Abstract
Objectives
The purpose of this study was to compare and determine whether there were any differences in clinical outcomes between pregnant and non-pregnant women who had been infected with COVID-19.
Methods
A literature search was performed in 9 databases on November 20, 2021. The relative risk (RR) with 95% confidence interval (95% CI) was used to estimate the effect of pregnancy on COVID-19 outcomes. The I square value was used to assess heterogeneity, and the random or the fixed-effects model were adopted. Sensitivity and publication bias analyses were performed.
Results
This study included 8 published studies with 859,278 COVID-19 female patients. The incidences of fever and cough among pregnant women with COVID-19 were 19.07% and 28.79%, respectively. Pregnancy was associated with significantly increased risks of intensive care unit (ICU) admission (RR = 2.23, 95% CI = 1.58–3.16) and ventilation (RR = 2.13, 95% CI = 1.06–4.28), but was not associated with a statistically significant increase in mortality.
Conclusions
Our results suggest that pregnant women with COVID-19 have a significantly higher probability of being hospitalized to the ICU and ventilation than non-pregnant women with COVID-19. To avoid these adverse outcomes, pregnant women should take precautions (for example, reduce going out, maintain social distance, and wear a mask) to avoid COVID-19 infection. Finally, additional research into the fetal outcomes is required to better investigate the impact of COVID-19 on pregnancy.
Keywords: SARS-COV-2, COVID-19, Pregnancy, Women, Meta-analysis
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and has become a global public health emergency. The World Health Organization has reported 394,381,395 confirmed cases of COVID-19 with 5,735,179 deaths by 8 February 2022 [1]. Although the current epidemic has reached a plateau phase, new mutant strains continue to arise, posing greater public health challenge worldwide. Meanwhile, it is evident that elderly individuals, immunosuppressed individuals, and those with pre-existing medical conditions are particularly vulnerable to the infection. On the one hand, it was also proposed that pregnant women may also be at higher risk [2,3]. On the other hand, several studies have reported that the clinical course, symptom characteristics, and severity of COVID-19 are similar in pregnant and non-pregnant women, with 86% mild cases, 9% severe cases, and 5% critical cases among all pregnant women who were diagnosed with COVID-19 [[4], [5], [6]]. These findings, however, appear to contradict the fact that pregnant women often develop serious illnesses after contracting a variety of respiratory pathogens (such as influenza A (H1N1) pdm09) [7].
It is well known that pregnancy alters the immune system and its response to viral infection, and infection with coronavirus, in theory, could lead to more severe symptoms, especially in the third trimester [8]. One study found that the incidence of SARS-CoV-2 infection in pregnant women was 3.02 times greater than that in the general population [9], and Peng-hui Wang et al. [10] pointed out that pregnant women with COVID-19 are more prone to develop severe conditions with a high mortality rate. Previous studies have shown that pregnant women infected with Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) or Middle East Respiratory Syndrome Coronavirus had a higher risk of developing adverse complications. There is a high mortality rate among pregnant women infected with SARS-CoV (25%) [11] and a high mortality rate of 40% among pregnant women infected with Middle East Respiratory Syndrome [12,13]. In addition, SARS-CoV-2 shares 79.6% sequence identity with SARS-CoV [14], and we should be alert to the possibility of serious adverse consequences for pregnant women with COVID-19. However, conclusions regarding clinical outcomes in pregnant women with COVID-19 have been inconsistent. Several studies have shown that pregnant women with COVID-19 did not have an increased risk for intensive care unit (ICU) admission or death compared to non-pregnant women [15,16]. In contrast, a Swedish study showed that pregnant and postpartum women with COVID-19 might have a higher risk (four to five times) of being admitted to the ICU than non-pregnant women of the same age [17]. Therefore, the outcome of pregnant women with COVID-19 is unclear.
The impact of COVID-19 on pregnant women should be investigated as additional data become available. Therefore, we conducted this systematic review and meta-analysis to explore whether pregnancy increases the risk of adverse outcomes of COVID-19 through a pooled analysis of the existing literature.
2. Methods
This systematic review and meta-analysis was registered in the International Prospective Register of Systematic Reviews on May 13, 2021. This systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses 2009 (PRISMA 2009) guidelines [18].
2.1. Search strategy
On November 20, 2021, two reviewers completed a literature search of all the following databases: (1) PubMed; (2) Cochrane library; (3) SinoMed (CBM); (4) Embase; (5) ScienceDirect; (6) Web of Science; (7) China National Knowledge Infrastructure (CNKI); (8) Wanfang Data Knowledge Service Platform and (9) China Science and Technology Journal VIP Database. We used the following combination of key words and mesh terms to search: Pregnant Women, Pregnancy, COVID-19, and SARS-CoV-2. The detailed retrieval strategy is provided in the appendix file. In addition, to avoid omitting any literature that might be included as much as possible, we also manually searched references of relevant literature to identify other eligible studies.
2.2. Study inclusion and exclusion criteria
The studies retrieved from the electronic database were imported into the literature management software (NoteExpress version 3.1), and the duplication checking function of the software and manual duplication checking were used to delete duplicates. The included studies that met the following criteria: (1) the exposed group were pregnant women confirmed with COVID-19; (2) the control group was non-pregnant women diagnosed with COVID-19; (3) the primary outcome events were death or ICU admission or ventilation (ventilator, intubation, mechanical or invasive ventilation); (4) relative risk (RR) or odds ratio (OR) or hazard ratio (HR) and 95% confidence intervals (95% CI) were provided; (5) for studies of the same population or the same dataset or database, only those with the largest number of patients and/or the most recent studies were selected. Exclusion criteria: conference abstracts, expert opinion or suggestion, systematic reviews and meta-analyses, comment, letter, correspondence, and case reports; publications in languages that are neither English nor Chinese; and non-human studies. Manual duplication checking and selection process for the study were carried out by two researchers, and any disagreements were resolved through discussion, with a third author consulted if necessary.
2.3. Data extraction
Two reviewers independently extracted the data from the original studies to ensure its accuracy. Any differences were resolved through discussion or consultation with others. We extracted the following data from the included studies: author's name, publication year, age, sample size, RR or OR or HR with corresponding 95% CI, etc.
2.4. Quality assessments
Two reviewers assessed the methodological quality of the studies included in this systematic review independently using the evaluation criteria for case-control and cohort studies provided in Nine-Star Newcastle Ottawa Scale (NOS) [19]. Disagreements were resolved through discussion or consultation with other co-authors. The quality of the included studies was rated as follows: low quality 0 to 4; moderate quality 5 to 6; and high quality 7 to 9.
2.5. Statistical analysis
In the systematic review and meta-analysis, we used the RR with 95% CI as a measure of effect size for all studies. For studies where RR was not provided, the data were converted according to the following methods: (1) HR was used to approximate RR [20,21]; (2) OR was transformed into RR, if applicable, with this formula: RR = OR/[(1-P0) + (P0 × OR)], in which P0 is the incidence of the outcome of interest in the non-exposed group [22]; the standard error of the resulting RR was calculated with the following formula: selog (RR) = selog (OR) × log (RR)/log (OR), which was also used to calculate the upper and lower limits of the CI by applying this formula to the upper and lower confidence limits of the adjusted odds ratio [23]. I square (I 2) statistics were used to assess heterogeneity [24]. Random- or fixed-effect models were selected according to the magnitude of the I 2 statistics of heterogeneity between studies. The more commonly used method of judgment is as follows: if I 2 ≥ 50%, the random effects model (REM) was used as the pooling method; otherwise, the fixed effects model (FEM) was used. If possible, we performed subgroup analysis by publication year, sample size, continent, etc. Publication bias was evaluated by funnel plots, Egger's test and Begg's test [25]. In addition, sensitivity analysis was performed to explore whether any study exerted substantial impact on the result. Finally, we performed chi-square tests for outcome incidence based on the data provided by the included literature. In all the analyses, P < 0.05 was deemed statistically significant. All statistical analyses were performed using STATA version 14.0 (Stata Corp, College Station, TX) software.
3. Results
3.1. Search results
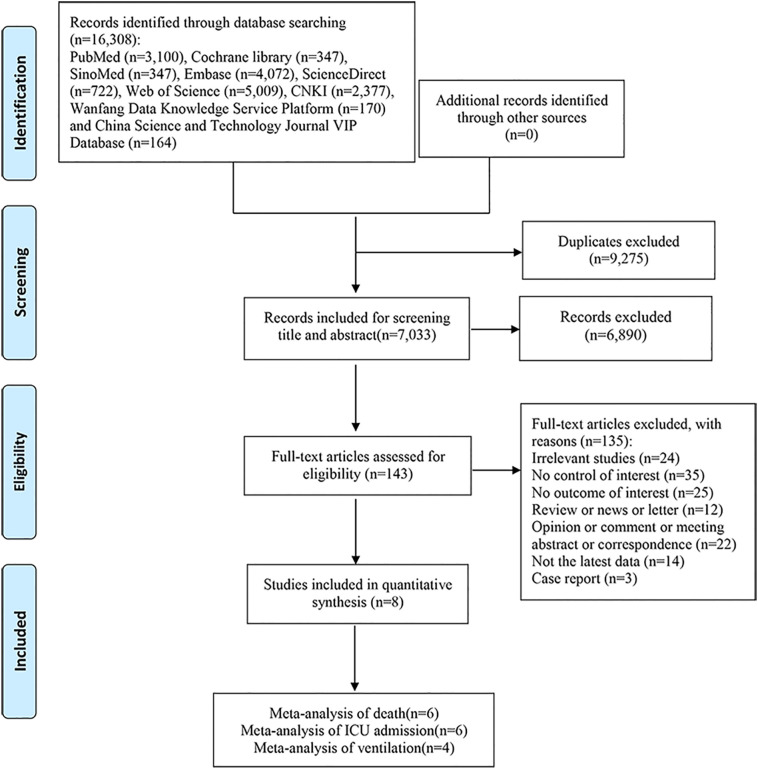
After a comprehensive search of each database, 16,308 articles were initially retrieved. After software and manual duplication checks, 7033 articles remained. After the initial screening of the titles and abstracts, 143 articles required further screening. Further screening was conducted by reading the full text, and 8 studies were eventually included in this systematic review and meta-analysis. The process of the study selection is shown in Fig. 1 .
Fig. 1.
Flow diagram of the literature search and selection process.
3.2. Characteristics of studies and study quality
We included 8 published studies [[26], [27], [28], [29], [30], [31], [32], [33]] with 859,278 COVID-19 female patients, of which 37,578 (4.37%) cases were pregnant patients. Most study designs are cohort studies or case-control studies. The mean age range of COVID-19 pregnant women is 28–32 year and the median gestational week range is 26–32 weeks.
The mortality rates for COVID-19 pregnant women and non-pregnant women were 0.90% and 0.55%, ICU admission rates were 4.31% and 1.68%, and ventilation rates were 2.13% and 0.77%, respectively. According to the results of the chi-square test, all P < 0.01 were statistically significant. The included studies all scored 7 points or higher according to NOS, indicative of high-quality studies. In terms of publication year, age, data source time and outcomes, the detailed characteristics of the included studies are shown in Table 1 . As shown in Table 2 , the most common comorbidities in pregnant and non-pregnant women with COVID-19 were diabetes (2.46% (925/37,578), 1.53% (12,206/796,608), respectively) and cardiovascular disease (2.18% (819/37,578), 2.17% (17,266/796,608), respectively). The incidences of fever and cough were 19.07% (5747/30,130) and 28.79% (8620/29,942), respectively, among pregnant women with COVID-19.
Table 1.
Characteristics of included studies.
| Author's name | Publication year | Country | Data source time | Study quality score | Sample size |
Age (mean ± SD) |
Outcomes⁎⁎⁎ |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Pregnant | Non-pregnant | Pregnant | Non-pregnant | Pregnant/Non-pregnant (N (%)) | RR (95% CI) | |||||
| Martinez-Portilla [29] | 2021 | Mexico | February 1–October 28, 2020 | 8 | 5183 | 5183 | 28.5 ± 5.9 | – | Death:(1.50)/ (0.80) ICU:(13.00)/ (7.40) Ventilation:(8.10)/ (8.60) |
Death:1.84(1.26–2.69) ⁎⁎ ICU:1.86(1.41–2.45) ⁎⁎ Ventilation:0.93(0.70–1.25) ⁎⁎ |
| Tug [31] | 2020 | Turkey | March 11–June 30, 2020 | 7 | 188 | 799 | 31 ± 12 | Death:0/3 ICU:6(3.2)/5(0.6) Ventilation:4(2.1)/5(0.6) |
ICU:5.1(1.57–16.53) Ventilation:2.125(1.25–3.60) |
|
| Vizheh [32] | 2021 | Iran | March–October 2020 | 7 | 110 | 234 | 32.02 ± 6.1 | 32.88 ± 6.3 | Death:6(5.5)/12(5.1) ICU:10(9.1)/19(8.1) |
Death:0.58(0.17–1.94) ⁎⁎ ICU:1.13(0.50–2.52) ⁎⁎ |
| Zambrano [33] | 2020 | USA | January 22–October 3, 2020 | 9 | 23,434 | 386,028 | – | Death:34(1.5)/447(1.2) ICU:245(10.5)/1492(3.9) Ventilation:67(2.9)/412(1.1) |
Death:1.7 (1.2–2.4) ICU:3.0 (2.6–3.4) Ventilation:2.9(2.2–3.8) |
|
| BahaaEldin [26] | 2021 | Egypt | February–July 2020 | 7 | 408 | 22,687 | 29.3 ± 8.1 | – | Death:10(2.5)/348(1.5) ICU:12(2.9)/281(1.2) Ventilation:11(2.7)/157(0.7) |
Death:1.6 (0.9–3.0) ⁎⁎ ICU:2.4 (1.3–4.3) ⁎⁎ Ventilation:3.9(2.1–7.4) ⁎⁎ |
| Rozo [30] | 2021 | Colombia | March 6–December 12, 2020 | 9 | 5614 | 365,749 | – | Death:54(1.0)/715(0.2) | Death:1.95 (1.71–2.22) | |
| Overtoom [28] | 2021 | Netherlands | March 1–August 31, 2020 | 9 | 376 | 19,110 | – | ICU:6(2)/122(6) | ICU:2.4 (1.3–4.3) ⁎⁎ | |
| Knobel [27] | 2021 | Brazil | until August 17, 2020 | 9 | 2265 | 21,910 | 29(11–44) ⁎ | 36(10–44) ⁎ | Death:151(6.7)/2858(13.0) ICU:(18.70)/ (24.40) Ventilation:(7.40)/ (10.70) |
Death:0.6228(0.5219–0.7432) |
Is the median age (range).
Is OR with 95% CI.
Is the data provided by the original text.
Table 2.
Comorbidities and symptoms of included studies.
| Author's name | Publication Year | Sample | Symptoms |
Comorbidities |
|---|---|---|---|---|
| Pregnant/Non-pregnant(N) | Pregnant/Non-pregnant(N) | |||
| Martinez-Portilla [29] | 2021 | 10,366 | – | Diabetes:174/− Cardiovascular diseases:174/− Asthma:112/− Obesity:477/− |
| Tug [31] | 2020 | 987 | Fever:24/35 | Diabetes:2/− Cardiovascular diseases:2/− Asthma:8/− Obesity:8/− |
| Vizheh [32] | 2021 | 344 | Fever:60/110 Cough:42/164 Headache:6/32 Diarrhea:7/20 |
Diabetes:9/15 Cardiovascular diseases:4/15 |
| Zambrano [33] | 2020 | 409,462 | Fever:3328/68536 Cough:5230/89,422 Headache:4447/95713 Diarrhea:1479/38,162 |
Diabetes:427/6119 Cardiovascular diseases:304/7703 Severe Obesity⁎⁎:174/1810 |
| BahaaEldin [26] | 2021 | 23,095 | Fever:328/354⁎ Cough:285/330 Headache:13/466 Diarrhea:40/2931 |
Diabetes:2.0%/5.4% Cardiovascular diseases:1.0%/1.9% |
| Rozo [30] | 2021 | 371,363 | Fever:1858/10,7994 Cough:2883/176,658 Headache:1176/84,208 Diarrhea:213/18,331 |
Diabetes:121/2895 Cardiovascular diseases:109/6154 Asthma:143/8809 Obesity:212/9261 |
| Overtoom [28] | 2021 | 19,486 | Fever:149/376 Cough:180/376 | Diabetes:6/− Cardiovascular diseases:6/− Obesity⁎⁎⁎:67/− |
| Knobel [27] | 2021 | 24,175 | – | Diabetes:178/1952 Cardiovascular diseases:216/2963 Respiratory diseases (including asthma):104/1385 Obesity:82/1912 |
The term used in the original text is “Temperature > 38 °C".
Defined as BMI ≥40 kg/m2.
Defined as BMI >30 kg/m2; other studies on the definition of obesity were not mentioned.
3.3. Results of meta-analysis
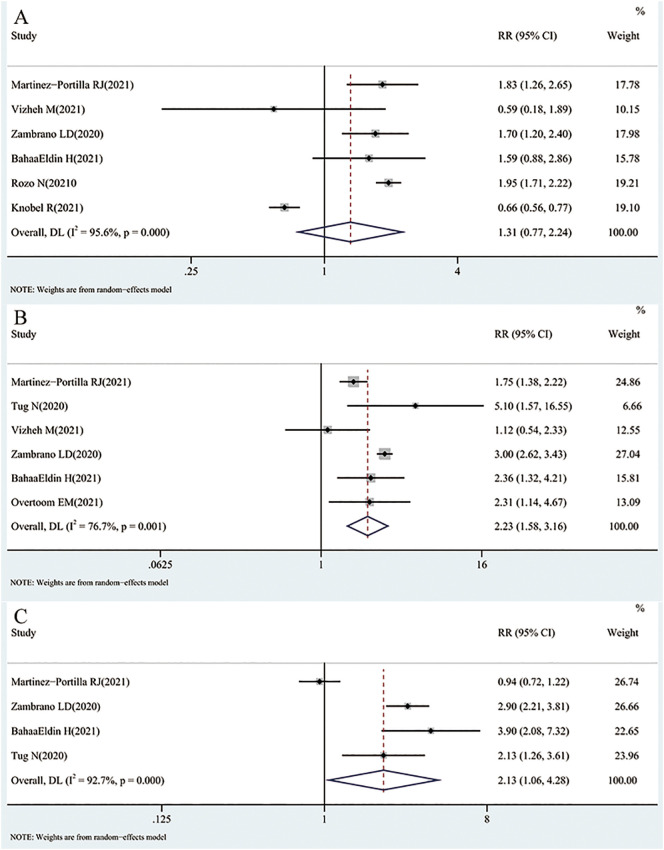
For death, we extracted RRs or ORs from 6 original studies [26,27,29,30,32,33]. Three studies suggest that pregnancy increases the risk of death, yet one study suggests that pregnancy is a protective factor against death. When pooled, the result of REM analysis showed that in COVID-19 patients, pregnancy was not associated with a statistically significant higher risk of death than non-pregnant women (pooled RR = 1.31; 95% CI = 0.77–2.24, I 2 = 95.6%). The result is shown in Fig. 2A.
Fig. 2.
Results of a meta-analysis of COVID-19 pregnant and non-pregnant death(A), ICU admission(B) and ventilation(C).
In total, 6 studies [26,28,29,[31], [32], [33]] provided data on ICU admission. All but one of the studies showed that pregnancy increased the risk of ICU admission. The pooled RR was 2.23 (95% CI = 1.58–3.16, I 2 = 76.7%, REM), indicating that pregnancy was associated with a statistically significant increased risk of ICU admission in COVID-19 patients. The result is shown in Fig. 2B.
Based on the extracted RR or OR from 4 studies [26,29,31,33] to calculate the pooled RR of ventilation (RR = 2.13, 95% CI = 1.06–4.28, I 2 = 92.7%, REM), the results showed that pregnancy was associated with a statistically significant increased risk of ventilation in COVID-19 patients. The result is shown in Fig. 2C.
3.4. Publication bias and sensitivity analyses
Funnel plots were not drawn due to the small number of included studies and the difficulty of assessing publication bias by visual assessment. For the three outcomes, both Begg's and Egger's tests showed that all P > 0.05 (Death: P = 0.348, P = 0.974; ICU admission: P = 0.851, P = 0.463; Ventilation: P = 0.497, P = 0.574; respectively); thus, publication bias may not exist. Sensitivity analyses were performed by removing each study at a time. The analyses showed that for all three outcomes, the pooled results did not differ significantly from the main results, indicating that the results of the meta-analysis were reasonably robust.
4. Discussion
Three concerns regarding pregnant women have arisen as a result of the present COVID-19 outbreak: (1) whether COVID-19 represents a risk for pregnant women, (2) whether pregnant women are more affected by COVID-19 than nonpregnant women, and (3) whether there is a risk of vertical mother-to-child transmission [34]. Therefore, we conducted this study to explore whether pregnancy increases the risk of certain adverse outcomes in COVID-19 compared to non-pregnant women with COVID-19.
Only a few systematic reviews and meta-analyses [[35], [36], [37]] related to COVID-19 and pregnant women have been published, however the majority of these studies only focused the different clinical manifestations between pregnant women with COVID-19 and uninfected pregnant women. Our systematic review and meta-analysis, on the other hand, aimed to compare the clinical outcomes of COVID-19 following confirmed viral exposure in pregnant women and non-pregnant women. The results of our analysis indicated that pregnancy was associated with a higher risk for ICU admission and ventilation, but not with death.
The most common symptoms reported by pregnant women with COVID-19 in this study were fever (19.07%) and cough (28.79%). The prevalence of these two symptoms in our study was much lower than those reported in another systematic review and meta-analysis [37] (fever (40%) and cough (41%)). This previous systematic review and meta-analysis [37] also showed that pregnant women with COVID-19 are less likely to have those symptoms than non-pregnant women of reproductive age with COVID-19. The lower incidence of fever in our study may be related to the different definitions and measurements of fever between the included studies; for example, BahaaEldin's study [26] only provided data for body temperature > 38 °C, which this study directly adopted as the data for fever.
Our systematic review and meta-analysis concluded that pregnancy is associated with an increased risk of ICU admission and ventilation, suggesting that pregnancy is a risk factor for ICU admission and ventilation in pregnant women with COVID-19. This is consistent with the findings of John et al. [37], who found that pregnant and newly pregnant women with COVID-19 had higher odds of admission to ICU compared with non-pregnant reproductive aged women with COVID-19 (OR = 2.13, 95% CI = 1.53–2.95). In addition, retrospective cohort studies also noted that women with COVID-19 had higher rates of intubation, ICU admission and preterm birth at the time of delivery compared to pregnant women without COVID-19 [38]. However, a letter [39] comparing ICU admissions of pregnant and non-pregnant women with COVID-19 disagreed, and stated that pregnant women were not at increased risk for ICU admission compared to non-pregnant women. It is said physiological and immunological changes that occur during pregnancy may increase the risk or severity of certain infections [40]. In this case, pregnant women maintain immunosuppression by inhibiting the activity of T cells during pregnancy, which makes them vulnerable to viral infection [41,42]. Throughout pregnancy, a woman's body is in a state of high immunosuppression, and there will be certain changes in anatomy, physiology, and biochemistry, such as increased oxygen consumption and heart rate. The enlarged uterus causes the diaphragm to move upward, etc. [[43], [44], [45], [46]]. When infected with the virus during pregnancy, especially respiratory infectious virus, immune changes may impair pathogen clearance, and other changes may also worsen clinical outcomes and increase the severity of infection, especially in the third trimester [[46], [47], [48], [49]]. Changes in the respiratory tract manifest significantly during pregnancy, influenced by high levels of estrogen and progesterone (as well as altered levels of other steroid hormones), and the restriction of lung expansion, making pregnant women more susceptible to infection by respiratory pathogens, which, once contracted, can develop into a more severe course of disease; and a prominent hallmark of pregnancy is the pro-inflammatory state, especially in early and late gestation, where SARS-CoV-2-induced cytokine storms may induce more severe inflammation [50], which may have deleterious effects on the mother and fetus. These changes predispose pregnant women to a more severe and prolonged course of the disease. Because pregnancy itself was a risk factor for ICU admission compared to non-pregnant women with COVID-19 [37], therefore, the risk of adverse outcomes may be greater when both pregnancy and COVID-19 co-exist. In addition, pregnant women often receive more attention in the case of viral infection, which might, to some extent, reduce the risk of severe adverse outcomes, such as the incidence of death.
Although a statistical association of our research was not found between pregnancy and death among COVID-19 patients, the mortality rate for pregnant women with COVID-19 was higher compared to non-pregnant women with COVID-19 (0.90% vs 0.55%). Cheng [51] pointed out that it was premature to conclude that the maternal mortality rate of SARS-CoV-2 infection may be lower, given that COVID-19 is poorly understood in the early days and that there is a lack of large sample studies. Although data on COVID-19 are accumulating, most of them come from small samples or case series, with widely varying clinical outcomes reported. For example, a case series of 9 pregnant women reported 7 deaths [52]. Also, a meta-analysis that included 20 studies of pregnant women with SARS-CoV-2 confirmed by reverse transcription polymerase chain reaction showed that the maternal ICU admission rate was 7.0%, the mortality rate was 0.9% [35], and the mortality rates were broadly consistent with our findings, but the ICU admission rate was higher than our result (3.21%). The global maternal mortality ratio in 2015 was 216 deaths per 100,000 live births [53], which was lower than the mortality rate of pregnant women with COVID-19 reported by our study. Our analysis of studies from several countries found that pregnant women with COVID-19 had a higher mortality rate but that pregnancy did not increase the risk of death compared to non-pregnant women with COVID-19. Some studies [34,37,54] have shown that pregnant women with COVID-19 have an increased risk of death and higher mortality rates compared to uninfected, that suggest the higher mortality of pregnant women with COVID-19 may be related to COVID-19 itself. Pregnancy is a physiological state that has multiple effects on the cardiovascular system. COVID-19 infection in pregnant women can lead to further alterations in the cardiovascular system, and elevated cardiac enzymes, such as pro-brain natriuretic peptide and cardiac troponin, are elevated, indicating evolving myocardial inflammation and injury. Studies have linked myocardial inflammation and injury to more severe disease and higher mortality in infected patients [[55], [56], [57]].
There are a few limitations of this systematic review and meta-analysis. First, the biggest limitation which is not described is cause and effect. It is unclear whether pregnant women were admitted to the ICU (and placed on ventilator) because they were pregnant or because their condition was severe enough to require more drastic measures by physicians. Meanwhile, it is also unclear whether COVID-19 patients are more prone to having poor outcomes because of pregnancy of if COVID-19 infection itself cases poor outcomes for pregnant patients. In light of the fact that the admission criteria for pregnant women vary from those for non-pregnant women at each institution, it would have been more informative if it had been clarified that these results were obtained by comparing only pregnant women with and without COVID. Nonetheless, the COVID variant acquired and the patient's vaccination status must also be disclosed for specification purposes. Second, many studies included in this review did not clearly define the adjusted confounders, such as age differences between two patient groups, demographical characteristics as well as vaccination status. For example, the pregnant women of reproductive age cannot be compared to the postmenopausal women. Finally, while there were very high levels of heterogeneity across the publications, the included studies were insufficient to perform meta-regression or subgroup analysis. It should be mentioned, however, that this systematic review and meta-analysis propose certain advantages. On the one hand, this study's inclusion and exclusion criteria were more stringent. On the other hand, using pooled RR data, we explored the impact of pregnancy status on mortality, ICU admissions, and ventilation status in COVID-19-infected women. Overall, the sensitivity analysis demonstrated that the meta-analysis' conclusions were stable, since the pooled results were not statistically different from the primary outcomes.
To minimize the chance of infection with COVID-19, pregnant women should be educated about the risks of COVID-19 virus and the signs of severe symptoms of COVID-19 infection. In particular, pregnant women should avoid contact with people who may have been exposed to or infected with COVID-19 and limit unnecessary public exposure. When out, pregnant women should wear a mask at all times, maintain a safe social distance, and sanitize their hands frequently. Nevertheless, pregnant women should also keep up with their scheduled influenza vaccination and prenatal care check-ups. Ideally, pregnant women and their caregivers should be immunized against COVID-19. However, if a pregnant woman has contracted COVID-19, she must receive rapid and standardized treatment as soon as possible to avoid serious complications. More importantly, a woman should consult a doctor when choosing drugs to treat her symptoms that are not contraindicated for pregnancy. Additionally, it is imperative that pregnant women receive adequate nutritional support in order to maintain homeostasis and avoid water-electrolyte disorders. Lastly, in the event of an emergency, terminate the pregnancy in a timely manner following a comprehensive evaluation of the severity of the disease, the gestational stage and the fetus's intrauterine condition.
In conclusion, this systematic review and meta-analysis suggests that pregnant women with COVID-19 have a higher risk of ICU admission and ventilation than non-pregnant women, but it was not associated with statistically significant increase in mortality. Pregnant women deserve closer attention and protection during the current COVID-19 pandemic. In view of the limitations of this analysis, more high-quality and confounder-adjusted studies, as well as studies investigating fetal outcomes, are needed to further validate or confirm these findings.
Authors' contributions
Hao Wang and Ning Li designed the research; Ning Li and Qiuxia Song conducted the literature search; Hao Wang, Ning Li and Xianwei Guo selected the study and extracted the data; Hao Wang, Qiwei Liang and Wanying Su analyzed the data; and Hao Wang and Ning Li wrote the paper. Mingming Liang and Dr. Chenyu Sun provided critical opinions. Dr. Yehuan Sun, Dr. Chenyu Sun, Mingming Liang, Xiuxiu Ding, Scott Lowe, and Rachel Bentley revised the paper. Hao Wang and Ning Li had primary responsibility for the final content. Dr. Yehuan Sun is the corresponding author. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Hao Wang: Writing – original draft, Formal analysis, Data curation. Ning Li: Writing – original draft, Data curation. Chenyu Sun: Writing – review & editing. Xianwei Guo: Data curation. Wanying Su: Formal analysis. Qiuxia Song: Data curation. Qiwei Liang: Formal analysis. Mingming Liang: Writing – review & editing. Xiuxiu Ding: Writing – review & editing. Scott Lowe: Writing – review & editing. Rachel Bentley: Writing - review & editing. Yehuan Sun: Writing – review & editing.
Declaration of Competing Interest
None.
Acknowledgments
We appreciate all authors for their contributions and support from Anhui Medical University and AMITA Health Saint Joseph Hospital Chicago. Preregistration of this study was undertaken (PROSPERO registration: CRD42021254730).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajem.2022.03.060.
Appendix A. Supplementary data
Supplementary material
References
- 1.World Health Organization WHO Coronavirus (COVID-19) Dashboard Data: World Health Organization. 2021. https://covid19.who.int/ [cited 2022 8 February]. Available from:
- 2.Yao W., Qiu J., Zhirong X., Ouyang S. Pregnancy and COVID-19: management and challenges. Rev Inst Med Trop Sao Paulo. 2020;62 doi: 10.1590/S1678-9946202062062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsaddig M., Khalil A. Effects of the COVID pandemic on pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. 2021 doi: 10.1016/j.bpobgyn.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K., et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2(2):100118. doi: 10.1016/j.ajogmf.2020.100118. Epub 2020/04/16. [PubMed PMID: 32292903; PubMed Central PMCID: PMCPMC7144599] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Molteni E., Astley C.M., Ma W., Sudre C.H., Magee L.A., Murray B., et al. SARS-CoV-2 (COVID-19) infection in pregnant women: characterization of symptoms and syndromes predictive of disease and severity through real-time, remote participatory epidemiology. medRxiv. 2020 doi: 10.1101/2020.08.17.20161760. Epub 2020/08/26. [PubMed PMID: 32839787; PubMed Central PMCID: PMCPMC7444306] [DOI] [Google Scholar]
- 7.World Health Organization Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf [cited 2021 23 October]. Available from:
- 8.Antonakou A. The latest update on the effects of COVID-19 infection in pregnancy. Eur J Midwifery. 2020;4:12. doi: 10.18332/ejm/120973. [PubMed PMID: MEDLINE:33537614] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artymuk N.V., Belokrinitskaya T.E., Filippov O.S., Frolova N.I., Surina M.N. Perinatal outcomes in pregnant women with COVID-19 in Siberia and the Russian Far East. J Maternal-Fetal Neonatal Med. 2021 doi: 10.1080/14767058.2021.1881954. [DOI] [PubMed] [Google Scholar]
- 10.Wang P.H., Lee W.L., Yang S.T., Tsui K.H., Chang C.C., Lee F.K. The impact of COVID-19 in pregnancy: part I. clinical presentations and untoward outcomes of pregnant women with COVID-19. J Chin Med Assoc. 2021 doi: 10.1097/jcma.0000000000000595. Epub 2021/08/10. [PubMed PMID: 34369462] [DOI] [PubMed] [Google Scholar]
- 11.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. Epub 2004/08/06. [PubMed PMID: 15295381; PubMed Central PMCID: PMCPMC7137614] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle east respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: Report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52(3):501–503. doi: 10.1016/j.jmii.2018.04.005. Epub 2018/06/17. [PubMed PMID: 29907538; PubMed Central PMCID: PMCPMC7128238] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East Respiratory Syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis. 2016;16:105. doi: 10.1186/s12879-016-1437-y. Epub 2016/03/05. [PubMed PMID: 26936356; PubMed Central PMCID: PMCPMC4776369] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blitz M.J., Grunebaum A., Tekbali A., Bornstein E., Rochelson B., Nimaroff M., et al. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol. 2020;223(2):290–291. doi: 10.1016/j.ajog.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rios-Silva M., Murillo-Zamora E., Mendoza-Cano O., Trujillo X., Huerta M. COVID-19 mortality among pregnant women in Mexico: A retrospective cohort study. J Glob Health. 2020;10(2) doi: 10.7189/jogh.10.020512. [PubMed PMID: WOS:000612476300165] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin J., Bystrom E., Carnahan A., Ahrne M. Public Health Agency of Sweden’s brief report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99(7):819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. w64. Epub 2009/07/23. [PubMed PMID: 19622511] [DOI] [PubMed] [Google Scholar]
- 19.Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2021 23 October]. Available from:
- 20.Stare J., D. M-B. Odds ratio, hazard ratio and relative risk. Metodoloski Zvezki. 2016;13(1):59–67. [Google Scholar]
- 21.A glossary of EBM terms: BMJ best practice. 2021. https://bestpractice.bmj.com/info/us/toolkit/ebm-tools/a-glossary-of-ebm-terms/ [cited 2021 24 October]. Available from:
- 22.Ronksley P.E., Brien S.E., Turner B.J., Mukamal K.J., Ghali W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342 doi: 10.1136/bmj.d671. [PubMed PMID: 21343207; PubMed Central PMCID: PMCPMC3043109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J., Yu K.F. What’s the relative risk?A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. Epub 2003/09/06. [PubMed PMID: 12958120; PubMed Central PMCID: PMCPMC192859] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. Epub 1997/10/06. [PubMed PMID: 9310563; PubMed Central PMCID: PMCPMC2127453] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BahaaEldin H., Sood H.A.E., Samy S., Khader Y., AbdelFatah M., Hassany M., et al. COVID-19 outcomes among pregnant and nonpregnant women at reproductive age in Egypt. J Public Health (Oxf) 2021;43(Suppl 3):iii12–iii18. doi: 10.1093/pubmed/fdab376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knobel R., Takemoto M.L.S., Nakamura-Pereira M., Menezes M.O., Borges V.K., Katz L., et al. COVID-19-related deaths among women of reproductive age in Brazil: the burden of postpartum. Int J Gynaecol Obstet. 2021;155(1):101–109. doi: 10.1002/ijgo.13811. Epub 2021/07/03. [PubMed PMID: 34213771] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overtoom E.M., Rosman A.N., Zwart J.J., Vogelvang T.E., Schaap T.P., van den Akker T., et al. SARS-CoV-2 infection in pregnancy during the first wave of COVID-19 in the Netherlands: a prospective nationwide population-based cohort study (NethOSS) BJOG. 2021;129(1):91–100. doi: 10.1111/1471-0528.16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Portilla R.J., Sotiriadis A., Chatzakis C., Torres-Torres J., Espino Y.S.S., Sandoval-Mandujano K., et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx) Ultrasound Obstet Gynecol. 2021;57(2):224–231. doi: 10.1002/uog.23575. Epub 2020/12/16. [PubMed PMID: 33320401] [DOI] [PubMed] [Google Scholar]
- 30.Rozo N., Valencia D., Newton S.M., Avila G., Gonzalez M.A., Sancken C.L., et al. Severity of illness by pregnancy status among laboratory-confirmed SARS-CoV-2 infections occurring in reproductive-aged women in Colombia. Paediatr Perinat Epidemiol. 2021 doi: 10.1111/ppe.12808. Epub 2021/09/02. [PubMed PMID: 34467554] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tug N., Yassa M., Köle E., Sakin Ö., Çakır Köle M., Karateke A., et al. Pregnancy worsens the morbidity of COVID-19 and this effect becomes more prominent as pregnancy advances. Turk J Obstet Gynecol. 2020;17(3):149–154. doi: 10.4274/tjod.galenos.2020.38924. Epub 2020/10/20. [PubMed PMID: 33072417; PubMed Central PMCID: PMCPMC7538816] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vizheh M., Muhidin S., Aghajani F., Maleki Z., Bagheri F., Hosamirudsari H., et al. Characteristics and outcomes of COVID-19 pneumonia in pregnancy compared with infected nonpregnant women. Int J Gynaecol Obstet. 2021;153(3):462–468. doi: 10.1002/ijgo.13697. Epub 2021/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., et al. Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. 10.15585/mmwr.mm6944e3 Epub 2020/11/06. [PubMed PMID: 33151921; PubMed Central PMCID: PMCPMC7643892] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epelboin S., Labrosse J., De Mouzon J., Fauque P., Gervoise-Boyer M.J., Levy R., et al. Obstetrical outcomes and maternal morbidities associated with COVID-19 in pregnant women in France: A national retrospective cohort study. PLoS Med. 2021;18(11) doi: 10.1371/journal.pmed.1003857. e1003857. Epub 2021/12/01. [PubMed PMID: 34847147; PubMed Central PMCID: PMCPMC8631654 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalil A., Kalafat E., Benlioglu C., O’Brien P., Morris E., Draycott T., et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:100446. doi: 10.1016/j.eclinm.2020.100446. Epub 2020/08/25. [PubMed PMID: 32838230; PubMed Central PMCID: PMCPMC7334039] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Y.J., Ye L., Zhang J.S., Yin Y.X., Liu M., Yu H.B., et al. Clinical features and outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):564. doi: 10.1186/s12879-020-05274-2. Epub 2020/08/05. [PubMed PMID: 32746801; PubMed Central PMCID: PMCPMC7396931] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. Bmj. 2020;370 doi: 10.1136/bmj.m3320. m3320. Epub 2020/09/03. [PubMed PMID: 32873575; PubMed Central PMCID: PMCPMC7459193] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinn J., Sedighim S., Kirby K.A., Hohmann S., Hameed A.B., Jolley J., et al. Characteristics and outcomes of women with COVID-19 giving birth at US academic centers during the COVID-19 pandemic. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.20456. e2120456. Epub 2021/08/12. [PubMed PMID: 34379123; PubMed central PMCID: PMCPMC8358731] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blitz M.J., Grünebaum A., Tekbali A., Bornstein E., Rochelson B., Nimaroff M., et al. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol. 2020;223(2):290–291. doi: 10.1016/j.ajog.2020.05.004. Epub 2020/05/11. [PubMed PMID: 32387323; PubMed Central PMCID: PMCPMC7204719] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathad J.S., Gupta A. Pulmonary infections in pregnancy. Semin Respir Crit Care Med. 2017;38(2):174–184. doi: 10.1055/s-0037-1602375. Epub 2017/06/01. [PubMed PMID: 28561248] [DOI] [PubMed] [Google Scholar]
- 41.Pazos M., Sperling R.S., Moran T.M., Kraus T.A. The influence of pregnancy on systemic immunity. Immunol Res. 2012;54(1–3):254–261. doi: 10.1007/s12026-012-8303-9. Epub 2012/03/27. [PubMed PMID: 22447351; PubMed Central PMCID: PMCPMC7091327] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longman R.E., Johnson T.R. Viral respiratory disease in pregnancy. Curr Opin Obstet Gynecol. 2007;19(2):120–125. doi: 10.1097/GCO.0b013e328028fdc7. Epub 2007/03/14. [PubMed PMID: 17353679] [DOI] [PubMed] [Google Scholar]
- 43.Wei L., Gao X., Chen S., Zeng W., Wu J., Lin X., et al. Clinical characteristics and outcomes of childbearing-age women with COVID-19 in Wuhan: retrospective, single-center study. J Med Internet Res. 2020;22(8) doi: 10.2196/19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberger S.E., Weiss S.T., Cohen W.R., Weiss J.W., Johnson T.S. Pregnancy and the lung. Am Rev Respir Dis. 1980;121(3):559–581. doi: 10.1164/arrd.1980.121.3.559. Epub 1980/03/01. [PubMed PMID: 6998334] [DOI] [PubMed] [Google Scholar]
- 45.Jamieson D.J., Theiler R.N., Rasmussen S.A. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12(11):1638–1643. doi: 10.3201/eid1211.060152. Epub 2007/02/08. [PubMed PMID: 17283611; PubMed Central PMCID: PMCPMC3372330] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sappenfield E., Jamieson D.J., Kourtis A.P. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol. 2013;2013:752852. doi: 10.1155/2013/752852. Epub 2013/08/13. [PubMed PMID: 23935259; PubMed Central PMCID: PMCPMC3723080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222(5):415–426. doi: 10.1016/j.ajog.2020.02.017. Epub 2020/02/28. [PubMed PMID: 32105680; PubMed Central PMCID: PMCPMC7093856] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Q., Shen J., Pan L., Huang L., Jiang X., Lu W., et al. Coronavirus disease 2019 in pregnancy. Int J Infect Dis. 2020;95:376–383. doi: 10.1016/j.ijid.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz D.A. The effects of pregnancy on women with COVID-19: maternal and infant outcomes. Clin Infect Dis. 2020;71(16):2042–2044. doi: 10.1093/cid/ciaa559. . Epub 2020/05/12. [PubMed PMID: 32392330; PubMed Central PMCID: PMCPMC7239237] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malinowski A.K., Noureldin A., Othman M. COVID-19 susceptibility in pregnancy: Immune/inflammatory considerations, the role of placental ACE-2 and research considerations. Reprod Biol. 2020;20(4):568–572. doi: 10.1016/j.repbio.2020.10.005. Epub 2020/11/14. [PubMed PMID: 33183974; PubMed Central PMCID: PMCPMC7832785] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng S.O., Khan S., Alsafi Z. Maternal death in pregnancy due to COVID-19. Ultrasound Obstet Gynecol. 2020;56(1):122. doi: 10.1002/uog.22111. Epub 2020/07/02. [PubMed PMID: 32608570; PubMed Central PMCID: PMCPMC7361716] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E., et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223(1) doi: 10.1016/j.ajog.2020.04.030. 109 e1- e16. [PubMed PMID: 32360108; PubMed Central PMCID: PMCPMC7187838] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alkema L., Chou D., Hogan D., Zhang S., Moller A.B., Gemmill A., et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2016;387(10017):462–474. doi: 10.1016/s0140-6736(15)00838-7. Epub 2015/11/21. [PubMed PMID: 26584737; PubMed Central PMCID: PMCPMC5515236] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ko J.Y., DeSisto C.L., Simeone R.M., Ellington S., Galang R.R., Oduyebo T., et al. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a coronavirus disease 2019 (COVID-19) diagnosis. Clin Infect Dis. 2021;73(Suppl. 1):S24–s31. doi: 10.1093/cid/ciab344. Epub 2021/05/13. [PubMed PMID: 33977298; PubMed Central PMCID: PMCPMC8136045] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hantoushzadeh S., Nabavian S.M., Soleimani Z., Soleimani A. COVID-19 disease during pregnancy and peripartum period: a cardiovascular review. Curr Probl Cardiol. 2022;47(1):100888. doi: 10.1016/j.cpcardiol.2021.100888. Epub 2021/06/16. [PubMed PMID: 34127288; PubMed Central PMCID: PMCPMC8106961] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. Epub 2020/03/27. [PubMed PMID: 32211816; PubMed Central PMCID: PMCPMC7097841] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. Epub 2020/03/29. [PubMed PMID: 32219356; PubMed Central PMCID: PMCPMC7101506] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material