Abstract
Acetyl-11-keto-beta-boswellic acid (AKBA), the major component of Boswellia serrata, exhibits anti-inflammatory activities. This in vitro study investigated the protective effects of AKBA against lipopolysaccharide (LPS)-induced cardiac dysfunction. In this study, the H9C2 cardiomyocytes were pretreated with AKBA (2.5, 5, and 10 μM for 24 h), and then cotreated with LPS for another 24 h. The MTT assay, ELISA test kits, and quantitative real-time PCR analysis assessed the cell viability, levels of proinflammatory factors (IL-β, IL-6, TNF- α, and PGE2), and the gene expression of IL-β, IL-6, TNF- α, iNOS, and COX-2, respectively. The nitric oxide (NO) and thiol levels were also measured using a biochemical assay. The results indicated that LPS exposure markedly reduced cell viability and total thiol content, but increased the inflammatory cytokines, NO metabolites, and gene expression of proinflammatory mediators in H9C2 cells. AKBA pretreatment significantly altered the mentioned factors induced by LPS. Our results demonstrated that AKBA might be a promising therapeutic agent for treating sepsis-related cardiac dysfunction in the future.
1. Introduction
Sepsis is a lethal condition caused by an overreaction of the immune system to infection that can lead to tissue damage, multiple organ failure, and even death [1–4]. Myocardial dysfunction is one of the prominent sepsis features that has proved to be associated with a high mortality risk for septic patients [5, 6]. Many studies have reported the crucial role of proinflammatory cytokines in myocardial oxidative damage that commonly occurs in the course of sepsis [7]. Throughout these severe inflammatory conditions, the elevating production of multiple proinflammatory mediators, such as interleukin-1beta (IL-1β), IL-6, tumour necrosis factor-α (TNF-α), and nitric oxide (NO), eventually lead to cardiac depression, oedema, and necrosis in the myocardial cells [2–4, 8]. According to previous studies, the systemic inflammatory response is stimulated by damage-associated molecular patterns (DAMPs) such as lipopolysaccharide (LPS) as a bacterial endotoxin, which can induce inflammatory signalling pathways through toll-like receptor-4 (TLR-4) in cardiomyocytes [3, 9, 10]. The LPS/TLR-4 complex formation can activate further inflammatory signalling cascades associated with several transcription factors, particularly the nuclear factor-kappa B (NF-κB) pathway, which stands as the primary signalling cascade in initiating the process of intracellular inflammation [3, 10, 11]. Considering the activation of inflammation by NF-κB, the intensified proinflammatory cytokines can accumulate intracellular oxygen free radicals (ROS) and automatically impair the structure and function of cardiomyocytes [7, 10, 12–14]. The available pharmacological approaches, such as using nonsteroidal anti-inflammatory drugs (NSAIDs), are minimal due to their controversial and various adverse effects [7, 10, 13–15]. It is consequently necessary to consider efficient therapeutic interventions to prevent sepsis-induced cardiomyopathy. There is growing evidence on natural compounds, especially plants' secondary metabolites, are capable of representing optimal therapeutic effects in alleviating LPS-induced cardiotoxicity [6, 16].
Acetyl-11-keto-β-boswellic acid (AKBA), as a plant-derived-bioactive pentacyclic triterpene, has been isolated from Boswellia serrata (BS) [4, 10, 17]. The remarkable biological features of AKBA have been repeatedly indicated, including antioxidant [18], antitumour [19], antimicrobial activity [20], anti-inflammatory [4, 10, 13, 14], neuroprotective [2, 14], and other beneficial qualities [13, 14, 21]. Recently, the cardioprotective activities of pentacyclic triterpenoid compounds have been paid the attention of many researchers. In one study, two natural compounds with a similar chemical structure (boswellic acid and oleanolic acid) have been investigated on high glucose-induced toxicity in the H9C2 cardiomyocyte cell line. This study showed that these compounds attenuate apoptosis by reducing the activity of NF-κB, lowering ROS production, and enhancing the glutathione redox cycle [22]. Further studies also reported the protective impacts of AKBA against the consequences of myocardial ischemia-reperfusion (I/R) injury in a rat model by modulating the oxidative-inflammatory cascades [23]. Based on the abovementioned data, this work aimed to investigate the effects of AKBA on lipopolysaccharide (LPS)-induced cell injury in H9C2 cells.
2. Methods and Materials
2.1. Chemicals and Reagents
AKBA (Calbiochem), DMEM culture media, fetal bovine serum (FBS), penicillin plus streptomycin (pen/strep), dimethyl sulfoxide (DMSO), LPS (Escherichia coli O55 : B5 purified by phenol extraction, L2880 SIGMA), and other chemicals used were of cell culture and analytical grade from Sigma-Aldrich (St. Louis, MO, USA). A proliferation assay kit (MTT) was provided from Roche Diagnostic (Mannheim, Germany). ELISA kits (PGE2, IL-6, IL-1β, and TNF-α) were supplied from IBL International (USA).
2.2. Cell Viability Assay
In this study, we first evaluated the viability of H9C2 cells in the presence of AKBA (2.5, 5, and 10 μM) to better understand the working solution selected based on nontoxic concentration. In brief, the cells were treated with different concentrations of AKBA (2.5, 5, and 10 μM) for 48 h, and then the cell viability was evaluated employing the MTT assay. Once the nontoxicity of the applied concentrations towards cells was ascertained, the protective effects of AKBA were assessed. In the next step, we evaluated the effects of the concentrations of AKBA (2.5, 5, and 10 μM) on LPS-induced (10 μg/ml) by culturing 7 × 103 H9C2 cells in a 96-well plate. In review, the cells were pretreated with different concentrations of AKBA (2.5, 5, and 10 μM) for 24 h, and then were cocultured with LPS (10 μg/ml) for another 24 h. In the end, the cell viability was measured by applying an MTT assay. For the MTT assay, briefly, 10 μL of MTT solution with a final concentration of 5 mg/ml was appended to each well to be incubated for 3 h. After discarding the medium culture (RPMI-1640), 100 μL of DMSO was used to dissolve the formed formazan crystals. The absorption of the 96-well plate was recorded by an ELISA reader (Awareness Inc., USA) at 570 nm and 620 nm [7].
2.3. Experimental Procedure and Grouping
The protective effects of AKBA against LPS-induced cardiomyocyte toxicity were evaluated as a model of septic shock. Initially, the cells were pretreated with AKBA (2.5, 5, and 10 μM) for 24 h, and then were coexposed with LPS (10 μg/ml) for another 24 h. After that, we assessed the changes in both gene expression (in the cell lysate, using real-time PCR) and protein (in the supernatant, using ELISA) levels of proinflammatory biomarkers, including TNF-α, IL-1β, IL-6, PGE2, iNOS, COX-2, and nitric oxide metabolites (NO). Total thiol content was also assessed as an antioxidant marker in the lysate. Experimental groups were as follows:
Group 1: control group, H9C2 cells received a complete media culture and the solvent of AKBA with neither AKBA nor LPS for 48 h
Group 2: LPS group, H9C2 cells received a complete media culture and the solvent of AKBA and LPS (10 μg/ml) for 48 h
Groups 3, 4, and 5: AKBA treated groups, H9C2 cells received a complete media culture and AKBA (2.5, 5, and 10 μM) for 24 h, and then coincubated with LPS (10 μg/ml) for another 24 h
AKBA was dissolved in DMSO, which was serially diluted with a complete medium that contained the final DMSO concentration at a lower percentage than 0.1% v/v throughout all of the experiments. We selected the concentrations of AKBA according to the preliminary results of the cell viability by the MTT assay, and a similar study evaluated the antioxidative effects of AKBA (2.5–10 μM) [16].
2.4. Measuring Total Protein Levels
The Bradford protein assay was carried out to quantify the total protein concentration in a sample using the Coomassie Brilliant Blue G-250 dye [24–27]. First, the dye (10 mg) was dissolved in 50 ml of ethanol (96%), and then phosphoric acid (85%) (10 ml) was added, and the volume of the solution reached 100 ml. Thereafter, bovine serum albumin (BSA, 4 mg/ml) solution was prepared as a standard curve. Then, after sample pouring (20 μl), a Bradford reagent (200 μl) was added to the 96-well microplate. Finally, after 5 minutes, the absorption was read out at 595 nm with a microplate reader [24–27].
2.5. Evaluation of the Protein Levels of Inflammatory Biomarkers
The ELISA assay evaluated the IL-1β, IL-6, TNF-α, and PGE2 levels as inflammatory mediators, which were carried out in accordance with the manufacturers' protocol, IBL company [6, 7, 24, 26, 28], USA. In summary, 1.5 × 106 cells were cultured in a 6-well plate overnight, and they were treated according to the experimental grouping section. The supernatant of cells was then used for the required measurements, and the lysates were collected for gene expression studies.
2.6. Gene Expression Assessment
A real-time PCR technique was performed through the SYBR Green procedure's employment to assess the possible impacts of different AKBA concentrations on the levels of TNF-α, IL-1β, IL-6, and COX-2 as well as iNOS related mRNA. Rotor-Gene 6000 was involved as a real-time reaction detection system, and GAPDH was considered a reference gene [7, 24, 26]. We procured the required real-time PCR primers in a similar design to those mentioned in previous studies [29]; the primer specificity was blasted and confirmed by applying NCBI Primer-BLAST. The primer sequences are detailed in Table 1. The real-time PCR reaction contained 5 μL of amplicon master mix, 0.4 μL of each primer (1 μM), 0.2 μL of DEPC water, and 50 ng of cDNA. Meanwhile, the prepared conditions for PCR were set at 95°C for 3 min and then followed by 45 cycles of 95°C for 20 sec, annealing temperature (55–65°C) for 5 sec, and 72°C for 10 sec. As the last step, we examined the values of gene expression levels by using the ∆Ct method and reported the fold-change values as 2−(∆∆Ct) to the control group.
Table 1.
| Gene name | 5′-3′ primer | Sequence Accession Number |
|---|---|---|
| tnf-α | FW CACCTCTCAAGCAGAGCACAG RW GGGTTCCATGGTGAAGTCAAC |
M98820 |
| cox-2 | FW AAATGGGCTCCCTCTCATCAGTTC RW TCTGCTTGGTGGTTTGCTACGAC |
X66539 |
| il-1β | FW TGTATGCTACCATCTGGCTTCGG RW GTTTGGAACAGTCGCTCGTCATC |
S67722 |
| il-6 | FW CATTGGAAGTGAAGCGTTTCG RW CAGCTGGGCTGTACAAACCTT |
L12562 |
| inos | FW TCCTACCCCAACTTCCAATGCTC RW TTGGATGGTCTTGGTCCTTAGCC |
E02522 |
| gapdh | FW GTATTGGGCGCCTGGTCACC RW CGCTCCTGGAAGATGGTGATGG |
AB017801 |
FW, forward primer; RW, reverse primer; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
2.7. Evaluation NO Metabolites and Thiol Content Level
The nitrite oxide metabolite levels were measured based on the measurement of nitrite (NO−2) as the stable and the final NO product by the Griess method described elsewhere [30]. The NO levels were assessed in the supernatant at 540 nm by using the standard curve of different nitrite concentrations [6, 7, 24, 26–28].
The total thiol content was evaluated through a colourimetric method, which was set according to the reaction of total thiol content with Ellman reagent (5,5′-dithiobis (2-nitrobenzoic acid) (Sigma-Aldrich) [31]. This particular reaction results in the formation of yellow-coloured TNB (5-thio-2-nitrobenzoic acid) that can be quantified at 412 nm [6, 7, 24, 26–28].
2.8. Statistical Analysis
We displayed the obtained results as mean ± SEM. The gathered data were analysed by GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA) software. Besides, the one-way analysis of variance (ANOVA) test was carried out with Tukey-Kramer's post hoc multiple comparisons test according to the variance's homogeneity. By statistics, probability (P) values of less than 0.001, 0.01, and 0.05 were considered significant differences in all of the performed calculations.
3. Results
3.1. AKBA Alleviates LPS-Induced Cytotoxicity in H9C2 Cells
As shown in Figure 1(a), in comparison to the control group, there were no significant changes in the level of cell viability of H9C2 cells incubated with various concentrations of AKBA (2.5, 5, and 10 μM) for 48 h. Incubation of the cells with LPS (10 μg/mL) for 48 h led to a significant reduction in the level of cell viability compared to the control group (P < 0.001; Figure 1(b)). However, treatment with AKBA (5 and 10 μM) notably increased the level of cell viability in the presence of LPS stimulation (P < 0.05 and 0.001, respectively; Figure 1(b)).
Figure 1.
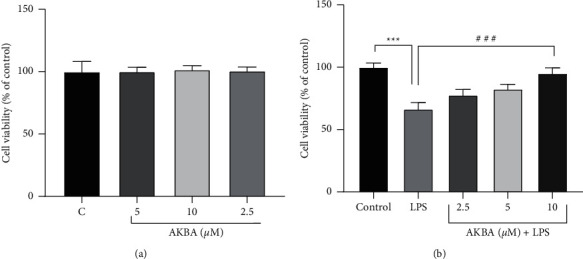
H9C2 cell viability in the presence of AKBA alone and with LPS. Effect of AKBA (2.5–10 uM) on cell viability (a). The survival of the H9C2 cell line was evaluated when treated with LPS (10 ug/ml) and 24 h of pretreatment with AKBA then exposure with LPS (b). n = six per group. Error bars indicate standard error mean (SEM) ∗∗∗P < 0.001 vs. control and ###P < 0.001 vs. LPS.
3.2. AKBA Inhibits LPS-Induced Inflammatory Cytokines Production in H9C2 Cells
To demonstrate the anti-inflammatory effects of AKBA, we assessed the production levels of proinflammatory cytokines, including TNF-α, IL-6, IL-1β, and PGE2, which contributed to LPS-induced cardiomyopathy. As illustrated in Figures 2(a)–2(d), the production levels of TNF-α, IL-6, IL-1β, and PGE2 were significantly elevated in H9C2 cells following the LPS (10 μg/mL) stimulation in comparison to the control group (P < 0.001 for all cases). However, pretreatment of the cells with AKBA (2.5, 5, and 10 μM) dramatically decreased the production of TNF-α (P < 0.001 for all cases; Figure 2(a)), IL-1β (P < 0.001 for all cases; Figure 2(b)), and IL-6 (P < 0.001 for all cases; Figure 2(c)) in a concentration-dependent manner, compared to the LPS group. Although AKBA exerted reducing effects on the level of PGE2, this effect was statistically significant only at two higher concentrations of AKBA (5 and 10 μM) in comparison to the LPS group (P < 0.001 for both cases; Figure 2(d)).
Figure 2.
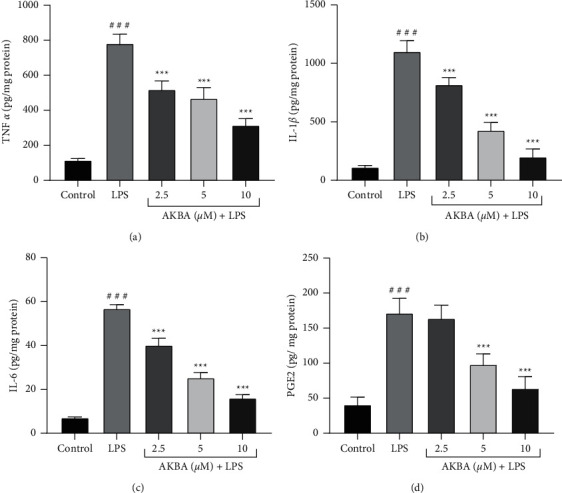
Effect of AKBA and LPS on the level of TNF-α (a), IL-1β (b), IL-6 (c), and PGE2 (d). The cells were pretreated with AKBA for 24 h, then incubated with LPS for another 24 h, and levels of TNF-α, IL-1β, IL-6, and PGE2 were evaluated by an ELISA assay. The data are the mean ± SEM (n = 6 per group). ###P < 0.001 vs. control and ∗∗∗P < 0.001 vs. LPS.
3.3. AKBA Attenuates the Gene Expression Levels of Inflammatory Cytokines in H9C2 Cells
Our study evaluated the capability of AKBA in suppressing the transcription of proinflammatory mediators in our study using the real-time PCR (qPCR) technique. According to Figures 3(a)–3(e), treatment of H9C2 cells with LPS (10 μg/mL) caused a significant enhancement in proinflammatory genes' expression levels (IL-1β, TNF-α, IL-6, iNOS, and COX-2). We revealed that three nontoxic concentrations of AKBA (2.5, 5, and 10 μM) exhibited notable suppressive impacts on the expression levels of proinflammatory mediators (IL-1β, TNF-α, IL-6, iNOS, and COX-2), in a concentration-dependent manner, compared to the LPS group (P < 0.001–0.01 for all cases; Figures 3(a)–3(e)).
Figure 3.
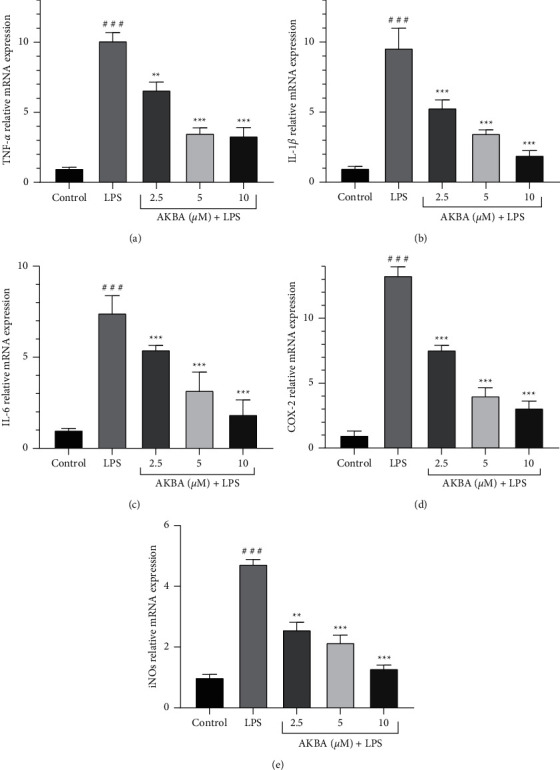
Effect of AKBA and LPS on the mRNA Level of TNF-α (a), IL-1β (b), IL-6 (c), Cox2 (d), and iNos (e). The cells were pretreated with AKBA for 24 h then incubated with LPS for another 24 h and gene expression of TNF-α, IL-1β, IL-6, Cox2, and iNos was evaluated by real time-PCR. The data are mean ± SEM (n = 6 per group). ###P < 0.001 vs. control, ∗∗P < 0.001 and ∗∗∗P < 0.001 vs. LPS.
3.4. AKBA Decreased Nitric Oxide Level and Increased Thiol Level
We investigated the antioxidant effect of AKBA in LPS-stimulated H9C2 cells by detecting the levels of nitric oxide (NO) and thiol [32] in the culture medium. According to the obtained results, LPS at a concentration of 10 μg/mL led to a significant enhancement in the level of NO (P < 0.001; Figure 4(a)) and a significant reduction in the level of total thiol content (P < 0.001; Figure 4(b)), compared to the control group.
Figure 4.

Cellular redox status including thiol state and NO state after treatment with AKBA and LPS. The cells were treated (24 hours) with various concentrations AKBA (2.5–10 μM) and then incubated with LPS (10 ug/ml) for 24 hours. The data are mean ± SEM (n = 6). ###P < 0.001 vs. control, ∗∗P < 0.001 and ∗∗∗P < 0.001 vs. LPS.
In contrast, pretreatment with AKBA (2.5, 5, and 10 μM) caused a significant inhibition of NO production compared to the LPS-treated group (P < 0.001–0.01 for all cases; Figure 4(a)). Moreover, preincubation of the cells with AKBA (2.5, 5, and 10 μM) resulted in a marked increase in the levels of total thiol content in a concentration-dependent manner compared to the LPS group (P < 0.001–0.01 for all cases; Figure 4(b)).
4. Discussion
To the best of our knowledge, the present work is the first study to investigate the potential protective effects of AKBA against LPS-induced cardiomyopathy. Here, we used an in vitro H9C2 model of myocardial injury to explore the protective mechanism of AKBA on LPS-induced cardiac dysfunction [33]. Our study results showed that LPS increased the levels of proinflammatory mediators (IL-1β, TNF-α, IL-6, and PGE2) and nitric oxide (NO), whereas it reduced the cell viability of H9C2 cells as well as the level of total thiol content. However, pretreatment with various concentrations of AKBA (2.5, 5, and 10 μM) could dramatically enhance the cell viability and the content of total thiol content. Furthermore, AKBA significantly suppressed the expression of proinflammatory factors and NO production, indicating that AKBA might be effective against LPS-induced inflammatory responses. We also found that the gene expression of COX-2 and iNOS was down-regulated by AKBA pretreatment.
It is well established that LPS, as an exogenous ligand of TLR4, induces inflammatory processes involved in severe cardiac injury [34]. LPS-mediated activation of initiates NF-κB dependent signalling pathways, resulting in the overproduction of various proinflammatory mediators, such as COX-2, iNOS, IL-1β, IL-6, and TNF-α [6, 24, 26–28, 35, 36]. Excessive production of proinflammatory cytokines plays a vital role in developing many inflammatory disorders, including sepsis-induced myocardial dysfunction [37]. Meanwhile, several studies have investigated the role of TNF-α in the pathogenesis of LPS -induced acute cardiac injury and have shown that high concentrations of TNF-α promoted the expression of specific cytokines and mediators involved in LPS-induced septic cardiomyopathy [6, 24, 26–28, 38, 39]. IL-6 is another important proinflammatory factor that can directly cause myocardial damage by stimulating nitric oxide synthase activity and NO production [40]. Besides, several published studies have reported that cytokines such as IL-1β may act as cardiodepressant inflammatory mediators during sepsis [6, 24, 26–28, 41]. Hence, inhibiting the production of inflammatory cytokines can be a critical molecular target for novel anti-inflammatory therapeutic approaches. In the present study, LPS notably stimulated the release of proinflammatory cytokines from H9C2 cardiomyocytes, consistent with previous reports [42]. The anti-inflammatory activity of AKBA has been investigated using several models of inflammation. Previous studies have reported that AKBA inhibits the generation of proinflammatory cytokines through down-regulation of the NF-κB pathway [43]. For example, Chao Wei et al. evaluated the neuroprotective function of AKBA in a mouse model of Alzheimer's disease. Their findings indicated that AKBA had a strong anti-inflammatory effect on APPswe/PS1dE9 mice by reducing inflammatory molecules' production through the inhibition of the NF-κB signalling pathway [44]. It has also been reported that the potential cardiac-protective of AKBA is likely associated with the activation of Nrf-2 related signalling cascades [23]. In another study, AKBA was shown to effectively alleviate oxygen-glucose deprivation (OGD)-induced neuroinflammation via the increased expression of Nrf2 [45].
Meanwhile, we explored the possible cardiac-protective effect of AKBA against LPS-induced inflammatory cytokine production in H9C2 cells. The results showed that AKBA pretreatment dramatically suppressed the protein and gene expression of IL-1β, IL-6, and TNF-α in LPS-exposed H9C2 cells in a concentration-dependent manner. Our results revealed that the protective effects of AKBA against LPS-induced cardiac injury were associated with inflammatory cytokine production inhibition. The obtained results were in accordance with a previous study in which AKBA markedly reduced TNF-α production in the target tissue against the LPS-induced neuroinflammatory model [13]. These findings suggest that the potent anti-inflammatory properties of AKBA may be related to its inhibitory activity on the expression of inflammatory factors.
COX-2 is an inducible isoform from cyclooxygenase that catalyses the formation of prostaglandin E2 (PEG2), which is involved in many processes leading to the inflammatory response [6, 24, 26–28, 46]. Many studies have shown that COX-2 enzymatic activity is significantly induced by proinflammatory stimuli such as TNF- α and LPS and high NO concentrations during various inflammatory conditions [47–49]. Thus, the level of COX-2 expression seems to play a pivotal role in multiple pathophysiological mechanisms, especially inflammation-related diseases [49, 50]. Likewise, several studies have proved that many natural products derived from medicinal plants with significant anti-inflammatory effects act as selective inhibitors of COX-2 activity [6, 24, 26–28, 49, 51]. In this research, we found that AKBA could efficiently suppress the mRNA expression of COX-2 in H9C2 cardiomyocytes activated with LPS. This outcome supports the hypothesis that the cardiac-protective effect of AKBA may also be attributed to the direct suppression of COX-2 gene expression. To further investigate the anti-inflammatory potential of AKBA, we measured the expression level of PEG2 in AKBA + LPS-treated H9C2 cells. Results indicated that pretreatment with AKBA meaningfully decreased the PGE2 level in LPS-activated H9C2 cells. As a result, inhibiting these inflammatory mediators' production can be an essential indicator for our anti-inflammatory agents.
Inducible nitric oxide synthase (iNOS) is commonly expressed in response to proinflammatory stimuli such as LPS and specific chemokines/cytokines in a wide range of cells. It has been found that the overproduction of NO by iNOS activity plays a crucial role in the pathophysiology of septic cardiomyopathy [52]. High NO levels lead to the induction of cellular oxidative damage via increasing the production of reactive nitrogen species (RNS) such as peroxynitrite [53]. Many previous studies have revealed that natural phytochemicals can show therapeutic effects against LPS-evoked inflammatory injury via reducing the iNOS expression and NO content [54]. In the current study, we also found a significant suppressive effect of AKBA on the expression of iNOS and the production of NO.
Similarly, antioxidant effects of AKBA have been previously reported. Manoj Kumar et al. have demonstrated that AKBA inhibits benzo (a) pyrene-induced liver toxicity by reducing NO generation [55]. Besides, Yu et al. investigated the nitric oxide inhibitory activity of bioactive compounds isolated from the resin of Boswellia carteri. Interestingly, their experiments showed that AKBA was one of the most potent antioxidant compounds in Boswellia carteri that could inhibit NO production in LPS-stimulated RAW264. 7 cells [56]. These findings provide novel evidence that AKBA may attenuate inflammatory responses by suppressing iNOS-mediated NO production.
Additionally, the amount of glutathione is another parameter used in our study to evaluate the antioxidant activity of AKBA. As a nonenzymatic antioxidant compound, total thiol content has a crucial role in protecting cells from oxidative stress and maintaining cellular redox homeostasis [57]. It has been proven that a decreased level of reduced total thiol content leads to activation of the NF-κB pathway and enhances the expression of proinflammatory cytokines during inflammation-related disorders [58]. Consequently, the increased level of total thiol content can be part of the antioxidant defence mechanism to inhibit sepsis-induced cardiomyopathy [59]. On the other hand, natural metabolites have been shown to protect against oxidative damage in many inflammatory conditions by enhancing intracellular total thiol content [6, 24, 26–28, 49, 60]. The results of a previous study revealed that AKBA possessed antioxidant effects through elevating the reduced total thiol content levels in an experimental model of colitis [61]. Consistent with these findings, we also observed a remarkable increase of reduced total thiol content in LPS-exposed H9C2 cardiomyocytes after pretreating with AKBA. Based on this result, it can be inferred that the beneficial effects of AKBA on LPS-induced inflammation in H9C2 cells may be due to its potent antioxidant potential.
Overall, our findings demonstrate the potent anti-inflammatory and antioxidant properties of AKBA, suggesting that it can be used as an effective therapeutic agent to lessen the inflammatory injuries caused by LPS in cardiomyocytes. However, additional in vivo experiments are required to further support the therapeutic potential of AKBA in sepsis-related myocardial dysfunction.
Acknowledgments
This study was financially supported by a nonspecific grant of the research council of Mashhad University of Medical Sciences (GN 991660, 991593, and 990961).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
This study does not require ethical approval.
Consent
This study does not require the need for informed consent.
Disclosure
Danial Taherzadeh, Vafa Baradaran Rahimi, Hamed Amiri, and Sajjad Ehtiati shared the first co-authorship.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors equally contributed to performing the project and preparing the manuscript.
References
- 1.Wei X., Meng X., Yuan Y., Shen F., Li C., Yang J. Quercetin exerts cardiovascular protective effects in LPS-induced dysfunction in vivo by regulating inflammatory cytokine expression, NF-κB phosphorylation, and caspase activity. Molecular and Cellular Biochemistry . 2018;446(1-2):43–52. doi: 10.1007/s11010-018-3271-6. [DOI] [PubMed] [Google Scholar]
- 2.Marefati N., Beheshti F., Etemadizadeh P., Hosseini M., Anaeigoudari A. Gum resin extract of Boswellia serrata attenuates lipopolysaccharide-induced inflammation and oxidative damage in hepatic and renal tissues of rats. Asian Pacific Journal of Tropical Biomedicine . 2022;12(1):20–25. [Google Scholar]
- 3.Marefati N., Beheshti F., Memarpour S., Rezaei M., Hosseini M. The effects of pre-treatment with olibanum and its constituent, boswellic acid on synaptic plasticity impairments induced by lipopolysaccharide in rats. Avicenna journal of phytomedicine . 2021;11(1):68–78. [PMC free article] [PubMed] [Google Scholar]
- 4.Marefati N., Beheshti F., Vafaee F., Barabadi M., Hosseini M. The effects of incensole acetate on neuro-inflammation, brain-derived neurotrophic factor and memory impairment induced by lipopolysaccharide in rats. Neurochemical Research . 2021;46(9):2473–2484. doi: 10.1007/s11064-021-03381-3. [DOI] [PubMed] [Google Scholar]
- 5.Fu C.-Y., Chen J., Lu X.-Y., et al. Dimethyl fumarate attenuates lipopolysaccharide-induced mitochondrial injury by activating Nrf2 pathway in cardiomyocytes. Life Sciences . 2019;235 doi: 10.1016/j.lfs.2019.116863.116863 [DOI] [PubMed] [Google Scholar]
- 6.Yahyazadeh R., Baradaran Rahimi V., Yahyazadeh A., Mohajeri S. A., Askari V. R. Promising effects of gingerol against toxins: a review article. Biofactors . 2021;47 doi: 10.1002/biof.1779. [DOI] [PubMed] [Google Scholar]
- 7.Baradaran Rahim V., Khammar M. T., Rakhshandeh H., Samzadeh-Kermani A., Hosseini A., Askari V. R. Crocin protects cardiomyocytes against LPS-Induced inflammation. Pharmacological Reports . 2019;71(6):1228–1234. doi: 10.1016/j.pharep.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T. Q. C., Duy Binh T., Pham T. L. A., et al. Anti-inflammatory effects of lasia spinosa leaf extract in lipopolysaccharide-induced RAW 264.7 macrophages. International Journal of Molecular Sciences . 2020;21(10):p. 3439. doi: 10.3390/ijms21103439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R., Li D., Ouyang J., et al. Leonurine alleviates LPS-induced myocarditis through suppressing the NF-кB signaling pathway. Toxicology . 2019;422:1–13. doi: 10.1016/j.tox.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Marefati N., Beheshti F., Mokhtari-Zaer A., et al. The effects of olibanum on oxidative stress indicators, cytokines, brain derived neurotrophic factor and memory in lipopolysaccharide challenged rats. Toxin Reviews . 2020:1–14. doi: 10.1080/15569543.2020.1855653. [DOI] [Google Scholar]
- 11.Ahmed N., El-Agamy D. S., Mohammed G. A., Abo-Haded H., Elkablawy M., Ibrahim S. R. M. Suppression of LPS-induced hepato-and cardiotoxic effects by pulicaria petiolaris via NF-κB dependent mechanism. Cardiovascular Toxicology . 2019;20:1–9. doi: 10.1007/s12012-019-09539-4. [DOI] [PubMed] [Google Scholar]
- 12.Al-Dossari M. H., Fadda L. M., Attia H. A., Hasan I. H., Mahmoud A. M. Curcumin and selenium prevent lipopolysaccharide/diclofenac-induced liver injury by suppressing inflammation and oxidative stress. Biological Trace Element Research . 2020;196(1):173–183. doi: 10.1007/s12011-019-01910-4. [DOI] [PubMed] [Google Scholar]
- 13.Marefati N., Beheshti F., Memarpour S., et al. The effects of acetyl-11-keto-β-boswellic acid on brain cytokines and memory impairment induced by lipopolysaccharide in rats. Cytokine . 2020;131 doi: 10.1016/j.cyto.2020.155107.155107 [DOI] [PubMed] [Google Scholar]
- 14.Marefati N., Khamse S., Khamse S., Mansouri S., Hosseini M., Anaeigoudari A. Effects of boswellia serrata resin on central nervous system: a mini review. Physiology and Pharmacology . 2021;25(4):288–295. doi: 10.52547/phypha.25.4.5. [DOI] [Google Scholar]
- 15.Molteni M., Bosi A., Rossetti C. Natural products with toll-like receptor 4 antagonist activity. International Journal of Inflammation . 2018;2018:9. doi: 10.1155/2018/2859135.2859135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajabian A., Sadeghnia H. R., Hosseini A., Mousavi S. H., Boroushaki M. T. 3-Acetyl-11-keto-β-boswellic acid attenuated oxidative glutamate toxicity in neuron-like cell lines by apoptosis inhibition. Journal of Cellular Biochemistry . 2020;121(2):1778–1789. doi: 10.1002/jcb.29413. [DOI] [PubMed] [Google Scholar]
- 17.Pang X., Yi Z., Zhang X., et al. Acetyl-11-Keto-β-Boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Research . 2009;69(14):5893–5900. doi: 10.1158/0008-5472.can-09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M., Wang M., Yang Q., et al. Antioxidant effects of hydroxysafflor yellow a and acetyl-11-keto-β-boswellic acid in combination on isoproterenol-induced myocardial injury in rats. International Journal of Molecular Medicine . 2016;37(6):1501–1510. doi: 10.3892/ijmm.2016.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W., Liu J., Fu W., et al. 3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. Journal of Experimental & Clinical Cancer Research . 2018;37(1):p. 132. doi: 10.1186/s13046-018-0805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iram F., Khan S. A., Husain A. Phytochemistry and potential therapeutic actions of boswellic acids: a mini-review. Asian Pacific Journal of Tropical Biomedicine . 2017;7(6):513–523. doi: 10.1016/j.apjtb.2017.05.001. [DOI] [Google Scholar]
- 21.Al-Dhubiab B. E., Patel S. S., Morsy M. A., et al. The beneficial effect of boswellic acid on bone metabolism and possible mechanisms of action in experimental osteoporosis. Nutrients . 2020;12(10):p. 3186. doi: 10.3390/nu12103186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan C. Y., Mong M. C., Liu W. H., Huang C. Y., Yin M. C. Three pentacyclic triterpenes protect H9c2 cardiomyoblast cells against high-glucose-induced injury. Free Radical Research . 2014;48(4):402–411. doi: 10.3109/10715762.2014.880113. [DOI] [PubMed] [Google Scholar]
- 23.Elshazly S. M., Abd El Motteleb D. M., Nassar N. N. The selective 5-LOX inhibitor 11-keto-β-boswellic acid protects against myocardial ischemia reperfusion injury in rats: involvement of redox and inflammatory cascades. Naunyn-Schmiedeberg’s archives of pharmacology . 2013;386(9):823–833. doi: 10.1007/s00210-013-0885-9. [DOI] [PubMed] [Google Scholar]
- 24.Baradaran Rahimi V., Rajabian A., Rajabi H., et al. The effects of hydro-ethanolic extract of capparis spinosa (C. spinosa) on lipopolysaccharide (LPS)-induced inflammation and cognitive impairment: evidence from in vivo and in vitro studies. Journal of Ethnopharmacology . 2020;256 doi: 10.1016/j.jep.2020.112706.112706 [DOI] [PubMed] [Google Scholar]
- 25.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry . 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Rahmanian-Devin P., Rakhshandeh H., Baradaran Rahimi V., et al. Intraperitoneal lavage with Crocus sativus prevents postoperative-induced peritoneal adhesion in a rat model: evidence from animal and cellular studies. Oxidative Medicine and Cellular Longevity . 2021;2021 doi: 10.1155/2021/5945101.5945101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaafari A., Baradaran Rahimi V., Vahdati-Mashhadian N., et al. Evaluation of the therapeutic effects of the hydroethanolic extract of portulaca oleracea on surgical-induced peritoneal adhesion. Mediators Inflamm . 2021;2021:18. doi: 10.1155/2021/8437753.8437753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghadiri M., Baradaran Rahimi V., Moradi E., et al. Standardised pomegranate peel extract lavage prevents postoperative peritoneal adhesion by regulating TGF-β and VEGF levels. Inflammopharmacology . 2021;29(3):855–868. doi: 10.1007/s10787-021-00819-6. [DOI] [PubMed] [Google Scholar]
- 29.Peinnequin A., Mouret C., Birot O., et al. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunology . 2004;5(1):p. 3. doi: 10.1186/1471-2172-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J., Zhang X., Broderick M., Fein H. Measurement of nitric oxide production in biological systems by using griess reaction assay. Sensors . 2003;3(8):276–284. doi: 10.3390/s30800276. [DOI] [Google Scholar]
- 31.Rahman I., Kode A., Biswas S. K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nature Protocols . 2006;1(6):3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 32.Lin H., Wang W., Lee M., Meng Q., Ren H. Current status of septic cardiomyopathy: basic science and clinical progress. Frontiers in Pharmacology . 2020;11:p. 210. doi: 10.3389/fphar.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., Liu P., Feng X., Ma C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. Journal of Cellular and Molecular Medicine . 2017;21(12):3178–3189. doi: 10.1111/jcmm.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan S., Long Z., Hou X., et al. H2 protects against lipopolysaccharide-induced cardiac dysfunction via blocking TLR4-Mediated cytokines expression. Frontiers in Pharmacology . 2019;10:p. 865. doi: 10.3389/fphar.2019.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong W., Chen X., Song X., et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Experimental and Therapeutic Medicine . 2020;19(3):1824–1834. doi: 10.3892/etm.2019.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng Y.-Y., Liu Y., Hu Z.-F., et al. Sanguinarine attenuates lipopolysaccharide-induced inflammation and apoptosis by inhibiting the TLR4/NF-κB pathway in H9c2 cardiomyocytes. Current Medical Science . 2018;38(2):204–211. doi: 10.1007/s11596-018-1867-4. [DOI] [PubMed] [Google Scholar]
- 37.Sun L.-j., Qiao W., Xiao Y.-j., Cui L., Wang X., Ren W.-d. Naringin mitigates myocardial strain and the inflammatory response in sepsis-induced myocardial dysfunction through regulation of PI3K/AKT/NF-κB pathway. International Immunopharmacology . 2019;75 doi: 10.1016/j.intimp.2019.105782.105782 [DOI] [PubMed] [Google Scholar]
- 38.An J., Du J., Wei N., Guan T., Camara A. K. S., Shi Y. Differential sensitivity to LPS-induced myocardial dysfunction in the isolated brown norway and DAHL S rat hearts. Shock . 2012;37(3):325–332. doi: 10.1097/shk.0b013e31823f146f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao P., Wang Y., Zeng S., Lu J., Jiang T.-M., Li Y.-M. Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacology and Immunotoxicology . 2015;37(5):428–433. doi: 10.3109/08923973.2015.1080266. [DOI] [PubMed] [Google Scholar]
- 40.Viswanadha V. P., Dhivya V., Beeraka N. M., et al. The protective effect of piperine against isoproterenol-induced inflammation in experimental models of myocardial toxicity. European Journal of Pharmacology . 2020;885 doi: 10.1016/j.ejphar.2020.173524.173524 [DOI] [PubMed] [Google Scholar]
- 41.Yousif N. G., Hadi N. R., Al-Amran F., Zigam Q. A. Cardioprotective effects of irbesartan in polymicrobial sepsis. Herz . 2018;43(2):140–145. doi: 10.1007/s00059-017-4537-6. [DOI] [PubMed] [Google Scholar]
- 42.Zeng M., Li M., Chen Y., et al. A new bisepoxylignan dendranlignan A isolated from chrysanthemum flower inhibits the production of inflammatory mediators via the TLR4 pathway in LPS-induced H9c2 cardiomyocytes. Archives of Biochemistry and Biophysics . 2020;690 doi: 10.1016/j.abb.2020.108506.108506 [DOI] [PubMed] [Google Scholar]
- 43.Sayed A. S., El Sayed N. S. E. D. Co-administration of 3-acetyl-11-keto-beta-boswellic acid potentiates the protective effect of celecoxib in lipopolysaccharide-induced cognitive impairment in mice: possible implication of anti-inflammatory and antiglutamatergic pathways. Journal of Molecular Neuroscience . 2016;59(1):58–67. doi: 10.1007/s12031-016-0734-7. [DOI] [PubMed] [Google Scholar]
- 44.Wei C., Fan J., Sun X., et al. Acetyl-11-keto-β-boswellic acid ameliorates cognitive deficits and reduces amyloid-β levels in APPswe/PS1dE9 mice through antioxidant and anti-inflammatory pathways. Free Radical Biology and Medicine . 2020;150 doi: 10.1016/j.freeradbiomed.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad S., Khan S. A., Kindelin A., et al. Acetyl-11-keto-β-boswellic acid (AKBA) attenuates oxidative stress, inflammation, complement activation and cell death in brain endothelial cells following OGD/reperfusion. NeuroMolecular Medicine . 2019;21(4):505–516. doi: 10.1007/s12017-019-08569-z. [DOI] [PubMed] [Google Scholar]
- 46.Lu L. Y., Loi F., Nathan K., et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. Journal of Orthopaedic Research . 2017;35(11):2378–2385. doi: 10.1002/jor.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S.-B., Lee W. S., Shin J.-S., Jang D. S., Lee K. T. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW 264.7 macrophages. International Immunopharmacology . 2017;49:21–29. doi: 10.1016/j.intimp.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Gamal El-Din M. M., El-Gamal M. I., Abdel-Maksoud M. S., et al. Inhibitory effects of triarylpyrazole derivatives on LPS-induced nitric oxide and PGE2 productions in murine RAW 264.7 macrophages. Bioorganic & Medicinal Chemistry Letters . 2020;30(4) doi: 10.1016/j.bmcl.2019.126884.126884 [DOI] [PubMed] [Google Scholar]
- 49.Rahmanian-Devin P., Baradaran Rahimi V., Jaafari M. R., Golmohammadzadeh S., Sanei-far Z., Askari V. R. Noscapine, an emerging medication for different diseases: a mechanistic review. Evidence-based Complementary and Alternative Medicine . 2021;2021:16. doi: 10.1155/2021/8402517.8402517 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Mukherjee D., Nissen S. E., Topol E. J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA . 2001;286(8):954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 51.Cao H., Yu R., Choi Y., et al. Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation. Pharmacological Research . 2010;61(6):519–524. doi: 10.1016/j.phrs.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franceschelli S., Pesce M., Ferrone A., et al. Biological effect of Licochalcone C on the regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO signaling pathways in H9c2 cells in response to LPS stimulation. International Journal of Molecular Sciences . 2017;18(4):p. 690. doi: 10.3390/ijms18040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durand A., Duburcq T., Dekeyser T., et al. Involvement of mitochondrial disorders in septic cardiomyopathy. Oxidative medicine and cellular longevity . 2017;2017:13. doi: 10.1155/2017/4076348.4076348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H. M.-D., Fu L., Cheng C. C., et al. Inhibition of LPS-induced oxidative damages and potential anti-inflammatory effects of Phyllanthus emblica extract via down-regulating NF-κB, COX-2, and iNOS in RAW 264.7 cells. Antioxidants . 2019;8(8):p. 270. doi: 10.3390/antiox8080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhardwaj P., Kumar M., Dhatwalia S. K., Garg M. L., Dhawan D. K. Acetyl-11-keto-β-boswellic acid modulates membrane dynamics in benzo pyrene-induced lung carcinogenesis. Molecular and Cellular Biochemistry . 2019;460:17–27. doi: 10.1007/s11010-019-03566-z. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P.-Y., Yu B., Men W.-J., et al. Acetyl-α-boswellic acid and Acetyl-β-boswellic acid protects against caerulein-induced pancreatitis via down-regulating MAPKs in mice. International Immunopharmacology . 2020;86 doi: 10.1016/j.intimp.2020.106682.106682 [DOI] [PubMed] [Google Scholar]
- 57.Bajic V. P., Van Neste C., Obradovic M., et al. Glutathione “redox homeostasis” and its relation to cardiovascular disease. Oxidative medicine and cellular longevity . 2019;2019:14. doi: 10.1155/2019/5028181.5028181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones J. T., Qian X., van der Velden J. L. J., et al. Glutathione S-transferase pi modulates NF-κB activation and pro-inflammatory responses in lung epithelial cells. Redox Biology . 2016;8:375–382. doi: 10.1016/j.redox.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsolaki V., Makris D., Mantzarlis K., Zakynthinos E. Sepsis-induced cardiomyopathy: oxidative implications in the initiation and resolution of the damage. Oxidative medicine and cellular longevity . 2017;2017:11. doi: 10.1155/2017/7393525.7393525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan H.-J., Tan Z.-B., Wu Y.-T., et al. The role of ginsenoside Rb1, a potential natural glutathione reductase agonist, in preventing oxidative stress-induced apoptosis of H9C2 cells. Journal of Ginseng Research . 2020;44(2):258–266. doi: 10.1016/j.jgr.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartmann R. M., Morgan Martins M. I., Tieppo J., Fillmann H. S., Marroni N. P. Effect of Boswellia serrata on antioxidant status in an experimental model of colitis rats induced by acetic acid. Digestive Diseases and Sciences . 2012;57(8):2038–2044. doi: 10.1007/s10620-012-2134-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.


