Abstract
The incidence of both type 1 and type 2 diabetes is increasing globally, most likely explained by environmental changes, such as changing exposures to foods, viruses, and toxins, and by increasing obesity. While cardiovascular disease (CVD) mortality has been declining recently, this global epidemic of diabetes threatens to stall this trend. CVD is the leading cause of death in both type 1 and type 2 diabetes, with at least a two- to fourfold increased risk in patients with diabetes. In this review, the risk factors for CVD are discussed in the context of type 1 and type 2 diabetes. While traditional risk factors such as dyslipidemia, hypertension, and obesity are greater in type 2 patients than in type 1 diabetes, they explain only about half of the increased CVD risk. The role for diabetes-specific risk factors, including hyperglycemia and kidney complications, is discussed in the context of new study findings.
Keywords: diabetes mellitus, type 1 diabetes, type 2 diabetes, epidemiology, cardiovascular disease, coronary artery disease, atherosclerosis, coronary heart disease
Introduction
Globally, the incidence of diabetes mellitus is on the rise [1, 2, 3•, 4, 5], and the prevalence of diabetes is expected to double over the next 20 years [6]. The increase in diabetes is likely explained by changes in the environment, since the change is too rapid to be attributed to genetic factors. Obesity is also increasing globally and likely accounts for much of the increase in type 2 diabetes [6]. Environmental factors may trigger the autoimmune destruction of beta cells in people with a genetic susceptibility to diabetes. While other environmental factors, such as changing exposures to foods, viruses, and toxins, no doubt play a role in the rising incidence of type 1 diabetes, rising obesity may explain some of the increasing incidence of type 1 diabetes in some children as well, since obesity is an increasing phenotype in children with type 1 diabetes [7].
Cardiovascular disease (CVD) is the leading cause of death among people with diabetes [8–11], and therefore, the increasing incidence of diabetes is of great public health concern. It has been previously reported that 75 % of patients with diabetes can expect to die from a cardiovascular event, posing a substantial challenge to clinicians and the health-care system as a whole [12]. Type 1 diabetes is associated with at least a two- to fourfold increased risk of subclinical coronary artery disease (CAD) [13, 14], and young adults with type 1 diabetes under the age of 40 have a risk of dying from ischemic heart disease that is 20-fold higher in men and 40-fold higher in women, as compared with nondiabetic adults. The relative increase in CVD is greater in type 1 diabetes than in type 2 diabetes, since individuals with type 1 diabetes are typically diagnosed under the age of 30 and the risk of CVD in the general population is relatively low among people in their 30s and 40s. Type 2 diabetes has traditionally been diagnosed in adults over 40, and so the relative increase in all-cause mortality is about twofold, while the relative increase in CVD mortality ranges from 1.5- to 4.6-fold, increasing with the duration of diabetes [15, 16]. As a result, the increasing incidence of type 2 diabetes among teens and young adults is of great concern, since these patients may be at even higher future risk for CVD than are young adults with type 1 diabetes. There is already evidence that youth with type 2 diabetes have a greater risk for renal complications than do youth with type 1 diabetes, and renal disease increases the risk for CVD.
The unfavorable macrovascular consequences of diabetes are well known, including an accelerated rate of atherosclerosis that predisposes individuals to revascularization, myocardial infarction (MI) [17, 18], and death [19]. In the United States alone, one third of all percutaneous coronary intervention (PCI) procedures are performed on patients with diabetes [20]. The risk and nature of CVD in relation to type 1 and type 2 diabetes have been studied extensively. The cause of the increased risk of CVD in diabetes is multifactorial, but important factors common to both types of diabetes include dyslipidemia, hypertension, poor glycemic control, and obesity
This review will discuss recent findings from studies addressing the risk and nature of CVD in type 1 and type 2 diabetes.
Type 1 Versus Type 2 Diabetes
To date, there is no gold standard definition of type 1 versus type 2 diabetes, and the diabetes type can be difficult to determine [7, 21]. Previously, type 1 diabetes was referred to as juvenile onset diabetes, but the age at diagnosis has been found to poorly differentiate the two types of diabetes. Type 2 diabetes, which traditionally developed in adults over age 40, is now increasing in adolescents and young adults [5, 22–24]. Difficulty in determining the type of diabetes can lead to misclassification and incorrect treatment, such as delaying insulin treatment in an adult with later onset type 1 diabetes. While the distinction between the two major forms of diabetes is less than perfect, the prevalence and incidence of both type 1 and type 2 diabetes have increased considerably over the past several decades [1, 2, 22, 24–26], increasing the public health importance of CVD prevention in diabetes.
CVD Risk Factors: Type 1 Versus Type 2 Diabetes
The main modifiable CVD risk factors for type 2 diabetes are dyslipidemia, hypertension, and obesity. In contrast, people with type 1 diabetes are less likely to be obese and usually have relatively normal lipid profiles. The major risk factors for CVD in type 1 diabetes are hyperglycemia, hypertension, and diabetic nephropathy.
Traditional CVD Risk Factors
The Role of Dyslipidemia in Type 1 and Type 2 Diabetes
Type 1 Diabetes
In contrast to patients with type 2 diabetes, people with type 1 diabetes typically do not have more dyslipidemia than do comparable nondiabetic individuals [27], and HDL-cholesterol and triglycerides are more favorable than in nondiabetic individuals [13]. However, in the EURODIAB study, a cross-sectional analysis of CVD risk factors demonstrated that type 1 diabetic patients with CVD had lower HDL-cholesterol and higher triglycerides than did those without CVD [28]. Longitudinally, over 10 years, higher levels of LDL-cholesterol and triglycerides and lower levels of HDL-cholesterol were associated with increased risk for CVD, mortality, and other complications among patients with type 1 diabetes in the Pittsburgh Epidemiology of Diabetes Complications study [29]. Therefore, while dyslipidemia is less common in type 1 diabetes, it remains a risk factor for CVD.
Type 2 Diabetes
Dyslipidemia, particularly low HDL-cholesterol and elevated triglycerides, is common in people with type 2 diabetes and is a risk factor for CVD in this population [30]. In the United Kingdom Prospective Diabetes Study (UKPDS), higher LDL-cholesterol, higher triglycerides, and lower HDL-cholesterol all predicted CVD among patients with type 2 diabetes [30]. In addition, higher LDL and non-HDL cholesterol were found to predict the future need for coronary artery bypass graft (CABG) among patients with type 2 diabetes who already had coronary artery disease [31].
The Role of Hypertension in Type 1 and Type 2 Diabetes
Type 1 Diabetes
Hypertension is increased in patients with type 1 diabetes when compared with nondiabetic individuals of similar age and gender, and hypertension is associated with greater prevalence of coronary artery calcium, a marker of subclinical atherosclerosis [13]. Studies in type 1 diabetes have demonstrated a high prevalence of hypertension, with 24 % of participants in the EURODIAB study hypertensive, but fewer than half of these patients were aware of their hypertension and fewer than 12 % of hypertensive patients were treated and controlled [32]. Higher pulse pressure, a consequence of systolic hypertension and diastolic dysfunction, was a significant predictor of mortality in the EURODIAB study [33].
Type 2 Diabetes
Higher systolic blood pressure increases the risk for CVD nearly twofold in type 2 diabetes [30]. The UKPDS tested whether treatment of hypertension with either the angiotensin converting enzyme (ACE) inhibitor captopril or the beta blocker atenolol would reduce macrovascular complications. Treatment with either ACE inhibitor or beta blocker lowered blood pressure to similar levels [34], and strokes were reduced by 44 %, while deaths related to diabetes were reduced by 32 % [35].
Gender Differences in Complications of Type 1 and Type 2 Diabetes
Type 1 Diabetes
The burden of diabetes-related complications have been widely demonstrated to vary by gender. In the general population, men are more likely to develop CVD [11] and kidney disease [36] than are women; however, diabetes disproportionately increases the risk for both complications in women. Women without diabetes have a lower risk for CVD during their premenopausal years than do males, but women with type 1 diabetes have a relatively greater increase in CVD than do men who have diabetes, with at least a fourfold increased risk in women, as compared with a twofold increased risk in men [14, 37] and similar CVD mortality risk in women as in men with type 1 diabetes [37].
Type 2 Diabetes
In a study of fatal CVD in Norway, 47,951 people identified in the National Cause of Death Registry were examined by diabetes status and gender [38], and the risk for fatal CVD was increased 2.5 times in women but only 1.8 times in men. While the relative increase in CVD risk is greater in women with type 2 diabetes, the absolute risk remains higher in men, in contrast to type 1 diabetes. The Detection of Ischemia in Asymptomatic Diabetics study reported that asymptomatic women with type 2 diabetes had a lower risk for a cardiac event (CVD death or nonfatal MI) when compared with men with type 2 diabetes [39], and in a case-control study of albuminuria and CVD in adults with type 2 diabetes, ischemic heart disease was more prevalent in men with albuminuria than in women [40].
The Role of Obesity
Type 1 Diabetes
While traditionally type 1 diabetes patients are not more obese than the general population, obesity is increasing in this former group just as in nondiabetic individuals, and there does appear to be a relationship between obesity and subclinical CVD in this group. Obesity was associated with progression of coronary artery calcium in the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study [41] and with the presence of coronary artery calcification (CAC) in the Pittsburgh EDC study [42]. However, the extent of CAC was inversely related to the degree of adiposity in adults with type 1 diabetes in the Pittsburgh EDC study, suggesting a more complex relationship between body fat and subclinical CVD in type 1 diabetes [42]. The consequences of the trend in increasing obesity among individuals with type 1 diabetes are therefore unclear, but it is likely that this trend will further increase CVD risk in this population.
Type 2 Diabetes
Obesity, especially central obesity, is associated with both type 2 diabetes and CVD. Through infiltration into many tissues and organs and formation of many potential toxic intermediates, fat accumulation is believed critical to the development of numerous CVD risk factors. The INSPIRE ME IAA study aimed to examine the relationship between visceral adiposity, type 2 diabetes, cardiometabolic risk, and CVD [43]. In this cross-sectional study, visceral adiposity was measured using computed tomography from June 2006 to May 2008. Visceral abdominal adiposity was strongly associated to cardiometabolic risk factors (ApoB, triglycerides, HDL-cholesterol, HbA1c, plasma glucose, and insulin) and the prevalence of CVD, adjusted for age, body mass index (BMI), and region, regardless of type 2 diabetes status. Examination of the relationship between overall adiposity as measured by prediagnosis BMI and mortality revealed a J-shaped relationship, with increased risk both among individuals with a normal BMI (<25 kg/m2) prior to diagnosis and among people who were obese (BMI 30 kg/m2 or higher) [44].
Diabetes-Specific Risk Factors
The Role of Glycemic Control
Type 1 Diabetes
Hyperglycemia has been demonstrated to increase CVD risk in type 1 diabetes [45], and intensive treatment during the DCCT was found to decrease CVD events by 42 % over the course of the EDIC follow-up study [46••]. Further intensive treatment and glycemic control were associated with the extent of coronary artery calcium, a subclinical marker of cardiovascular risk in the DCCT cohort [47], and baseline HbA1c levels were associated with coronary artery calcium and CVD events, independently of intensive treatment during the DCCT.
Type 2 Diabetes
In new onset type 2 diabetes, intensive blood glucose lowering with either insulin or sulfonylureas decreased both CVD and microvascular complications [48]. The UKPDS study demonstrated that improved glycemic control, using a combination of treatments including sulfonylureas and insulin, was associated with significantly fewer CVD events [48]. A recent report from the Diabetes Mellitus and Diastolic Dysfunction study showed that glycemic normalization improved skin microvascular function in patients with type 2 diabetes [49]. Microcirculatory and endothelial dysfunction are signs of cardiovascular engagement in patients with diabetes, and so this study demonstrates an earlier effect of normalizing glucose levels on the vascular system in diabetes. Furthermore, abnormal glucose tolerance has been shown to be associated with greater arterial stiffness, an early sign of atherosclerosis.
However, recent trials designed to achieve near-normal glycemia in type 2 diabetes have not shown such promise [50, 51••, 52]. In fact, the Action to Control Cardiovascular Risk in Diabetes trial of improved glycemic control in subjects with type 2 diabetes was stopped prematurely due to excess mortality in the active arm of the study [51••]. Intensive glycemic control, as compared with standard control, resulted in significantly higher death from any cause and from CVD. While intensive therapy reduced the risk of nonfatal MI by 24 %, if a CVD event occurred it was more likely to be fatal.
The Role of Diabetic Nephropathy
Type 1 Diabetes
In people with type 1 diabetes, diabetic nephropathy is a clear risk factor for CVD. The presence of overt nephropathy predicts hard CVD events, independently of hypertension, estimated insulin resistance, and dyslipidemia [53]. In fact, several recent reports suggest that the excess risk for CVD in type 1 diabetes may be minimal in the absence of diabetic nephropathy. The FinnDiane study reported that there was no increase in mortality over 7 years among patients with normal renal function, but there was a graded increase in mortality risk with the extent of renal complications [54].Similarly, in the Pittsburgh Epidemiology of Diabetes Complications study, over 20 years of follow-up, the standardized mortality rate was not significantly higher in people with type 1 diabetes and normal levels of albuminuria [55], and most deaths in this group were not attributed to diabetes or CVD.
Type 2 Diabetes
Estimated glomerular filtration rate (eGFR) is a strong predictor of mortality in patients with diabetes. The Taichung Diabetes Study looked at the relationship between renal function and proteinuria and mortality among 6,533 patients with type 2 diabetes for greater than 30 years duration [56]. Renal function was assessed by eGFR, and proteinuria was measured in urine. It was concluded that the risks of all-cause and CVD mortality were independently associated with proteinuria and eGFR, and so these measures could increase the accuracy of risk stratification [56]. Only a few studies have explored the relationship between decreasing renal function and mortality in patients with diabetes, and even fewer studies have observed the relationship between eGFR and CVD-specific mortality. Furthermore, current guidelines for classifying and staging chronic kidney disease are based only on eGFR, without taking into account proteinuria.
The Role of Novel Risk Factors
Since only about half of CVD risk is explained by traditional CVD risk factors, identifying novel risk factors is essential for developing new interventions for CVD prevention. Here, we review several of these novel markers and their possible roles in the development of CVD in type 1 and type 2 diabetes.
Lipoprotein-Associated Phospholipase A2
Type 1 Diabetes
Elevated levels of lipoprotein-associated phospholipase A2 (Lp-PLA2), a vascular-specific inflammatory enzyme, have been reported to be associated with CVD risk factors and outcomes. Elevated Lp-PLA2 levels are associated with an increased risk of CVD equivalent in magnitude to the association observed with non-HDL cholesterol or systolic blood pressure. Lp-PLA2 levels may potentially be used to better determine a patient’s risk of suffering a heart attack or ischemic stroke. In the CACTI study, Lp-PLA2 mass and activity were measured in 506 adults with type 1 diabetes and 591 nondiabetic controls, and Lp-PLA2 activity was significantly associated with progression of coronary artery calcium, a marker of subclinical atherosclerosis [57].
Type 2 Diabetes
Several studies have indicated that Lp-PLA2 may be higher among patients with type 2 diabetes than among nondiabetic controls [58]. Lp-PLA2 mass and activity were measured in baseline plasma in both prevalent and incident cases of diabetes, with a total of 5,474 men and women adults from the Cardiovascular Health Study analyzed. Linear and Cox proportional hazards were used to adjust for confounding variables, and multivariable relative risks were calculated. Lp-PLA2 activity was positively associated with insulin resistance, which has been associated with increased risk for CVD [58]. Furthermore, in the Risk Factors, Atherosclerosis, and Clinical Events in Diabetes substudy of the VADT, 197 individuals with type 2 diabetes had CAC measured at two visits, and higher Lp-PLA2 mass was associated with a higher likelihood of CAC progression [59].
Sex Hormones
Type 1 Diabetes
One hypothesis is that estrogen and other sex hormones may be affected by diabetes and may influence the gender differences in complications [36, 60, 61]. Men with type 1 diabetes have been reported to have lower levels of total testosterone and estradiol, and low testosterone appears to be associated with nephropathy [62]. Women with type 1 diabetes have more irregular menstrual cycles, which have been shown to increase the risk of coronary calcium [60, 63–66], and there is a much higher prevalence of ovarian hyperandrogenism consistent with polycystic ovary syndrome in women with type 1 diabetes than in the nondiabetic population [67].
Type 2 Diabetes
In patients with type 2 diabetes, there is stronger evidence for a role of low testosterone in CVD risk among men than in type 1 diabetes [68, 69]. In the Cheshire Primary Care cohort, a medical record search demonstrated that 4.4 % of men with type 2 diabetes who had testosterone measured were hypogonadal, and nearly a third were borderline or frankly hypogonadal [70]. Furthermore, testosterone levels were significantly lower in men with type 2 diabetes than in men with type 1 diabetes, and testosterone levels were associated with obesity in both groups of men with diabetes, although an increase in BMI had a greater effect on testosterone levels in men with type 1 diabetes than in men with type 2 diabetes,
Proneurotensin
Proneurotensin, which is a precursor of the hormone neurotensin, which is produced in the brain, has been found to be associated with the development of diabetes mellitus, as well as with CVD and mortality, and is associated with a higher risk for CVD mortality in women than in men [71]. Proneurotensin was measured in plasma from 4,632 fasting subjects who took part in the population-based Malmo Diet and Cancer Study. Multivariate Cox proportional hazards models examined baseline proneurotensin and first event, which included death during a follow-up ranging from 13.2 to 15.7 years. Fasting proneurotensin was significantly associated with the development of incident diabetes, as well as with total and cardiovascular mortality, but only in women [71]. More research is needed to understand the relationship of hormones in the development of complications of diabetes.
Inflammation
Type 1 Diabetes
Inflammation is central to the development of atherosclerosis and has been investigated as a risk factor for CVD [72]. In type 1 diabetes, inflammatory markers, including soluble interleukin-2 receptor [73] and fibrinogen [74], have been associated with increased CVD risk, and greater inflammation has been observed in youth with type 1 diabetes, as compared with nondiabetic youth [75].
Type 2 Diabetes
C-reactive protein (CRP) in the highest versus lowest quartile was reported to increase the risk of developing type 2 diabetes more than 15-fold in the Women’s Health Study [76], supporting a role for inflammation in this disease. Furthermore, levels of CRP and HbA1c appear to interact to increase the risk for CVD. In the Intervention Project on Cerebrovascular Diseases and Dementia in the Community of Ebersberg, Bavaria study, 3,534 subjects (882 with diabetes) had CRP measured and carotid intima-media thickness (cIMT) assessed at baseline and after 2 years of follow-up [77]. Higher levels of HbA1c predicted progression of cIMT, and there was a significant interaction between HbA1c and CRP, such that people with both values in the highest two quartiles had a 4.3-fold increased risk for cIMT progression. This interaction was observed in both people with and those without diabetes. Among patients with advanced vascular disease as manifested by symptomatic peripheral artery disease (n=454), increasing quartiles of CRP predicted major CVD events and interacted with HbA1c quartiles to jointly increase the risk for a CVD event [78]. Patients with both CRP (>0.44 mg/dL) and HbA1c (>6.2 %) in the highest two quartiles had a nearly threefold increased risk for a CVD event as patients with both parameters in the lower two quartiles. An association between higher CRP and CVD events was confirmed in 746 men with type 2 diabetes who were free of CVD and were followed for 5 years [79]. Higher quartiles of CRP were associated with greater risk for a CVD event in a dose-response manner (OR 1.0, 1. 51, 2.52, 2.62 for CRP quartiles 1–4), and this increase was independent of LDL-cholesterol, HDL-cholesterol, apolipoprotein B, non-HDL-cholesterol, glycemic control, body mass index, and fibrinogen levels. In 1999, Jager and colleagues from the Hoorn Study reported that CRP and the von Willebrand factor in the highest tertile were associated with two- and threefold increases in CVD mortality, respectively, in 631 adults with type 2 diabetes and free of diabetes [80]. In 2012, a much larger study of over 25,000 adults in England and Scotland demonstrated that each standard deviation increase in CRP was associated with a 53% increase in CVD mortality and a 43% increase in overall mortality in adults both with and without diabetes [81]. In addition, in the Diabetes Heart Study, higher levels of CRP predicted greater mortality risk in patients with type 2 diabetes [82].
The Role of Multiple Traditional Risk Factors
Type 1 Diabetes
In a recent report from the Scottish Care Information-Diabetes Collaboration database, CVD risk factors and mortality were examined in this population-based registry of 21,789 people with type 1 diabetes. Compliance with treatment guidelines was relatively low, with only 13 % meeting guidelines for hemoglobin A1c (<7 %); 28 % were current smokers, only 39 % of people >40 years of age were treated with statins, and over a third had significant hypertension (BP> 140/90) [83]. In this population, women had a threefold increased risk for a CVD event, as compared with the nondiabetic population, and men had a 2.3-fold increased CVD event risk. These results demonstrate that there remains a need for improved traditional risk factor management in patients with type 1 diabetes, despite the generally lower levels of dyslipidemia and obesity in this population.
Type 2 Diabetes
With the global diabetes epidemic, integrated CVD risk reduction intervention models are urgently needed to target the known, modifiable risk factors. The ADDITION-Europe study group recently published results from a 5-year cluster-randomized trial that examined an intervention to promote early intensive management of patients with type 2 diabetes. Subjects were randomized at 343 general practice offices, and endpoints were available in 3,055 patients. The intervention modestly but significantly improved HbA1c, cholesterol levels, and blood pressure. Griffin and colleagues reported a small, nonsignificant decline in the incidence of CVD (hazard ratio 0.83, 95 % CI 0. 65–1.05) and death (hazard ratio 0.91, 95 % CI 0.9–1.21) with the intervention.
The CARRS multicenter translational trial is examining a multifactorial strategy to reduce risk in people with diabetes in South Asia [84]. The 1,146 adults with diabetes from 10 urban clinic sites were randomly assigned to receive standard care versus the multicomponent CVD risk reduction intervention, which consists of an electronic medical record with a decision-support system that sends prompts to the health-care providers and a care coordinator to facilitate patient and provider adherence to evidence-based guidelines. They plan to examine the effectiveness of the intervention in controlling individual CVD risk factors (blood glucose, blood pressure, and cholesterol), cost effectiveness, and feasibility. Once completed, this study hopes to demonstrate the effectiveness of a low-cost diabetes care delivery model for high-risk patients, among whom the greatest gains in CVD prevention can be realized.
Differences and Similarities in CVD Risk Factors in Type 1 and Type 2 Diabetes
A comparison of traditional and diabetes-related risk factors by type 1 and type 2 diabetes is summarized in Table 1. There appear to be important differences in the effect of risk factors (and in the benefits of treating such risk factors), such as dyslipidemia and hyperglycemia, on CVD risk in type 1, as compared with type 2, diabetes [85]. Furthermore, women with type 1 diabetes have a significantly greater relative increase in CVD, as compared with nondiabetic women, than do women with type 2 diabetes, perhaps explained by the younger age at which type 1 diabetes typically develops and the very low incidence of CVD in nondiabetic women in this younger age group. In type 1 diabetes, there is evidence that the risk for CVD is equivalent in men and women with the disease [37], whereas the absolute risk for CVD remains higher in men than in women with type 2 diabetes. However, the development of type 2 diabetes at younger ages may potentially change the relationship of gender and CVD to one that is more similar to that for type 1 diabetes.
Table 1.
Cardiovascular disease (CVD) risk factors in type 2 diabetes and type 1 diabetes: Contrasts and commonalities
| Type 1 diabetes | Type 2 diabetes | |
|---|---|---|
| • Dyslipidemia | • More favorable lipids than nondiabetic individuals | • Low HDL-cholesterol and high triglycerides common |
| • Modest CVD risk factor | • Low HDL-cholesterol and elevated triglycerides and LDL-cholesterol associated with CVD | |
| • Hypertension | • High prevalence of hypertension | • Hypertension is common |
| • Poorly treated and controlled | • Component of the metabolic syndrome that contributes to type 2 diabetes risk | |
| • Higher systolic blood pressure and pulse pressure associated with CVD | • Increases the risk of CVD | |
| • Gender | • Women have at least a fourfold increased CVD risk, as compared with a twofold risk in men | • Women have an increased relative risk of CVD, but absolute risk remains lower than in men with type 2 diabetes |
| • CVD mortality is the same in men and women with type 1 diabetes | • | |
| • Obesity | • Typically not more obese than the general population | • Obesity is a risk factor for type 2 diabetes |
| • Obesity increases risk for CVD | • Visceral adiposity is associated with CVD, independent of diabetes status | |
| • Extent of atherosclerosis associated with lower adiposity, so relationship not straightforward | ||
| • Hyperglycemia | • Intensive treatment and improved blood sugar levels decrease CVD events | • Intensive treatment to normalize blood sugar did not decrease CVD in several trials among type 2 diabetic patients |
| • Renal disease | • Potent CVD risk factor | • Renal disease often present at time of diagnosis |
| • May explain all excess CVD risk in type 1 diabetes | • Lower GFR or proteinuria increase CVD risk |
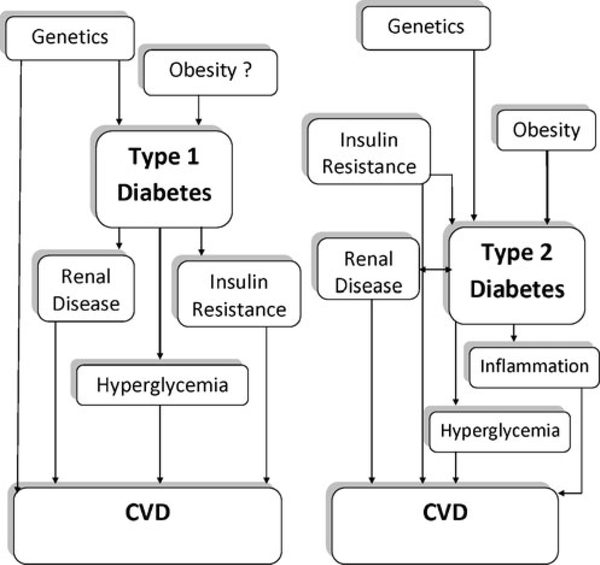
Nevertheless, the risk factors for CVD in type 1 and type 2 diabetes demonstrate a great deal of overlap and include obesity, hyperglycemia, inflammation, insulin resistance, and renal disease, as is shown in Fig. 1. The interesting distinction between the two types of diabetes is that many of these risk factors, particularly obesity and insulin resistance, also contribute to the development of type 2 diabetes, and many people already have renal complications and greater inflammation at diagnosis of type 2 diabetes. The greater levels of obesity and traditional CVD risk factors in type 2 diabetes may also contribute to a higher risk for CVD at a given age, as was demonstrated in the SEARCH for diabetes in youth study when they examined arterial stiffness in youth with type 1 and type 2 diabetes [86••].
Fig. 1.
The hypothesized relationship of common risk factors for cardiovascular disease (CVD)
CVD Treatment in Type 1 Versus Type 2 Diabetes
CVD in people with diabetes often first manifests as a fatal event [87], highlighting the need for prevention. Furthermore, patients with diabetes have more extensive coronary disease than do those without diabetes, resulting in more challenging PCI or CABG [20]. The recommendation of coronary revascularization for patients with diabetes remains controversial. Several issues remain to be addressed by clinical studies, such as the type of treatment, adequacy of glycemic control, aggressive management of other atherosclerotic risk factors, adjunctive therapy with statins and angiotensin converting enzyme inhibitors, and status of distal vessel bed [88]. A meta-analysis of percutaneous intervention (PCI) versus CABG in patients with diabetes and left main and/or multivessel coronary artery disease was conducted, which confirmed that PCI significantly reduced stroke risks and, despite high rates of revascularization, PCI with drug-eluting stent had better outcomes for combined outcomes of death, MI, or CVA [89].
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial has examined different strategies for treating cardiac ischemia among subjects with type 2 diabetes [90]. In this randomized trial with 2,368 patients with type 2 diabetes and stable ischemic heart disease, health outcomes after prompt revascularization versus medical therapy were compared. Subjects were stratified by type of revascularization (PCI vs. CABG), and linear mixed models were fit to evaluate the outcomes by treatment. The results showed that prompt coronary revascularization, as compared with medical therapy, improved several measures of activity, energy, and self-rated health in patients with type 2 diabetes and stable ischemic heart disease, although there was no significant difference in rates of death or major CVD events between these treatment groups [90].
The BARI 2D trial also recently published results from an examination of the long-term clinical impact of completeness of revascularization in patients with diabetes [20]. In this study, they performed a post hoc, nonrandomized analysis in 751 patients who were randomly assigned to early revascularization, in which 264 underwent CABG and 487 underwent PCI. The completeness of revascularization was characterized by the residual postprocedure myocardial jeopardy index. The results showed that subjects with type 2 diabetes and less complete revascularization had an increase in the risk for long-term cardiovascular events and decreased event-free survival.
It has been shown that the incidence of adverse outcomes post PCI or CABG remains increased among patients with diabetes. Last year, a study examined the association between diabetes and all-cause mortality following primary percutaneous coronary intervention (PPCI) [19]. This was a retrospective cohort analysis of patients undergoing PPCI for ST-segment elevation myocardial infarction (STEMI). The results showed that all-cause mortality following STEMI was nearly twofold higher in patients with diabetes, as compared with those without [19], perhaps due to greater comorbidity, presence of more advance coronary disease, poorer postprocedural coronary reperfusion, longer door-to-balloon times, and more interhospital transfer in patients with diabetes.
CVD Events in Type 1 Versus Type 2 Diabetes
CVD risk is clearly increased in diabetes, and in treatment guidelines, diabetes has been described as a CVD equivalent. However, recently, this tenet has been questioned. A meta-analysis of 13 studies was performed to compare diabetes with prior history of coronary heart disease (CHD), to determine whether diabetes is actually a CHD equivalent [91•]. There were 45,108 subjects across all studies. The results demonstrated that having diabetes was associated with a lower risk of a CHD event than was a prior CHD and that those with diabetes without a prior MI had a 43 % lower risk of having a CHD event, in comparison with nondiabetic individuals who had had a previous MI. The results from this study therefore suggest that diabetes may not be a CHD equivalent.
CVD events in type 1 diabetes are increased when compared with nondiabetic individuals. In a study utilizing data from a general practice database in the U.K., the risk for a major CVD event (stroke, MI, revascularization, or CVD death) was increased 3.6-fold in men and 7.7-fold in women, as compared with nondiabetic individuals [10]. The question of whether type 1 diabetes is a CHD equivalent has not been studied, perhaps in part due to the younger ages at which CVD events occur in people with type 1 diabetes. Fewer studies have examined stroke risk in type 1 diabetes. Stroke is found to occur at least 20 years earlier with type 1 diabetics than in the general population, and so this is an important area for investigation. The Pittsburgh EDC study recently reported on the predictors of survival after incident stroke in type 1 diabetes [92]. They studied 658 study participants with childhood-onset type 1 diabetes, followed biennially for 18 years. Multivariable Cox modeling was performed, which showed that diabetes duration, systolic blood pressure, non-high-density lipoprotein cholesterol, white blood cells, and pulse significantly predicted ischemic stroke. More than two thirds of the incident strokes were ischemic and were usually not preceded by either CAD or TIA, similar to the general population [92].
Mortality Trends in Type 1 Versus Type 2 Diabetes
CVD is the leading cause of morbidity and mortality in patients with type 1 diabetes [10, 11], and all-cause mortality is increased in type 2 diabetes [93]. Half of patients with type 1 diabetes develop calcified plaques in the coronary arteries by age 40 [13], and coronary artery calcium is a potent predictor of CVD events [94•] and mortality [95]. Mortality rates from ischemic CHD in type 1 diabetes have been described [11] in the U.K., where 369 (1.6 %) of 23,751 patients with type 1 diabetes under the age of 30 died from ischemic CHD between 1972–1993 and 2000. Current trends in mortality among people with diabetes is therefore of great public health importance.
Trends in all-cause mortality among people with type 1 diabetes were recently reported by Harjutsalo and colleagues among a registry of 17,306 Finnish patients diagnosed between 1970 and 1999 [96••]. Mortality was examined by diagnosis before age 15 and from 15 to 29, and the risk for mortality was higher in the early diagnosis group (standardized mortality ratio [SMR] =3.6) than in the later onset group (SMR =2.8). Furthermore, the mortality risk was increased more in women with type 1 diabetes (SMR =5.5 for the early onset and 3.6 for the later onset group). Over time, there was a trend toward decreased mortality among patients with earlier onset but an increase in mortality among those patients diagnosed between 15 and 19 years of age.
Among patients with type 2 diabetes, rates of CVD mortality appear to have decreased over recent years, according to data from the Framingham Heart Study [97]. While rates decreased in all groups, there remained at least a twofold increased risk for mortality associated with diabetes, and there appeared to be a greater decline in mortality among men than among women with diabetes [97]. A recent meta-analysis that examined mortality in patients with type 2 diabetes without prior CVD, as compared with nondiabetic individuals with previous CVD, also found evidence that the impact of diabetes on CVD may differ by gender [91•]. The risk for CVD and CAD outcomes was lower in men with diabetes than in men with prior CVD, but in women there was a nonsignificantly higher risk for CVD and CAD outcomes in women with diabetes than in women with previous CVD.
Conclusions
CVD remains the main cause of mortality in both type 1 and type 2 diabetes, and the incidence of diabetes is increasing. While CVD mortality has been dropping in the overall population, the increasing global burden of diabetes mellitus may reverse that trend.
Interestingly, while type 1 and type 2 diabetes are often thought of as very different diseases, the risk factors for CVD are similar in both groups and include insulin resistance, female gender, obesity, inflammation, and renal disease. However, there are differences in how these risk factors relate to the development of type 1 versus type 2 diabetes, with obesity and insulin resistance preceding the development of type 2 diabetes and renal disease often diagnosed concurrently, as compared with type 1 diabetes, where insulin resistance and renal disease appear to develop secondary to diabetes and obesity can develop in response to intensive insulin therapy.
More research is needed into the implications of these differences in risk factors for CVD and into how risk factor detection and modification can best be accomplished in people with diabetes.
Footnotes
Conflict of Interest
Lindsey Duca declares that she has no conflict of interest.
Rachel Sippl declares that she has no conflict of interest.
Janet K. Snell-Bergeon declares that she has no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bruno G, Novelli G, Panero F, Perotto M, Monasterolo F, Bona G, et al. The incidence of type 1 diabetes is increasing in both children and young adults in Northern Italy: 1984–2004 temporal trends. Diabetologia. 2009;52(12):2531–5. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RBJ, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–24. [DOI] [PubMed] [Google Scholar]
- 3. Derraik JG, Reed PW, Jefferies C, Cutfield SW, Hofman PL, Cutfield WS. Increasing incidence and age at diagnosis among children with type 1 diabetes mellitus over a 20-year period in Auckland (New Zealand). PLoS One. 2012;7(2):e32640. • This important article not only highlights the rising incidence of type 1 diabetes in Auckland, as has been reported globally, but also demonstrates that the increase is occurring to a greater extent amoung older age groups (10–14 years of age). Studies such as this may help to pinpoint the factors that are leading to the ever-increasing incidence of type 1 diabetes.
- 4.Evertsen J, Alemzadeh R, Wang X. Increasing incidence of pediatric type 1 diabetes mellitus in Southeastern Wisconsin: relationship with body weight at diagnosis. PLoS One. 2009;4(9):e6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammi N, Blomstedt PA, Moltchanova E, Eriksson JG, Tuomilehto J, Karvonen M. Marked temporal increase in the incidence of type 1 and type 2 diabetes among young adults in Finland. Diabetologia. 2008;51(5):897–9. [DOI] [PubMed] [Google Scholar]
- 6.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19(2):93–6. [DOI] [PubMed] [Google Scholar]
- 7.Pozzilli P, Guglielmi C, Caprio S, Buzzetti R. Obesity, autoimmunity, and double diabetes in youth. Diabetes Care. 2011;34 Suppl 2:S166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abi Khalil C, Roussel R, Mohammedi K, Danchin N, Marre M. Cause-specific mortality in diabetes: recent changes in trend mortality. Eur J Prev Cardiol. 2012;19(3):374–81. [DOI] [PubMed] [Google Scholar]
- 9.Astrup AS. Cardiovascular morbidity and mortality in diabetes mellitus: prediction and prognosis. Dan Med Bull. 2011;58(8): B4152. [PubMed] [Google Scholar]
- 10.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29 (4):798–804. [DOI] [PubMed] [Google Scholar]
- 11.Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46(6):760–5. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120(13):1266–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52(11):2833–9. [DOI] [PubMed] [Google Scholar]
- 14.Colhoun HM, Rubens MB, Underwood SR, Fuller JH. The effect of type 1 diabetes mellitus on the gender difference in coronary artery calcification. J Am Coll Cardiol. 2000;36(7):2160–7. [DOI] [PubMed] [Google Scholar]
- 15.Silbernagel G, Rosinger S, Grammer TB, Kleber ME, Winkelmann BR, Boehm BO, et al. Duration of type 2 diabetes strongly predicts all-cause and cardiovascular mortality in people referred for coronary angiography. Atherosclerosis. 2012;221(2):551–7. [DOI] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med. 2011;171 (5):404–10. [DOI] [PubMed] [Google Scholar]
- 17.Hong YJ, Jeong MH, Choi YH, Song JA, Kim DH, Lee KH, et al. Impact of diabetes mellitus on plaque vulnerability and clinical outcome in patients with acute myocardial infarction with plaque rupture. Int J Cardiol. 2012;154(2):197–8. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald MR, Petrie MC, Home PD, Komajda M, Jones NP, Beck-Nielsen H, et al. Incidence and prevalence of unrecognized myocardial infarction in people with diabetes: a substudy of the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes (RECORD) study. Diabetes Care. 2011;34 (6):1394–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn MB, Cubbon RM, Mercer B, Wheatcroft AC, Gherardi G, Aziz A, et al. Association of diabetes with increased all-cause mortality following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction in the contemporary era. Diab Vasc Dis Res. 2012;9(1):3–9. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz L, Bertolet M, Feit F, Fuentes F, Sako EY, Toosi MS, et al. Impact of completeness of revascularization on long-term cardiovascular outcomes in patients with type 2 diabetes mellitus: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D). Circ Cardiovasc Interv. 2012;5 (2):166–73. [DOI] [PubMed] [Google Scholar]
- 21.Dabelea D, Pihoker C, Talton JW, D’Agostino RBJ, Fujimoto W, Klingensmith GJ, et al. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34(7):1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haines L, Wan KC, Lynn R, Barrett TG, Shield JP. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30(5):1097–101. [DOI] [PubMed] [Google Scholar]
- 23.Kousa A, Puustinen N, Karvonen M, Moltchanova E. The regional association of rising type 2 diabetes incidence with magnesium in drinking water among young adults. Environ Res. 2012;112:126–8. [DOI] [PubMed] [Google Scholar]
- 24.Jefferies C, Carter P, Reed PW, Cutfield W, Mouat F, Hofman PL, et al. The incidence, clinical features, and treatment of type 2 diabetes in children <15 yr in a population-based cohort from Auckland, New Zealand, 1995–2007. Pediatr Diabetes. 2012;13(4):294–300. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364(9446):1699–700. [DOI] [PubMed] [Google Scholar]
- 26.Gyurus EK, Patterson C, Soltesz G. Twenty-one years of prospective incidence of childhood type 1 diabetes in Hungary—the rising trend continues (or peaks and highlands?). Pediatr Diabetes. 2012;13(1):21–5. [DOI] [PubMed] [Google Scholar]
- 27.Wadwa RP, Kinney GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, et al. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes Care. 2005;28 (5):1051–6. [DOI] [PubMed] [Google Scholar]
- 28.Koivisto VA, Stevens LK, Mattock M, Ebeling P, Muggeo M, Stephenson J, et al. Cardiovascular disease and its risk factors in IDDM in Europe. EURODIAB IDDM Complications Study Group. Diabetes Care. 1996;19(7):689–97. [DOI] [PubMed] [Google Scholar]
- 29.Orchard TJ, Forrest KY, Kuller LH, Becker DJ. Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2001;24(6):1053–9. [DOI] [PubMed] [Google Scholar]
- 30.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316(7134):823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosevski M, Borozanov V, Vavlukis M, Pemovska G. Georgievska-Ismail Lj. Carotid ultrasound, blood lipids and waist determination can predict a future coronary revascularisation in the type 2 diabetic cohort. Prilozi. 2007;28(2):127–36. [PubMed] [Google Scholar]
- 32.Collado-Mesa F, Colhoun HM, Stevens LK, Boavida J, Ferriss JB, Karamanos B, et al. Prevalence and management of hypertension in type 1 diabetes mellitus in Europe: the EURODIAB IDDM Complications Study. Diabet Med J Br Diabet Assoc. 1999;16(1):41–8. [DOI] [PubMed] [Google Scholar]
- 33.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care. 2008;31(7):1360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. Bmj. 1998;317(7160):713–20. [PMC free article] [PubMed] [Google Scholar]
- 35.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998;317 (7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 36.Maric C. Sex, diabetes and the kidney. Am J Physiol Renal Physiol. 2009;296(4):F680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol. 1996;16 (6):720–6. [DOI] [PubMed] [Google Scholar]
- 38.Madssen E, Vatten L, Nilsen TI, Midthjell K, Wiseth R, Dale AC. Abnormal glucose regulation and gender-specific risk of fatal coronary artery disease in the HUNT 1 study. Scand Cardiovasc J. 2012;46(4):219–25. [DOI] [PubMed] [Google Scholar]
- 39.Tandon S, Wackers FJ, Inzucchi SE, Bansal S, Staib LH, Chyun DA, et al. Gender-based divergence of cardiovascular outcomes in asymptomatic patients with type 2 diabetes: results from the DIAD study. Diab Vasc Dis Res. 2012;9(2):124–30. [DOI] [PubMed] [Google Scholar]
- 40.Nakhjavani M, Morteza A, Jenab Y, Ghaneei A, Esteghamati A, Karimi M, et al. Gender difference in albuminuria and ischemic heart disease in type 2 diabetes. Clin Med Res. 2012;10(2):51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues TC, Veyna AM, Haarhues MD, Kinney GL, Rewers M, Snell-Bergeon JK. Obesity and coronary artery calcium in diabetes: the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Diabetes Technol Ther. 2011;13(10):991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Double-edged relationship between adiposity and coronary artery calcification in type 1 diabetes. Diab Vasc Dis Res. 2007;4(4):332–9. [DOI] [PubMed] [Google Scholar]
- 43.Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R, et al. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab. 2012;97 (5):1517–25. [DOI] [PubMed] [Google Scholar]
- 44.Logue J, Walker JJ, Leese G, Lindsay R, McKnight J, Morris A et al. The Association Between BMI Measured Within a Year After Diagnosis of Type 2 Diabetes and Mortality. Diabetes care. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjarnegard N, Arnqvist HJ, Lindstrom T, Jonasson L, Jonsson A, Lanne T. Long-term hyperglycaemia impairs vascular smooth muscle cell function in women with type 1 diabetes mellitus. Diab Vasc Dis Res. 2009;6(1):25–31. [DOI] [PubMed] [Google Scholar]
- 46. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353 (25):2643–53. •• This article reports on CVD outcomes from the landmark Diabetes Control and Complications Trial and the follow-up Epidemiology of Diabetes Interventions and Complications Study and provides definitve proof that lowering hemoglobin A1c decreases CVD in type 1 diabetes.
- 47.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55(12):3556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 49.Jarnert C, Kalani M, Ryden L, Bohm F. Strict glycaemic control improves skin microcirculation in patients with type 2 diabetes: a report from the Diabetes mellitus and Diastolic Dysfunction (DADD) study. Diab Vasc Dis Res. 2012;9(4):287–95. [DOI] [PubMed] [Google Scholar]
- 50.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. [DOI] [PubMed] [Google Scholar]
- 51. Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT et al. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. •• The ACCORD trial was designed to test whether reducing blood glucose levels to near normal would decrease CVD and mortality in people with type 2 diabetes, but this trial instead found an increased risk for mortality in the intensive treatment group.
- 52.Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358 (24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 53.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26(5):1374–9. [DOI] [PubMed] [Google Scholar]
- 54.Groop PH, Thomas MC, Moran JL, Waden J, Thorn LM, Makinen VP, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58 (7):1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin CC, Chen CC, Kung PT, Li CI, Yang SY, Liu CS, et al. Joint relationship between renal function and proteinuria on mortality of patients with type 2 diabetes: the Taichung Diabetes Study. Cardiovasc Diabetol. 2012;11(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinney GL, Snell-Bergeon JK, Maahs DM, Eckel RH, Ehrlich J, Rewers M, et al. Lipoprotein-associated phospholipase A(2) activity predicts progression of subclinical coronary atherosclerosis. Diabetes Technol Ther. 2011;13(3):381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson TL, Biggs ML, Kizer JR, Cushman M, Hokanson JE, Furberg CD, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) and future risk of type 2 diabetes: results from the Cardiovascular Health Study. J Clin Endocrinol Metab. 2012;97(5):1695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saremi A, Moritz TE, Anderson RJ, Abraira C, Duckworth WC, Reaven PD. Rates and determinants of coronary and abdominal aortic artery calcium progression in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care. 2010;33(12):2642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Codner E. Estrogen and type 1 diabetes mellitus. Pediatr Endocrinol Rev. 2008;6(2):228–34. [PubMed] [Google Scholar]
- 61.Gaete X, Vivanco M, Eyzaguirre FC, Lopez P, Rhumie HK, Unanue N, et al. Menstrual cycle irregularities and their relationship with HbA1c and insulin dose in adolescents with type 1 diabetes mellitus. Fertil Steril. 2010;94(5):1822–6. [DOI] [PubMed] [Google Scholar]
- 62.Maric C, Forsblom C, Thorn L, Waden J, Groop PH. Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids. 2010;75(11):772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samara-Boustani D, Colmenares A, Elie C, Dabbas M, Beltrand J, Caron V, et al. High prevalence of hirsutism and menstrual disorders in obese adolescent girls and adolescent girls with type 1 diabetes mellitus despite different hormonal profiles. Eur J Endocrinol. 2012;166(2):307–16. [DOI] [PubMed] [Google Scholar]
- 64.Schweiger BM, Snell-Bergeon JK, Roman R, McFann K, Klingensmith GJ. Menarche delay and menstrual irregularities persist in adolescents with type 1 diabetes. Reprod Biol Endocrinol. 2011;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snell-Bergeon JK, Dabelea D, Ogden LG, Hokanson JE, Kinney GL, Ehrlich J, et al. Reproductive history and hormonal birth control use are associated with coronary calcium progression in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2008;93(6):2142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strotmeyer ES, Steenkiste AR, Foley TPJ, Berga SL, Dorman JS. Menstrual cycle differences between women with type 1 diabetes and women without diabetes. Diabetes Care. 2003;26(4):1016–21. [DOI] [PubMed] [Google Scholar]
- 67.Roldan B, Escobar-Morreale HF, Barrio R, de La Calle H, Alonso M, Garcia-Robles R, et al. Identification of the source of androgen excess in hyperandrogenic type 1 diabetic patients. Diabetes Care. 2001;24(7):1297–9. [DOI] [PubMed] [Google Scholar]
- 68.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93(5):1834–40. [DOI] [PubMed] [Google Scholar]
- 69.Tomar R, Dhindsa S, Chaudhuri A, Mohanty P, Garg R, Dandona P. Contrasting testosterone concentrations in type 1 and type 2 diabetes. Diabetes Care. 2006;29(5):1120–2. [DOI] [PubMed] [Google Scholar]
- 70.Anderson SG, Heald A, Younger N, Bujawansa S, Narayanan RP, McCulloch A, et al. Screening for hypogonadism in diabetes 2008/9: results from the Cheshire Primary Care cohort. Prim Care Diabetes. 2012;6(2):143–8. [DOI] [PubMed] [Google Scholar]
- 71.Melander O, Maisel AS, Almgren P, Manjer J, Belting M, Hedblad B, et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA. 2012;308 (14):1469–75. [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto K, Sera Y, Abe Y, Ueki Y, Tominaga T, Miyake S. Inflammation and insulin resistance are independently related to all-cause of death and cardiovascular events in Japanese patients with type 2 diabetes mellitus. Atherosclerosis. 2003;169(2):317–21. [DOI] [PubMed] [Google Scholar]
- 73.Wadwa RP, Kinney GL, Ogden L, Snell-Bergeon JK, Maahs DM, Cornell E, et al. Soluble interleukin-2 receptor as a marker for progression of coronary artery calcification in type 1 diabetes. Int J Biochem Cell Biol. 2006;38(5–6):996–1003. [DOI] [PubMed] [Google Scholar]
- 74.Rodrigues TC, Snell-Bergeon JK, Maahs DM, Kinney GL, Rewers M. Higher fibrinogen levels predict progression of coronary artery calcification in adults with type 1 diabetes. Atherosclerosis. 2010;210(2):671–3. doi: 10.1016/j.atherosclerosis.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snell-Bergeon JK, West NA, Mayer-Davis EJ, Liese AD, Marcovina SM, D’Agostino RBJ, et al. Inflammatory markers are increased in youth with type 1 diabetes: the SEARCH Case-Control study. J Clin Endocrinol Metab. 2010;95(6):2868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. [DOI] [PubMed] [Google Scholar]
- 77.Sander D, Schulze-Horn C, Bickel H, Gnahn H, Bartels E, Conrad B. Combined effects of hemoglobin A1c and C-reactive protein on the progression of subclinical carotid atherosclerosis: the INVADE study. Stroke; J Cereb Circ. 2006;37(2):351–7. doi: 10.1161/01.STR.0000199034.26345.bc. [DOI] [PubMed] [Google Scholar]
- 78.Schillinger M, Exner M, Amighi J, Mlekusch W, Sabeti S, Rumpold H, et al. Joint effects of C-reactive protein and glycated hemoglobin in predicting future cardiovascular events of patients with advanced atherosclerosis. Circulation. 2003;108(19):2323–8. doi: 10.1161/01.CIR.0000095267.24234.00. [DOI] [PubMed] [Google Scholar]
- 79.Schulze MB, Rimm EB, Li T, Rifai N, Stampfer MJ, Hu FB. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004;27(4):889–94. [DOI] [PubMed] [Google Scholar]
- 80.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, et al. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19(12):3071–8. [DOI] [PubMed] [Google Scholar]
- 81.Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care. 2012;35(2):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cox AJ, Agarwal S, Herrington DM, Carr JJ, Freedman BI, Bowden DW. C-reactive protein concentration predicts mortality in type 2 diabetes: the Diabetes Heart Study. Diabet Med: J British Diabet Assoc. 2012;29(6):767–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Livingstone SJ, Looker HC, Hothersall EJ, Wild SH, Lindsay RS, Chalmers J, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9(10):e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shah S, Singh K, Ali MK, Mohan V, Kadir MM, Unnikrishnan AG et al. Improving diabetes care: Multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia-The CARRS Multi-center Translation Trial. Diabetes Res Clin Pract. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vyssoulis G, Pietri P, Vlachopoulos C, Alexopoulos N, Kyvelou SM, Terentes-Printzios D, et al. Early adverse effect of abnormal glucose metabolism on arterial stiffness in drug naive hypertensive patients. Diab Vasc Dis Res. 2012;9(1):18–24. [DOI] [PubMed] [Google Scholar]
- 86. Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM, Rodriguez BL, et al. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2010;33(4):881–6. •• Arterial stiffness is an early sign of CVD and is associated with events. The SEARCH study is one of the few studies to examine CVD risk concurretly in type 1 and type 2 diabetes. Among youth with diabetes, type 2 diabetes increased arterial stiffness more than did type 1 diabetes.
- 87.Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56(17):1397–406. [DOI] [PubMed] [Google Scholar]
- 88.Standards of medical care in diabetes. Diabetes Care. 2011;34(1): S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao F, Zhou YJ, Shen H, Wang ZJ, Yang SW, Liu XL. Meta-analysis of percutaneous coronary intervention versus coronary artery bypass graft surgery in patients with diabetes and left main and/or multivessel coronary artery disease. Acta Diabetol. 2012. [DOI] [PubMed] [Google Scholar]
- 90.Brooks MM, Chung SC, Helmy T, Hillegass WB, Escobedo J, Melsop KA, et al. Health status after treatment for coronary artery disease and type 2 diabetes mellitus in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial. Circulation. 2010;122(17):1690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee C, Joseph L, Colosimo A, Dasgupta K. Mortality in diabetes compared with previous cardiovascular disease: A gender-specific meta-analysis. Diabetes Metab. 2012. June 7. • Diabetes has been described as a coronary heart disease (CHD) equivalent, but this study examined whether people with type 2 diabetes and no history of CHD actually have the same risk for a CHD event as people with prior CHD. It was found that diabetes did not increase the risk for a CHD event as much as prior CHD, calling into question the dogma that diabetes is a CHD equivalent.
- 92.Secrest AM, Prince CT, Costacou T, Miller RG, Orchard TJ. Predictors of and survival after incident stroke in type 1 diabetes. Diab Vasc Dis Res. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med: J British Diabet Assoc. 2006;23 (5):516–21. [DOI] [PubMed] [Google Scholar]
- 94. Rana JS, Gransar H, Wong ND, Shaw L, Pencina M, Nasir K, et al. Comparative value of coronary artery calcium and multiple blood biomarkers for prognostication of cardiovascular events. Am J Cardiol. 2012;109(10):1449–53. • In this prospective cohort study, CAC was examined as an independent predictor of CVD, and was found to improve net reclassification and area under the curve more than multiple biomarkers, suggesting this could be a powerful method for improving risk stratification.
- 95.Agarwal S, Morgan T, Herrington DM, Xu J, Cox AJ, Freedman BI, et al. Coronary calcium score and prediction of all-cause mortality in diabetes: the diabetes heart study. Diabetes care. 2011;34(5):1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. Bmj. 2011;343:d5364. •• In this recent study, the trends in mortality among people with type 1 diabetes were examined in the Finnish registry of all people diagnosed between 1970 and 1999. Differences in survival trends were found between individuals with diagnosis of type 1 diabetes early in life, as compared with those diagnosed at older ages.
- 97.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RBS, Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119(13):1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]