Abstract
Introduction:
Many patients use mobile devices to track health conditions by recording patient-generated health data. However, patients and clinicians may disagree how to use these data.
Objective:
To systematically review the literature to identify how patient-generated health data and patient-reported outcomes collected outside of clinical settings can affect patient–clinician relationships within surgery and primary care.
Methods:
Six research databases were queried for publications documenting the effect of patient-generated health data or patient-reported outcomes on patient–clinician relationships. We conducted thematic synthesis of the results of the included publications.
Results:
Thirteen of the 3204 identified publications were included for synthesis. Three main themes were identified: patient-generated health data supported patient–clinician communication and health awareness, patients desired for their clinicians to be involved with their patient-generated health data, which clinicians had difficulty accommodating, and patient-generated health data platform features may support or hinder patient–clinician collaboration.
Conclusion:
Patient-generated health data and patient-reported outcomes may improve patient health awareness and communication with clinicians but may negatively affect patient–clinician relationships.
Keywords: patient-generated health data, patient reported outcomes, primary care, professional-patient relations [Mesh], surgery
Introduction
Many people in the United States have access to smartphones and may be interested in health tracking. In 2018, 77% of US adults owned smartphones,1 and in 2016, at least 325,000 mobile health applications (mHealth apps) were available to help smartphone owners track their health.2 In the same year, 64 percent of teens and young adults reported using mHealth apps to track their health or address a health concern.3 In addition, Krebs and Duncan 2015 found that more than half of smartphone owners had downloaded a health-related mobile app, with most using the app at least once per day. The most common reasons for downloading an app were to track physical activity levels, record diet intake, or to learn more about exercising.4
The data collected by patients are examples of patient-generated health data (PGHD) and patient reported outcomes (PROs). PGHD and PROs are “health-related data—including health history, symptoms, biometric data, treatment history, lifestyle choices, and other information—created, recorded, gathered, or inferred by or from patients or their designees (i.e. care partners or those who assist them) to help address a health concern.”5 PROs are a form of PGHD captured in a patient’s home, clinic, or hospital.6 The impetus for collecting these data may be due to a patient’s self-motivation to track their health or at the direction of a clinician.5 Examples of PGHD and PROs include a patient’s health history, symptoms, biometric data, treatment history, health behaviors, satisfaction, and quality of life.5–7 These data can be passively or actively collected by patients using mHealth apps, wearable devices (e.g. activity trackers), medical devices (e.g. continuous glucose monitoring systems),8 or validated questionnaires9 administered using mobile devices.10
Almost half of the patients or caregivers who collect the PGHD report that the practice changed their approach to maintaining their health.11 For example, they asked their clinicians new questions, sought second opinions, or reflected about their healthcare decision-making. While PGHD is a useful tool for patients, patients and clinicians may disagree about how PGHD should be used to address health concerns, and these disagreements could negatively impact patient–clinician relationships.12 Patients may expect their healthcare team to review their PGHD and respond within a short time frame with detailed explanations on how to interpret the data. This could be disruptive to existing clinical workflows, and clinicians have expressed concerns about the time needed to discuss PROs during short clinic appointments.13
Some research has begun to identify how PGHD and PROs affect patient–clinician relationships. However, previous systematic reviews have focused on: clinician perceptions of PGHD quality,14 PGHD to support diabetes self-management and education,15 the use of PROs in randomized clinical control trials,16,17 strategies to improve PRO data collection,18 and assessing PRO implementations in specific health domains.19–21 Our objective was to systematically review the literature to identify the effect of PGHD and PROs on patient–clinician relationships within surgery and primary care.
Methods
Focus and search strategy
In this review, we focused on how PGHD and PROs collected by surgery and primary care patients in everyday life (i.e. outside the clinic) can affect patient–clinician relationships. We initially focused on surgery because of the potential tensions between patients and clinicians using PGHD as identified in a previous study.12 In this study, patients and clinicians disagreed about the use of unstructured PGHD, the frequency of recording PGHD, electronic messaging about PGHD, and their goals for using PGHD. We included primary care in our search strategy, in addition to surgery, due to the large number of mHealth apps developed to facilitate collecting PGHD for chronic conditions,22–27 which are managed by primary care clinicians.28,29 We also limited our scope to data collected outside of clinical settings to align with the definition of PGHD and PROs.5
With the assistance of a health sciences librarian, we developed our search strategy and terms by identifying keywords and MeSH terms associated with the focus of this review. We queried six research databases that focused on health or information technology domains: MEDLINE, Embase, CINAHL Plus, PsychINFO, IEEE Xplore, and the ACM Digital Library for publications published between January 1, 2006, and October 13, 2017 (the date on which databases were queried). We chose this timeframe for two reasons. First, we wanted to focus on recent PGHD and PRO developments during the transition of mobile devices from predominantly cellular phones to smartphones. Smartphones can be used to collect PGHD or PROs and share these data with clinicians.8–10 The US smartphone ownership in 2006 comprised approximately 2% of all mobile phones30 and increased to 77% in 2018.1 Second, the 2009 Health Information Technology for Economic and Clinical Health Act31 required hospitals to accept digital PGHD from a subset of their patients by 2015.32 The search strategy was supplemented by hand searching the citations contained within systematic reviews that were retrieved by the initial search queries. The search strategies used for this review can be found in Supplemental Table 1.
Eligibility criteria and screening
We included primary research publications describing or documenting the effects of PGHD or PROs on patient–clinician relationships in surgery and primary care when the data were collected outside of clinical settings. Patients could have recorded PGHD or PROs at the direction of a clinician, as part of a research study, or initiated by the patient. Publications retrieved by the queries that incorporated other health domains (e.g. gastroenterology) in addition to surgery or primary care were also included. We excluded publications that only focused on patient or clinician satisfaction using PGHD or PRO platforms as they did not address how PGHD and PROs affect the relationships between patients and clinicians.
Retrieved publications were uploaded into Covidence,33 a platform that facilitates abstract screening and full-text eligibility assessment activities for systematic reviews. Duplicates were identified and removed prior to abstract screening using Covidence and manual review. RL and SM independently screened titles and abstracts against the inclusion and exclusion criteria using Covidence. Disagreements were resolved by consensus. Full-text publications were then reviewed and independently assessed against the inclusion and exclusion criteria by RL and SM. Disagreements were resolved with an independent assessment by LK. Cohen’s Kappa was calculated using STATA34 to assess RL and SM’s inter-rater full-text study eligibility agreement.
Data extraction
The following information was extracted from the included publications: objective, participant demographics, the types of PGHD and PROs collected by patients, and the published results or findings. All text-labeled results or findings from the included publications were copied verbatim and uploaded into Dedoose.35
Synthesis of findings
We followed the Enhancing Transparency in Reporting the Synthesis of Qualitative Research (ENTREQ) statement,36 which informed our thematic synthesis of the publication results and findings.37 The ENTREQ statement consists of 21 items to promote transparency in qualitative synthesis research and is analogous to the quantitatively focused Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement. We chose to use thematic synthesis because it is widely used in healthcare systematic reviews36 and has been previously used in a review focusing on patient perspectives of patient–physician relationships.38
A hybrid approach using both inductive and deductive methods was used to code the text included for final synthesis.39 An initial code book was developed based on the previous research regarding the potential effects of PGHD on patient–clinician relationships.12 RL, SM, and LK initially coded a subset of the studies line-by-line independently and met to resolve coding discrepancies. The subsequent studies were all coded by RL and half were coded by SM and LK. SM and LK resolved coding discrepancies with RL.
Once coding was complete, the authors met to develop descriptive themes regarding how PGHD and PROs impact on patient–clinician relationships. The ENTREQ statement calls for the comparison method within and across the included studies to be explicitly identified. We chose the Systems Engineering Initiative for Patient Safety (SEIPS) Implementation Model 2.040 to guide our comparisons. The model was developed to facilitate the comparison of multiple health information system-specific socio-technical factors, work processes, and outcomes. The authors used the SEIPS Implementation Model’s components to document each included publication’s characteristics. Specifically, the authors summarized each included article according to the persons, tasks, tools, technology, organization, internal and external environments, processes, outcomes, and adaptations. The SEIPS implementation model publication summaries were used by the authors to generate descriptive themes describing the effect of PGHD and PROs on patient–clinician relationships across the included publications. Final descriptive theme summaries for each publication were agreed upon by the researchers. Once the descriptive theme summaries were developed; RL, SM, and LK independently drafted analytical themes and met to develop a final set of analytical themes. The final analytical themes reflected the codes and descriptive themes identified during the first two phases of the thematic synthesis.
Finally, RL assessed the quality of the included publications using the Mixed Methods Appraisal Tool (MMAT),41 which is designed to assess the quality of a heterogeneous body of the literature utilizing qualitative, quantitative, or mixed methods.42 MMAT quality scores range from 1 (one criteria component met) to 4 (all criteria met). The components assess the studies to determine if criteria, such as the presence of clear research questions or appropriate analysis methods, are incorporated into the included research publications for synthesis.
Results
Identification and selection
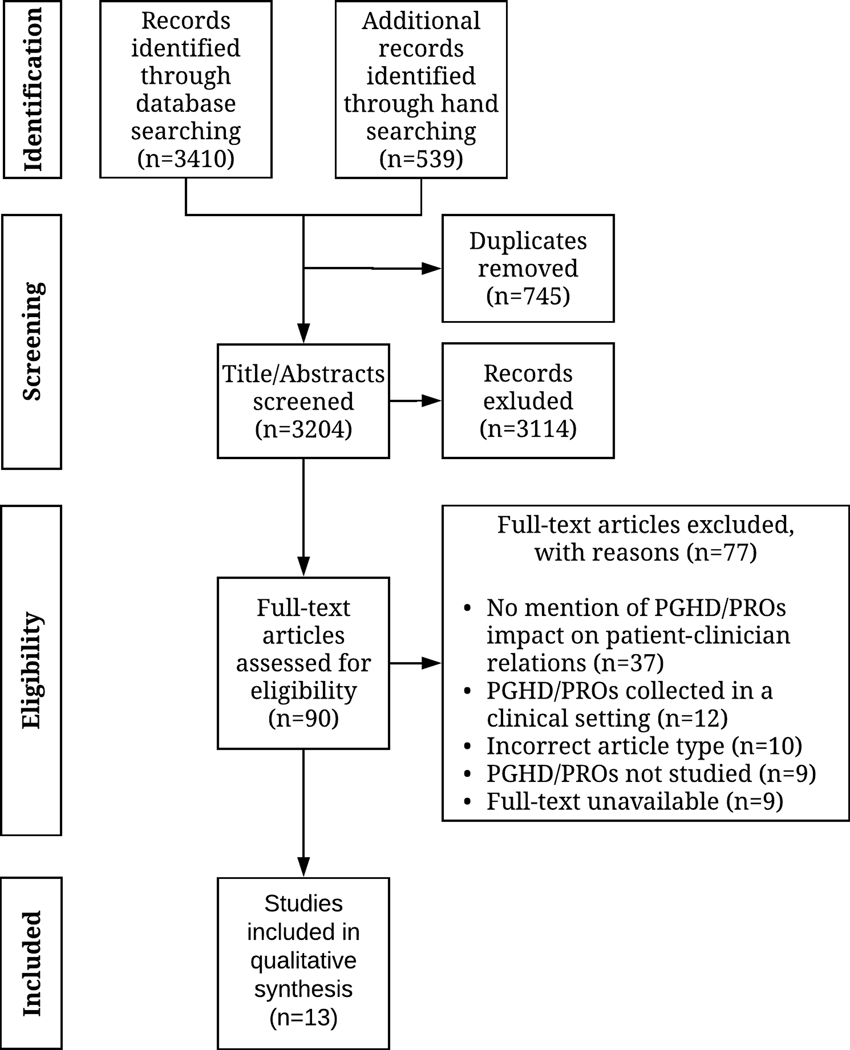
We identified 3204 publications; 3114 (97.2%) did not meet the inclusion criteria based on their title and abstract. Ninety (2.8%) publications were included in the full-text review. Of the 90 full texts, 77 (85.6%) did not meet the inclusion criteria (see Figure 1 for a list of exclusion reasons). Thirteen (14.4%) of the 90 publications were included for final qualitative synthesis.43–55 None of the included publications were excluded based on MMAT quality ratings. Figure 1 depicts the PRISMA flow diagram which illustrates the number of records identified and included and excluded through the screening process.56 Inter-rater agreement for the full-text screening process was 0.89, indicating almost perfect agreement.57
Figure 1.
Preferred reporting items for systematic review and meta-analyses (PRISMA) diagram depicting the flow of information through the systematic review.
Included publication characteristics
Table 1 provides summary information about the 13 publications included in the final synthesis. All included publications described or documented the effect of patients collecting PGHD or PROs on patient–clinician relationships. However, only one of the 13 publications included this as an explicit research objective.46 One included publication focused on surgical patients,43 and the remaining 12 publications pertained to medical conditions managed by primary care clinicians.44–55 The publications were published between 2007 and 2017 and conducted in the United States (n = 6), Denmark (n = 1), South Korea (n = 1), Finland (n = 1), Slovakia (n = 1), the United Kingdom (n = 1), Italy (n = 1), and Canada (n = 1). The publications used qualitative (n = 8),43–45,48,50,52,53,55 quantitative (n = 1),49 or mixed methods (n = 4).46,47,51,54 Sample sizes ranged from 2 to 800 patients and from 1 to 21 clinicians. All publications described or documented patients recording PGHD and/or PROs for personal use outside the clinic. Three publications47,51,53 incorporated parents or caregivers tracking PGHD or PROs on behalf of a child. The PGHD collected by patients and/or caregivers can be found in Table 1. The MMAT quality scores of the included publications ranged from 1 (one criteria component met) to 4 (all criteria met).
Table 1.
Characteristics of included publications.
| Title | Authors | Quality | Year | Country | Patient Population | Provider Population | Caregiver Population | PGHD Types |
|---|---|---|---|---|---|---|---|---|
| Barriers and benefits to using mobile health technology after operation: a qualitative study | Abelson et al.43 | 4 | 2017 | USA | 800 Phone survey respondents | N/A | N/A | N/A |
| Beyond self-monitoring: understanding non-functional aspects of home-based healthcare technology | Gronvall and Verdezoto44 | 3 | 2013 | Denmark | 6 Pregnant women with complications 7 Older adults with heart conditions 6 Healthy self-monitoring older adults | 1 midwife for pregnant patients Unknown number hospital nurses for patients with heart conditions | N/A | Pregnant women: Weight, blood pressure, pulse, CTG, urine protein levels, online questionnaire Heart condition patients: Weight, blood pressure, pulse, symptom survey, ECG data (subset of participants) Health older adults: Blood pressure |
| “My Doctor is Keeping an Eye on Me!”: exploring the clinical applicability of a mobile food logger | Kim et al.45 | 3 | 2016 | South Korea | 20 Patients with lifestyle diseases (e.g. hypertension, diabetes, heart disease) | otorhinolarynologist family medicine physicians 1 OBGYN 1 rehabilitation physician 1 urologist | N/A | Food intake, perceptions of post-meal fullness, meal contexts, meal time, activity levels, and activity trackers |
| Boundary negotiating artifacts in personal informatics: patient- provider collaboration with patient-generated data | Chung et al.46 | 3 | 2016 | USA | 21 1 Surveyed patients who were overweight, obese, or diagnosed with IBS Interviews: 7 Overweight/obese patients 2 Patients diagnosed with IBS 9 Overweight/obese patients diagnosed with IBS | family medicine physicians 5 gastroenterologists dieticians 1 behavioral psychologist 1 APRN 1 health navigator | N/A | Food intake, calorie intake, physical activity levels, weight, heart rates, sleep quality, pain levels, medication use, bowel movement, stress, fatigue, nausea. |
| Evaluation of a web-based asthma self-management system: a randomized controlled pilot trial | Wiecha et al.47 | 3 | 2015 | USA | 58 Children ages 9–17 diagnosed with persistent asthma | Unknown number of primary care providers Unknown number of asthma nurses or asthma specialists | Parent or guardian of children participants | Peak flow readings, symptoms (e.g. cough, wheeze, shortness of breath), contextual data (e.g. activity limitations, missed school, ED visits), medication use |
| Information technology supporting diabetes self-care: a pilot study | Halkoaho et al48 | 2 | 2007 | Finland | 3 Type 1 diabetics 6 Type 2 diabetics | 3 nurses | N/A | Blood glucose levels and treatment goals |
| Yet another hypertension telehealth solution? the rules will tell you | Lehocki et al.49 | 2 | 2014 | Slovakia | 2 Patients diagnosed with hypertension and unspecified comorbidities | Unspecified providers | N/A | Blood pressure, pulse |
| Nurses’ and community support workers’ experience of telehealth: a longitudinal case study | Sharma and Clarke50 | 4 | 2014 | United Kingdom | Patients diagnosed with asthma, diabetes, COPD, or CHF (not recruited for study participation) | Nurses treating patients with asthma, diabetes, COPD, or CHF Community support workers | N/A | Blood glucose level, weight, blood pressure, oxygen level and heart rate. |
| Using a mobile app to manage type 1 diabetes: the case of TreC diabetes | Miele et al.51 | 2 | 2015 | Italy | 15 Children aged 4–12 diagnosed with type 1 diabetes | Diabetes specialist | Parent or guardian of children participants | Blood glucose values, meal compostion, carbohydrate content, and physical activity levels |
| Improving diabetes management with a patient portal: a qualitative study of diabetes self-management portal | Urowitz et al.52 | 3 | 2012 | Canada | 1 Patient diagnosed with type 1 diabetes 6 Patients diagnosed with type diabetes | Unspecified number of: General practitioners Dieticians APRNS Diabetes educators | N/A | All participants recorded blood glucose levels Additional data collected at provider discretion on a per patient basis (e.g. weight, blood pressure) |
| Integrating patient-generated health data into clinical care settings or clinical decision-making: lessons learned from project HealthDesign | Cohen et al.53 | 3 | 2016 | USA | Patients diagnosed with moderate-to-severe asthma Older adults at risk for cognitive decline Adolescent receiving behavioral health interventions Patient’s diagnosed with Crohn’s disease Premature infants with medical complications (not recruited for study participation) | Primary care providers Nurses Gastroenterologists High-risk infant case managers | Parent or guardian of infant participants (Not recruited for study participation) | Asthma patients: medication use, peak flow measurements, environmental factors Older adults: task completion (data not shared with provider) Adolescents: Food intake, physical activity, mood Crohn’s disease patients: Weight, physical activity, mood, relevant symptoms Premature infants: infant weight, food consumption, elimination patterns |
| Using patient-generated health data from mobile technologies for diabetes self-management support: provider perspectives from an academic medical center | Nundy et al.54 | 3 | 2014 | USA | Unspecified number of type 1 or type 2 diabetic patients | Unspecified number of nursecare managers 10 primary care providers 2 endocrinologists & diabetes specialists | N/A | Medication use, blood glucose levels, barriers to diabetes self-care |
| More than telemonitoring: health provider use and nonuse of life-log data in irritable bowel syndrome and weight management | Chung et al.55 | 3 | 2015 | USA | Patients who are overweight/ obese and/or diagnosed with IBS | family medicine physicians 5 gaste nterologists 1 APRN dieticians 1 behavioral psychologist 1 health navipator | N/A | Physical activity levels, food/diet data, stress logs, sleep logs, mood diaries |
N/A: not applicable; APRN: Advanced practice registered nurse; CHF: Congestive heart failureCOPD: Chronic obstructive pulmonary disease; CTG: Cardiotocograph; ECG: Electrocardiogram; ED: Emergency department; IBS: Irritable bowel syndrome; OBGYN: Obstetrics and gynecology; PGHD: patient-generated health data.
Synthesis of findings
We identified three main themes and six subthemes. The main themes were: (1) PGHD supported patient–clinician communication and health awareness, (2) patients desired for their clinicians to be involved with their PGHD, which clinicians had difficulty accommodating, and (3) PGHD platform features may support or hinder patient–clinician collaboration. Table 2 lists the analytical themes and subthemes.
Table 2.
Major analytical themes and subthemes.
| Major analytical theme | Subtheme |
|---|---|
| PGHD supported patient-clinician communication and health awareness | PGHD fostered patient-clinician communication PGHD improved the clinicians understanding of their patients’ health |
| Patients desired for their clinicians to be involved with their PGHD, which clinicians had difficulty accommodating | Patients desired clinician involvement with their PGHD Clinicians had varied interest, encountered barriers, and identified workarounds when integrating PGHD into clinical encounters |
| PGHD platform features may support or hinder patient-clinician collaboration | Trends, summary measures, and education supported PGHD clinical integration and use Some PGHD platforms negatively impacted patient-clinician collaboration |
PGHD: patient-generated health data.
Theme 1: PGHD supported patient–clinician communication and health awareness
PGHD fostered patient–clinician communication.
Patients and clinicians in eight publications43,45–48,50,53,55 viewed PGHD as a tool to enhance patient–clinician communication. In one publication, clinicians perceived PGHD as a tool to support clinician–clinician communication.52 Five publications documented or described improved patient–clinician communication when collaboratively using PGHD as a discussion tool, such as identifying opportunities to improve patient health.45–47,54,55 In two publications, clinicians explicitly informed patients when to expect communication from the healthcare team about their PGHD.52,53 Specifically, the clinicians let them know they would not be contacted if their data appeared normal. In two publications, clinicians used PGHD to provide emotional support to patients,46,53 such as providing empathy regarding a patient’s health experiences.46
PGHD improved the clinicians understanding of their patients’ health.
In seven publications, clinicians modified their patients’ treatment plans after reviewing their PGHD.45,46,48,52–55 In six publications, clinicians used PGHD to identify patient treatment or goal barriers.48,50,51,53–55 PGHD was also utilized by clinicians in six publications to gain a greater understanding of a patient’s health between clinic visits.44–47,53,55 Patients in three publications could record additional PGHD about the context of their health condition in relation to their daily lives that they would share with their clinicians.47,49,52 Clinicians would use PGHD to set agendas with patients during clinical encounters in two publications.54,55 Physicians in turn would use these agendas to focus clinical encounters on pertinent patient issues identified in PGHD such as poor blood sugar monitoring54 or specific concerns patients have difficulty articulating.55 Two publications reported that clinicians used PGHD to identify whether patients’ personally identified goals were being achieved.51,55
Theme 2: patients desired for their clinicians to be involved with their PGHD, which clinicians had difficulty accommodating
Patients desired clinician involvement with their PGHD.
In four publications, patients wanted their clinicians to make the review of PGHD a central component of their clinic visits or expressed a N/A: not applicable; APRN: Advanced practice registered nurse; CHF: Congestive heart failureCOPD: Chronic obstructive pulmonary disease; CTG: Cardiotocograph; ECG: Electrocardiogram; ED: Emergency department; IBS: Irritable bowel syndrome; OBGYN: Obstetrics and gynecology; PGHD: patient-generated health data.desire for greater clinician involvement with their data during clinic visits.45,46,49,52 In two publications, patients wanted clinicians to provide empathy or emotional support after clinician PGHD review.45,46 In addition, in two publications, patients wanted clinicians to acknowledge their efforts in recording PGHD.45,49 In one publication, some patients perceived clinician acknowledgment as a reward for collecting the data.45 Clinician acknowledgment also affected patient health management. In three publications, patients had increased accountability and treatment adherence when clinicians asked about their tracking behaviors and emphasized the importance of tracking PGHD.45,46,55 In three publications, some patients were unable to draw actionable insights from their PGHD because they were unable to make sense of their data on their own. This prompted them to seek greater clinician involvement to aid in interpreting PGHD;45,46,52 one publication emphasized that patients wanted a clinician review of PGHD review to result in personalized treatment and action plans.46 While some patients desired their clinicians to be involved with interpreting their PGHD, in four publications, other patients could make sense of their PGHD and generate actionable insights independent of clinician review.44,45,51,52
Clinicians had varied interest, encountered barriers, and identified workarounds when integrating PGHD into clinical encounters.
Across publications, clinicians had differing views about their roles regarding the collaborative use of PGHD during their clinical encounters with their patients. In 11 publications, clinicians would review PGHD and discuss the data with their pat ients.44–50,52–55 Alternately, in eight publications, PGHD was identified by clinicians as an important educational tool to improve patient self-awareness about their health conditions. This self-awareness in turn could promote patient self-care and support goal attainment, potentially without clinician involvement.44–46,48,49,52,53,55 In three publications, physicians had varied levels of interest in using PGHD,45 with some delegating PGHD review to other clinicians50,55 and others questioning whether additional health benefits would result from the clinician review of PGHD.55
Clinicians also encountered barriers when integrating PGHD into clinical encounters. In five publications, clinicians reported PGHD review barriers such as clinic appointment time constraints, a lack of formal workflow integration policies, information overload, or an absence of reimbursement incentives.45,47,52,53,55 Clinicians varied in their confidence in their ability to effectively interpret PGHD during clinical encounters, which negatively affected care planning and the suggestions they provided to their patients.45 Some clinicians noted that reviewing PGHD within an online portal reduced the amount of time they had to interact with patients in the clinic.52 In addition, when clinicians direct a patient to record PGHD, either a lack of reimbursement incentives or poor integration of PGHD into clinical workflow could result in clinicians being reluctant to engage with the collected data, which can send patients mixed messages about the value of collecting or reviewing the data.55
Clinicians employed or identified different methods to overcome PGHD review barriers and facilitate the use of PGHD during clinical encounters. In two publications, clinicians reviewed brief summaries of PGHD prior to meeting with patients, which facilitated PGHD clinical integration.45,54 Clinicians in three publications asked patients to verbally summarize their PGHD to reduce the effort required to interpret PGHD during clinic appointments.46,53,55 In six publications, clinicians identified certain types of patients who benefited from PGHD more than others, which allowed them to recommend PGHD collection to specific subsets of patients, potentially decreasing the overall burden of assisting patients with data interpretation.44,47–49,52,54 Examples of such patients are those who are starting new treatments, who travel frequently, or who have severe/chronic conditions.
Theme 3: PGHD platform design choices both supported and hindered collaboration
Trends, summary measures, and education supported PGHD clinical integration and use.
In eight publications, clinicians and patients used trends and summary measures depicted in graphs or charts to help make sense of PGHD.45,47–49,51–54 Two publications described designing the trends and summary measures to be quickly interpreted by clinicians prior to seeing patients in the clinic.45,54 Clinicians and patients in four publications received in-person training on how to use the PGHD platforms44,47,48,52 and, in one of those publications, patients were financially incentivized to complete an online tutorial.47 PGHD platforms in two publications incorporated patient-focused educational materials about health conditions or data.47,52 Patients and clinicians in four publications expressed a desire for an automated PGHD analysis, the ability to interact with PGHD to highlight areas of interest, or additional data incorporated into trends and summary measure tools to facilitate data review.44–46,52
Some PGHD platforms negatively impacted patient–clinician collaboration.
Three publications identified specific PGHD tracking tools or PGHD collection methods that hindered patient–clinician collaboration.46,52,55 The clinicians in one publication expressed concerns that using a diabetes PGHD platform could result in time-consuming, redundant work and decrease the time they had to spend with patients.52 Another publication identified that PGHD platforms may lack enough flexibility to meet patient–clinician needs, standardized data presentation to make the data useful for patients and clinicians, and mechanisms for patients to easily share data with clinicians.55 In four publications, some patients and clinicians perceived that use of PGHD platforms would result in reduced face-to-face interaction and a negative impact on patient–clinician relationships.43,44,48,50 In particular, some patients expressed a preference for in-person communication.43 In addition, clinicians thought that not all of their patients-specific health conditions would benefit from collecting PGHD;44 preferred interacting with patients not computers;44 identified they had to trust the authenticity of patient data;48 desired to engage with patients during clinic appointments in addition to remotely monitoring PGHD;48 feared contributing to the social exclusion of patients by not directly interacting with their patients;50 and perceived it would be challenging to accurately diagnose patient conditions without subjective information such as patient appearance.50
Discussion
In this systematic review, we synthesized the existing literature to identify common themes concerning the effects of PGHD and PROs on patient–clinician relationships within surgery and primary care. We identified that PGHD and PROs facilitated patient–clinician communication, and these data provided additional context which improved clinician awareness of their patient’s health states in between clinical encounters. In addition, we found patients desired for their clinicians to be involved with their PGHD during clinic visits, which clinicians had difficulty accommodating. Finally, specific PGHD platform features either supported or hindered PGHD collaboration between patients and clinicians.
This research provides a novel synthesis of current research regarding how PGHD and PROs affect patient–clinician relationships. Several of the included publications described situations where patients who collected PGHD desired for their clinicians to assist with analyzing their PGHD and provide insights as to how the patients could improve their health based on the data. These same publications described instances in which clinicians had difficulty accommodating these requests alongside their existing work obligations and practices. This finding is congruent with previous research we conducted12 and shows potential implications for patient–clinician relationships. Unmet patient expectations during clinical encounters, such as unsuccessful efforts to request medical information from clinicians, can negatively affect patient satisfaction, treatment adherence, symptom improvement, and relationships with clinicians.58,59 Our synthesis describes how the unmet needs of patients collecting PGHD when collaborating with their physicians can contribute to relationship tensions for both parties. In addition, this review characterizes how PGHD can contribute to enhanced communication and shared understanding between patients and clinicians. In general, medical information is focused on population-level knowledge and not on individual patients and their priorities,60 which could result in differing patient and clinician health perspectives.61,62 In addition, enhanced patient–clinician communication has been associated with improved health outcomes and patient satisfaction.63–66 PGHD has the potential to support shared understanding between patients and clinicians, which could increase satisfaction,67 promote patient participation during clinical encounters,68 patient trust of their clinicians,69 and treatment adherence.70 Finally, this review is congruent with the few studies that exist that have identified the existence of workflow barriers when integrating PGHD into clinical encounters5,12,13 and identifies specific opportunities for clinicians and healthcare organizations to promote the use and integration of PGHD into clinical encounters.
The findings of this review establish guidance for mitigating negative impacts on patient–clinician relationships when integrating PGHD into clinical encounters.
Establishing goals and setting expectations when using PGHD
We recommend clinicians work to communicate expectations for how patients and providers will each use PGHD to support care. In our review, multiple publications suggested that explicit conversations concerning the goals of both parties for collecting and using PGHD may be needed before initiating data collection. Two included publications52,53 developed a clinician communication algorithm to indicate when a clinician should reach out to patients. Patients were taught about this algorithm to set expectations concerning patient–clinician PGHD communication. Another publication55 reports that failing to set expectations may result in mixed messages from physicians to patients as to the purpose and role of PGHD in their relationships. Setting expectations can help both parties understand why and how PGHD will be used to address patient health concerns.
In addition to setting expectations, we recommend patients and clinicians engage in an ongoing, collaborative process incorporating input and agreement from both parties to achieve the full potential of PGHD. Jahng and colleagues demonstrated that when patients and physicians have congruent beliefs about how involved a patient is in their health decision making, patients have better outcomes and higher levels of satisfaction.71 Patient–physician disagreement about how involved patients are in their own care may result in lower rates of satisfaction.72 Furthermore, research has demonstrated patient–clinician collaboration for expectations setting is desired by both groups and feasible.73,74 Our review of the literature complements this research by demonstrating patients and clinicians may have differing viewpoints or preferences as to how PGHD could be collaboratively used to address health concerns. This was exemplified by one publication which identified patient–clinician PGHD collaboration ceased due to a mismatch of each party being unable to agree which tool works best to meet their needs.46
We recommend clinicians openly share their rationale for encouraging patients to record these data and the level of involvement patients can expect from their healthcare team. Multiple publications report that patients and clinicians often do not explicitly discuss their roles and expectations when using PGHD to address a health concern. During these conversations, clinicians could ask questions to ascertain the patient’s preferred role in healthcare according to the Match Model.75 Patients are categorized as members of one of four categories depending on their health literacy and desired level of involvement in their health. Patient–clinician conversations may need to be revisited during subsequent clinical encounters and conversations because patients may shift to different Match Model quadrants over time75 or develop higher levels of autonomy when using PGHD. In addition, this process may result in contextualized and personalized patient plans of action, which can directly affect a patient’s adherence.76–80 This practice may help clinicians adapt their level of collaboration to be congruent with the patient’s needs and preferences.
Integrating PGHD into clinical encounters
Patients who desire or need assistance with data review may encounter challenges or limitations when engaging with their healthcare team. An example was patients may desire greater involvement with their physicians to make sense of their data,45,46,49,52 which clinicians may have difficulty accommodating.
Publications in our review identified two strategies to improve patient–clinician PGHD collaboration. Two of the included publications45,54 gave clinicians access to PGHD in the form of summarized reports that could be interpreted quickly immediately prior to patient encounters, which worked well for existing clinician workflows. In one study,45 clinicians reported sufficient time for data review as a result of the summaries. Another strategy to improve PGHD collaboration, identified in three included publications,46,53,55 involved clinicians asking patients to verbally summarize their PGHD during clinic visits. We recommend PGHD platform designers consider developing visualizations of trends and summary measures using PGHD-focused applications such as Apple Health Kit,81 Google Fit,82 or Samsung Health.83 These applications also have the potential to support electronic health record integration, which could facilitate clinician access to the data using their preferred platform. If clinicians or patients opt to use a PGHD platform that does not incorporate trends or summary measures, we recommend clinicians ask patients to verbally summarize their data during clinical encounters or request patients to prepare questions about their PGHD prior to clinic appointments. Incorporating these three strategies into PGHD platforms may improve PGHD clinical integration and collaboration during patient clinic visits.
Augmenting the data review process with automated data analysis may reduce the burden on clinicians to perform data review tasks on behalf of patients. For example, the web-based PGHD aggregation platform exist.io supports the exploration of correlations between patient self-tracking attributes and behaviors.84 Users can integrate multiple data sources, such as Apple Health Kit, activity trackers, email, calendar, social media, and weather. Exist.io analyzes how the data are interconnected and associated with health outcomes, such as weight gain. Alternatively, trained clinicians could assist with annotating PGHD, which could subsequently be analyzed to identify correlations between the data and the patient’s health condition.85 These tools in turn may reduce the need for clinicians to assist with patient PGHD review and could also be used to automatically identify patterns in PGHD to aid clinician interpretation. Other recent work, however, cautions against overreliance on quantification and automation, as qualitative, contextual data are often valuable information for action planning and empathy.86
Opportunities to improve PGHD data collaboration
In this review, we identified how PGHD platform components may support or hinder patient–clinician PGHD collaboration. One strategy to overcome the barriers of specific PGHD platforms would be to create resources, such as physical spaces or websites, to help identify PGHD platforms that better meet the needs of both patients and clinicians. For example, the Ochsner Health System has created dedicated physical spaces in their hospitals where patients can learn more about the various clinician-preferred mHealth apps and devices to address their health concerns.87
Another strategy to address PGHD platform barriers could be to conduct future design work using participatory design methods. Participatory design incorporates all of the stakeholders (e.g. clinicians and patients) in the design process88 and has been previously used to design a clinician focused PGHD dashboard for use during clinic visits.89 Involving patients and clinicians in the design process has the potential to create PGHD platforms that better meet the needs and preferences of both groups.
Recommendations for future research
While all of the publications included for final synthesis in this review described or documented the effect of PGHD and PROs on patient–clinician relationships, only one publication explicitly had an objective to study how these data affect patient–clinician relationships.46 Additional research is needed to explicitly identify how these data and technological platforms can positively or negatively impact the relationships between patients and their healthcare team. For example, future research should consider how PGHD has an impact on patient–clinician communication, patient trust of clinicians, satisfaction, and treatment adherence. In addition, the majority of the included publications had a clinician perspective bias, for example, one publication reported changes to patient interactions based only on data from nurses and community support workers.50 Researchers will need to include patient perspectives when assessing these technologies to reduce the potential for informatics generated inequalities.90 Finally, as only one included publication pertained to the surgical domain, we recommend additional research to identify how PGHD and PROs could support surgery patient relationships with their clinicians.
Limitations
Our review results primarily focused on PGHD collected for conditions treated by primary care clinicians, and so we do not know the extent to which our findings transfer to other specialties or care domains. Despite this limitation, the patient and clinician participants included for final synthesis in this review represented a wide range of illnesses, diseases, and clinical roles. This provided a rich dataset for final synthesis.
In addition, the use of a wide range of search terms in the research database queries reflects the lack of a unified language around PGHD and PROs within the literature. While the authors collaborated with a health sciences librarian to develop effective search strategies and queries; it is possible that publications fitting the inclusion criteria were not captured in our queries.
Finally, only one member of the research team assessed the included publications’ quality. However, none of the included publications were excluded from the qualitative synthesis based on quality.
Conclusion
Using PGHD and PROs during clinical encounters may promote patients taking a more active role in their healthcare, improve patient–clinician communication, and support clinician work activities. However, patients and clinicians may disagree about how these data should be used to collaboratively address health concerns, which could be affected by how the PGHD platforms are designed. Future research needs to be conducted to measurably assess how PGHD and PROs affect patient–clinician relationships and identify opportunities to improve collaboration using these data.
Supplementary Material
Acknowledgements
We thank and acknowledge the University of Washington Biomedical Health Informatics Librarian Diana Louden for her assistance in developing the search strategies and queries used in this systematic review.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Institutes of Health, National Library of Medicine (NLM) Biomedical and Health Informatics Training Program at the University of Washington (Grant No. T15LM007442). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
Contributor Information
Ross J Lordon, University of Washington School of Medicine, USA.
Sean P Mikles, University of Washington School of Medicine, USA.
Laura Kneale, University of Washington School of Medicine, USA; University of Washington–Bothell, USA.
Heather L Evans, Medical University of South Carolina, USA.
Sean A Munson, University of Washington College of Engineering, USA.
Uba Backonja, University of Washington School of Medicine, USA; University of Washington–Tacoma, USA.
William B Lober, University of Washington School of Medicine, USA; University of Washington, USA.
References
- 1.Pew Research Center. Inquiries D—20036 U—419—4300—M—419—4349—F—419—4372—M. Demographics of Mobile Device Ownership and Adoption in the United States, http://www.pewinternet.org/fact-sheet/mobile/, (2018, accessed 20 September 2018). [Google Scholar]
- 2.Research2Guidance. mHealth App Economics 2017/2018 Current Status and Future Trends in Mobile Health. Berlin, 2017. November, https://research2guidance.com/wp-content/uploads/2017/11/R2G-mHealth-Developer-Economics-2017-Status-And-Trends.pdf [Google Scholar]
- 3.Rideout V and Fox S. Digital health practices, social media use, and mental well-being among teens and young adults in the U.S. Hopelab and Well Being Trust, 2018. Summer, https://digitalcommons.psjhealth.org/cgi/viewcontent.cgi?article=2092&context=publications [Google Scholar]
- 4.Krebs P and Duncan DT. Health app use among US mobile phone owners: a national survey. JMIR Mhealth Uhealth 2015; 3(4): e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro M, Johnston D, Wald J, et al. Patient-generated health data. Research Triangle Park, NC: RTI International, 2012, https://www.rti.org/sites/default/files/resources/patientgeneratedhealthdata.pdf [Google Scholar]
- 6.Howie L, Hirsch B, Locklear T, et al. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood) 2014; 33(7): 1220–1228. [DOI] [PubMed] [Google Scholar]
- 7.Cochrane Patient Reported Outcomes Methods Group, https://methods.cochrane.org/pro/ (accessed 19 August 2019).
- 8.Cortez A, Hsii P, Mitchell E, et al. Conceptualizing a data infrastructure for the capture, use, and sharing of patient-generated health data in care delivery and research through 2024. Accenture, January 2018, https://www.healthit.gov/sites/default/files/onc_pghd_final_white_paper.pdf [Google Scholar]
- 9.Deshpande PR, Rajan S, Sudeepthi BL, et al. Patient-reported outcomes: a new era in clinical research.Perspect Clin Res 2011; 2(4): 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coons SJ, Eremenco S, Lundy JJ, et al. Capturing patient-reported outcome (PRO) data electronically: the past, present, and promise of ePRO measurement in clinical trials. Patient Patient Cent Outcome Res 2014; 8: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox S and Duggan M. Tracking for Health. Pew Research Center, January 2013, http://www.pewinter-net.org/2013/01/28/tracking-for-health/ [Google Scholar]
- 12.Sanger P, Hartzler A, Armstrong C, et al. A patient-centered system in a provider-centered world: challenges of incorporating post-discharge wound data into practice. J Am Med Inform Assoc 2016; 23(3): 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones JB, Snyder CF and Wu AW. Issues in the design of Internet-based systems for collecting patient-reported outcomes. Qual Life Res 2007; 16(8): 1407–1417. [DOI] [PubMed] [Google Scholar]
- 14.West P, Kleek MV, Giordano R, et al. Information quality challenges of patient-generated data in clinical practice. Front Public Health 2017; 5: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwod D, Gee PM, Fatkin K, et al. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabet Sci Tech 2017; 11(5): 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch E, Rogak L and Dueck A. Methods for implementing and reporting patient-reported outcome (PRO) measures of symptomatic adverse events in cancer clinical trials. Clin Ther 2016; 38(4): 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcnair A, Macefield R, Blencowe N, et al. “Trial Exegesis”: methods for synthesizing clinical and patient reported outcome (PRO) data in trials to inform clinical practice. A Systematic Review. PLoS One 2016; 11(8): e0160998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercieca-Bebber R, Palmer M, Brundage M, et al. Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: a systematic review. BMJ Open 2016; 6(6): e010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antunes B, Harding R, Higginson IJ, et al. Implementing patient-reported outcome measures in palliative care clinical practice: a systematic review of facilitators and barriers. Palliat Med 2014; 28(2): 158–175. [DOI] [PubMed] [Google Scholar]
- 20.Younossi Z and Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C—the impact of liver disease and new treatment regimens. Aliment Pharmacol Therapuetics 2015; 41(6): 497–520. [DOI] [PubMed] [Google Scholar]
- 21.Johnston D, Sung L, Stark D, et al. A systematic review of patient-reported outcome measures of neuropathy in children, adolescents and young adults. Support Care Cancer 2016; 24(9): 3723–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosa ASM, Yoo I and Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inf Decis Mak 2012; 12(1): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnhold M, Quade M and Kirch W. Mobile applications for diabetics: a systematic review and expertbased usability evaluation considering the special requirements of diabetes patients age 50 years or older. J Med Internet Res 2014; 16(4): e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateo GF, Granado-Font E, Ferré-Grau C, et al. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta-analysis. J Med Internet Res 2015; 17(11): e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKay FH, Cheng C, Wright A, et al. Evaluating mobile phone applications for health behaviour change: a systematic review. J Telemed Telecare 2018; 24(1): 22–30. [DOI] [PubMed] [Google Scholar]
- 26.Matthews J, Win KT, Oinas-Kukkonen H, et al. Persuasive technology in mobile applications promoting physical activity: a systematic review. J Med Syst 2016; 40(3): 72. [DOI] [PubMed] [Google Scholar]
- 27.Chiarini G, Ray P, Akter S, et al. mHealth technologies for chronic diseases and elders: a systematic review. IEEE J Select Areas Commun 2013; 31(9): 6–18. [Google Scholar]
- 28.Reynolds R, Dennis S, Hasan I, et al. A systematic review of chronic disease management interventions in primary care. BMC Fam Pract 2018; 19(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham HH, O’Malley A, Bach PB, et al. Primary care physicians’ links to other physicians through Medicare patients: The scope of care coordination. Ann Int Med 2009; 150(4): 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lella A. U.S. smartphone penetration surpassed 80 percent in 2016. comScore, Inc., http://www.comscore.com/Insights/Blog/US-Smartphone-Penetration-Surpassed-80-Percent-in-2016 [Google Scholar]
- 31.111th Congress. Health Information Technology for Economic and Clinical Health (HITECH) Act— Title XIII of Division A and Title IV of Division B of the American Recovery and Reinvestment Act of 2009 (ARRA). United States Government, 2009, https://www.govinfo.gov/content/pkg/PLAW-111publ5/pdf/PLAW-111publ5.pdf [Google Scholar]
- 32.Medicare Promoting Interoperability Program Stage 3. Eligible Hospitals, Critical Access Hospitals, and Dual-Eligible Hospitals Attesting to CMS. Objectives and Measures for 2018. Objective 4 of 6. Updated: July 2018, https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/MedicareEHStage3_Obj4.pdf [Google Scholar]
- 33.Covidence better systematic review management, https://www.covidence.org/home
- 34.Stata Software for Statistics Data Science, https://www.stata.com/
- 35.Dedoose, https://www.dedoose.com/
- 36.Tong A, Flemming K, McInnes E, et al. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Method 2012; 12(1): 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas J and Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Method 2008; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridd M, Shaw A, Lewis G, et al. The patient–doctor relationship: a synthesis of the qualitative literature on patients’ perspectives. Br J Gen Pract 2009; 59(561): e116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fereday J and Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Method 2006; 5(1): 80–92. [Google Scholar]
- 40.Holden RJ, Carayon P, Gurses AP, et al. SEIPS 2.0: a human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics 2013; 56(11): 1669–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pluye P, Robert E, Cargo M, et al. Proposal: a mixed methods appraisal tool for systematic mixed studies reviews, 2011, http://mixedmethodsappraisaltoolpublic.pbworks.com. [Google Scholar]
- 42.Pace R, Pluye P, Bartlett G, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud 2012; 49(1): 47–53. [DOI] [PubMed] [Google Scholar]
- 43.Abelson JS, Kaufman E, Symer M, et al. Barriers and benefits to using mobile health technology after operation: a qualitative study. Surgery 2017; 162(3): 605–611. [DOI] [PubMed] [Google Scholar]
- 44.Grönvall E and Verdezoto N. Beyond self-monitoring: understanding non-functional aspects of home-based healthcare technology. In: Proceedings of the 2013 ACM international joint conference on Pervasive and ubiquitous computing—UbiComp’13. Zurich, Switzerland: ACM Press, 2013. p. 587, http://dl.acm.org/citation.cfm?doid=2493432.2493495 [Google Scholar]
- 45.Kim Y, Ji S, Lee H, et al. “My Doctor is Keeping an Eye on Me!”: exploring the clinical applicability of a mobile food logger. In: Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems (CHI’16). New York: ACM, 2016, pp. 5620–5631, 10.1145/2858036.2858145 (accessed 20 March 2018). [DOI] [Google Scholar]
- 46.Chung C-F, Dew K, Cole AM, et al. Boundary negotiating artifacts in personal informatics: patient-provider collaboration with patient-generated data. In: Proceedings of the 19th ACM conference on computer-supported cooperative work & social computing—CSCW’16. San Francisco, CA: ACM Press, 2016. pp. 768–84, http://dl.acm.org/citation.cfm?doid=2818048.2819926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiecha JM, Adams WG, Rybin D, et al. Evaluation of a web-based asthma self-management system: a randomised controlled pilot trial. BMC Pulmon Med 2015; 15: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halkoaho A, Kavilo M and Pietilä A-M. Information technology supporting diabetes self-care: a pilot study. Eur Diabet Nurs 2007; 4(1): 14–17. [Google Scholar]
- 49.Lehocki F, Kossaczky I, Homola M, et al. Yet another hypertension telehealth solution? The rules will tell you. In: 2014 IEEE conference on biomedical engineering and sciences (IECBES). Kuala Lumpur, Malaysia: IEEE, 2014, pp. 510–515, http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=7047554 [Google Scholar]
- 50.Sharma U and Clarke M. Nurses’ and community support workers’ experience of telehealth: a longitudinal case study. BMC Health Serv Res 2014; 14: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miele F, Eccher C and Piras EM. Using a mobile app to manage type 1 diabetes: the case of TreC diabetes. In: Proceedings of the 5th international conference on digital health 2015—DH’15. Florence: ACM Press, 2015, pp. 113–4, http://dl.acm.org/citation.cfm?doid=2750511.2750541 [Google Scholar]
- 52.Urowitz S, Wiljer D, Dupak K, et al. Improving diabetes management with a patient portal: a qualitative study of diabetes self-management portal. J Med Int Res 2012; 14(6): e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen DJ, Keller SR, Hayes GR, et al. Integrating patient-generated health data into clinical care settings or clinical decision-making: lessons learned from project HealthDesign. JMIR Hum Factors. 2016; 3(2): e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nundy S, Lu Hogan P, Mishra A, et al. Using patient-generated health data from mobile technologies for diabetes self-management support. J Diabet Sci Tech 2014; 8(1): 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung C-F, Cook J, Bales E, et al. More than telemonitoring: health provider use and nonuse of life-log data in irritable bowel syndrome and weight management. J Med Internet Res 2015; 17(8): e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 2009; 16(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landis JR and Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33(1): 159–174. [PubMed] [Google Scholar]
- 58.Bell RA, Kravitz RL, Thom D, et al. Unmet expectations for care and the patient-physician relationship.J Gen Intern Med 2002; 17(11): 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Like R and Zyzanski SJ. Patient satisfaction with the clinical encounter: Social psychological determinants. Soc Sci Med 1987; 24(4): 351–357. [DOI] [PubMed] [Google Scholar]
- 60.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making 2007; 27(5): 696–713. [DOI] [PubMed] [Google Scholar]
- 61.Klienman A. Patients and Healers in the Context of Culture. (Comparative Studies of Health Systems and Medical Care; ), https://www.ucpress.edu/book/9780520045118/patients-and-healers-in-the-context-of-culture (accessed 23 August 2019). [Google Scholar]
- 62.Makoul G, Clayman ML, Lynch EB, et al. Four concepts of health in America: results of national surveys. J Health Commun 2009; 14(1): 3–14. [DOI] [PubMed] [Google Scholar]
- 63.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995; 52(9): 1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan SH, Greenfield S and Ware JE. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care 1989; 27(3 Suppl): S110–S127. [DOI] [PubMed] [Google Scholar]
- 65.Jackson JL. Communication about symptoms in primary care: impact on patient outcomes. J Altern Complement Med 2005; 11 (Suppl 1): S51–S56. [DOI] [PubMed] [Google Scholar]
- 66.Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract 2000; 9(9): 796–804. [PubMed] [Google Scholar]
- 67.Davidson R and Mills ME. Cancer patients’ satisfaction with communication, information and quality of care in a UK region. Eur J Cancer Care (Engl) 2005; 14(1): 83–90. [DOI] [PubMed] [Google Scholar]
- 68.Charles C, Gafni A and Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999; 49(5): 651–661. [DOI] [PubMed] [Google Scholar]
- 69.Gordon HS, Street RL, Sharf BF, et al. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J Clin Oncol 2006; 24(6): 904–909. [DOI] [PubMed] [Google Scholar]
- 70.DiMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: results from the Medical Outcomes Study. Health Psychol 1993; 12(2): 93–102. [DOI] [PubMed] [Google Scholar]
- 71.Jahng KH, Martin LR, Golin CE, et al. Preferences for medical collaboration: patient–physician congruence and patient outcomes. Patient Educ Couns 2005; 57(3): 308–314. [DOI] [PubMed] [Google Scholar]
- 72.Krupat E, Rosenkranz SL, Yeager CM, et al. The practice orientations of physicians and patients: the effect of doctor–patient congruence on satisfaction. Patient Educ Couns 2000; 39(1): 49–59. [DOI] [PubMed] [Google Scholar]
- 73.American Medical Informatics Association. Redefining our Picture of Health: Towards a Person-Centered Integrated Care, Research, Wellness, and Community Ecosystem, https://www.amia.org/sites/default/files/API-2017-White-Paper-Redefining-our-Picture-of-Health.pdf
- 74.Cronin RM, Conway D, Condon D, et al. Patient and healthcare provider views on a patient-reported outcomes portal. J Am Med Inform Assoc 2018; 25(11): 1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters RM. Matching physician practice style to patient informational issues and decision-making preferences. An approach to patient autonomy and medical paternalism issues in clinical practice. Arch Fam Med 1994; 3(9): 760–763; discussion 764. [DOI] [PubMed] [Google Scholar]
- 76.Gomez G and Aillach E. Ways to improve the patient-physician relationship. Curr Opin Psychiatry 2013; 26(5): 453–457. [DOI] [PubMed] [Google Scholar]
- 77.Tinsel I, Buchholz A, Vach W, et al. Implementation of shared decision making by physician training to optimise hypertension treatment. Study Protocol of a Cluster-RCT. BMC Cardiovasc Disord 2012; 12: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Langenberg DR and Andrews JM. Satisfaction with patient-doctor relationships in inflammatory bowel diseases: examining patient-initiated change of specialist. World J Gastroenterol 2012; 18(18): 2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCabe R, Bullenkamp J, Hansson L, et al. The therapeutic relationship and adherence to antipsychotic medication in schizophrenia. Plos One 2012; 7(4): e36080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schoenthaler AM, Schwartz BS, Wood C, et al. Patient and physician factors associated with adherence to diabetes medications. Diabetes Educ 2012; 38(3): 397–408. [DOI] [PubMed] [Google Scholar]
- 81.HealthKit—Apple Developer, https://developer.apple.com/healthkit/ (accessed 23 August 2019).
- 82.Google Fit, https://www.google.com/intl/en_us/fit/ (accessed 25 August 2019).
- 83.Samsung Health. Samsung Electronics America, https://www.samsung.com/us/support/owners/app/samsung-health/ (accessed 23 August 2019). [Google Scholar]
- 84.Exist, https://exist.io (accessed 23 August 2019).
- 85.Schroeder J, Hoffswell J, Chung C-F, et al. Supporting patient-provider collaboration to identify individual triggers using food and symptom journals. CSCW 2017; 17: 1726–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chung C-F, Wang Q, Schroeder J, et al. Identifying and planning for individualized change: patient-provider collaboration using lightweight food diaries in healthy eating and irritable bowel syndrome. Proc ACM Interact Mob Wearable Ubiquitous Technol 2019; 3(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ochsner Health. The O Bar—Ochsner Health System, 2016, https://www.ochsner.org/io/the-o-bar
- 88.Schuler D and Namioka A. Participatory design: principles and practices. Boca Raton, FL: CRC Press, 1993, 338 pp. [Google Scholar]
- 89.Kim Y, Heo E, Lee H, et al. Prescribing 10,000 steps like aspirin: designing a novel interface for data-driven medical consultations. In: Proceedings of the 2017 CHI conference on human factors in computing systems—CHI’17. Denver, CO: ACM Press, 2017, pp. 5787–5799, Available from: http://dl.acm.org/citation.cfm?doid=3025453.3025570 [Google Scholar]
- 90.Veinot TC, Mitchell H and Ancker JS. Good intentions are not enough: how informatics interventions can worsen inequality. J Am Med Inform Assoc 2018; 25(8): 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.