the vaccine for emergency use on 1 June 2021.1 The most important factor for choosing the CoronaVac vaccine when starting vaccination in Turkey was its ability to be stored without the need for freezing, in addition to the opinion that the more traditional technology using the whole virus that was employed for its manufacture would be more acceptable by the public. Side effects of CoronaVac are rare and those reported have generally been mild, the most common ones being fatigue, injection site pain, and sore muscles.2
The vaccine was found to be 50.7–83.5% effective against symptomatic COVID-19 and 100% protective against severe disease in phase 3 studies conducted in Brazil and Turkey.2,3 However, there is little data about its effectiveness in real-world conditions. The SARS-CoV-2 antibody levels have been shown to decrease 5 months after vaccination in adults vaccinated with CoronaVac, just like the decrease in post-infection antibody levels but studies on how long vaccine protection lasts are inadequate.4 There is also increasing concern about the vaccine’s effectiveness against the new variants.
The current study aimed to investigate the protective effect of the CoronaVac vaccine in HCWs in Turkey, a country where CoronaVac is widely used. Our study was carried out 7.5 months after the first vaccination with CoronaVac in Turkey and aims to provide information about the long-term protective effects of this vaccine.
Materials and methods
The study was conducted between 27 July and 16 August 2021, 7.5 months after COVID-19 vaccination was started, on HCWs working at Ankara City Hospital, a health campus with a capacity of 3810 beds and consisting of 8 separate hospitals. The questionnaire was sent to all employees in the form of a survey link by using a telephone application. In total, 628 healthcare workers volunteered to participate in the study. Each volunteer could complete the questionnaire only once.
The questionnaire consisted of 12 questions on the following topics: (1) demographic characteristics (age, gender, profession); (2) history of or current work in units that care for COVID-19 patients (inpatient service, intensive care, emergency room, outpatient clinic); (3) whether vaccinated with 2 doses of CoronaVac (Sinovac®); (4) whether a third dose of the COVID-19 vaccine had been administered; (5) which vaccine had been used for the third dose, if any (CoronaVac vs. BioNTech); (6) whether there was a history of a COVID-19 infection (confirmed by SARS-CoV-2 PCR); (7) whether this infection was before or after the CoronaVac vaccination; (8) if the infection was after the vaccination, whether it was after the 1st dose or the 2nd dose or the 3rd dose, if any; (9) the time between being vaccinated and the COVID-19 infection, if any, in the post-vaccine period; (10) which of the four clinical pictures of COVID-19 infection had been present (asymptomatic; only loss of taste and smell; flu-like picture with coryza and/or coughing and/or nasal discharge and/or bone-joint pain; physician-diagnosed lower respiratory tract infection; (11) history of hospitalization during the COVID-19 infection; (12) history of intensive care unit stay during the COVID-19 infection.
Cases with COVID-19 infection ≥14 days after the vaccination were considered breakthrough cases. Those who experienced COVID-19 before vaccination or those who were not vaccinated and experienced COVID-19 were compared with the breakthrough cases in terms of demographic and clinical features. The rate of past COVID-19 infection in subjects who were and were not vaccinated was also compared. Ethics committee approval was obtained for this study (Approval number E2-21-709).
Results
A total of 628 subjects, consisting of 158 (25.2%) males and 470 (74.8%) females, volunteered to complete the questionnaire. The mean age of the volunteers was 35.5 ± 8.9 years (4 subjects were aged ≥60 years). There were 432 (68.8%) subjects who had worked or continued to work in units that cared for COVID-19 patients. A total of 536 volunteers had been vaccinated and 92 (14.6%) had not been vaccinated against COVID-19 with CoronaVac. Of the 92 unvaccinated subjects, only one had been vaccinated with two doses of BioNTech in June 2021. This healthcare worker was excluded because she was fully vaccinated, and the total number of unvaccinated personnel was found to be 91 (14.5%). A third dose had been administered to 355 subjects; this was BioNTech in 302 (85.1%) and CoronaVac in 53 (14.9%) (Figure 1).
Figure 1.
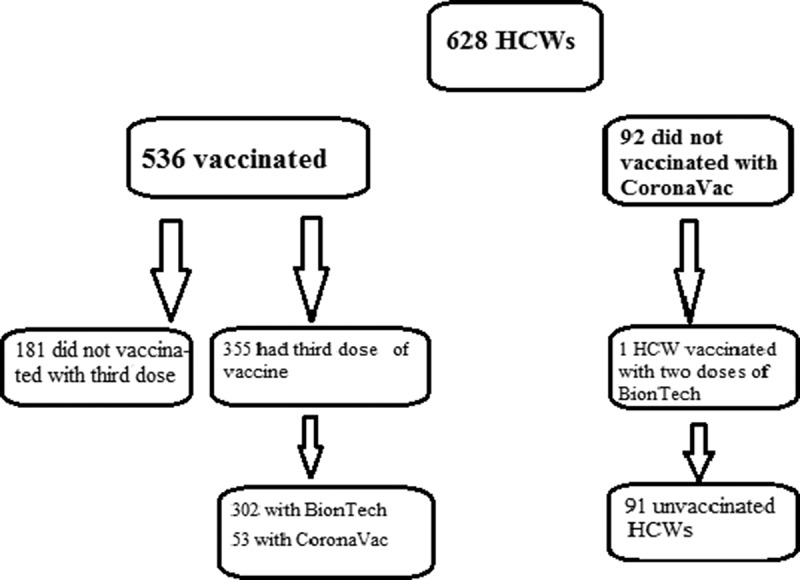
The vaccination status of health care workers.
There were a history of COVID-19 infection in 234 (37.2%) subjects and 188 (35%) had been vaccinated and 46 (50%) not vaccinated. The rate experiencing COVID-19 disease was significantly lower in the vaccinated than the unvaccinated volunteers (p = .017). Among the subjects who had a history of COVID-19 disease and vaccination, 146 (77.7%) had the COVID-19 infection before the CoronaVac vaccination, and 42 (22.3%) afterward. Of the latter 42 subjects, 38 (90.2%) had experienced the COVID-19 infection after the second dose and 4 (9.8) after the third dose (Figure 2). In the group that experienced COVID-19 infection after the third dose, 3 (75%) were vaccinated with BioNTech and 1 with CoronaVac; of these four HCWs, 1 HCW had received the third dose of CoronaVac vaccine experienced COVID-19 infection one month after the last dose of CoronaVac. Among the other three HCWs who had received the BioNTech vaccine, one experienced COVID-19 7 days after the third dose while the other two had the disease one month afterward.
Figure 2.
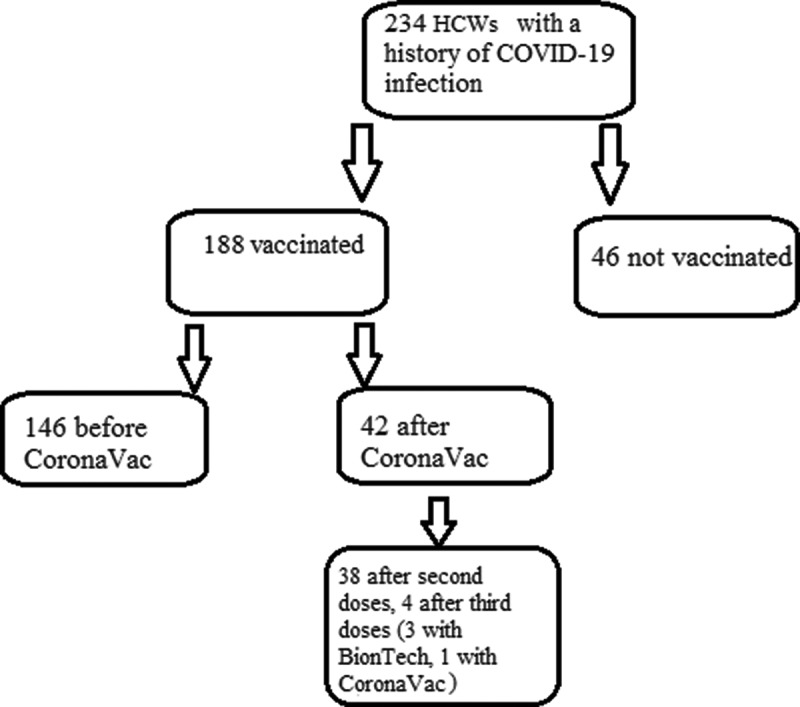
Vaccination history of HCWs who had experienced the COVID-19 infection.
The median interval from the second vaccine dose to the COVID-19 diagnosis was two months (min. 7 days-max 6 months). One HCW had COVID-19 seven days after receiving second dose of the CoronaVac vaccine. After this subject and 3 subject who experienced COVID-19 after third doses of vaccination with BioNTech were excluded, the rate of breakthrough cases was found to be 7% (38/536). The comparison of the demographic and clinical characteristics of the HCWs with breakthrough infection and the HCWs with COVID-19 infection before vaccination or HCWs who were not vaccinated and experienced COVID-19 infection is shown in Table 1.
Table 1.
The comparison of HCWs who experienced COVID-19 after two doses of the CoronaVac vaccine with HCWs who did not vaccinated and experienced COVID-19 or experienced COVID-19 before two doses of the CoronaVac vaccine as regards the demographic features and COVID-19 clinical picture, hospitalization during COVID-19, and vaccination with the third dose
| COVID-19 before vaccination or COVID-19 in unvaccinated n: 192 | COVID-19 after vaccination n: 38 | p | |
|---|---|---|---|
| Age (mean ± SD) | 34.8 ± 9.4 | 36.6 ± 7.4 | .20 |
| Gender (female percentage) n (%) | 102 (69.8) | 30 (78.9) | .34 |
Occupation n (%)
|
70 (36.5) | 13 (34.2) | .98 |
| 51 (26.5) | 10 (26.3) | ||
| 71 (37.0) | 15 (39.5) | ||
| Working at COVID-19 clinics | 138 (71.9) | 27 (71) | .15 |
Clinical picture of COVID-19
|
18 (9.4) | 4 (10.5) | .86 |
| 23 (12) | 6 (15.8) | ||
| 118 (61.5) | 20 (52.6) | ||
| 33 (17.1) | 8 (21.1) | ||
| Hospitalization during COVID-19 | 20 (10.4) | 4 (10.5 | .93 |
The rate of vaccination with the third dose lower in those who had COVID-19 after vaccination (12, 31.6%) than those who had COVID-19 before vaccination (83, 54.8%) (p = .008). None of the HCW with COVID-19 history in the study group required ICU admission during COVID-19. According to hospital records, a pharmacist working in our hospital died before the vaccination started, there were no other HCW who died.
Discussion
The results of our study indicate that CoronaVac provides protection against COVID-19. However, there was no difference in disease severity or hospitalization rate between those who had COVID-19 before or after vaccination.
The results of Phase 3 studies conducted in Turkey have shown vaccination with two doses of CoronaVac vaccine to reduce the risk of developing symptomatic COVID-19 in the 18–59 years age group by 83.5% and to prevent COVID-19-related hospitalization by 100% when compared to placebo.2 In a Phase-3 study conducted in Brazil, the protection rate against symptomatic COVID-19 was found to be 50.7%, while protection against a severe clinical picture was 100%, similar to the Turkish study.3 In a prospective observational cohort study involving approximately 10.2 million persons and conducted in Chile, the effectiveness was 65.9% in preventing COVID-19, 87.5% in preventing hospitalization, and 90.3% in preventing ICU admission.5 The rate of being sick with COVID-19 was significantly lower in vaccinated HCWs than those who were not vaccinated in the current study. Considering that approximately 80% of the cases of COVID-19 were before CoronaVac vaccination in the vaccinated group in our study, we believe that CoronaVac is even more effective in preventing COVID-19 infection than the above numbers indicate. In our study, the median time between vaccination and COVID-19 infection was 2 months. This period also coincides with an increase in the number of cases in Turkey. In addition, after the two doses of CoronaVac vaccination, there was an increase in the behaviors of the health personnel to spend time together without the mask and social distance rules with the belief that those who have been vaccinated will no longer get COVID-19.
Some of the most important expectations from the COVI9-19 vaccines are reduced hospitalization rates and hospital occupancy rates to ensure the maintenance of the quality of service in the healthcare system. It has been reported that CoronaVac prevents hospitalization due to COVID-19 by 87.5% to 100%.2,5 Approximately one-fifth of the breakthrough cases had been hospitalized in the current study, and the hospitalization rate was similar in the breakthrough cases and those who had COVID-19 before CoronaVac vaccination or those who were unvaccinated. The demographic features of those with unvaccinated/pre-vaccine and post-vaccine COVID-19 were also similar. We are unable to comment on the impact of co-morbidity on the hospitalization rate as it was not included in the questionnaire. In our county, when healthcare workers get COVID-19, decision of hospitalization can be made even if there is no indication for hospitalization, often to protect the household. This may be the reason for the high hospitalization rate in this study.
The most significant benefits of the CoronaVac vaccine are reported to be preventing a severe clinical picture and intensive care unit hospitalization.3,5 None of the breakthrough cases or those who had COVID-19 before CoronaVac vaccination was admitted to intensive care in the current study. The absence of intensive care admissions in our study cohort may be due to the fact that it consisted of a relatively young age group with very few subjects aged >60 years. Another reason may be the low rate of subjects with co-morbid conditions, but we did not question the co-morbidity status and cannot reach a definite conclusion.
Breakthrough infections have been reported after other COVID-19 vaccines as well.6 In a study conducted on HCWs, the rate of breakthrough cases among those vaccinated with BioNTech was 0.4%. The majority of breakthrough cases have been reported to be asymptomatic.7 The rate of breakthrough cases in the current study was 7%, higher than the rate reported with the BioNTech vaccine, and most cases were symptomatic.
The emergence of new variants has resulted in contemplating whether the vaccines would be effective against these new variants as well. There are only a few studies on the effectiveness of the CoronaVac vaccine against the new variants. The P.1 lineage or Gamma variant virus has been shown to evade neutralizing antibodies induced by the inactivated SARS-CoV-2 vaccine.4 In another study conducted in Brazil at a time when 86% of the genotypes were found to be of the gamma variant, being vaccinated with two doses of CoronaVac was 37.1% effective in preventing COVID-19.8 While B.1.1.7 (Alpha) was previously the dominant variant in Turkey, the delta variant was first detected in April 2021 and became the predominant variant by August 2021.9,10 The variant of SARS-CoV-2 that infected the HCWs was not investigated in the current study. Considering that the median time between vaccination and getting COVID-19 in the HCWs who stated that they were infected after the vaccination was 2 months, the delta variant was present in Turkey during that period. There is therefore a possibility that these individuals were infected with the delta variant. However, the date range of our study includes only a small part of the period in which the delta variant became the dominant variant in the community. The delta variant possesses enhanced infectivity and replication ability and it has been found to be associated with an increase in symptomatic breakthrough infections following mRNA vaccines. However, the upper respiratory tract SARS-CoV-2 IgG level has been found at low levels or to be absent in all subjects who had a breakthrough infection with the delta variant. Such infection is therefore believed to be the result of waning immunity in vaccinated individuals.11,12 It was reported that neutralizing antibody titers against the delta variant in CoronaVac recipients were significantly lower than unvaccinated, naturally infected patients.13Additional studies are needed on breakthrough infections associated with the delta variant in subjects vaccinated with CoronaVac in countries where the delta variant has become predominant, as in Turkey.
The rate of vaccination with the third dose lower in those who had COVID-19 after vaccination than those who had COVID-19 before vaccination. This may be due to the awareness about protection from reinfection after recovery.14 It has been reported that subjects who recovered and produced antibodies to SARS-CoV-2 were protected from reinfection for at least six months.
The strength of our study is that it was carried out in healthcare workers, who are at high risk of getting COVID-19 and who have a high probability of undergoing a PCR test when they suspect COVID-19 infection in themselves. In support of this notion, one-third of our study population had experienced COVID-19. A limitation of our study was that co-morbidity was not investigated and we were therefore unable to reveal whether the similarity of hospitalizations and clinical presentations was due to a difference in the co-morbidity distribution between the groups. Another limitation of our study is that it did not cover the period when the delta variant was predominant in Turkey. About half of our study group had been vaccinated with a single dose of BioNTech six months after CoronaVac vaccination. Our study period covered approximately 2 months after BioNTech vaccination. A third dose of vaccination with BioNTech possibly compensated for the decreased effectiveness of CoronaVac 6 months after vaccination. This was another limitation of our study.
In conclusion, CoronaVac is protective against SARS-CoV-2 infection. Further research is needed on the protection provided by CoronaVac in the elderly and those with co-morbid conditions and against emerging variants, in addition to how long this protection lasts.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.WHO . [accessed 2021 Sept 16]. https://extranet.who.int/pqweb/sites/default/files/documents/Status%20of%20COVID-19%20Vaccines%20within%20WHO%20EUL-PQ%20evaluation%20process%20-%203%20June%202021r.pdf.
- 2.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–5. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palacios R, Batista AP, Albuquerque CSN, Patiño EG, Santos JP, Conde MTRP, Piorelli RO, Júnior LCP, Raboni SM, Ramos F, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV study. SSRN. published online 2021. Apr 14. doi: 10.2139/ssrn.3822780. (preprint). [DOI] [Google Scholar]
- 4.Souza WM, Amorim MR, Sesti-Costa R, Coimbra LD, Brunetti NS, Toledo-Teixeira DA, de Souza GF, Muraro SP, Parise PL, Barbosa PP, et al. Neutralisation of SARS-CoV-2 lineage P.1 by antibodies elicited through natural SARS-CoV-2 infection or vaccination with an inactivated SARS-CoV-2 vaccine: an immunological study. Lancet Microbe. 2021. July 8;2:e527–e535. doi: 10.1016/S2666-5247(21)00129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 285 . 2021. :875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duerr R, Dimartino D, Marier C, Zappile P, Wang G, Lighter J, Elbel B, Troxel AB, Heguy A.. Dominance of alpha and Iota variants in SARS-CoV-2 vaccine breakthrough infections in New York City. J Clin Invest. 2021. Aug 10;131:152702. doi: 10.1172/JCI152702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. July 28;385:1474–84. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, Borg R, Schulz WL, de Oliveira RD, Da Silva PV, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am. 2021. Sept;1:100025. doi: 10.1016/j.lana.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.[accessed 2021 Sept 16]. https://www.dw.com/tr/delta-varyant%C4%B1-t%C3%BCrkiyede-ne-kadar-yayg%C4%B1n/a-57984629.
- 10.[accessed 2021 Sept 16]. https://www.aa.com.tr/tr/koronavirus/koronavirus-bilim-kurulu-uyesi-korukluoglu-agustosta-vakalarin-yuzde-80i-delta-varyanti-olacak/2298422.
- 11.Luo CH, Morris CP, Sachithanandham J, Amadi A, Gaston D, Li M, Swanson NJ, Schwartz M, Klein EY, Pekosz A, et al. Infection with the SARS-CoV-2 delta variant is associated with higher infectious virus loads compared to the alpha variant in both unvaccinated and vaccinated individuals. medRxiv. 2021. Aug 20;8(15):21262077. doi: 10.1101/2021.08.15.21262077. (Preprint). [DOI] [Google Scholar]
- 12.Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, Sabo RT, Hall N, Foreman A, Schubert PL, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021. Aug 6;70(31):1059–62. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vacharathit V, Aiewsakun P, Manopwisedjaroen S, Srisaowakarn C, Laopanupong T, Ludowyke N, Phuphuakrat A, Setthaudom C, Ekronarongchai S, Srichatrapimuk S, et al. CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis. 2021. Aug 26;S1473-3099(21):00568–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, Warren F, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–40. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]


