Abstract
Objective:
To characterize and compare the neuropsychological profiles of patients with primary progressive apraxia of speech (PPAOS) and apraxia of speech with progressive agrammatic aphasia (AOS-PAA).
Method:
Thirty-nine patients with PPAOS and 49 patients with AOS-PAA underwent formal neurological, speech, language, and neuropsychological evaluations. Cognitive domains assessed included immediate and delayed episodic memory (Wechsler Memory Scale-Third edition; Logical Memory; Visual Reproduction; Rey Auditory Verbal Learning Test), processing speed (Trail Making Test A), executive functioning (Trail Making Test B; Delis-Kaplan Executive Functioning Scale – Sorting), and visuospatial ability (Rey-Osterrieth Complex Figure copy).
Results:
The PPAOS patients were cognitively average or higher in the domains of immediate and delayed episodic memory, processing speed, executive functioning, and visuospatial ability. Patients with AOS-PAA performed more poorly on tests of immediate and delayed episodic memory and executive functioning compared to those with PPAOS. For every 1 unit increase in aphasia severity (e.g. mild to moderate), performance declined by 1/3 to 1/2 a standard deviation depending on cognitive domain. The degree of decline was stronger within the more verbally mediated domains, but was also notable in less verbally mediated domains.
Conclusion:
The study provides neuropsychological evidence further supporting the distinction of PPAOS from primary progressive aphasia and should be used to inform future diagnostic criteria. More immediately, it informs prognostication and treatment planning.
Keywords: Primary progressive apraxia of speech, Primary progressive aphasia, Agrammatic aphasia, Frontotemporal dementia, Motor speech disorder, Nonfluent primary progressive aphasia
INTRODUCTION
In 1967, Darley described a motor speech disorder characterized by varying combinations of slow speaking rate, syllable segmentation, abnormal prosody, distorted sound substitutions, additions, and prolongations, sometimes accompanied by groping and trial and error articulatory movements (Darley, 1967, 1969). This disorder was termed apraxia of speech (AOS). It reflects abnormal planning and/or programming of speech production and is distinct from aphasia (a disorder of language) and dysarthria (disorders of neuromuscular execution). In adults, AOS is well known as an acquired, focal impairment in the context of stroke with a course that eventually improves or becomes chronic and stable. AOS that has an insidious onset and progresses over time because of neurodegeneration is the subject of more recent investigation (Duffy, 2006; Josephs et al., 2005; Josephs et al., 2012).
In the context of neurodegenerative disease, AOS can be embedded within a broader dysfunction of cognition, language, or motor symptoms (Duffy, 2006). It is part of the most recent criteria for progressive supranuclear palsy (PSP) (Hoglinger et al., 2017) and corticobasal syndrome (CBS) (Armstrong et al., 2013). It is also a core criterion for the nonfluent/agrammatic variant of primary progressive aphasia (referred to in the literature as nfvPPA, nfPPA, PPA-G, or agPPA) and can present with or without accompanying aphasia (Gomo-Tempini et al., 2011; Ogar, Dronkers, Brambati, Miller, & Gomo-Tempini, 2007). The Gorno-Tempini et al. (2011) criteria specify that either aphasia (in the form of agrammatism) or AOS is sufficient for a diagnosis of nfvPPA (in addition to other diagnostic criteria). However, as many in the scientific community acknowledge, motor speech disorders (i.e., AOS) should not be considered PPA (Duffy & Josephs, 2012; Josephs et al., 2012; Mesulam, 2001, 2003). Our group demonstrated that in about 20% of nfvPPA cases, AOS was the primary and sometimes only deficit with little to no evidence of aphasia (Duffy, Strand, & Josephs, 2014). To recognize this and help determine whether the distinction is necessary, the term primary progressive apraxia of speech (PPAOS) is used when progressive AOS is the primary and only deficit (Duffy, 2006; Josephs et al., 2014; Josephs et al., 2012). When patients meet the language-driven requisite of PPA, the terms apraxia of speech and progressive agrammatic aphasia (AOS-PAA) are used to reflect the combination of both speech and language disorder. When discussing prior literature, we use the term nfvPPA to refer to individuals where no specific distinction about the combination of speech vs. language symptoms is reported.
On imaging, patients with PPAOS show specific involvement of the precentral cortex and supplementary motor area (SMA), with hypometabolism seen on [18F]fluorodeoxyglucose positron emission tomography imaging (Josephs et al., 2012; Josephs et al., 2006), gray matter atrophy on magnetic resonance imaging (MRI) (Josephs et al., 2013), reduced connectivity to speech, language, and face sensorimotor networks on functional MRI (Botha et al., 2018), and white matter tract degeneration on diffusion tensor imaging (Whitwell et al., 2013). Although not necessarily specific to PPAOS, prominent AOS in the context of progressive speech and language disorders (e.g., progressive nonfluent aphasia) has been linked to CBD, PSP, and Pick’s disease, which are all tauopathies (Deramecourt et al., 2010; Josephs et al., 2012; Josephs et al., 2006; Tetzloff, Duffy, et al., 2018). In vivo imaging of tau, with tau-PET, showed uptake bilaterally throughout the premotor and precentral cortices, involving inferior, middle, and superior frontal gyri and the SMA in PPAOS (Utianski, Whitwell, Schwarz, Senjem, et al., 2018). A similar pattern was seen in patients with AOS-PAA; however, when agrammatic aphasia occurred without AOS (herein referred to as progressive agrammatic aphasia [PAA]), uptake was predominantly in left frontal regions as well as temporal regions (Utianski, Whitwell, Schwarz, Duffy, et al., 2018). These and other studies (Tetzloff et al., 2019) provide support showing that AOS and agrammatic aphasia, when present in isolation, have separable neuroanatomical substrates. Exploring the neuropsychological profiles of patients with AOS in isolation (PPAOS) compared to AOS with agrammatic aphasia could contribute to an understanding of these syndromes as possibly representing separate entities.
Neurocognitively, preliminary work suggests that cognitive functioning in PPAOS is mostly intact, while patients with both AOS and aphasia appear more cognitively compromised. Josephs et al. (2014) found that of the 13 PPAOS patients studied, all were within or above normal limits on a measure of executive functioning, only one individual demonstrated mildly lower than normal delayed word list memory, and only two demonstrated mild slowing on a processing speed task. Using a patient sample with some overlap with Josephs et al. (2014), Utianski, Whitwell, Schwarz, Senjem, et al. (2018) also found patients with PPAOS were neurocognitively normal and it was only if they later developed aphasia (i.e., 7/14 patients) that they demonstrated any sign of neurocognitive difficulties. While performance on the Visual Object and Space Perception Letters and Cube subtests (VOSP; n = 14) and Camden word recognition test (n = 11) were within normal limits in this group, five of the seven with aphasia fell below the normal cutoff (scoring below 26/30) on the Montreal Cognitive Assessment (MoCA), a verbally mediated cognitive assessment, and two fell below cutoff (scoring below 12/18) on the Frontal Assessment Battery. These studies had small samples and relatively limited reporting of cognitive measures, necessitating follow-up studies expanding on the generalizability of results. Additionally, as the findings from Utianski, Whitwell, Schwarz, Senjem, et al. (2018) suggest, there may be neurocognitive differences beyond the speech and language domains in PPAOS and AOS patients with aphasia. Indeed, several studies and meta-analyses demonstrate that when aphasia is present with AOS, it is associated with disruption in frontally mediated cognitive functions. Butts et al. (2015) reported that these patients had deficits in the domain of executive functioning. Similarly, in a recent meta-analysis, patients with nfvPPA perform lower on measures of working and episodic memory compared to healthy controls (Eikelboom et al., 2018). Interestingly, these deficits in frontally mediated cognitive functions extend into the visuospatial domain as well. Watson et al. (2018) showed patients with nfvPPA performed worse than healthy controls on measures of visuospatially based episodic memory and executive functioning. Consistent across these studies, however, was that AOS was not recorded, and the contribution of AOS or aphasia severity to the neuropsychological profile was not examined. Group studies that do not sufficiently describe patient characteristics may not accurately reflect the degree of impairment in the group as a whole; that is, impairment may be underreported if all patients have AOS and do not have aphasia (Bettcher & Sturm, 2014; Butts et al., 2015; Harris, Saxon, Jones, Snowden, & Thompson, 2019). As of yet, the potential cognitive differences between patients with PPAOS and patients with AOS and aphasia is unknown. It is also unclear how aphasia severity might be related to degree of cognitive impairment.
Present Study
We aimed to characterize and compare the neuropsychological profiles of patients with PPAOS (i.e. progressive isolated AOS) and those who present with a combination of AOS-PAA (term and classification discussed in more detail below). We also examined whether severity of aphasia, independent of disease duration, was associated with performance in five cognitive domains (immediate and delayed episodic memory, processing speed, executive functioning, and visuospatial functioning). It was hypothesized that (1) PPAOS patients would be grossly cognitively normal in the five cognitive domains, (2) patients with AOS-PAA would have poorer cognition than those with PPAOS, particularly in executive functioning, and (3) increasing aphasia severity would be associated with lower cognitive performance particularly on tests that are highly verbally mediated.
METHOD
Standard Protocol Approvals and Patient Consents
This study was approved by the Mayo Institutional Review Board. All participants provided written informed consent before participating in research. Research was completed in accordance with the Helsinki Declaration.
Participants
Ninety-nine patients were followed as part of a larger study of degenerative disorders of language and speech. Forty of these patients (PPAOS n = 13; AOS-PAA n = 27) were reported on in prior work discussed above (Butts et al., 2015; Utianski, Whitwell, Schwarz, Duffy, et al., 2018; Utianski, Whitwell, Schwarz, Senjem, et al., 2018). Patients were recruited from the Mayo Clinic Department of Neurology by the Neurodegenerative Research Group (NRG) between July 2010 and April 2018. Procedures and measures of this larger study have been previously reported (Duffy et al., 2017; Duffy et al., 2015; Josephs et al., 2013; Josephs et al., 2014; Josephs et al., 2012). Participants underwent a detailed speech and language examination, neurological evaluation, and neuropsychological testing over a span of 48–72 hours. All participants presented with a chief complaint of speech and/or language dysfunction and were included if they were at least 18 years of age, spoke English as their primary language, and had evidence of AOS on exam.
Clinical classification was made via consensus after two experienced speech-language pathologists (J.R.D., H.M.C., and R.L.U.) made independent judgments of the presence, nature, and severity of AOS, aphasia, and dysarthria without any knowledge of the neurological, neuroimaging, or neuropsychological results. Interrater reliability was not assessed, but agreement about presence/absence of aphasia and AOS was reached without the need for discussion in all but a few cases. In the few cases not reaching consensus, a third speech-language pathologist was the tie-breaker.
It is important at this juncture to clarify that both groups technically met diagnostic criteria (Gomo-Tempini et al., 2011) for nfvPPA; however, this is because motor speech is considered an element of language for the root PPA criteria, which is not universally accepted. As our research group considers motor speech to be separable from language, we do not consider patients with PPAOS to have met necessary root criteria for a nonfluent/agrammatic PPA diagnosis. This is why we opted not to use this terminology (i.e., nfvPPA). Given that we are exploring the implications for this distinction, we have used the term “PPAOS” to recognize AOS as the initial and sole problem at onset and “AOS-PAA” to recognize those that meet the language-driven requisite of PPA.
Patients were classified as PPAOS if there was evidence of progressive impairment in speech (i.e., AOS), which was the first noted symptom, and any other neurological deficits, including aphasia, were no more than equivocal (Botha & Josephs, 2019; Josephs et al., 2014; Josephs et al., 2012). Patients were classified as AOS-PAA if there was evidence of AOS and agrammatic aphasia (see below for assessment procedures). Neuropsychological performance was not considered during the adjudication process for diagnostic classification. The presence of dysarthria did not preclude a classification of PPAOS or AOS-PAA; if present, dysarthria must have been less severe than both the AOS and agrammatic aphasia at study enrollment. Eleven patients presented with only agrammatic aphasia (i.e., no AOS). These patients were classified as PAA to distinguish them from patients with both agrammatism and AOS. Unfortunately, due to the smaller and disproportionate sample size (compared to the PPAOS and AOS-PAA groups), we could not include this group as part of our analyses. We do, however, include their data here to allow the reader a qualitative comparison.
Speech & Language Assessment
Formal speech and language assessment of all participants were audio and video recorded. Judgments about motor speech abilities were based on all spoken language tasks of the Western Aphasia Battery-Revised (WAB-R) (Kertesz, 2007) plus additional speech tasks that included vowel prolongation, speech alternating motion rates (e.g., rapid repetition of puhpiih-puh), speech sequential motion rates (e.g., rapid repetition of puh-tuh-kuh), word and sentence repetition tasks, and a conversational speech sample. Speech characteristics were rated on the Apraxia of Speech Rating Scale, (Josephs et al., 2012; Strand, Duffy, Clark, & Josephs, 2014). A subjective index of AOS severity (1–4; 1 =mild, 4 = severe) was also assigned. The same speech tasks were also judged for the presence or absence of dysarthria, again rated on a 0–4 severity scale (0 = normal/no dysarthria, 4 = severe).
Presence or absence of aphasia was determined, and severity of aphasia was quantified with several measures of language abilities. The WAB-R aphasia quotient (WAB-AQ (Kertesz, 2007)) served as a composite measure of global language ability. It includes measures of word and sentence repetition, confrontation naming, spontaneous speech fluency, word finding, grammatical competence, verbal and reading comprehension, and writing. A WAB-AQ score of 93.8 or above is considered normal, consistent with standardized test guidelines. Additional supplementary reading and writing tasks from the WAB were also administered. Grammar (and therefore agrammatism) was assessed by review of conversational speech and oral and written picture description tasks. Examples of agrammatism include telegraphic written and/or spoken language, grammatical simplification, and omission of function words or morphological markers noted on formal (e.g. Northwestern Anagram Test) and/or informal assessment. The influence of grammatical complexity on comprehension was assessed with formal measures (e.g., subtests of the Boston Diagnostic Aphasia Examination). A consensus determination was made as to whether a patient qualified as agrammatic based on the combination of these informal (e.g., conversational speech) and formal (e.g., NAT) measures described above. The 15-item Boston Naming Test (BNT) (Lansing, Ivnik, Cullum, & Randolph, 1999) served as a sensitive measure of confrontation naming ability; a score of 13 or above is considered normal. As some of the WAB-R subtests can be influenced by non-aphasic deficits, including but not limited to AOS, participants were required to perform below normal on at least two measures of language to establish the presence of aphasia. The composite of the aforementioned tests was utilized in the judgment of overall aphasia severity (0–4; 0 = normal/no aphasia; 4 = severe). Of note, severity and nature of agrammatism, specifically, were not quantified in this study’s sample. For quantification of elements of agrammatism in a subset of our larger study participants with agrammatism alone and with additional AOS see Tetzloff, Utianski, et al. (2018). See Table 1 for speech and language scores.
Table 1.
Median (1st and 3rd interquartile range) or n (percent) of patient demographics
| PPAOS (n = 39) | AOS-PAA (n = 49) | PAA (n = 11) | p-value | |
|---|---|---|---|---|
| Age (years) | 71 (62, 78) | 70 (64, 74) | 69 (65, 75) | .4682 |
| Sex (female) | 21 (53.8%) | 23 (46.9%) | 7 (63.6%) | .5201 |
| Education (years) | 16 (14, 18) | 15 (12, 16) | 14 (12, 16) | .0242 |
| Disease duration (years) | 3 (2, 5) | 3 (2, 4) | 2 (1, 2) | .5662 |
| Apraxia of speech severity | 2 (1, 2) | 2 (1, 3) | 0 (0, 0) | .0132 |
| ASRS total score version 3 | 15 (11, 22) | 15 (12, 23) | 2 (1, 4) | .4752 |
| Dysarthria present | 14 (35.9%) | 17 (34.7%) | 0 (0.0%) | .9071 |
| Dysarthria severity | 1 (1, D | 1 (1, 2) | 0 (0, 0) | .7752 |
| Aphasia severity | 0 (0, 0) | 1 (1, 2) | 2 (1, 2) | < .0012 |
| WAB-AQ | 98 (96, 99) | 86 (82, 94) | 89 (81, 90) | < .0012 |
| BNT-short form | 14 (13, 15) | 13 (11, 14) | 13 (10, 14) | < .0012 |
| UPDRS III | 11 (5, 18) | 10 (6, 20) | 4 (2, 10) | .3962 |
| Neuropsychiatric Inventory | 1 (0, 3) | 2 (0, 4) | 2 (2, 5) | .4482 |
| Montreal Cognitive Assessment | 28 (26, 29) | 24 (21, 25) | 23 (22, 24) | < .0012 |
ASRS = Apraxia of Speech Rating Scale; BNT = Boston Naming Test; UPDRS-III = Unified Parkinson’s disease rating scale – part 3; WAB-AQ = Western Aphasia Battery – Aphasia Quotient.
Pearson’s Chi-squared test of PPAOS and AOS-PAA only (excluding PAA), not adjusted for multiple comparisons
Linear Model ANOVA of PPAOS and AOS-PAA only (excluding PAA), not adjusted for multiple comparisons.
Neurologic and Neuropsychological Evaluation
All participants underwent a detailed neurologic examination, including general cognitive testing (MoCA (Nasreddine et al., 2005)) and an index of motor parkinsonism (Movement Disorder Society revised Unified Parkinson’s Disease Rating Scale-III (Goetz et al., 2007)).
The neuropsychological evaluation was performed separately from the comprehensive speech and language evaluation by a trained psychometrist and was supervised by a neuropsychologist (M.M.M.). Cognitive domains assessed included immediate and delayed episodic memory (Wechsler Memory Scale-Third edition [WMS-III] Logical Memory [LM] and Visual Reproduction [VR] (Wechsler, 1997); Rey Auditory Verbal Learning Test [AVLT] (Rey, 1964)), processing speed (Trail Making Test A [TMT A] (Spreen & Strauss, 1998)), executive functioning (Trail Making Test B [TMT B] and Delis-Kaplan Executive Functioning Scale –Sorting subtest [DKEFS Sorting] (Delis, Kaplan, & Kramer, 2001)), and visuospatial ability (Rey-Osterrieth Complex Figure copy [Rey-O] (Osterrieth, 1944; Rey, 1964)). Of note, although patients completed the letter and cube subtests of the VOSP battery (Warrington & James, 1991), these data were not included in the visuospatial composite due to significant skewing and ceiling effect.
Raw scores of the neuropsychological tests were converted to age-adjusted scaled scores (SS) using either the Mayo Older Americans Normative Study (MOANS) in the case of the AVLT (Ivnik et al., 1992), Rey-O (Machulda et al., 2007), and TMT A&B (Ivnik, Malec, Smith, Tangalos, & Petersen, 1996) or published norms in the case of the WMS-III subtests and DKEFS Sorting (scaled score; M = 10; SD = 3). Of note, six patients were unable to complete TMT B in the allotted time and received a default MOANS score of ‘1’. Scaled score descriptives for each test are presented in Table 1. Scaled scores were averaged, with missing scores omitted from the average, to create composite scores for the following cognitive domains: 1) immediate episodic memory (WMS-III LM I and VR I, AVLT 1st learning trial), 2) delayed episodic memory (WMS-III LM II and VR II, AVLT delayed recall), and 3) executive functioning (TMT B, D-KEFS Sorting). Domains 4 (processing speed) and 5 (visuospatial) were based on a single measure and did not require a composite score. The raw scores on the VOSP letter perception subtest were used for additional description of performance, as the Rey-O, while a visuospatial test, also can be impacted by executive dysfunction. Due to changes in the testing protocol over time, individual cognitive tests were missing from some cognitive domain composites: 19 for immediate and delayed episodic memory (7 PPAOS; 12 AOS-PAA), two for processing speed (2 AOS-PAA), one for executive functioning (1 AOS-PAA), and three for visuospatial abilities (3 AOS-PAA).
Statistical Analysis
For hypothesis 1 (patients with PPAOS will be grossly cognitively normal), we examined age-corrected scaled scores for the five cognitive composite domains within the PPAOS group. We used the following identifiers for ranges of scores: SS < 7 = low; SS = 7 – 11 = average; SS > 11 = above average. For hypothesis 2 (patients with AOS-PAA will perform more poorly than patients with PPAOS on cognitive measures), we used penalized logistic regression to predict whether patients were PPAOS or AOS-PAA based on the five cognitive domains detailed above. The outcome of the model was group membership, PPAOS or AOS-PAA. We employed an elasticnet regularization to prevent overfitting and shrink parameter estimates to be more generalizable. We used a penalty that was 80% lasso and 20% ridge and used leave-one-out cross validation to determine lambda that is at most one standard deviation above the optimal solution. The result is a parsimonious solution from what would ordinarily be an overspecified model (more predictors than typically allowed in maximum likelihood estimation). In this way, we could quantify the evidence of the marginal (not adjusted for confounders) discriminative power of these five cognitive domains while avoiding multiple comparisons (and the need to correct for such).
For hypothesis 3 (aphasia severity will be associated with cognitive performance), we fit a single, comprehensive, hierarchical Bayesian linear model to describe whether aphasia severity influences performance across the five cognitive domains. In this model, the cognitive domain composite score was the dependent variable that was predicted by aphasia severity, diagnosis (PPAOS vs. AOS-PAA), age at evaluation, disease duration, and education. We also included coefficients estimated in batches (Gelman & Hill, 2006) for intercept shifts for each domain, batched coefficients for domain aphasia severity interaction terms, and batched intercepts per person. Hierarchical models address the problem of multiple comparisons by creating a single comprehensive model and benefit from partial pooling of variances as well as shrinking coefficients, resulting in more stable and generalizable estimates.
The prior distributions for each of the unbatched terms were assumed to be Student t-distributions centered at zero and a standard deviation of three with four degrees of freedom. The batched coefficients were each assumed to follow independent normal distributions with prior distributions on the mean and standard deviation. The prior distributions for both the mean and standard deviation had a mean of zero and standard deviation of three, with the standard deviation prior distribution being truncated to be strictly positive.
The model was fit using the statistical software R version 3.6.2 using the package rjags version 4–10 running JAGS version 4.3. The posterior estimates of effects were based on four parallel chains of 20,000 samples each, resulting in posterior samples of 80,000 per parameter. These posterior samples were generated after 100 initial adaptation iterations and 10,000 burn in samples that were discarded. After this fitting process, the Gelman Rubin diagnostic was approximately 1, not indicating lack of convergence of any parameters. Parameter estimates are generated by summarizing these posterior samples using medians and quantile intervals.
RESULTS
Clinical Consensus Group Classification
Thirty-nine patients (39%) were classified as PPAOS and 49 patients (49%) were classified as AOS-PAA. The groups did not differ on most demographic variables (e.g., age, sex, disease duration) but did differ in education (PPAOS > AOS-PAA), AOS severity (AOS-PAA > PPAOS), and MoCA score (PPAOS > AOS-PAA). Consistent with classification criteria, the AOS-PAA group had significantly lower scores on the WAB-AQ and BNT than the PPAOS group. See Table 1 for demographic and clinical information of all groups, including PAA (n = 11, 11% of total sample; not included in statistical analyses).
Patients with PPAOS were Generally Cognitively Average to above Average
As seen in Table 2, on average, those classified as PPAOS fell within the average or above average range (SS range = 9 – 12) in all cognitive domains. On the individual level, two patients with PPAOS had low executive functioning (SS = 3 and 6.5), four patients had low immediate episodic memory (SS range = 4 – 6.5), eight patients had low processing speed (SS range = 3–6), and four patients had low visuospatial performance (SS range = 2 – 6). No patients had low scores on the delayed episodic memory composite. For patients who had low Rey-O scores, VOSP letter (raw scores = 10/10), and cube (raw score range = 19–20/20) scores were within normal limits, suggesting the low Rey-O performance may be secondary to planning and organization difficulty.
Table 2.
Median (1st and 3rd quartiles) of patient cognitive test and composite scores and penalized odds ratios from the logistic model
| Cognitive Assessment | PPAOS (n = 39) | AOS-PAA (n = 49) | PAA (n = 11) | p-value | Penalized Odds Ratio |
|---|---|---|---|---|---|
| Immediate Episodic Memory | n = 32 | n = 38 | n = 7 | 1.08* | |
| composite average | 11 (10,14) | 8 (6, 10) | 7 (6, 10) | < .0012 | |
| AVLT 1st trial (MOANS) | 8 (7, 10) | 8 (5, 9) | 7 (6, 8) | .0831 | |
| WMS-III LM I (SS) | 12 (10, 14) | 8 (6, 9) | 5 (3, 7) | < .0011 | |
| WMS-III VR I (SS) | 11 (8, 14) | 8 (6, 10) | 10 (8, 10) | .0061 | |
| Delayed Episodic Memory | n = 32 | n = 38 | n = 7 | 1.05* | |
| composite average | 13 (11,14) | 10 (8, 12) | 7 (7, 8) | < .0012 | |
| AVLT delay (MOANS) | 11 (10, 14) | 9 (8, 11) | 8 (5, 8) | < .0011 | |
| WMS-III LM II (SS) | 13 (10, 15) | 10 (8, 11) | 9 (4, 10) | < .0011 | |
| WMS-III VR II (SS) | 13 (11, 15) | 10 (8, 12) | 8 (6, 9) | .0041 | |
| Processing Speed | n = 39 | n = 48 | n = 10 | ||
| TMT A (MOANS) | 9 (7, 11) | 8 (6, 10) | 6 (5, 10) | .0102 | |
| Executive Functioning | n = 39 | n = 48 | n = 8 | 1.08* | |
| composite average | 10 (8, 12) | 8 (6, 9) | 8 (5, 9) | < .0012 | |
| TMT B (MOANS) | 9 (7, 11) | 7 (5, 9) | 7 (4, 10) | .0021 | |
| D-KEFS Card Sort (SS) | 12 (10, 14) | 8 (6, ID | 7 (6, 8) | < .0011 | |
| Visuospatial | n = 39 | n = 47 | n = 10 | ||
| Rey-Osterrieth (MOANS) | 10 (7, 12) | 9 (6, 11) | 8 (6, 11) | .0061 |
AVLT = Rey Auditory Verbal Learning Test; D-KEFS = Delis-Kaplan Executive Functioning System; LM = Logical Memory; MOANS = Mayo Older American Normative Studies (M = 10; SD = 3); SS = scaled score (M = 10; SD = 3); TMT = Trail Making Test; VR = Visual Reproduction; WMS-III = Wechsler Memory Scale – 3rd edition.
Data in bold are the values included in the penalized logistic regressions model with elastic-net regularization.
Linear Model ANOVA of PPAOS and AOS-PAA only (excluding PAA), not adjusted for multiple comparisons
independent t-test of PPAOS and AOS-PAA only (excluding PAA), not adjusted for multiple comparisons.
Given the group differences in education, we re-ran the model with education as a predictor. Education was dropped from the model and resulted in insignificant changes in odds ratios for Delayed Episodic Memory (1.04) and Executive Functioning (1.06).
Worse Performance on Immediate and Delayed Episodic Memory and Executive Functioning Distinguished AOS-PAA from PPAOS
The penalized logistic regression model demonstrated good classification separation for PPAOS and AOS-PAA with area under the Receiver Operating Characteristic curve (AUC) = 0.83, p < 0.001. Specifically, the immediate and delayed episodic memory and executive functioning domains distinguished between the two groups, with the AOS-PAA group performing worse in these domains than the PPAOS group (see Table 2). Processing speed and visuospatial abilities were dropped by the model; that is, they were less important in predicting group classification. Despite worse performance in the AOS-PAA group, the groups’ median scores fell mostly within the low average range.
Aphasia Severity Predicted Poorer Cognitive Performance Across All Domains
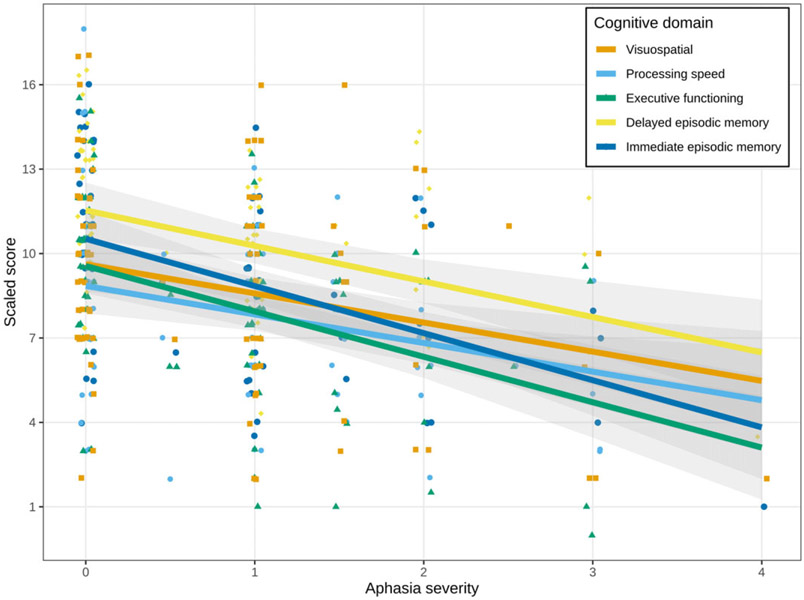
Controlling for age, education, and disease duration, performance in each cognitive domain decreased with increasing aphasia severity (Figure 1). Aphasia severity ranged from 0 (PPAOS) to 4 (ratings of 1–4 correspond to AOS-PAA).
Fig. 1.
Change in performance (scaled scores) with increasing severity of aphasia (aphasia rating scale 0–4; 0 = PPAOS and 1–4 = AOS-PAA) within each cognitive domain: Immediate episodic memory, delayed episodic memory, executive functioning, processing speed, and visuospatial abilities. Dots represent individual scores. Lines represent fitted values in each domain across the range of aphasia severity for a 70-year-old individual with 16 years of education and 3-year disease duration.
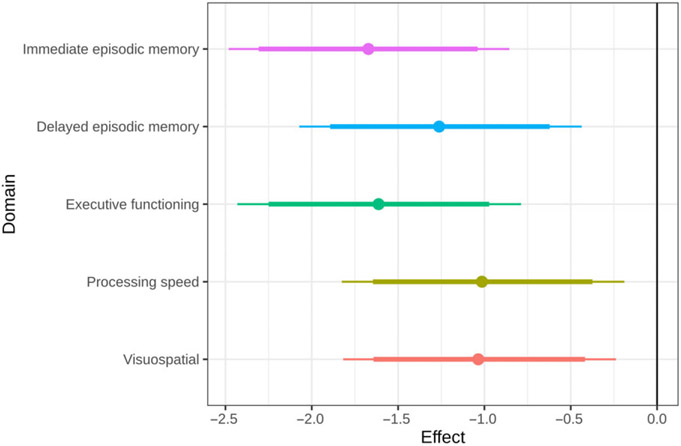
There was strong evidence (probability > 0.95) for an association between aphasia severity and performance on all five cognitive domains (see Figure 2). For every 1 unit increase in aphasia severity, immediate episodic memory declined by 1.64 scaled scores, delayed episodic memory declined by 1.24 scaled scores, executive functioning declined by 1.58 scaled scores, processing speed declined by 1.02 scaled scores, and visuospatial abilities declined by 1.01 scaled scores.
Fig. 2.
Change in scaled score within each cognitive domain for every one-unit change in aphasia from the hierarchical model.
Comparisons between cognitive domains of the strength of the association between aphasia severity and cognitive performance revealed moderate evidence of a difference between immediate episodic memory vs. (a) visuospatial abilities and (b) processing speed (posterior probability > .90). There was also moderate evidence of a difference between executive functioning and (a) visuospatial abilities and (b) processing speed (posterior probability > .90). There was otherwise weak evidence of differences for all other domain comparisons. Of note, the Spearman correlation between aphasia severity and disease duration was r(89) = −.11, p = .44. This low correlation suggests that the association between aphasia severity and cognition is not driven by or confounded by length of disease course.
DISCUSSION
The present study examined the neuropsychological performance of patients with PPAOS and AOS-PAA. Consistent with our hypotheses, cognitive functioning in the PPAOS group fell in at least the average range. Performance was lower in the AOS-PAA group. Immediate and delayed episodic memory and executive functioning were particularly useful for distinguishing between the groups, even though the AOS-PAA groups’ median scores were in the low average range. Additionally, increasing aphasia severity was associated with poorer performance in all cognitive domains, independent of age, education, and disease duration.
The neuropsychological profile of PPAOS revealed that these patients, as a whole, performed at or above average on measures across five domains of cognitive functioning. These data are consistent with the conceptualization of this syndrome as an isolated deficit in motor speech programming (i.e., AOS) and past imaging data that demonstrated limited cortical involvement (i.e., restricted mainly to left lateral superior motor cortex and SMA; Josephs et al., 2012), at least in the early stages of the disease. However, it is possible that our measures were not sensitive enough to capture more subtle cognitive change in this group. For example, previous work demonstrated that scores on verbal fluency measures, such as letter and action fluency, were lower in patients with PPAOS compared to controls and frequently abnormal (Hoglinger et al., 2017; Josephs et al., 2012). Performance on fluency measures might be confounded by motor speech problems, but it is also possible that these tests are more sensitive to subtle changes in executive functioning as well.
Examining individual-level scores in the current study found that if patients with PPAOS had difficulty on any task, it was most likely reduced processing speed (17%, n = 8). In a longitudinal study, Josephs et al. (2014) demonstrated processing speed was the only cognitive domain to decline over an average of 2.4 years in a group of 13 patients with PPAOS. The relative vulnerability of processing speed may reflect the propensity for many of these patients to eventually develop extrapyramidal signs, particularly bradykinesia, axial rigidity, and a masked face, as the underlying disease process progresses. Although none of the patients with PPAOS in our study met criteria for any other neurological disorder at presentation, we speculate that slowing might at least partially reflect greater motor symptomatology (as measured by the UPDRS, patients with low TMT A scores: median UPDRS = 16 [13.5, 25.5]; patients with normal or above TMT A scores: median UPDRS = 8.5 [5, 16.5]), impacting performance on this speeded, motor-mediated task. This is compatible with the motor features of the neurologic syndromes that frequently eventually emerge in these patients, i.e., PSP or CBS.
In contrast to the PPAOS group, the AOS-PAA group performed worse across all cognitive domains, and group differences were particularly pronounced in executive functioning and episodic memory. Group differences in delayed episodic memory appeared largely driven by exceptionally strong performance in the PPAOS group rather than poor performance in the AOS-PAA group. In fact, on average, delayed episodic memory was the AOS-PAA groups’ strongest cognitive domain. In contrast group differences in immediate episodic memory and executive functioning appeared driven more by the AOS-PAA group’s poorer functioning, with 25% of patients falling below the normal (low average or higher) range. Lower performance in these domains suggests greater disruption of frontally mediated systems and processes (i.e., executive functioning and encoding) in patients with AOS-PAA compared to patients with PPAOS. This is perhaps not surprising given that AOS-PAA is associated with extramotor regions and more widespread frontal involvement (Botha et al., 2015; Whitwell et al., 2013). The finding is also consistent with prior work showing that patients with AOS-PAA tend to have low levels of executive functioning and working memory (Bettcher & Sturm, 2014; Butts et al., 2015; Harris et al., 2019). Although we were unable to include the agrammatic-only group (PAA) in the analyses, directly comparing normed scores across groups suggest that they may have even more cognitive difficulty than the group with AOS and agrammatism (i.e., AOS-PAA). Future work with larger sample sizes allowing statistical comparisons could help demonstrate these potentially meaningful differences between groups.
These data – in combination with prior work in imaging, pathology, and clinical syndromes – argue for a recognition of PPAOS as a unique syndrome separable from PAA (Tetzloff et al., 2019; Utianski, Whitwell, Schwarz, Duffy, et al., 2018). While PPAOS and AOS-PAA may share the same underlying neuropathology, how their clinical courses differ continues to be a subject of investigation with mounting evidence that prognosis and trajectories may be separable. Several other studies have already demonstrated differences in the emergence of other speech and neurologic features in these patients as well as differences in survival (Tetzloff et al., 2019; Utianski, Duffy, Clark, Strand, Boland, et al., 2018; Whitwell et al., 2020; Whitwell et al., 2017). For example, Whitwell et al. (2020) found better survival and lower risk of death for individuals with PPAOS than AOS-PAA. In fact, individuals with AOS-PAA were three times more likely to die before an individual with PPAOS. Additionally, Whitwell et al. (2017) showed that within the PPAOS group, there were notable differences among individuals in whether they developed aphasia (i.e., PAA) within a 2-year follow-up. In their study, only 40% of PPAOS patients developed aphasia by 2 years post-diagnosis. Though not specific to PPA or PPAOS, Vöglein et al. (2021) demonstrated the diagnostic and prognostic importance of identifying first symptoms (FS) in neurodegenerative disease. For example, a cognitive FS compared to non-cognitive FS was associated with longer survival in frontotemporal lobar degeneration. This work demonstrates that initial symptoms have value in diagnosis and in predicting the course of disease, even among those with the same underlying pathology.
Clinically, these features also suggest different therapeutic approaches for maintaining functions or compensating for losses. For example, patients with PPAOS have a pure motor speech disorder and can much more easily use complex technology to supplement their speech; they may also benefit from intervention with the principles of motor learning. On the other hand, when aphasia is present, the complexity of such a system must be calibrated to the language and cognitive impairments. Compensation and adaptation also have implications for professional accommodations; patients with PPAOS, for example, may be able to continue to work, given appropriate support for augmentative or alternative means of communication.
In the context of the neuropsychological evaluation, these results may also assist the clinician in diagnostic accuracy. While it is clear that a comprehensive speech and language evaluation is fundamental to the diagnosis of AOS and aphasia (and by extension – PPAOS and PPA), it is often the case that the patient has not seen a speech language pathologist prior to a neuropsychological evaluation. We recommend that when a patient presents with an insidious onset of AOS, the clinical neuropsychological evaluation cover not only appropriate language measures (especially measures of agrammatism in speech and writing) but also ensure appropriate assessment of executive functioning and episodic learning. In the case where the neuropsychological profile is average or above (possibly with the exception of mild slowing), and there is no more than equivocal evidence of aphasia, a diagnosis of PPAOS should be considered. If there is evidence of more cognitive impairment, other diagnostic classifications may need consideration (e.g., CBS, PSP, PPA).
In the current study, the AOS-PAA group was quite heterogeneous with respect to the severity of aphasia relative to AOS. Some patients had more prominent AOS than aphasia (n = 25), some had equivalent AOS and aphasia (n = 6), and some had more prominent aphasia than AOS (n = 15). It has been proposed that the group with more prominent AOS than aphasia is more similar clinically and pathologically to PPAOS than to AOS-PAA, where aphasia is more prominent, and may represent patients on a broader spectrum of “progressive apraxia of speech” (Botha et al., 2015; Josephs et al., 2013). Future studies may wish to examine potential differences in neuropsychological profiles, with systematic evaluation of differences relative to symptom predominance.
Examining associations between cognitive performance and aphasia severity reveals that these associations are unlikely to be driven by age, education, or disease duration. In fact, to this latter point there was no substantial correlation between disease duration and aphasia severity. Similar illness durations regardless of aphasia severity in the AOS-PAA group suggest we are not simply capturing the artifact of illness progression with increasing aphasia severity and that aphasia itself was associated with performance in these domains. Even within an AOS-PAA sample with mild–moderate aphasia, aphasia severity predicted performance across all cognitive domains. For every 1 unit increase in aphasia severity, performance declined by 1/3 to 1/2 a standard deviation (SD) depending on cognitive domain. The association was strongest within more verbally mediated domains, but was also notable in less verbally mediated domains. While this could demonstrate that neuropsychological performance is influenced by language impairment (though the current data cannot establish direction of causation), associations with less verbally mediated domains suggest it would not be the entire explanation. Although speculative, this lends potential support for unbiased involvement of these other cognitive domains, rather than poorer neuropsychological performance simply representing an artifact of poorer language skills.
As this was a cross-sectional study, future work should examine intraindividual changes in aphasia and cognition in a longitudinal design, in patients with varying combinations of AOS and aphasia. We also did not examine cognitive profiles with respect to AOS subtype. A speech pattern dominated by distorted sound substitutions and additions, and articulatory errors is identified as the “phonetic” subtype, whereas a speech pattern dominated by slow, prosodically segmented speech is the “prosodic” subtype (Utianski, Duffy, Clark, Strand, Botha, et al., 2018). Preliminary work with patients with PPAOS suggests possible group differences in processing speed (measured with graphomotor tasks) between the phonetic (faster) and prosodic (slower) groups, but these data need replication in a larger sample with a more comprehensive neuropsychological and neurological battery (Utianski, Duffy, Clark, Strand, Botha, et al., 2018). Additionally, prior work suggests that the phonetic subtype develops more severe language impairment (Hoglinger et al., 2017; Josephs et al., 2014; Utianski, Duffy, Clark, Strand, Botha, et al., 2018) and earlier in the disease course (Whitwell et al., 2017) compared to the prosodic subtype. In examining the neuropsychological profiles of the different presentations of AOS, it would be important to properly account for unique contributions of subtype and language impairment on cognitive performance. A longitudinal study, controlling for language change over time, would be helpful to examine whether the subtype of AOS predicts cognitive change. Finally, it is important to note that there were few patients with aphasia severity scores ≥3. This is at least partially because people with severe aphasia are difficult to test. Thus, when aphasia is very severe, cognition is a bit of a “black box.” For example, it is possible that visuospatial abilities are relatively preserved or that we could see a difference between verbal and nonverbal episodic memory performance. However, we cannot determine this with any certainty until we have tests that are able to tap into these processes while minimizing the impact of language impairment.
CONCLUSIONS
This study characterized the neuropsychological performance of patients with PPAOS and provides a comparison with patients with AOS-PAA, extending findings from previous studies in a large sample of patients who were evaluated with the same language and cognitive battery. The results suggest that patients with PPAOS are typically cognitively average or above in the domains of immediate and delayed episodic memory, processing speed, executive functioning, and visuospatial ability. Further, patients with AOS-PAA performed more poorly on tests of cognition compared to those with PPAOS. Finally, severity of aphasia contributes to, but does not entirely account for, cognitive performance across cognitive domains. This study builds on prior work supporting the possibility that PPAOS represents a unique syndrome and can be used to inform future diagnostic criteria. More immediately, it may inform prognostication and treatment planning.
FINANCIAL SUPPORT
This work was supported by the National Institutes of Health (K.A.J. grant numbers R01-DC010367, R01-DC14942, and R21-NS94684), (J.L.W. grant number R01-DC12519).
CONFLICTS OF INTEREST
Angelina J. Polsinelli – None. Mary M. Machulda – NIH funding: R01-DC12519, U01-AG06786, R01-AG50603, P50-AG16574, AG49810. Peter Martin – None. Joseph R. Duffy – NIH funding: R01-DC12519, R21-NS94684. Heather M. Clark – NIH funding: R01-DC12519, R01-NS89757, R21-NS94684. Alissa M. Butts – None. Hugo Botha –NIH funding: AG 62677, AG 63911, DC 14942-3. Val J. Lowe serves as a consultant for Bayer Schering Pharma, Philips Molecular lmaging, Life Molecular lmaging, AVID Radiopharamceuticals, and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI). NIH grants P50 AG016574, R01 NS89757, R01 NS089544, R01 DC10367, R01 AG011378, R01 AG041851, R01 AG034676, R01 AG054449, R01 NS097495, U01 AG006786, and R21 NS094489. Jennifer L. Whitwell – NIH funding: R01-DC12519, R01-NS89757, R01-AG50603, R01-DC14942, R01-AG37491. Keith A. Josephs – NIH funding: R01-AG37491, R01-DC14942, R01-DC12519, R01-NS89757, R01-AG50603. Rene L. Utianski – NIH funding: R01-DC12519.
REFERENCES
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, … Hallett M (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80(5), 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM & Sturm VE (2014). Neuropsychological assessment of primary progressive aphasia (PPA). Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 24(4), 128–136. [Google Scholar]
- Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG, … Josephs KA (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. doi: 10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H & Josephs KA (2019). Primary progressive aphasias and apraxia of speech. Continuum: Lifelong Learning in Neurology, 25(1), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Utianski RL, Whitwell JL, Duffy JR, Clark HM, Strand EA, … Jones DT (2018). Disrupted functional connectivity in primary progressive apraxia of speech. Neuroimage Clinical, 18, 617–629. doi: 10.1016/j.nicl.2018.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, & Josephs KA (2015). Neuropsychological profiles differ among the three variants of primary progressive aphasia. Journal of the International Neuropsychological Society, 21(6), 429–435. doi: 10.1017/S1355617715000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley F (1967). Lacunae and research approaches to them. Brain Mechanisms Underlying (pp. 236–240). New York, NY: Grune & Stratton. [Google Scholar]
- Darley F (1969). Aphasia: Input and output disturbances in speech and language processing. Paper presented to the annual convention of the American Speech and Hearing Association, Chicago [unpublished]. [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). The Dells Kaplan Executive Function System: Examiner’s Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani M, Bombois S, Kerdraon O, … Pasquier F (2010). Prediction of pathology in primary progressive language and speech disorders. Neurology, 74(1), 42–49. [DOI] [PubMed] [Google Scholar]
- Duffy JR (2006). Apraxia of speech in degenerative neurologic disease. Aphasiology, 20(6), 511–527. doi: 10.1080/02687030600597358 [DOI] [Google Scholar]
- Duffy JR, Hanley H, Utianski R, Clark H, Strand E, Josephs KA, & Whitwell JL (2017). Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain and Language, 168, 84–94. doi: 10.1016/j.bandl.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR & Josephs KA (2012). The diagnosis and understanding of apraxia of speech: Why including neurodegenerative etiologies may be important. Journal of Speech, Language, and Hearing Research, 55(5), S1518–S1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Strand E, & Josephs K (2014). Motor speech disorders associated with primary progressive aphasia. Aphasiology, 28(8–9), 1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, & Josephs KA (2015). Primary progressive apraxia of speech: clinical features and acoustic and neurologic correlates. American Journal of Speech and Language Pathology, 24(2), 88–100. doi: 10.1044/2015_AJSLP-14-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelboom WS, Janssen N, Jiskoot LC, van den Berg E, Roelofs A, & Kessels RP (2018). Episodic and working memory function in Primary Progressive Aphasia: A meta-analysis. Neuroscience & Biobehavioral Reviews, 92, 243–254. [DOI] [PubMed] [Google Scholar]
- Gelman A, & Hill J (2006). Data Analysis Using Regression and Multilevel/hierarchical Models. New York, NY: Cambridge University Press. [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, … Dubois B (2007). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Movement Disorders, 22(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Saxon JA, Jones M, Snowden JS, & Thompson JC (2019). Neuropsychological differentiation of progressive aphasic disorders. Journal of Neuropsychology, 13(2), 214–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, … Movement Disorder Society-endorsed PSP Study Group (2017). Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Movement Disorders, 32(6), 853–864. doi: 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, & Petersen RC (1996). Neuropsychological tests’ norms above age 55: COW AT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist, 10(3), 262–278. [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, & Kurland LT (1992). Mayo’s Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. The Clinical Neuropsychologist, 6(S1), 83–104. [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, … Dickson DW (2005). Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase, 11(4), 283–296. doi: 10.1080/13554790590963004 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda M, Senjem ML, Lowe V, … Whitwell JL (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81, 337–345. doi: 10.1212/WNL.0b013e31829c5ed5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, … Whitwell JL (2014). The evolution of primary progressive apraxia of speech. Brain, 137(Pt 10), 2783–2795. doi: 10.1093/brain/awu223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, … Whitwell JL (2012). Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain, 135(Pt 5), 1522–1536. doi: 10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, … Petersen RC (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129(Pt 6), 1385–1398. doi: 10.1093/brain/awl078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery (Revised). San Antonio, TX: PsychCorp. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, & Randolph C (1999). An empirically derived short form of the Boston Naming Test. Archives of Clinical Neuropsychology, 14, 481–487. [PubMed] [Google Scholar]
- Machulda MM, Ivnik R, Smith G, Ferman TJ, Boeve BF, Knopman D, … Tangalos EG (2007). Mayo’s older Americans normative studies: Visual form discrimination and copy trial of the Rey–Osterrieth complex figure. Journal of Clinical and Experimental Neuropsychology, 29(4), 377–384. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. [PubMed] [Google Scholar]
- Mesulam MM (2003). Primary progressive aphasia—a language-based dementia. New England Journal of Medicine, 349(16), 1535–1542. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, & Gorno-Tempini ML (2007). Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Disease & Associated Disorders, 21(4), S23–S30. [DOI] [PubMed] [Google Scholar]
- Osterrieth P (1944). Test of copying a complex figure: Contribution to the study of perception and memory. Archives de Psychologie, 30, 286–356. [Google Scholar]
- Rey A (1964). The Clinical Psychological Examination. Paris, Prance: Presses Universitaires de Prance. [Google Scholar]
- Spreen O, & Strauss E (1998). Compendium of neuropsychological tests: Administration, norms and commentary (2nd ed.). New York, NY: Oxford University Press. [Google Scholar]
- Strand EA, Duffy JR, Clark HM, & Josephs K (2014). The Apraxia of Speech Rating Scale: a tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders, 51, 43–50. doi: 10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff KA, Duffy JR, Clark HM, Utianski RL, Strand EA, Machulda MM, … Senjem ML (2019). Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain, 142(8), 2466–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff KA, Duffy JR, Strand EA, Machulda MM, Boland SM, Utianski RL, … Josephs KA (2018). Clinical and imaging progression over 10 years in a patient with primary progressive apraxia of speech and autopsy-confirmed corticobasal degeneration. Neurocase, 24(2), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzloff KA, Utianski RL, Duffy JR, Clark HM, Strand EA, Josephs KA, & Whitwell JL (2018). Quantitative Analysis of Agrammatism in Agrammatic Primary Progressive Aphasia and Dominant Apraxia of Speech. Journal of Speech, Language, and Hearing Research, 61(9), 2337–2346. doi:doi: 10.1044/2018_JSLHR-L-17-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Strand EA, Boland SM, Machulda MM, … Josephs KA (2018). Clinical Progression in Four Cases of Primary Progressive Apraxia of Speech. American Journal of Speech-Language Pathology, 27(4), 1303–1318. doi: 10.1044/2018_ajslp-17-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Strand EA, Botha H, Schwarz CG, … Josephs KA (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain & Language, 184, 54–65. doi: 10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Whitwell JL, Schwarz CG, Duffy JR, Botha H, Clark HM, … Petersen RC (2018). Tau uptake in agrammatic primary progressive aphasia with and without apraxia of speech. European Journal of Neurology, 25(11), 1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Whitwell JL, Schwarz CG, Senjem ML, Tosakulwong N, Duffy JR, … Josephs KA (2018). Tau-PET imaging with [18F]AV-1451 in primary progressive apraxia of speech. Cortex, 99, 358–374. doi: 10.1016/j.cortex.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vöglein J, Kostova I, Arzberger T, Röber S, Schmitz P, Simons M, … & Levin J (2021). First symptom guides diagnosis and prognosis in neurodegenerative diseases – A retrospective study of autopsy proven cases. European Journal of Neurology, doi: 10.1111/ene.14800 [DOI] [PubMed] [Google Scholar]
- Warrington EK, & James M (1991). The Visited Object and Space Perception Battery. Bury St Edmunds, England: Thames Valley Test Company. [Google Scholar]
- Watson CL, Possin K, Allen IE, Hubbard HI, Meyer M, Welch AE, … Miller Z (2018). Visuospatial functioning in the primary progressive aphasias. Journal of the International Neuropsychological Society, 24(3), 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler Memory Scale (3rd ed.). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, … Josephs KA (2013). Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang, 125(3), 245–252. doi: 10.1016/j.bandl.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Martin P, Duffy JR, Clark HM, Utianski RL, Botha H, … Josephs KA (2020). Survival analysis in primary progressive apraxia of speech and agrammatic aphasia. Neurology: Clinical Practice, doi: 10.1212/CPJ.0000000000000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Duffy JR, Clark HM, Strand EA, Machulda MM, … Josephs KA (2017). Predicting clinical decline in progressive agrammatic aphasia and apraxia of speech. Neurology, 89(22), 2271–2279. doi: 10.1212/wnl.0000000000004685 [DOI] [PMC free article] [PubMed] [Google Scholar]