Abstract
The efficacy of SCH27899, a new everninomicin antibiotic, against replicative Legionella pneumophila lung infections in an immunocompromised host was evaluated using a murine model of Legionnaires' disease. A/J mice were immunocompromised with cortisone acetate and inoculated intratracheally with L. pneumophila serogroup 1 (105 CFU per mouse). At 24 h postinoculation, mice were administered either SCH27899 (6 to 60 mg/kg [MPK] intravenously) or a placebo once daily for 5 days, and mortality and intrapulmonary growth of L. pneumophila were assessed. In the absence of SCH27899, there was 100% mortality in L. pneumophila-infected mice, with exponential intrapulmonary growth of the bacteria. In contrast, administration of SCH27899 at a dose of ≥30 MPK resulted in ≥90% survival of infected mice, which was associated with inhibition of intrapulmonary growth of L. pneumophila. In subsequent studies, the efficacy of SCH27899 was compared to ofloxacin (OFX) and azithromycin (AZI). Administration of SCH27899, OFX, or AZI at a dose of ≥30 MPK once daily for 5 days resulted in ≥85% survival of infected mice and inhibition of intrapulmonary growth of the bacteria. However, L. pneumophila CFU were recovered in lung homogenates following cessation of therapy with all three antibiotics. These studies demonstrate that SCH27899 effectively prevents fatal replicative L. pneumophila lung infection in immunocompromised A/J mice by inhibition of intrapulmonary growth of the bacteria. However, in this murine model of pulmonary legionellosis, SCH27899, like OFX and AZI, was bacteriostatic.
Legionella pneumophila, the causative agent of Legionnaires' disease, is a facultative intracellular pathogen of mononuclear phagocytic cells (7, 9, 10). While all persons are susceptible to legionellosis, the frequency and severity of the disease are greatest in immunocompromised patients, with transplant recipients having the highest risk (2, 11). These patients frequently develop severe disseminated L. pneumophila infections, which may persist and/or recur despite antibiotic therapy (6). Furthermore, administration of antibiotics such as macrolides and rifampin, used to treat L. pneumophila-infected immunocompetent patients, may be contraindicated in similarly infected transplant patients, as these agents can alter the efficacy of drugs used to counter rejection (2). Therefore, identification of new antimicrobials which are effective against L. pneumophila infections in immunocompromised patients is needed.
SCH27899 is a newly described everninomicin antibiotic with a wide spectrum of activity against gram-positive bacteria, including methicillin-resistant staphylococci, vancomycin-resistant enterococci, and penicillin-resistant streptococci (R. S. Hare and F. J. Sabatelli, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., E-119, 1998). Recent in vitro studies using a cell-free system have demonstrated that SCH27899 is also active against a narrow spectrum of gram-negative organisms, including L. pneumophila (4). However, the efficacy of SCH27899 against L. pneumophila in vivo has not been assessed.
We have previously developed a model of replicative L. pneumophila lung infection in immunocompetent A/J mice which mimics the pathogenesis of legionellosis in immunocompetent humans (1). Because immunosuppression induced by cortisone acetate (CA) predisposes humans to legionellosis, we hypothesized that CA administration to A/J mice would result in enhanced susceptibility to a severe replicative L. pneumophila infection. Furthermore, because of their small size (thereby requiring relatively little experimental compound), ease of handling, and low cost, we hypothesized that this model of legionellosis in CA-treated A/J mice would provide a valuable tool to evaluate the efficacy of antimicrobials, including SCH27899, in the treatment of legionellosis in an immunocompromised host.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
A virulent strain of L. pneumophila serogroup 1 (strain AA100), a rederivation of a primary clinical isolate from the Wadsworth Veterans Administration Hospital, was provided by Paul Edelstein. The bacteria were maintained and passaged on buffered charcoal-yeast extract (BCYE) agar (Becton Dickinson, Cockeysville, Md.) (3, 5). For preparation of inocula, L. pneumophila was grown for 48 h on BCYE agar plates and resuspended in the appropriate media (buffered yeast extract broth for in vitro studies; saline for in vivo studies) at the desired concentration, based on optical density at 595 nm. The number of CFU per inoculum was confirmed by enumeration of the bacteria on BCYE agar.
Antimicrobial agents.
Erythromycin lactobionate USP (ERY), azithromycin (AZI), and ofloxacin (OFX) were obtained from Neuman Distributors, Inc. SCH27899 was manufactured at Schering Plough Research Institute. ERY, AZI, and OFX were diluted in sterile water; SCH27899 was diluted in its placebo.
In vitro susceptibility testing.
Microbroth dilution susceptibility testing of extracellular L. pneumophila to SCH27899, ERY, OFX, and AZI was performed using buffered yeast extract broth with a final volume of 200 μl and a bacterial concentration of 5 × 105 CFU/well. Plates were read after a 48-h incubation at 37°C. All testing was done in duplicate.
Animal care.
Female pathogen-free 8-week-old A/J mice (Jackson Laboratories, Bar Harbor, Maine) were used in all experiments. Animals were housed in microisolator cages and were cared for in accordance with standard guidelines.
Induction of immunosuppression in A/J mice.
Beginning one day prior to intratracheal (IT) inoculation of bacteria, mice were administered CA (100 mg/kg [MPK] subcutaneously once daily [OD] for 3 consecutive days), followed by a similar single injection at 6 days postinfection (D. Loebenberg, F. Menzel, E. Corcoran, K. Raynor, J. Halpern, A. F. Cacciapuoti, and R. S. Hare, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-063, 1998). Control mice were inoculated with an equivalent volume of saline.
Intratracheal inoculation of A/J mice with L. pneumophila.
Immunocompetent and CA-compromised A/J mice were inoculated IT with L. pneumophila as previously described (1, 12). Briefly, mice were anesthetized with ketamine (2.5 mg/mouse intraperitoneally) and tethered. An incision was made through the skin of the ventral neck. The trachea was isolated and the desired concentration of L. pneumophila (104 to 106 CFU/mouse) in 25 μl of phosphate-buffered saline, followed by 10 μl of air, was injected directly into the trachea using a 26-gauge needle. This range of inocula was chosen since it has been previously demonstrated that the maximal nonlethal IT dose of L. pneumophila in immunocompetent A/J mice is 106 CFU/mouse (1). The incision was closed with a sterile wound clip.
Antibiotic therapy.
Twenty-four hours after IT inoculation, L. pneumophila-infected immunocompromised A/J mice were administered SCH27899 (6, 9, 15, 30, or 60 MPK intravenously [i.v.] via the tail vein), OFX (3 or 30 MPK orally [p.o.] via gavage), or AZI (3 or 30 MPK p.o. via gavage) for the desired dose interval and duration. Control mice were administered an equivalent volume of SCH27899 placebo. All mice were assessed for morbidity, mortality, and intrapulmonary growth of L. pneumophila.
Quantification of L. pneumophila in lung homogenates from immunocompromised A/J mice.
At specific time points postinoculation (p.i.), L. pneumophila-infected mice were humanely euthanized and lungs were removed. Lung tissue was finely minced in sterile water (10 ml/lung) and homogenized. Lung homogenates were serially diluted in sterile water and cultured on BCYE agar containing polymyxin B, anisomycin, and cefamandole (BCYE plus PAC; Becton Dickinson) for 72 h (1). The lower limit of detection of L. pneumophila in this system was 102 CFU/lung.
Statement of animal care and use.
These studies were carried out in accordance with the NIH Guide to the Care and Use of Laboratory Animals (10a) and the Animal Welfare Act in a program accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Statistical analysis.
The t test, the one-way analysis of variance, and the Wilcoxon test were used to compare differences between treatment groups. A value of P <0.05 was considered significant.
RESULTS
SCH27899 inhibits growth of L. pneumophila in vitro.
In initial assays, the effect of SCH27899 on growth of L. pneumophila was assessed in vitro by broth microdilution susceptibility testing. In agreement with previous studies (4), SCH27899 inhibited growth of L. pneumophila, with a MIC of 0.008 μg/ml, as compared to those of ERY (0.032 μg/ml), OFX (0.016 μg/ml), and AZI (0.032 μg/ml).
CA-mediated immunosuppression enhances A/J mouse susceptibility to fatal legionellosis.
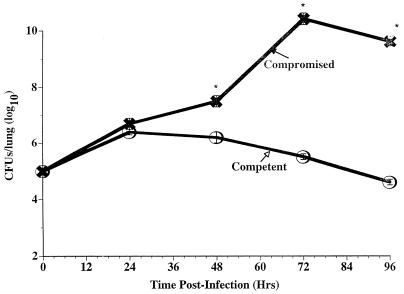
Mortality and intrapulmonary growth of L. pneumophila were compared in infected immunocompetent and immunocompromised A/J mice. While there was 100% survival in L. pneumophila-infected immunocompetent mice administered ≤106 bacteria IT, inoculation of CA-treated A/J mice with ≥105 bacteria resulted in 100% mortality within 7 days postinfection. Furthermore, as shown in Fig. 1, survival of L. pneumophila-infected immunocompetent mice (inoculated IT with 105 CFU of L. pneumophila) was associated with a decrease in CFU in lung homogenates within 96 h p.i., while mortality in similarly infected immunocompromised mice was associated with a ≥4 log increase in CFU in the lung within 4 days p.i. Results of these studies established a minimum lethal dose of 105 L. pneumophila CFU IT in immunocompromised A/J mice and confirmed that mortality in CA-treated A/J mice was associated with exponential intrapulmonary growth of bacteria. All subsequent studies were conducted using an IT inoculum of 105 L. pneumophila CFU/mouse.
FIG. 1.
Intrapulmonary growth of L. pneumophila in immunocompetent and immunocompromised A/J mice. A/J mice were administered CA (100 MPK) or an equivalent volume of saline subcutaneously and inoculated IT with L. pneumophila (105 CFU/mouse). At specific time points p.i. mice were humanely euthanized, lungs were excised, and intrapulmonary L. pneumophila was quantified by culture of lung homogenates. Results represent the mean of bacteria in the lungs of 4 to 6 mice per time point. ∗, significantly enhanced growth compared to similarly infected competent mice.
SCH27899 therapy enhances survival of L. pneumophila-infected A/J mice by inhibition of intrapulmonary growth of bacteria.
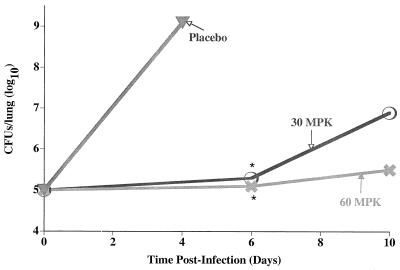
In subsequent in vivo studies, the effect of SCH27899 on the pathogenesis of replicative L. pneumophila lung infection was assessed. As shown in Table 1, treatment of mice with ≥30 MPK of SCH27899 resulted in ≥90% survival at 6 days p.i., compared to 100% mortality in similarly infected mice administered placebo. In subsequent studies, the effect of SCH27899 on intrapulmonary growth of L. pneumophila was determined. As shown in Fig. 2, L. pneumophila grew exponentially in mice administered placebo and was associated with 100% mortality within 5 days p.i. In contrast, administration of SCH27899 (30 or 60 MPK OD for 5 days) to similarly infected mice resulted in inhibition of intrapulmonary growth of the bacteria and 100% survival. Furthermore, as shown in Table 2, administration of SCH27899 (total daily dose, 60 MPK) at either a 12- or 24-h dosing interval significantly enhanced the survival of mice compared to similarly infected mice administered placebo. There was no significant difference in recovery of intrapulmonary L. pneumophila from the different treatment groups, indicating that the dosing interval had a minimal effect on SCH27899 activity.
TABLE 1.
| Treatment | % Survival |
|---|---|
| Placebo | 0 |
| SCH (6 MPK) | 0 |
| SCH (9 MPK) | 20 |
| SCH (15 MPK) | 60 |
| SCH (30 MPK) | 90 |
| SCH (60 MPK) | 100 |
A/J mice were immunocompromised with CA and inoculated IT with L. pneumophila (105 CFU/mouse). At 24 h p.i., mice were administered either SCH27899 (SCH) (6 to 60 MPK OD i.v.) or an equivalent volume of placebo (OD i.v.) for 5 days and observed for mortality. Results represent the percentage of survival among 10 mice per treatment group at 6 days p.i.
FIG. 2.
Effect of SCH27899 (30 or 60 MPK OD for 5 days) on intrapulmonary growth of L. pneumophila. A/J mice were administered CA (100 MPK) subcutaneously and inoculated IT with L. pneumophila (105 CFU/mouse). At 24 h p.i., immunocompromised infected mice were administered SCH27899 (30 or 60 MPK i.v. OD) for 5 days or an equivalent volume of placebo for 5 days. Mice were subsequently humanely euthanized at 6 or 10 days p.i., lungs were excised and homogenized, and intrapulmonary L. pneumophila was quantified by culture of lung homogenates. Results represent the mean of bacteria in the lungs of 6 to 8 mice per time point. ∗, significantly less growth compared to similarly infected compromised untreated mice.
TABLE 2.
Evaluation of dosing intervals of SCH27899 on survival and intrapulmonary growth of L. pneumophila in immunocompromised A/J micea
| Treatment | Dosing interval (h) | % Survival | CFU/lung (log10) |
|---|---|---|---|
| SCH | 12 | 85 | 5.53 ± 0.2b |
| SCH | 24 | 100 | 5.40 ± 0.2b |
| Placebo | 12 | 14 | 9.22 ± 0.1 |
| Placebo | 24 | 14 | 9.55 ± 0.2 |
A/J mice were immunocompromised and inoculated IT with L. pneumophila (105 CFU/mouse). At 24 h p.i., mice were administered a total daily dose of SCH27899 (SCH) (60 MPK i.v.), either as a single dose (60 MPK every 24 h) or as a divided dose (30 MPK every 12 h) for 3 days. Control mice were administered an equivalent volume of placebo. Mice were observed for 4 days for mortality, surviving mice were humanely euthanized, lungs were excised, and intrapulmonary L. pneumophila CFU were quantified by culture of lung homogenates. Results represent the percentage of survival and mean ± standard error of the mean of bacteria in the lungs of 7 to 8 mice per time point.
Significantly decreased growth when compared to similarly infected mice administered placebo.
Efficacy of SCH27899 as compared to OFX or AZI in the treatment of legionellosis in the immunocompromised host.
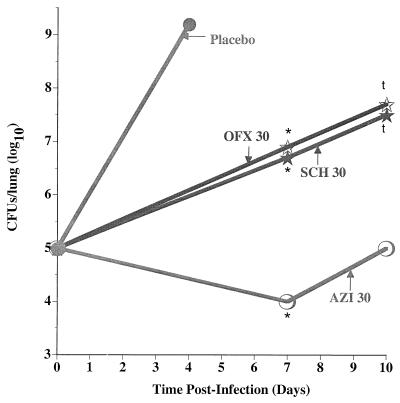
The relative efficacy of SCH27899 in the treatment of replicative L. pneumophila lung infections in immunocompromised A/J mice was compared to that of OFX and AZI. As shown in Table 3, administration of SCH27899, OFX, or AZI significantly enhanced survival in a dose-dependent manner. In subsequent studies, the effects of SCH27899, OFX, and AZI therapy on intrapulmonary growth of L. pneumophila were assessed. As shown in Fig. 3, administration of either SCH27899, OFX, or AZI resulted in significant inhibition of intrapulmonary growth of bacteria at 7 days p.i. (compared to similarly infected mice administered placebo), with significantly fewer CFU recovered from mice treated with AZI. Five days following cessation of antibiotic therapy (i.e., day 10 p.i.), significantly fewer CFU were recovered in lung homogenates from mice treated with AZI than from mice administered either OFX or SCH27899. However, there was regrowth of L. pneumophila in lung homogenates from mice administered all three drugs at 10 days p.i. compared to day 7 p.i., indicating that all three antibiotics were bacteriostatic.
TABLE 3.
Evaluation of SCH27899, OFX, and AZI therapy on survival of L. pneumophila-infected immunocompromised A/J micea
| Treatment | % Survival |
|---|---|
| Placebo | 0 |
| SCH (30 MPK) | 85 |
| SCH (60 MPK) | 92 |
| OFX (3 MPK) | 57 |
| OFX (30 MPK) | 92 |
| AZI (3 MPK) | 64 |
| AZI (30 MPK) | 92 |
Mice were immunocompromised and inoculated IT with L. pneumophila (105 bacteria/mouse). At 24 h p.i., mice were administered either SCH27899 (SCH) (30 or 60 MPK i.v.), OFX (3 or 30 MPK p.o.), or AZI (3 or 30 MPK p.o.) once daily for 5 days. Survival of mice was compared at 10 days p.i. Results represent the percentage of survival among 14 mice per treatment group.
FIG. 3.
Effect of SCH27899, AZI, or OFX on intrapulmonary growth of L. pneumophila. A/J mice were administered CA (100 MPK) subcutaneously and inoculated IT with L. pneumophila (105 CFU/mouse). At 24 h p.i., infected mice were administered either SCH27899 (30 MPK i.v.), OFX (30 MPK p.o.), or AZI (30 MPK p.o.) for 5 days or an equivalent volume of placebo for 5 days. Mice were subsequently humanely euthanized at 7 or 10 days p.i., lungs were excised and homogenized, and intrapulmonary L. pneumophila was quantified by culture of lung homogenates. Results represent the mean of bacteria in the lung of 7 to 8 mice per time point. ∗, significantly less growth compared to similarly infected mice administered placebo; t, significantly enhanced growth compared to AZI.
DISCUSSION
The efficacy of SCH27899, a new everninomicin antibiotic, against replicative L. pneumophila lung infection was evaluated with a murine model of legionellosis in immunocompromised A/J mice. While the activity of SCH27899 against a wide spectrum of gram-positive organisms is well documented (Hare and Sabatelli, 38th ICAAC), results of recent in vitro studies by our laboratory and others (4) have demonstrated that SCH27899 also effectively inhibits replication of selective gram-negative pathogens, including L. pneumophila. However, the therapeutic efficacy of SCH27899 in treatment of replicative L. pneumophila infections in vivo has not been previously investigated.
To evaluate the efficacy of SCH27899 against L. pneumophila infection in vivo, an animal model of legionellosis was developed in A/J mice immunocompromised by CA administration. We have previously demonstrated that immunocompetent A/J mice inoculated IT with L. pneumophila develop replicative lung infections which resolve spontaneously in the absence of antibiotic therapy (1). Results of the current study demonstrate that similarly infected immunocompromised A/J mice, like immunocompromised humans, have enhanced susceptibility to fatal replicative L. pneumophila infections. This animal model of legionellosis in immunocompromised A/J mice, therefore, provides a reliable and reproducible system to evaluate antibiotic efficacy against L. pneumophila infections in vivo.
Using this animal model, the efficacy of SCH27899 in the treatment of replicative L. pneumophila lung infections in an immunocompromised host was evaluated. SCH27899 (at a dose of ≥30 MPK) effectively enhanced survival of L. pneumophila-infected immunocompromised A/J mice by inhibiting intrapulmonary growth of the bacteria. Results of additional studies evaluating the effect of dosing interval on drug efficacy (Table 2) support the case of OD dosing of SCH27899 in the immunocompromised host. Subsequent comparative studies with OFX and AZI demonstrated that SCH27899 was as effective as these antibiotics in preventing fatal legionellosis in immunocompromised A/J mice. Enhanced survival in mice treated with all three antibiotics was associated with inhibition of intrapulmonary growth of the bacteria. However, significantly fewer CFU were recovered in lung homogenates from mice administered AZI than similarly infected mice administered SCH27899 or OFX. While the reason for this apparent enhanced efficacy of AZI has not been thoroughly investigated, AZI has previously been shown to achieve high concentrations in tissues, due in part to its direct uptake by, and targeted delivery to, inflammatory sites by recruited phagocytes (8). Furthermore, due to the drug's long half-life, high concentrations of AZI in tissue are maintained for prolonged periods of time (8). Therefore, it is possible that decreased recovery of viable bacteria from lungs of AZI-treated mice may be due, at least in part, to exposure of the bacteria to relatively large concentrations of residual drug in lung homogenates. Of potentially greater significance was the recovery of L. pneumophila CFU in lung homogenates from mice euthanized several days after cessation of antibiotic therapy with either SCH27899, OFX, or AZI. These results demonstrated that in this murine model system, all three antibiotics were bacteriostatic, rather than bactericidal.
The pharmacokinetics of SCH27899 in mice have recently been evaluated by several investigators (C. Lin, S. Gupta, D. Loebenberg, and M. N. Cayen, unpublished data; O. Vesga and W. A. Craig, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-032, 1997; E. Wang, Y. Bergeron, M. Simard, M. Cote-Richer, and M. G. Bergeron, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-040, 1998). These studies demonstrated that SCH27899 (i) has a half-life of 2 to 4 h, (ii) exhibited high protein binding in serum (>95%), (iii) attained concentrations in plasma and lung (>1 μg/ml or >1 μg/g for >24 h following a single dose of SCH27899 at 30 MPK i.v.), (iv) has a pharmacokinetic profile that was not altered significantly by infection or immunosuppression, and (v) is the pharmacokinetic/pharmacodynamic parameter area under the concentration-time curve/MIC ratio that showed the best correlation with in vivo efficacy. Based on results of these studies, it is likely that the efficacy of SCH27899 (at a dose of ≥30 MPK) in the murine model of legionellosis is due, at least in part, to the low MIC, the high area under the concentration-time curve/MIC ratio, and/or concentrations in the lung which exceeded the MIC for more than 24 h.
In summary, replicative L. pneumophila lung infections remain a significant cause of morbidity and mortality in immunocompromised patients. Choices of antibiotic therapy for this patient population are limited. Results of the current study with an animal model of Legionnaires' disease in immunocompromised A/J mice demonstrate that administration of SCH27899 significantly enhances survival in the immunocompromised infected host by inhibiting intrapulmonary growth of L. pneumophila.
ACKNOWLEDGMENTS
This study was supported by Schering Plough Research Institute.
We thank Bruce Belanger for his expertise in biostatistics.
REFERENCES
- 1.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires' disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 2.Chow J W, Yu V L. Legionella: a major opportunistic pathogen in transplant recipients. Semin Respir Infect. 1998;13:132–139. [PubMed] [Google Scholar]
- 3.Edelstein P H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelstein P H, Edelstein M A C. In vitro activity of SCH 27899 (Ziracin) against 104 Legionella species. Diagn Microbiol Infect Dis. 1999;33:59–62. doi: 10.1016/s0732-8893(98)00106-0. [DOI] [PubMed] [Google Scholar]
- 5.Feeley J C, Gibson R J, Gorman G W, Langford N C, Raheed J K, Mackel D C, Baine W B. Charcoal-yeast extract agar: primary medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington R D, Woolfrey A E, Bowden R, McDowell M G, Hackman R C. Legionellosis in a bone marrow transplant center. Bone Marrow Transplant. 1996;18:361–368. [PubMed] [Google Scholar]
- 7.Horwitz M A. Phagocytosis of Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1984;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lode H, Borner K, Koeppe P, Schaberg T. Azithromycin—review of key chemical, pharmacokinetic and microbiological features. J Antimicrob Chemother. 1996;37(Suppl. C):1–8. doi: 10.1093/jac/37.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 9.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 10.Nash T W, Libby D M, Horwitz M A. Interaction between Legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines and hydrocortisone. J Clin Investig. 1984;74:771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.National Research Council. NIH guide to the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 11.Schlossberg D, Bonan J. Legionella and immunosuppression. Semin Respir Infect. 1998;13:128–131. [PubMed] [Google Scholar]
- 12.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–1441. [PubMed] [Google Scholar]