Abstract
Aims
REVEAL was the first randomized controlled trial to demonstrate that adding cholesteryl ester transfer protein inhibitor therapy to intensive statin therapy reduced the risk of major coronary events. We now report results from extended follow-up beyond the scheduled study treatment period.
Methods and results
A total of 30 449 adults with prior atherosclerotic vascular disease were randomly allocated to anacetrapib 100 mg daily or matching placebo, in addition to open-label atorvastatin therapy. After stopping the randomly allocated treatment, 26 129 survivors entered a post-trial follow-up period, blind to their original treatment allocation. The primary outcome was first post-randomization major coronary event (i.e. coronary death, myocardial infarction, or coronary revascularization) during the in-trial and post-trial treatment periods, with analysis by intention-to-treat. Allocation to anacetrapib conferred a 9% [95% confidence interval (CI) 3–15%; P = 0.004] proportional reduction in the incidence of major coronary events during the study treatment period (median 4.1 years). During extended follow-up (median 2.2 years), there was a further 20% (95% CI 10–29%; P < 0.001) reduction. Overall, there was a 12% (95% CI 7–17%, P < 0.001) proportional reduction in major coronary events during the overall follow-up period (median 6.3 years), corresponding to a 1.8% (95% CI 1.0–2.6%) absolute reduction. There were no significant effects on non-vascular mortality, site-specific cancer, or other serious adverse events. Morbidity follow-up was obtained for 25 784 (99%) participants.
Conclusion
The beneficial effects of anacetrapib on major coronary events increased with longer follow-up, and no adverse effects emerged on non-vascular mortality or morbidity. These findings illustrate the importance of sufficiently long treatment and follow-up duration in randomized trials of lipid-modifying agents to assess their full benefits and potential harms.
Trial registration
International Standard Randomized Controlled Trial Number (ISRCTN) 48678192; ClinicalTrials.gov No. NCT01252953; EudraCT No. 2010-023467-18.
Keywords: CETP inhibitor therapy, Randomized trial
Structured Graphical Abstract
See the editorial comment for this article ‘CETP inhibitors revisited’, by Ulrich Laufs and Thimoteus Speer, https://doi.org/10.1093/eurheartj/ehab889.
Introduction
HPS3/TIMI55-REVEAL was the first randomized controlled trial to demonstrate that adding cholesteryl ester transfer protein (CETP) inhibitor therapy to intensive statin therapy reduced the risk of major coronary events. Compared with placebo, anacetrapib produced a 9% [95% confidence interval (CI) 3–15%; P = 0.004] proportional reduction in the incidence of major coronary events during a median follow-up period of 4.1 years.1 , 2 Anacetrapib is a potent inhibitor of CETP that increases circulating levels of high-density lipoprotein (HDL) cholesterol and lowers the levels of non-HDL cholesterol [particularly low-density lipoprotein (LDL) cholesterol]. It is highly lipophilic and accumulates in adipose tissue during continued dosing, creating a reservoir that leads to its slow elimination from the peripheral circulation.3 As a consequence, residual lipid-modifying effects—albeit substantially reduced—are observed for some years after stopping prolonged treatment.4 , 5
We now report efficacy and safety results from an additional 2 years of follow-up following the discontinuation of the randomized anacetrapib treatment or matching placebo in REVEAL.
Methods
Patients
The design of REVEAL has been reported previously1 , 2 and is described in detail, together with the plan for post-trial follow-up, in Supplementary material online, Appendix S1. Between August 2011 and October 2013, men and women aged 50 years or older with pre-existing atherosclerotic vascular disease were randomly allocated to receive anacetrapib 100 mg daily or a matching placebo after having been given an open-label atorvastatin regimen intended to lower their pre-randomization LDL cholesterol to below 77 mg/dL (2.0 mmol/L). At their final follow-up visits between July 2016 and January 2017, the randomized anacetrapib or placebo and the study atorvastatin were stopped, and participants were advised to discuss their future non-trial LDL-lowering treatment regimen with their family doctor or cardiologist.
All surviving participants who had a final follow-up visit in person or by telephone were eligible to take part in the post-trial follow-up study. Participants who withdrew consent or were lost to follow-up during the in-trial period, and those followed at the time of their final follow-up visit via a relative, carer, or medical record review, were excluded. In addition, 70 of the 431 study sites declined involvement in the extended follow-up study. We were able to follow participants who attended 58 of those sites via another local study site or their regional co-ordinating centre; however, it was not possible to follow-up 194 participants (95 in the anacetrapib group vs. 99 participants in the placebo group) from the remaining 12 sites.
In order to yield unbiased assessments of the longer-term effects of anacetrapib, participants and their doctors (as well as trial personnel) remained blinded to the previous randomized study treatment unless there was a particular requirement to know it. During the combined follow-up period, only 112/30 449 (0.4%) participants were unblinded (55 in the anacetrapib arm vs. 57 in the placebo arm).
Procedures and follow-up
During the in-trial on-treatment period, routine follow-up visits were scheduled at 2 and 6 months after randomization, and every 6 months thereafter until the end of the treatment phase of the trial. Information was sought about all serious adverse events (including trial outcomes), non-serious adverse events attributed to the study treatment that resulted in its discontinuation, and any symptoms suggestive of hepatitis or myopathy. Other non-serious adverse events were recorded only for participants in North America. Blood samples were checked at every visit for evidence of liver or muscle injury. At selected visits, samples were sent for central analysis (including blood lipids) and archiving to allow subsequent assays. During the in-trial period, it emerged that LDL cholesterol levels measured by a direct assay may be underestimated in those treated with anacetrapib compared with the gold standard beta-quantification method.1 , 6 Therefore, further descriptions in this manuscript are framed in the context of non-HDL cholesterol rather than LDL cholesterol. More information on the sampling schedule can be found in the protocol.1 Those participants who were not able to attend clinic visits were contacted by telephone, or followed indirectly via their relatives or carers, or by reviewing their medical records.
During the post-trial period after cessation of study treatment, trained research co-ordinators collected information for consenting participants by telephone interview or medical record review once every 6 months from May 2017 to April 2019. Information was sought only about serious adverse events and the use of LDL-lowering therapy, in the same way as for the main trial, by taking a medical history from the patient, albeit this was done remotely rather than in-person. Participants remained blind to their treatment during both phases of the study and therefore any effect of this difference in event ascertainment was the same in both treatment groups, which minimizes any bias in between-treatment comparisons. No biological samples or physical measurements were collected during the post-trial period.
Adjudication of study outcomes
The pre-specified primary outcome for this report was the first major coronary event (i.e. the composite of coronary death, myocardial infarction, or coronary revascularization) during the combined in-trial and post-trial follow-up periods.
During the in-trial treatment period, reports of possible myocardial infarction, coronary revascularization, stroke, cancer, death, and serious liver or muscle events were centrally adjudicated by clinicians blinded to the treatment allocation using pre-specified definitions.1 , 2 Endpoint adjudication was completed for more than 99.9% of relevant in-trial event reports.1 After the database was finalized for analyses of the in-trial follow-up period, the impact of adjudication was assessed (Supplementary material online, Appendix S2 and Table S1). The adjudication process resulted in relatively little re-categorization of the reported coronary deaths (91% confirmed), myocardial infarctions (87% confirmed), and coronary revascularizations (91% confirmed), with modest re-categorization of strokes (81% of ischaemic strokes and 70% of haemorrhagic strokes were confirmed), and causes of death (80% of cardiovascular deaths and 79% of non-cardiovascular deaths were confirmed). As a consequence, the adjudication process had a little material impact on analyses of the effect of anacetrapib on the primary endpoint of major coronary event during the in-trial period (rate ratio 0.88; 95% CI 0.82–0.95; P < 0.001 using pre-adjudicated data compared with rate ratio 0.91; 95% CI 0.85–0.97; P = 0.004 using adjudicated data) or on any of the components of the primary outcomes or on other cardiovascular outcomes (Supplementary material online, Appendix S2 and Figure S1).
For the post-trial follow-up period, the main outcomes of interest were cardiovascular events, cancers (by site and overall), deaths (cause-specific and overall), and other serious adverse events (see Supplementary material online, Appendix S1). In view of the minimal impact of adjudication on the assessment of the effect of anacetrapib on the primary outcome of first major coronary event during the in-trial period, as well as the high confirmation rate of other cardiovascular events and cancers, only deaths and strokes reported in the post-trial phase were adjudicated (with 97.8% of such events successfully adjudicated). Subtypes of ischaemic stroke were not ascertained in either phase of the study.
Statistical analysis
The data analysis plan for the extended follow-up period was published on the trial website (www.revealtrial.org) before any members of the Steering Committee were unblinded to the post-trial results. Supplementary material online, Appendix S1 provides the data analysis plan that contains a detailed description of the statistical methods. The log-rank method was used to conduct intention-to-treat comparisons of the time to the first event of interest during the overall follow-up period between participants who had originally been randomly allocated anacetrapib and those who had been allocated placebo. Recurrent events were analysed using the negative binomial. Two-sided P-values <0.05 were regarded as statistically significant, although allowance for multiple hypothesis testing was made in their interpretation. Minor differences in the results of in-trial analyses between the previous publication1 and the present report are due to the inclusion of a small number of events reported after the previous analysis was done.
Funding and organization
The trial was designed, conducted, analysed, and interpreted by independent investigators in the Clinical Trial Service Unit (CTSU) at the University of Oxford (the regulatory trial sponsor), Oxford, UK, in collaboration with the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, USA, along with other members of the Steering Committee and Merck & Co, Inc., NJ, USA. Merck funded the trial and provided trial drugs. The writing committee prepared the manuscript, which was reviewed and approved by the trial Steering Committee. The decision to submit it for publication was independent of all funding sources.
Results
Between August 2011 and October 2013, a total of 30 449 men and women were randomized at 431 sites in Europe, North America, and China. They had a mean age of 67 years, 84% were male, 88% had a history of coronary heart disease, 22% had cerebrovascular disease, and 37% had diabetes mellitus.2 By the end of the pre-randomization run-in period, blood cholesterol levels were well-controlled on the study atorvastatin regimen: mean LDL cholesterol was 61 mg/dL (1.58 mmol/L), non-HDL cholesterol was 92 mg/dL (2.37 mmol/L), HDL cholesterol was 40 mg/dL (1.04 mmol/L), and apolipoprotein B was 62 mg/dL (0.0012 mmol/L). Baseline characteristics of participants who entered the post-trial follow-up study (Supplementary material online, Appendix S2 and Table S2) were not notably different to those of the randomized participants in the trial as a whole.1 , 2
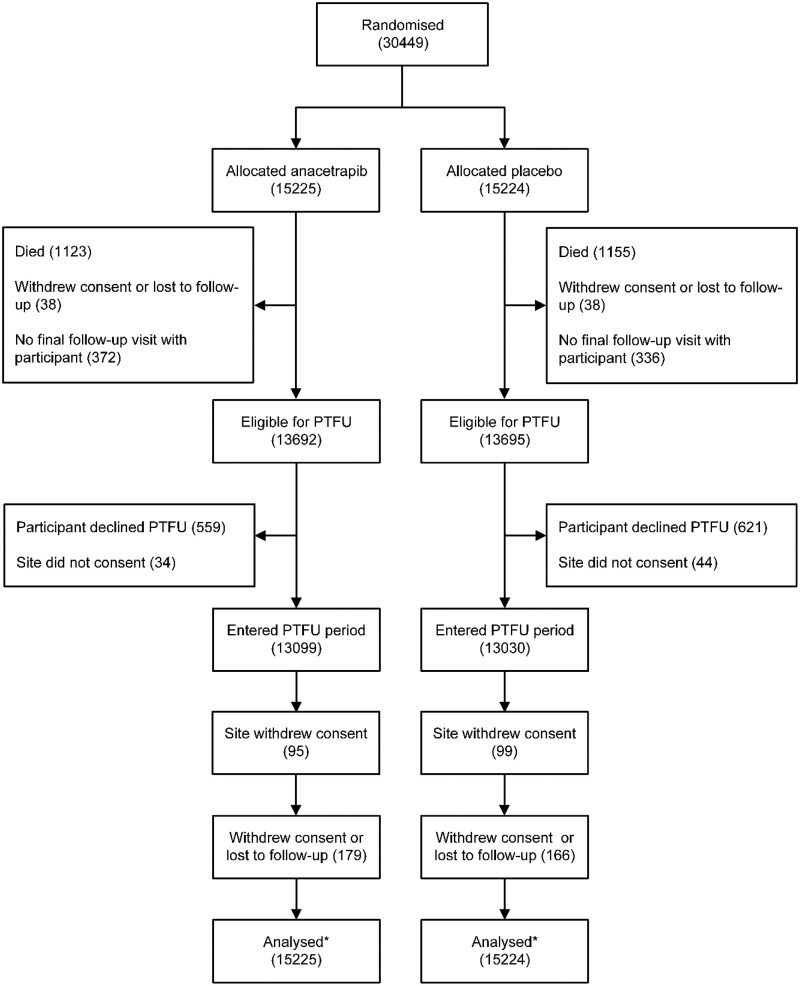
During the in-trial period, 2278 (7.5%) of randomized participants died, 33 (0.1%) withdrew consent, and 43 (0.1%) were lost to follow-up (Figure 1). A further 708 (2.3%) were excluded from post-trial follow-up because their final follow-up visit was not conducted in person or by telephone. Of 27 387 (89.9%) participants who were eligible for post-trial follow-up, 1180 did not give consent and 78 had previously attended 1 of the 12 sites that did not take part in the extended follow-up study. Consequently, 26 129 (85.8%) individuals from the original cohort entered the post-trial follow-up phase: of these, 970 died during the post-trial period and 539 withdrew consent or were lost to follow-up. Follow-up was a median of 4.1 years for the in-trial period, 2.3 years for the post-trial period, and 6.3 years for the combined periods.
Figure 1.
Consort diagram for randomized in-trial and post-trial cohorts. PTFU, post-trial follow-up.
During the in-trial period, use of statin treatment was 95% at the study midpoint in both groups and 92% at the final visit, and it was still high at the end of the post-trial period (89% in those originally allocated anacetrapib vs. 90% in the placebo group; Supplementary material online, Appendix S2 and Table S3). Adherence to the randomized treatment was also high: 90% at the study midpoint in both groups and 85% at the final visit.2 Compared with placebo, mean HDL cholesterol at the in-trial study midpoint was 43 mg/dL (1.12 mmol/L) higher (85 mg/dL vs. 42 mg/dL), and non-HDL cholesterol was 17 mg/dL (0.44 mmol/L) lower (80 mg/dL vs. 97 mg/dL) among the participants randomly assigned anacetrapib.2 The lipid effects of anacetrapib persisted at the final follow-up visit; mean HDL cholesterol was 44 mg/dL (1.13 mmol/L) higher and non-HDL cholesterol was 17 mg/dL (0.44 mmol/L) lower in those allocated the active drug.
Effects on first vascular events
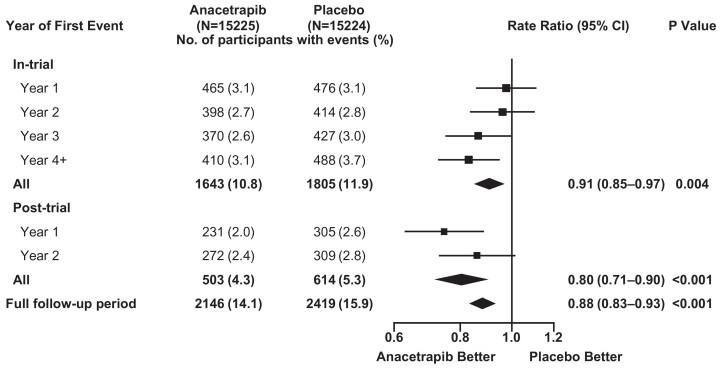
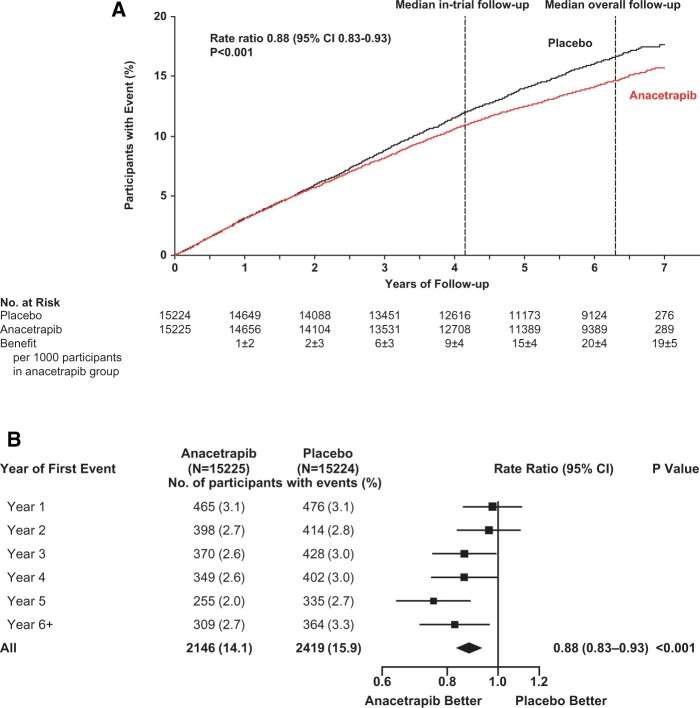
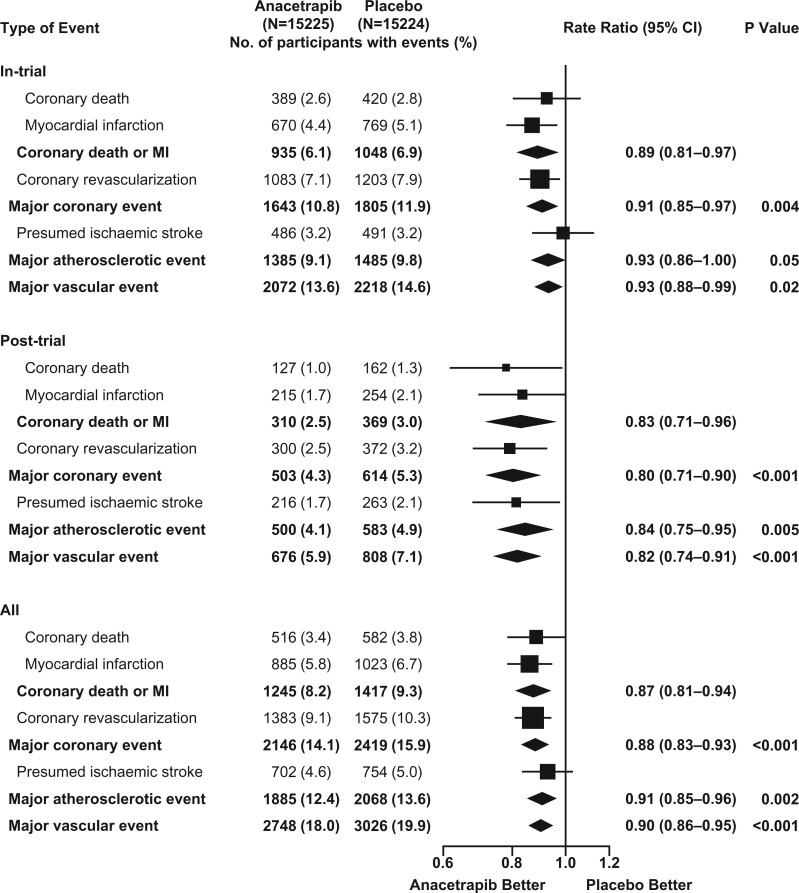
During the in-trial period, allocation to anacetrapib was associated with significantly fewer first major coronary events [1643 (10.8%) of 15 225 anacetrapib-allocated vs. 1805 (11.9%) of 15 224 placebo-allocated participants; rate ratio 0.91; 95% CI 0.85–0.97; P = 0.004: Figure 2]. Subsequently, during the post-trial follow-up period, there was a further significant reduction in first major coronary events among the participants who had originally been allocated anacetrapib [503 of 11 777 (4.3%) vs. 614 of 11 617 (5.3%); rate ratio 0.80; 95% CI 0.71–0.90; P < 0.001]. Consequently, during combined follow-up for a median of 6.3 years, allocation to anacetrapib treatment for a median of 4.1 years resulted in a significant 12% proportional reduction in the rate of first major coronary events [2146 (14.1%) vs. 2419 (15.9%); rate ratio 0.88; 95% CI 0.83–0.93; P < 0.001; Figure 3A; Graphical Abstract], with larger proportional reductions observed in the later years of follow-up (P for trend across rate ratios by year of first event = 0.01; Figure 3B). The absolute reduction in major coronary events of 1.1% (95% CI 0.4–1.8%) at the end of the in-trial treatment period increased to 1.8% (95% CI 1.0–2.6%) by the end of the combined follow-up period. The effects of anacetrapib allocation on major coronary events appeared to be similar among various patient subgroups (Supplementary material online, Appendix S2 and Figure S2).
Figure 2.
Effects of anacetrapib on first major coronary event by year during the in-trial, post-trial, and overall follow-up periods. Rate ratios are shown for the first major coronary event among patients in the anacetrapib group vs. those in the placebo group according to period of follow-up. The numbers at risk decline with each year of follow-up because of censoring, and the percentages are the number of events as a proportion of the number at risk. For each year of follow-up, rate ratios are plotted as squares (with the size of each square proportional to the amount of statistical information) and their 95% confidence intervals are represented as horizontal lines. For pre-specified composite periods of follow-up (indicated in bold text), the rate ratios and their corresponding 95% confidence intervals are represented by diamonds, and P-values are shown. Squares or diamonds to the left of the vertical line indicate benefit with anacetrapib, and the comparison is significant (P < 0.05) if the horizontal line or diamond does not overlap the solid vertical line. CI, confidence interval.
Figure 3.
(A) Effects of anacetrapib on first major coronary event during the in-trial, post-trial, and overall follow-up periods. (B) Effect of anacetrapib on major coronary events by year of follow-up. Figure legend as for Figure 2. P-value for trend across rate ratios by year of first event = 0.01. CI, confidence interval.
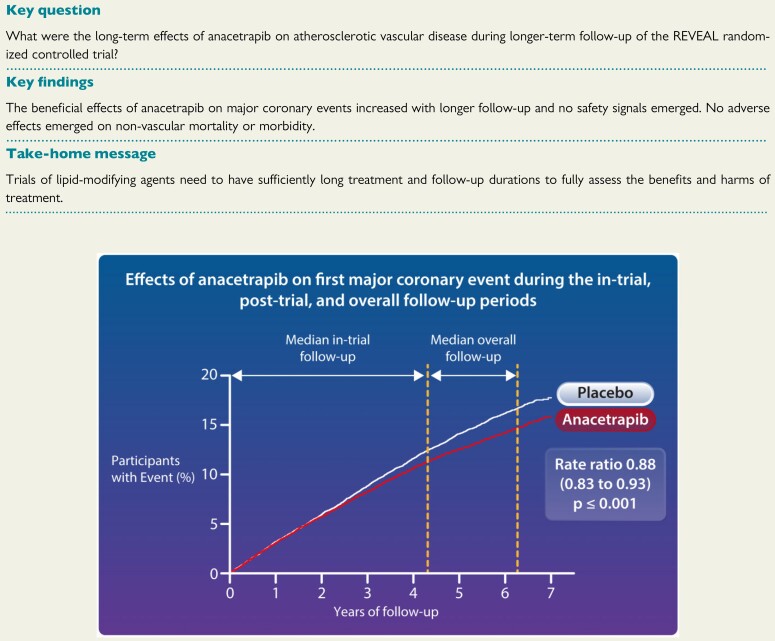
Structured Graphical Abstract.
Larger reductions in the rates of secondary cardiovascular outcomes were also observed with extended follow-up (Figure 4). A non-significant 7% proportional reduction in the incidence of first major atherosclerotic event during the in-trial period (rate ratio 0.93; 95% CI 0.86–1.00; P = 0.052), was followed by a 16% reduction in the post-trial period (rate ratio 0.84; 95% CI 0.75–0.95; P = 0.005), yielding a significant overall risk reduction of 9% (rate ratio 0.91; 95% CI 0.85–0.96; P = 0.002). Likewise, a 7% proportional reduction in the incidence of major vascular events during the in-trial period (rate ratio 0.93; 95% CI 0.88–0.99; P = 0.02) was followed by an 18% reduction in the post-trial period (rate ratio 0.82; 95% CI 0.74–0.91; P < 0.001), resulting in a significant overall risk reduction of 10% (rate ratio 0.90; 95% CI 0.86–0.95; P < 0.001).
Figure 4.
Effect of anacetrapib on other pre-specified vascular outcomes during the in-trial, post-trial, and overall follow-up periods. Figure legend as for Figure 2. The primary outcome was major coronary event (composite of coronary death, myocardial infarction, or coronary revascularization). Secondary outcomes were presumed ischaemic stroke (i.e. not known to be haemorrhagic), major atherosclerotic event (coronary death, myocardial infarction, or presumed ischaemic stroke), and major vascular event (major coronary event or presumed ischaemic stroke). The composite of coronary death or myocardial infarction was a pre-specified tertiary outcome. A single patient may have had multiple events and so may contribute information to more than one row. CI, confidence interval; MI, myocardial infarction.
There was no clear beneficial effect of anacetrapib on stroke (either overall or by subtype; Supplementary material online, Appendix S2 and Figure S3) or on non-coronary revascularization procedures (Supplementary material online, Appendix S2 and Figure S4) during the in-trial treatment period or at the end of the combined follow-up period.
Effect on recurrent vascular events
During the combined follow-up period, the incidence of first and subsequent major coronary events combined was reduced by 14% in those allocated anacetrapib (rate ratio 0.86; 95% CI 0.81 to 0.92; P < 0.001). Treatment of 1000 people with anacetrapib for a median of 4.1 years resulted in 17.9 (95% CI 9.9–26.0) people avoiding 27.5 (95% CI 18.5–36.5) major coronary events during a median of 6.3 years of follow-up (Table 1). There were similar results for first and subsequent major atherosclerotic events and major vascular events (Supplementary material online, Appendix S2 and Table S4).
Table 1.
Effect on first and total number of major coronary events
| First events | Total events | |||||
|---|---|---|---|---|---|---|
| Follow-up period | Anacetrapib | Placebo | Events avoided per 1000 patients | Anacetrapib | Placebo | Events avoided per 1000 patients |
| In-trial | 1643 | 1805 | 10.6 | 2142 | 2408 | 17.5 |
| Post-trial | 503 | 614 | 10.1 | 719 | 872 | 12.0 |
| Combined | 2146 | 2419 | 17.9 | 2861 | 3280 | 27.5 |
Effects on mortality, cancer, and other serious adverse events
During the in-trial treatment period, there were no significant effects of anacetrapib on death from coronary disease, other vascular disease, non-vascular causes, or all causes combined.1 Subsequently, during the post-trial follow-up period, a beneficial effect of anacetrapib on coronary death did emerge (rate ratio 0.78; 95% CI 0.62–0.98) such that, by the end of the combined follow-up period, there were marginally significantly fewer coronary deaths among participants originally allocated anacetrapib [516 (3.4%) vs. 582 (3.8%); rate ratio 0.88; 95% CI 0.79–1.00; P = 0.04; Figure 4]. Overall, there were also fewer deaths from cardiovascular causes among participants who had been allocated anacetrapib [722 (4.7%) vs. 796 (5.2%); P = 0.05], but no apparent effect on deaths due to all non-vascular causes [860 (5.6%) vs. 870 (5.7%); P = 0.76] or any particular non-vascular cause (Supplementary material online, Appendix S2 and Figure S5).
No significant effects of anacetrapib were found during extended follow-up on the incidence of cancer at all sites combined [1437 (9.4%) vs. 1418 (9.3%); P = 0.79] or at any pre-specified site (Supplementary material online, Appendix S2 and Figure S6). Nor was there evidence of adverse effects on the incidence of hypertension-related or other serious adverse events (Supplementary material online, Appendix S2 and Figure S7).
Discussion
We have previously reported that adding anacetrapib to intensive statin therapy for a median of 4 years reduced the incidence of major coronary events, with the proportional reduction in risk appearing to increase with treatment duration.1 We now report that the risk reduction continued to increase during the 2-year period after the anacetrapib treatment had stopped, such that the absolute reduction at 6 years was more than 50% greater than at the end of the treatment period. In addition, there was a significant reduction in the risk of death from coronary heart disease during extended follow-up. Importantly, no safety signal emerged with prolonged follow-up; in particular, there was no evidence of any adverse effects of anacetrapib on cancer, non-vascular mortality or other serious adverse events.
The results of REVEAL contrast with those reported from the large clinical outcome trials of other CETP inhibitor agents. The ILLUMINATE (Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events) trial was terminated because of excesses of cardiac events and death with torcetrapib, which have been attributed to various off-target drug effects on blood pressure.7 The Dal-OUTCOMES (Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome)8 and ACCELERATE (Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High Risk for Vascular Outcomes)9 trials were both stopped early after only about 2 years of treatment because of a perceived lack of efficacy. Dalcetrapib is a relatively weak CETP inhibitor that does not lower LDL cholesterol at the dose tested in Dal-OUTCOMES, whereas evacetrapib has effects on blood lipid levels similar to those of anacetrapib. However, ACCELERATE involved fewer patients than REVEAL (12 092 vs. 30 449), had fewer primary cardiovascular outcomes (1555 vs. 3443), and shorter treatment duration (median of 26 vs. 50 months).
Analyses of genetic variants in CETP indicate that differences in coronary disease risk are due largely to the genotype-related differences in LDL cholesterol levels.10 As previously reported, the 9% proportional reduction in major coronary events observed during the in-trial period of REVEAL1 is consistent with the risk reduction expected from the Cholesterol Treatment Trialists’ meta-analysis of randomized trials of 4–5 years of statin therapy with the 17 mg/dL (0.44 mmol/L) reduction in non-HDL cholesterol that was achieved in REVEAL.11 These observations reduce the likelihood that other actions of anacetrapib played a major role in modifying the risk of coronary events. In particular, the 43 mg/dL (1.12 mmol/L) increase in HDL cholesterol does not appear to have had an effect on major coronary events as large as would be anticipated from observational epidemiological studies.12 As observed with anacetrapib in REVEAL, the reductions in major coronary events in the trials of statin therapy tended to increase with increasing duration of therapy, and these beneficial effects persisted or even increased after study treatment had been stopped.11 , 13–18 In a subset of participants in the Determining the DEFINE (Efficacy and Tolerability of CETP Inhibition with Anacetrapib) trial, low plasma concentrations of anacetrapib were still detectable 2–4 years after the treatment had stopped.4 , 19 Consequently, the prolonged half-life of anacetrapib may have contributed to some extent to the benefit seen in REVEAL during the 2 years of extended follow-up after the study treatment phase had ended.
Our study has several strengths that facilitate a robust assessment of the persistent effects of previous treatment with anacetrapib. In particular, a high proportion of randomized participants completed the extended follow-up phase (blind to their initial treatment allocation), use of statin therapy was equally high in both randomized groups, and large numbers of vascular and non-vascular events occurred during the extended follow-up period. Only those deaths and strokes that were reported during post-trial follow-up were adjudicated, but analyses of the impact of adjudication during the in-trial period (Supplementary material online, Appendix S2, Table S1, and Figure S1) indicate that this would not materially alter the findings.
There are some limitations to our study. We did not collect blood samples for lipid analysis during the post-trial follow-up study and therefore, we are limited in the mechanistic insights we can provide for anacetrapib effects on major coronary events. Data on the time course of lipid parameters after the cessation of treatment have been published elsewhere.3 , 4 , 19 Unlike adipose tissue, evidence from other studies has shown a relatively quick elimination of anacetrapib in plasma after the cessation of treatment. Plasma concentrations of anacetrapib were reduced to ∼40% of their on-treatment level 12 weeks after stopping treatment in the 76-week DEFINE trial.4 The effect on LDL cholesterol was 18.6% and on HDL cholesterol was 73% of that seen on treatment.4 Estimates of the effect of anacetrapib on lipid parameters after the cessation of chronic dosing, over a longer time course, are not available from the REVEAL trial or other studies. The effect of anacetrapib on blood pressure was presented in the main REVEAL results paper.1 Blood pressure measurements were slightly higher among those randomized to anacetrapib compared with placebo (by 0.7 mmHg and 0.3 mmHg for systolic and diastolic readings, respectively); however, there was no significant difference in the rates of serious adverse events attributed to hypertension.1 We did not perform physical measurements on patients during the post-trial period, but similarly, no hazard for hypertension-related events emerged. Finally, we have not assessed effects that might take longer than 5–6 years to emerge (e.g. adverse effects on cancer or neurocognitive disorders, or beneficial effects on heart failure17), so it is intended to continue long-term follow-up through linkage to routine data on causes of hospitalization, cancer, and death in countries where these are available.
In conclusion, extended follow-up of the REVEAL trial showed that the absolute reduction in major coronary events produced by adding anacetrapib to effective statin therapy was about 50% larger at 6 years than it had been at the end of the 4-year treatment period. Although the manufacturers of anacetrapib decided not to pursue regulatory approval or commercialization of anacetrapib,20 these results still have important implications. In particular, they indicate that randomized trials of the effects of lipid-modifying drugs on clinical outcomes need to involve prolonged treatment and follow-up in order to avoid under-estimating treatment effects (as, e.g. may have been the case in the trials of proprotein convertase subtilisin/kexin 9 monoclonal antibodies21). Moreover, if the benefits seen with anacetrapib in REVEAL were largely due to the reduction in non-HDL cholesterol, then CETP inhibitor agents that produce larger reductions in non-HDL cholesterol22 may produce larger benefits that would be considered clinically worthwhile.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank the participants, the local clinical centre staff, regional and national co-ordinators, and the members of the Data Monitoring Committee.
Funding
The REVEAL study is funded by Merck & Co, Inc. The Clinical Trial Service Unit at the University of Oxford receives support from the UK Medical Research Council (which funds the MRC Population Health Research Unit in a strategic partnership with the University of Oxford), the British Heart Foundation (which also directly supports JC Hopewell FS/14/55/30806 and R Collins), and Cancer Research UK. The work described in this article is also supported by Health Data Research UK and the National Institute of Health Research Oxford Biomedical Research Centre. Disclosure of Interest forms for authors are provided with the full-text version of this article.
Conflict of interest: The Clinical Trial Service Unit at the University of Oxford (regulatory sponsor of REVEAL) has a staff policy of not accepting honoraria or consultancy fees directly or indirectly from industry (see https://www.ndph.ox.ac.uk/files/forms/ctsu_honoraria_25june14.pdf).
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Bowman L, Hopewell JC, Chen F et al. ; HPS37TIMI55-REVEAL Collaborative Group . Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 2. Bowman L, Chen F, Sammons E et al. ; REVEAL Collaborative Group . Randomized Evaluation of the Effects of Anacetrapib through Lipid-modification (REVEAL)—a large-scale, randomized, placebo-controlled trial of the clinical effects of anacetrapib among people with established vascular disease: trial design, recruitment, and baseline characteristics. Am Heart J 2017;187:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krishna R, Gheyas F, Liu Y et al. Chronic administration of anacetrapib is associated with accumulation in adipose and slow elimination. Clin Pharmacol Ther 2017;102:832–840. [DOI] [PubMed] [Google Scholar]
- 4. Gotto AM, Cannon CP, Li XS et al. ; DEFINE Investigators . Evaluation of lipids, drug concentration, and safety parameters following cessation of treatment with the cholesteryl ester transfer protein inhibitor anacetrapib in patients with or at high risk for coronary heart disease. Am J Cardiol 2014;113:76–83. [DOI] [PubMed] [Google Scholar]
- 5. Barter PJ, Waters DD. Variations in time to benefit among clinical trials of cholesterol-lowering drugs. J Clin Lipidol 2018;12:857–862. [DOI] [PubMed] [Google Scholar]
- 6. Davidson M, Liu SX, Barter P et al. Measurement of LDL-C after treatment with the CETP inhibitor anacetrapib. J Lipid Res 2013;54:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barter PJ, Caulfield M, Eriksson M, Grundy SM et al. ; ILLUMINATE Investigators . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz GG, Olsson AG, Abt M et al. dal-OUTCOMES Investigators . Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 9. Lincoff AM, Nicholls SJ, Riesmeyer JS et al. ; ACCELERATE Investigators . Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 10. Nomura A, Won H-H, Khera AV et al. Protein-truncating variants at the cholesteryl ester transfer protein gene and risk for coronary heart disease. Circ Res 2017;121:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fulcher J, O'Connell R, Voysey M et al. ; Cholesterol Treatment Trialists’ Collaboration . Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 12. Di Angelantonio E, Sarwar N, Perry P et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins R, Reith C, Emberson J et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 14. Baigent C, Keech A, Kearney PM et al. ; Cholesterol Treatment Trialists' Collaboration . Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen TR, Wilhelmsen L, Faergeman O et al. Follow-up study of patients randomized in the Scandinavian Simvastatin Survival Study (4S) of cholesterol lowering. Am J Cardiol 2000;86:257–262. [DOI] [PubMed] [Google Scholar]
- 16. Heart Protection Study Collaborative Group . Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet 2011;378:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ford I, Murray H, McCowan C, Packard CJ. Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy. Circulation 2016;133:1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LIPID Study Group (Long-term Intervention with Pravastatin in Ischaemic Disease) . Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet 2002;359:1379–1387. [DOI] [PubMed] [Google Scholar]
- 19. Gotto AM, Kher U, Chatterjee MS et al. ; DEFINE Investigators . Lipids, safety parameters, and drug concentrations after an additional 2 years of treatment with anacetrapib in the DEFINE study. J Cardiovasc Pharmacol Ther 2014;19:543–549. [DOI] [PubMed] [Google Scholar]
- 20. Merck Provides Update on Anacetrapib Development Program . https://www.merck.com/news/merck-provides-update-on-anacetrapib-development-program/ (8 September 2021).
- 21. Waters DD, Hsue PY. PCSK9 inhibition to reduce cardiovascular risk: tempering expectations. Circ Res 2017;120:1537–1539. [DOI] [PubMed] [Google Scholar]
- 22. Hovingh GK, Kastelein JJ, van Deventer SJ et al. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2015;386:452–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.