Abstract
Background
Primary membranous nephropathy (PMN) is associated with the highest risk for developing venous thrombosis compared with other nephrotic diseases. The aim of the study was to assess the predictive value of the pathognomonic anti-phospholipase A2 receptor (PLA2R) antibody with regard to incidence of venous thrombosis in PMN.
Methods
A total of 365 in-hospital patients diagnosed with PMN were enrolled in the study. Anti-PLA2R antibody was detected by commercial enzyme-linked immunosorbent assay. Multivariate logistic regression was used to detect the independent risk factors for venous thrombosis.
Results
Thirty-seven patients (10.14%) had venous thrombosis. Patients with venous thrombosis had higher levels of cholesterol (CHOL), low-density lipoprotein (LDL), and D-dimer than those without venous thrombosis (p < .05). Patients with venous thrombosis had significantly lower levels of albumin (23.95 ± 5.53 vs. 26.18 ± 6.59 g/L, p = .049). No significant differences were found in proteinuria, serum creatinine, estimated glomerular filtration rate, platelets, and fibrinogen between patients with and without thrombosis. Anti-PLA2R antibody levels in patients with venous thrombosis were significantly higher than in patients without it (p = .002). In the univariate logistic regression, Ln anti-PLA2R antibody (OR: 1.340; p = .004), albumin (OR: 0.945; p = .050), CHOL (OR: 1.191; p = .006), and LDL (OR: 1.271, p = .006) were associated with venous thrombosis. Ln anti-PLA2R antibody (OR = 1.269; 95%CI: 1.032–1.561), and LDL (OR = 1.213; 95%CI: 1.017–1.448) were the independent risk factors for venous thrombosis (p < .05) in multivariate analysis.
Conclusions
Anti-PLA2R antibody was the independent risk factor for venous thrombosis in PMN. Larger prospective studies were warranted to verify the results in future.
Keywords: Primary membranous nephropathy, anti-PLA2R antibody, deep vein thrombosis, risk factors
Background
Deep vein thrombosis (DVT), renal vein thrombosis (RVT), and pulmonary embolism (PE) are collectively known as venous thromboembolism (VTE), which is a common complication and a major cause of morbidity and mortality in patients with nephrotic syndrome (NS) [1,2].
The underlying pathophysiological mechanisms of VTE in patients with NS have not been fully elucidated. Hypercoagulability is postulated to due to a variety of elements, including the imbalance of glomerular loss of coagulation factors with increased liver procoagulants synthesis, altered platelet activity, intravascular volume contraction, venous stasis accompanying edema [3,4]. Proteinuria and serum albumin levels, which reflect the severity of NS, are the most frequently studied VTE risk factors in patients with NS [5–7]. Higher proteinuria and lower serum albumin levels have been suggested to be risk factors of VTE in some but not all studies [7–10]. Compared with other nephrotic diseases, membranous nephropathy (MN) is associated with the highest risk for developing venous thrombosis, especially RVT [11,12], even adjusted for gender, proteinuria, and serum albumin by multivariable analysis [13]. The reason for the increased thromboembolic risk in MN has yet to be unraveled.
Identification of antibody to phospholipase A2 receptor (PLA2R) in 70–80% of adult patients with primary MN (PMN) is the major breakthrough, which shows PMN is an autoimmune disease [14,15]. Anti-PLA2R antibody is highly specific for MN, rarely being detected in other nephropathies, autoimmune diseases, or healthy individuals. Furthermore, a number of studies have shown that PLA2R autoantibody correlate closely with disease activity and progression [16,17] and can be used to monitor response to immunosuppressive therapy [18,19]. Since anti-PLA2R antibody was considered to be pathogenic for MN, some researchers have postulated the association between anti-PLA2R antibody and the particularly high VTE risk [13]. Moreover, in our clinical experience, we observed some patients with serum albumin level around 30 g/L and extremely high level of anti-PLA2R antibody had extensive VTE. To the best of our knowledge, there was no clinical study to evaluate whether anti-PLA2R antibody was the risk factor for VTE in patients with PMN till now.
In this study, we added anti-PLA2R antibody to the common thrombophilic risk factors, such as proteinuria, hypoalbuminemia, and serum creatinine, to analyze the predictors of venous thrombosis in a large Chinese PMN cohort.
Methods
Design
We retrospectively reviewed all the in-hospital patients diagnosed with PMN in Shandong Provincial Hospital affiliated to Shandong First Medical University between January 2018 and January 2021. The prevalence of venous thrombosis and its risk factors was investigated at the time of diagnosis. Follow-up data were collected by review of the hospital's electronic medical records.
Participants
The inclusion criteria for PMN were as follows: biopsy-diagnosed MN or patients with positive anti-PLA2R antibody test, except for clinical factors such as systemic lupus erythematosus (SLE), hepatitis B virus (HBV), tumor, and drug-induced secondary MN [20]. Major exclusion criteria included: (1) without the result of anti-PLA2R antibody test; (2) without a venous examination; (3) use of immunosuppressive drugs prior to the study enrollment; (4) exposure to classic risk factors for venous thrombosis such as antiphospholipid syndrome, major surgery, prolonged immobilization.
The Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University approved the study.
Venous thrombosis examination
All the patients enrolled in the study received renal vascular and lower extremity vascular ultrasounds for venous thrombosis screening. Venous ultrasound was screened by sonologists with 10-year experience in vessel ultrasound. Two patients received computed tomography pulmonary angiography (CTPA) and diagnosed with PE. Both of them had extensive renal venous and inferior vena cava thrombosis.
Clinical evaluation
Clinical data were obtained at the time of venous examination by reviewing the patient's previous medical records, including age, sex, blood pressure measurements, history of smoking, hemoglobin, platelet count, serum albumin, serum lipids profile, plasma fibrinogen, D-dimer, urinalysis, quantification of proteinuria, blood urea nitrogen, serum creatinine, estimated glomerular filtration rate (eGFR), anti-PLA2R antibody, and pathological results. In our cohort, anti-PLA2R antibody was detected by commercial enzyme-linked immunosorbent assay (ELISA), and the manufacturer’s recommended cutoff value is 20 RU/mL.
Complete remission (CR) was defined as ≤0.3 g/day proteinuria plus stable renal function, partial remission (PR) as a 50% reduction of initial proteinuria, and less than 3.5 g/day with stable renal function.
Statistical analysis
Statistical software SPSS 25.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY) was employed for statistical analysis. Data with a normal distribution were expressed as mean ± standard deviation (SD) and compared by t-tests, and data with a non-normal distribution were presented as the median and quartile and compared by nonparametric test. The categorical variables were expressed as rates and were compared by the χ2 test. The correlation between two parameters (nonparametric distributions) was analyzed by Spearman’s rank coefficient of correlation. Univariate analysis followed by multivariate logistic regression analysis with stepwise variable selection procedure was applied to identify independent factors associated with vein thrombosis event. All baseline variables entered the initial model and were maintained if p < .05. Statistical significance was considered as p < .05.
Results
Patient population
A total of 365 in-hospital patients diagnosed with PMN at Shandong Provincial Hospital affiliated to Shandong First Medical University from January 2018 to August 2021 were enrolled in the study, including 355 biopsy-proven PMN and 10 anti-PLA2R antibody positive patients who did not receive renal biopsy because of extensive venous thrombosis. All of the 10 patients without renal biopsy received associated evaluation to exclude clinical conditions such as SLE, HBV, tumor, and drug-induced secondary MN. Of the 10 patients, the average albumin levels were 22.0 ± 4.4 g/L, the average 24 h urine protein were 7.56 ± 4.14 g, and the anti-PLA2R antibody levels ranged from 77.22 to 1500 RU/mL, with a median value of 173.89 RU/mL at time of diagnosis.
General baseline characteristics of the study patients
General baseline clinical profiles of the patients are listed in Table 1. Of the 365 patients, 135 (36.99%) were females and 230 (63.01%) were males. The mean age of the patients was 47.69 ± 12.01 years. 67.67% (247/365) of the patients had nephrotic-level proteinuria. At a cut-off value of 20 RU/mL for anti-PLA2R antibody, 83.33% (293/365) of the patients had either elevated anti-PLA2R antibody in the serum or enhanced PLA2R in glomeruli. In Spearman’s correlation analysis, anti-PLA2R antibody correlated positively with proteinuria (r = 0.296, p < .001), total cholesterol (CHOL) (r = 0.255, p < .001), and serum creatinine (r = 0.112, p = .033). The anti-PLA2R antibody level correlated negatively with serum albumin (r = −0.337, p < .001).
Table 1.
The clinical parameters of the studied PMN patients.
| Parameters | n = 365 |
|---|---|
| Gender (male/female) | 230/135 |
| Age (mean ± SD) (years) | 47.90 ± 12.01 |
| Level of PLA2R Abs (median, quartile) (RU/mL) | 37.55 (4.17, 136.40) |
| <2 (n, %) | 52 (14.25) |
| 2–20 (n, %) | 102 (27.95) |
| >20 (n, %) | 211 (57.81) |
| Ln PLA2R Abs (mean ± SD) | 3.36 ± 1.87 |
| Glomerular PLA2R antigen positive (n, %) | 265 (72.59) |
| PLA2R-related MNa (n, %) | 293 (83.33) |
| Urinary protein (mean ± SD) (g/24 h) | 6.43 ± 4.82 |
| Nephrotic proteinuria (n, %) | 247 (67.67) |
| Albumin (mean ± SD) (g/L) | 25.95 ± 6.52 |
| SCR (mean ± SD) (μmol/L) | 67.75 ± 30.53 |
| eGFR (mean ± SD) (mL/min/1.73 m2) | 107.37 ± 20.69 |
| CHOL (mean ± SD) (mmol/L) | 7.98 ± 2.46 |
| LDL (mean ± SD) (mmol/L) | 5.16 ± 1.83 |
| Combined with DM (n, %) | 39 (10.68) |
PMN: primary membranous nephropathy; SD: standard deviation; PLA2R: phospholipase A2 receptor; Abs: antibodies; SCR: serum creatinine; eGFR: estimated glomerular filtration rate; CHOL: cholesterol; LDL: low-density lipoprotein; DM: diabetes.
PLA2R-related MN was defined as either positive for serum anti-PLA2R antibody (cutoff value of 20 RU/mL) or glomerular PLA2R antigen.
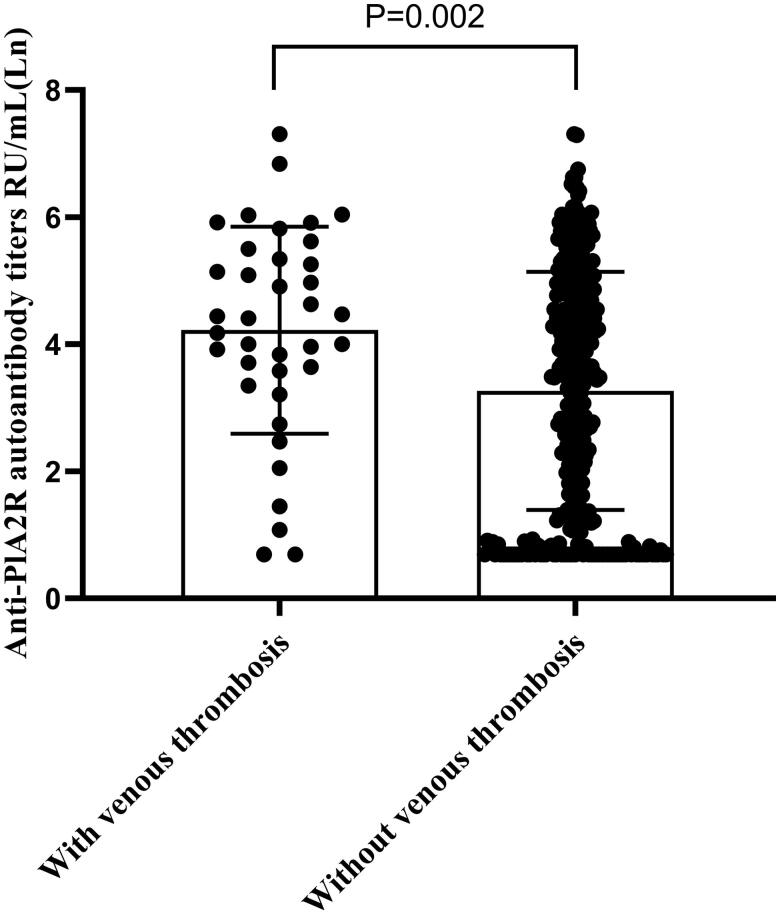
Among the studied patients, 37 patients (10.14%) had venous thrombosis. A total of 46 anatomic site venous thrombosis were detected in these patients, and seven patients had simultaneous venous thrombosis at more than one site. Twenty-two patients had a DVT; 11 patients had a RVT; 10 patients had an inferior vena cava thrombosis; two patients had a PE. The clinical characteristics of patients with and without venous thrombosis at the time of vascular ultrasounds examination are shown in Table 2. There were no statistical differences in the distributions of age and sex between patients with and without venous thrombosis. Patients with venous thrombosis had significantly higher level of CHOL, low-density lipoprotein (LDL), D-dimer, and lower level of albumin than those without venous thrombosis (p = .025, p = .037, p < .001, p = .049, respectively). No significant differences were found in urine proteinuria, SCR, eGFR, platelets, and fibrinogen between patients with and without thrombosis. Anti-PLA2R antibody was highly skewed, in Mann–Whitney’s U-test anti-PLA2R antibody level in patients with venous thrombosis were significantly higher than in patients without it (p = .004). The distributions of Ln anti-PLA2R antibody of the two groups are shown in Figure 1.
Table 2.
Laboratory findings of PMN with or without venous thrombosis.
| Parameters | With venous thrombosis (n = 37) | Without venous thrombosis (n = 328) | p Value |
|---|---|---|---|
| Age (mean ± SD) (years) | 48.97 ± 10.94 | 47.78 ± 12.14 | .567 |
| Gender (female, %) | 10 (27.03%) | 125 (38.11%) | .186 |
| PLT (mean ± SD) (×109/L) | 274.47 ± 67.43 | 266.31 ± 62.51 | .462 |
| Ln PLA2R Abs (mean ± SD) | 4.22 ± 1.63 | 3.26 ± 1.87 | .002 |
| Urinary protein (mean ± SD) (g/24 h) | 7.20 ± 4.25 | 6.34 ± 4.87 | .320 |
| Albumin (mean ± SD) (g/L) | 23.95 ± 5.53 | 26.18 ± 6.59 | .049 |
| SCR (mean ± SD) (μmol/L) | 71.19 ± 18.97 | 67.36 ± 31.56 | .470 |
| eGFR (mean ± SD) (mL/min/1.73 m2) | 104.03 ± 18.15 | 107.75 ± 20.95 | .301 |
| CHOL (mean ± SD) (mmol/L) | 9.07 ± 3.00 | 7.86 ± 2.37 | .025 |
| LDL (mean ± SD) (mmol/L) | 5.98 ± 2.46 | 5.07 ± 1.72 | .037 |
| D-dimer (median, quartile) (μg/mL) | 1.41 (0.57, 3.41) | 0.57 (0.34, 1.06) | <.001 |
| Fib (median, quartile) (g/L) | 4.09 (3.53, 4.86) | 3.97 (3.50, 4.67) | .518 |
| Combined with DM (n, %) | 3 (8.11%) | 36 (10.98%) | .592 |
PMN: primary membranous nephropathy; SD: standard deviation; PLT: platelet; PLA2R: phospholipase A2 receptor; Abs: antibodies; SCR: serum creatinine; eGFR: estimated glomerular filtration rate; CHOL: cholesterol; LDL: low-density lipoprotein; Fib: fibrogen; DM: diabetes.
Bold values are statistical significance.
Figure 1.
Scatter plot shows the levels of anti-PLA2R antibody in PMN patients with and without venous thrombosis. Anti-PLA2R antibody was highly skewed, so natural log transformation was used for the analysis.
Risk factors of venous thrombosis in patients with PMN at time of diagnosis
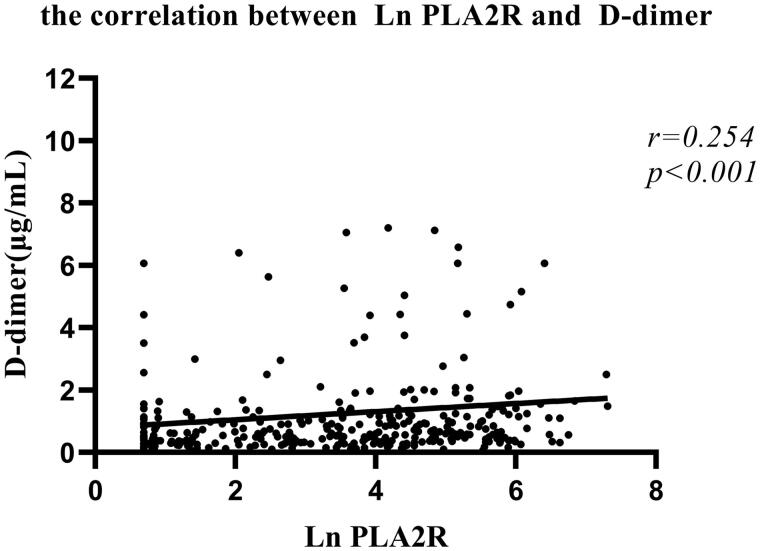
D-dimer is a fragment of degraded fibrin reflecting the activation of fibrinolysis and thrombosis, which was used as the marker of hypercoagulable state in patients with MN [21]. In our patients, Spearman’s correlation analysis showed that higher anti-PLA2R antibody level was positively and significantly correlated with the plasma D-dimer level (Figure 2; r = 0.254, p < .001).
Figure 2.
Scatter plot shows the correlation between the levels of anti-PLA2R antibody and the levels of plasma D-dimer in Spearman’s correlation analysis. Anti-PLA2R antibody was highly skewed, so natural log transformation was used for the analysis.
Since plasma D-dimer level was influenced by many factors, correlation of anti-PLA2R antibody and venous thrombosis was further analyzed. Results of the univariate and multivariate logistic regression analyzing risk factors for venous thrombosis in PMN are shown in Table 3. Anti-PLA2R antibody was highly skewed, so natural log transformation was used for the analysis. In the univariate logistic regression, Ln anti-PLA2R antibody (OR: 1.340; p = .004), albumin (OR: 0.945; p = .050), CHOL (OR: 1.191, p = .006), and LDL (OR: 1.271, p = .006) were associated with venous thrombosis. CHOL was highly colinear with LDL, and then albumin, LDL, and Ln anti-PLA2R antibody were used in the further multivariate logistic regression with forward-conditional method. We found that only Ln anti-PLA2R antibody (OR = 1.269; 95%CI: 1.032–1.561), and LDL (OR = 1.213; 95%CI: 1.017–1.448) were the independent risk factors for venous thrombosis (p < .05).
Table 3.
Risk factors in predicting venous thrombosis in patients with PMN.
| Parameters | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| p Valuea | OR (95%CI) | p Value | OR (95%CI) | |
| Age per year | .566 | 1.008 (0.980, 1.038) | ||
| Sex (female) | .189 | 0.601 (0.282, 1.285) | ||
| Ln PLA2R Abs per Ln change | .004 | 1.340 (1.098, 1.636) | .024 | 1.269 (1.032, 1.561) |
| Albumin per g/L | .050 | 0.945 (0.892, 1.000) | ||
| SCR per μmol/L | .477 | 1.003 (0.994, 1.012) | ||
| eGFR per mL/min/1.73 m2 | .301 | 0.992 (0.977, 1.007) | ||
| PLT per ×109/L | .461 | 1.002 (0.997, 1.007) | ||
| CHOL per mmol/L | .006 | 1.191 (1.050, 1.351) | ||
| LDL per mmol/L | .006 | 1.271 (1.073, 1.506) | .032 | 1.213 (1.017, 1.448) |
| Proteinuria per g/day | .321 | 1.033 (0.968, 1.103) | ||
| Fib per g/L | .730 | 1.028 (0.877, 1.206) | ||
| Combined with DM | .594 | 0.716 (0.209, 2.449) | ||
PMN: primary membranous nephropathy; PLA2R: phospholipase A2 receptor; Abs: antibodies; SCR: serum creatinine; eGFR: estimated glomerular filtration rate; PLT: platelet; CHOL: cholesterol; DM: diabetes; Fib: fibrogen.
Only variables with p≤ .05 in the univariate logistic regression analysis were used in the multiple logistic regression.
Bold values are statistical significance.
Follow up
Follow-up data were collected by review of the hospital's electronic medical records. Two hundred and thirty-five of the 365 patients were followed up in our in-patient or out-patient clinics for at least 3 months, with a median follow-up time of 11 months (3–42 months).
Among the 235 patients, 29 patients were diagnosed with venous thrombosis at baseline (two with PE, 13 with RVT and/or inferior vena cava thrombosis, 14 with DVT), with a median follow-up time of 16 months (3–39 months). All of the 29 patients received immunosuppressive therapy, and low-molecular-weight heparin (LMWH) for 2–4 weeks followed by oral warfarin or rivaroxaban planning for at least six months. During the follow-up, 25 patients reached CR or PR with a significant decline of the level of anti-PLA2R antibody (269.44 ± 380.22 vs. 10.8 ± 19.7 RU/mL; p < .01). Among the 25 patients, thrombosis was absent in six patients, partially resolved in eight patients, 11 did not receive a second ultrasound examination. Four of the 29 patients experienced no response in proteinuria, but the thrombosis was partially resolved with persistently anti-coagulation therapy. Among the four patients, anti-PLA2R antibody of one patient turned negative, while anti-PLA2R antibody of the other three patients maintained positive.
One hundred and ninety-six patients without thrombosis at baseline had follow-up data. One hundred and forty-one patients received immunosuppressive therapy, while the others received supportive care only at baseline. According to local clinical practice, patients with albumin lower than 30 g/L received anti-coagulation therapy, such as aspirin, LMWH, warfarin, and rivaroxaban. During the follow-up, 131 patients reached CR or PR. Thirteen patients received a second venous thrombosis examination, and one patient presented with new thromboembolic events, who experienced no response in proteinuria after 4 month immunosuppressive treatment with anti-PLA2R antibody level increasing from 288.12 to 345.14 RU/mL.
Discussion
Compared with other nephrotic diseases, PMN is associated with the highest risk for developing venous thrombosis [11–13]. The aim of this study was to explore whether the pathognomonic anti-PLA2R antibody contributes to venous thrombosis risk in PMN.
In this large PMN cohort, venous thrombosis occurred in 10.14% of the patients, and DVT occurred more frequently than RVT. The frequency of venous thrombosis in our patients was consistent with some reports [13,22], but substantially lower than studies using more sensitive examination for VTEs screening, such as CTPA or lung ventilation and perfusion scintigraphy [10,23]. In our local clinical practice, considering the risk of contrast induced nephropathy, CTPA was not performed unless patients presented with clinical signs and symptoms of PE, such as dyspnea, hemoptysis, chest pain, and syncope. Lung ventilation and perfusion scintigraphy was neither routinely performed. Hence, in this study, we focused on venous thrombosis and symptomatic PE. The other explanation for the relatively lower prevalence of venous thrombosis was that non-nephrotic patients were also included and accounted for 32.33% in our cohort. Although the prevalence of thrombosis was lower in patients without NS than in patients with it, two venous thrombosis events were indeed detected in patients without NS. Non-nephrotic patients should also be involved to explore the risk predictors of thrombosis in PMN.
In our cohort, we found that hypoalbuminemia, CHOL, and LDL were the risk factors for venous thrombosis in univariate analysis. Proteinuria was not the predictive of thrombotic events in this cohort. Anti-PLA2R antibody is an organ-specific autoantibody which targets the kidney podocytes, and is considered to be pathogenic in PMN. The levels of anti-PLA2R antibody correlate closely with disease activity and progression in PMN [16,17]. In the cross-sectional analysis, anti-PLA2R antibody was correlated with proteinuria and hypoalbuminemia at baseline. More interestingly, the levels of anti-PLA2R antibody were significantly higher in patients with venous thrombosis than that of patients without venous thrombosis. Furthermore, in multivariate logistic regression analysis, anti-PLA2R antibody was the independent risk factor for venous thrombosis, even adjusted for albumin and LDL. The results of our study indicated that anti-PLA2R antibody is superior to albumin and proteinuria in relation to the assessment of the risk of venous thrombosis in PMN. In this retrospective study, only 27 patients received a second venous thrombosis examination during follow up. Among the 27 patients, only one new thromboembolic event was detected. In spite of the limited follow-up data, the lower frequency of new thrombosis might be due to extensive anti-coagulation therapy in patients with PMN to some extent. The dynamic effect of anti-PLA2R antibody on venous thrombosis needs to be identified in larger long-term prospective studies.
The high risk of VTE in individuals with nephrotic range proteinuria was assumed to be secondary to loss of anticoagulant proteins. However, microalbuminuria and declined eGFR were also found to be independently associated with increased risk for VTE [24–27]. Pang et al. performed urine proteomics of PMN and found that these proteins are mainly involved in immune response and coagulation cascades [28]. These results indicated that there might be direct links between renal injury and thrombosis. The correlation of anti-PLA2R antibody induced renal injury and activation of coagulation deserves further investigation. On the other hand, a recent study demonstrated that MN patients with Th17-mediated inflammation had more VTEs [29]. Th17-immune response is a proinflammatory immune pathway associated with autoimmune diseases [30,31]. Whether the development of thrombosis will be induced by the activation of autoimmune response against PLA2R is another question for future consideration.
This study has several limitations. First, to explore the role of anti-PLA2R antibody in the thrombosis risk, it would be the best to investigate it in PLA2R-related MN. The optimum cut-off value for the diagnostic accuracy of the commercially available ELISA assay in different populations was still under discussion [32,33]. Moreover, in patients who are anti-PLA2R antibody negative, the positive staining of the renal biopsy for PLA2R antigen may also disclose PLA2R-related MN [34]. It is hard to accurately define the PLA2R-related MN. However, in our cohort, using a relatively conservative cut-off value of 20 RU/mL for anti-PLA2R antibody, 83.33% of the patients had either elevated anti-PLA2R antibody in the serum or enhanced PLA2R in glomeruli. That is, PLA2R-related MN accounted for the majority of the population in our cohort. Second, despite the large size of our cohort, there were relatively few events, which might limit the ability to identify predisposing risk factors. Finally, based on a retrospective analysis of passively captured clinical events, the true frequency might be underestimated and the dynamic effect of anti-PLA2R antibody on venous thrombosis cannot be determined.
Conclusions
In summary, we were the first to explore the risk of anti-PLA2R antibody in the development of venous thrombosis in PMN, and found anti-PLA2R antibody to be the independent risk factor. Larger prospective studies are warranted to verify the results in future.
Acknowledgements
We thank the nurses of Department of Nephrology of Shandong Provincial Hospital Affiliated to Shandong First Medical University for their support in collecting data from this work.
Glossary
Abbreviations
- CHOL
cholesterol
- CTPA
computed tomography pulmonary angiography
- CR
Complete remission
- DVT
deep vein thrombosis
- eGFR
estimated glomerular filtration rate
- ELISA
enzyme-linked immunosorbent assay
- HBV
hepatitis B virus
- LDL
low-density lipoprotein
- MN
membranous nephropathy
- NS
nephrotic syndrome
- PE
pulmonary embolism
- PLA2R
phospholipase A2 receptor
- PMN
primary membranous nephropathy
- SLE
systemic lupus erythematosus
- RVT
renal vein thrombosis
- VTE
venous thromboembolism
- PR
partial remission
- SD
standard deviation
Funding Statement
This work was supported by grants of Taishan Scholars Program of Shandong Province (No. ts201712090), Academic promotion programme of Shandong First Medical University (No. 2019QL022), and China international medical foundation (No. Z-2017-24-2037).
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University (approval number: SZRJJ:NO.2021-172).
Authors contributions
Huizi Zhu and Liang Xu: acquisition of data, analysis, and interpretation of data. Xiang Liu and Bing Liu: acquisition of data, and article revision. Chunjuan Zhai: statistical analysis. Rong Wang: text revision. Xiaowei Yang: the conception and design of the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used during the current study are available from the corresponding author on reasonable request.
References
- 1.Singhal R, Brimble KS.. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006;118(3):397–407. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Mecca G, Marchesi D, et al. . Platelet hyperaggregability and the nephrotic syndrome. Thromb Res. 1979;16(3–4):345–354. [DOI] [PubMed] [Google Scholar]
- 3.Llach F. Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int. 1985;28(3):429–439. [DOI] [PubMed] [Google Scholar]
- 4.Robert A, Olmer M, Sampol J, et al. . Clinical correlation between hypercoagulability and thrombo-embolic phenomena. Kidney Int. 1987;31(3):830–835. [DOI] [PubMed] [Google Scholar]
- 5.Lionaki S, Derebail VK, Hogan SL, et al. . Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007;18(8):2221–2225. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Chapagain A, Nitsch D, et al. . Proteinuria and hypoalbuminemia are risk factors for thromboembolic events in patients with idiopathic membranous nephropathy: an observational study. BMC Nephrol. 2012;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li SJ, Tu YM, Zhou CS, et al. . Risk factors of venous thromboembolism in focal segmental glomerulosclerosis with nephrotic syndrome. Clin Exp Nephrol. 2016;20(2):212–217. [DOI] [PubMed] [Google Scholar]
- 9.Gyamlani G, Molnar MZ, Lu JL, et al. . Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol Dial Transplant. 2017;32(1):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Lv J, Zhou F, et al. . Risk factors of pulmonary thrombosis/embolism in nephrotic syndrome. Am J Med Sci. 2014;348(5):394–398. [DOI] [PubMed] [Google Scholar]
- 11.Llach F, Papper S, Massry SG.. The clinical spectrum of renal vein thrombosis: acute and chronic. Am J Med. 1980;69(6):819–827. [DOI] [PubMed] [Google Scholar]
- 12.Velasquez FF, Garcia PN, Ruiz MN.. Idiopathic nephrotic syndrome of the adult with asymptomatic thrombosis of the renal vein. Am J Nephrol. 1988;8(6):457–462. [DOI] [PubMed] [Google Scholar]
- 13.Barbour SJ, Greenwald A, Djurdjev O, et al. . Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81(2):190–195. [DOI] [PubMed] [Google Scholar]
- 14.Beck LJ, Bonegio RG, Lambeau G, et al. . M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y, Li J, He F, et al. . The diagnosis accuracy of PLA2R-AB in the diagnosis of idiopathic membranous nephropathy: a meta-analysis. PLOS One. 2014;9(8):e104936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofstra JM, Beck LJ, Beck DM, et al. . Anti-phospholipase A(2) receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6(6):1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanigicherla D, Gummadova J, McKenzie EA, et al. . Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83(5):940–948. [DOI] [PubMed] [Google Scholar]
- 18.Beck LJ, Fervenza FC, Beck DM, et al. . Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22(8):1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Debiec H, Ruggiero B, et al. . Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26(10):2545–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276. [DOI] [PubMed] [Google Scholar]
- 21.Wang GH, Lu J, Ma KL, et al. . The release of monocyte-derived tissue factor-positive microparticles contributes to a hypercoagulable state in idiopathic membranous nephropathy. J Atheroscler Thromb. 2019;26(6):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou PM, Li H, Cai JF, et al. . A cohort study of incidences and risk factors for thromboembolic events in patients with idiopathic membranous nephropathy. Chin Med Sci J. 2018;33(2):91–99. [DOI] [PubMed] [Google Scholar]
- 23.Li SJ, Guo JZ, Zuo K, et al. . Thromboembolic complications in membranous nephropathy patients with nephrotic syndrome—a prospective study. Thromb Res. 2012;130(3):501–505. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoodi BK, Gansevoort RT, Veeger NJ, et al. . Microalbuminuria and risk of venous thromboembolism. JAMA. 2009;301(17):1790–1797. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Fang MC, Udaltsova N, et al. . Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folsom AR, Lutsey PL, Astor BC, et al. . Chronic kidney disease and venous thromboembolism: a prospective study. Nephrol Dial Transplant. 2010;25(10):3296–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoodi BK, Gansevoort RT, Naess IA, et al. . Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation. 2012;126(16):1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang L, Li Q, Li Y, et al. . Urine proteomics of primary membranous nephropathy using nanoscale liquid chromatography tandem mass spectrometry analysis. Clin Proteomics. 2018;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cremoni M, Brglez V, Perez S, et al. . Th17-immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front Immunol. 2020;11:574997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwakura Y, Nakae S, Saijo S, et al. . The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Yang H, Shi W, et al. . MicroRNA-mediated regulation of T helper type 17/regulatory T-cell balance in autoimmune disease. Immunology. 2018;155(4):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tampoia M, Migliucci F, Villani C, et al. . Definition of a new cut-off for the anti-phospholipase A2 receptor (PLA2R) autoantibody immunoassay in patients affected by idiopathic membranous nephropathy. J Nephrol. 2018;31(6):899–905. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Li X, Ma C, et al. . Serum anti-PLA2R antibody as a diagnostic biomarker of idiopathic membranous nephropathy: the optimal cut-off value for Chinese patients. Clin Chim Acta. 2018;476:9–14. [DOI] [PubMed] [Google Scholar]
- 34.Chadban SJ, Ahn C, Axelrod DA, et al. . KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104(4 Suppl. 1):S11–S103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.