Abstract
The methylation and expression of DNA repair system genes has been studied in several tumor types. These genes have been associated with resistance to chemotherapy treatments by epigenetic regulation. Studies have yet to show the effects of combined therapy using an epigenetic drug (5-aza-2CdR) and cisplatin (CDDP) on DNA repair genes in oral squamous cell carcinoma (OSCC). This study proposed to investigate the effects of CDDP in combination with 5-aza-2CdR on the methylation of MGMT and MLH1 genes in oral cancer cells. Oral squamous cell carcinoma cell lineages (SCC-9, SCC-15, and SCC-25) were submitted to 72 hours of treatment: 0.1 μM CDDP (or 4.44 μM SCC-9), 0.1 μM and 0.3 μM 5-aza-2CdR (or 1 μM and 3 μM SCC-9), and the drugs in combination. Cell viability was assessed by MTT, DNA methylation of MGMT and MLH1 genes by Methylation Sensitivity High-Resolution Melting (MS-HRM), and the relative expression of the genes by RT-qPCR. The results show that all treatments reduced cell viability; however, in SCC-15 and SCC-9 (IC50 value), 5-aza-2CdR promotes cell sensitization to cytotoxic effect of cisplatin. The MGMT promoter region was 100% demethylated in the SCC-15 and SCC-25 cells but partially (50%) methylated in SCC-9 before drug treatment. Treatment with IC50 CDDP value kept the methylation status and decreased MGMT expression in SCC-9; MGMT gene in SCC-15 and SCC-25 cells became downregulated after treatment with 5-aza-2CdR. MLH1 was demethylated, but the treatments with low-doses and combined drugs decreased the expression in SCC-9 and SCC-25; however high doses of 5-aza-2CdR and drug combination with IC50 value CDDP increased expression of MLH1 in SCC-9. The data presented suggest that epigenetic drugs associated with chemotherapy have clinical translational potential as a therapy strategy to avoid or reverse cancer resistance, requiring further investigation.
Keywords: Oral squamous cell carcinoma, DNA methylation, DNA repair, cisplatin, combination therapy
Introduction
Oral cancer is one of the most frequent types of cancer, with a high worldwide mortality rate and high incidence in low- and middle-income countries [1] and more than 90% of these cases represented by oral squamous cell carcinoma (OSCC) [2-4]. OSCC frequently affects the anterior two-thirds of the tongue, floor of the mouth, retromolar region, gingiva and palate region [5]. OSCC occurrence is more common on the tongue and is associated with a poor prognosis [6]. Such an association may be related to the frequent appearance of OSCC in the mobile region of the tongue, a highly vascularized site that can facilitate the appearance of tumor metastasis [7].
Oral cancer’s etiology and main risk factors include: chemical factors such as tobacco and alcohol consumption; biological factors such as certain viral infections, including the human papilloma virus (HPV), often associated with the appearance of tumors in the oropharyngeal region [8]; syphilis; and genetic and epigenetic factors [9-11].
Cisplatin (CDDP) is an alkylating agent and a member of the platinum complex group (carboplatin, oxaloplatin), being the first-line drug for postoperative chemotherapy in OSCC patients with potent and effective antitumor activity against various types of solid tumors, including head and neck cancer [12]. Several molecular mechanisms are associated with its cytotoxic activity such as the induction of oxidative stress, action on the p53 protein pathway that generates alterations in the cell cycle, blocking cell division by formation of adducts in DNA that, in general, generate signaling and induction of cell death by apoptosis [13]. However, CDDP therapeutic efficacy is limited and compromised by the emergence of intrinsic or acquired drug resistance that can result from degradation of the copper membrane transporter (CTR1) [14-17], or overexpression of the transmembrane protein 205 (TMEM205) [18] and ABC type ATPases proteins such as the multiple drug resistance protein (MRP) [18-20]. Other factors are described in the literature, including epigenetic silencing of critical genes in DNA repair pathway for response to drugs by DNA methylation [21-23].
The DNA mismatch repair (MMR) system that correct the DNA mismatches is required to cell cycle arrest or apoptosis and participate in the DNA damage response after treatment with chemotherapeutic agents which forms adducts resulting in the generation of mismatched bases [24,25]. MMR loss has been related with resistance mechanisms [26]. In addition, MLH1 gene has also been found to be epigenetically silenced by promoter hypermethylation, down-regulating MMR, which may play a role in maintaining drug sensitivity [27-29]. Another gene involved in DNA repair activity is O6-methylguanine-DNA methyltransferase (MGMT). The protein encoded by this gene acts to protect cells from the toxic and carcinogenic effects of alkylating agents, such as nitrosamines present in cigarette smoke [30] and also, of chemotherapeutic agents such as cisplatin [31], removing the adducts from the O6 position of the guanine. Many studies show that hypermethylation in the promoter region of this gene is associated with decreased expression, which is related to the increased risk of brain tumor, colon cancer, lung cancer, head and neck cancer and other tumors [32-34]. However, the action of this gene may also be correlated with the resistance mechanism to treatment by chemotherapy, an unfavorable bias [35-38].
Some strategies, including combination therapies, have been applied in patients to prevent chemotherapy resistance [39]. This study aims to associate cisplatin with the demethylating agent 5-aza-2’-deoxycytidine (5-aza-2CdR or decitabine). The possible action to reverse the silencing of key genes in the cell cycle, through demethylation by the action of demethylating drugs, has emerged as a therapeutic strategy to be used in severe cases and unresponsiveness to conventional therapies [40,41].
In a previous study by our research group, it was shown that cells of the SCC-9 tumor lineage present methylation in the MGMT gene in at least half of the tumor cell population. Moreover, treatment with low concentrations of 5-aza-2CdR promoted partial demethylation of MGMT and increase its expression [42]. In addition, the promoter hypermethylation in MLH1 gene has been reversed with the use of 5-aza-2CdR, allowing gene expression and normal MMR capability [43,44]. However, the effect of cisplatin in association with 5-aza-2CdR was not described in OSCC lineages studied here: SCC-9, SCC-15, and SCC-25.
Numerous studies have shown that the use of 5-aza-2CdR in combination with chemotherapeutic agents could represent an interesting therapeutic approach, due to the anti-proliferative and pro-apoptotic effect against tumor cells as well as the recovery of sensitivity to chemotherapeutic agents, such as cisplatin [45-49]. Our objective was to analyze the effect of combined therapy between 5-aza-2CdR and cisplatin in three OSCC cell lines, with MGMT and MLH1 as the main target genes, while also assessing the epigenetic profile.
Materials and methods
Cell culture
SCC-9, SCC-15, and SCC-25 cells (ATCC® CRL-1629TM, ATCC® CRL-1623TM, ATCC® CRL-1628TM) were gently provided by Dr. Edgar Graner (Piracicaba Dental School, University of Campinas, Piracicaba, Brazil). Cells were cultured in DMEM/Ham’s F12 medium (Dulbecco’s Modified Eagle’s Medium e Ham’s F12) (Sigma, USA) supplemented with 400 ng/mL hydrocortisone (Sigma, USA), 10% fetal bovine serum (FBS) (Sigma, USA), antibiotic solution (100 U/mL penicillin and 100 μg/mL streptomycin) (Invitrogen, USA) and incubated at 37°C and 5% CO2 until the pre-confluence.
Combined-treatment with cisplatin and 5-Aza-2CdR
Cisplatin (Takara Bio USA, Inc.) was diluted in 0.9% saline solution and 5-aza-2CdR (Sigma-Aldrich) in 1 × phosphate-buffered saline solution (PBS). SCC-9 cells were treated with 0.1 μM or 4.44 μM (IC50 value) of cisplatin and 0.1 μM, 0.3 μM, 1 μM or 3 μM 5-aza-2CdR (Sigma-Aldrich) for 72 h. SCC-15 and SCC-25 cells were treated with 0.1 μM cisplatin and 0.1 μM or 0.3 of 5-aza-2CdR. Cisplatin and 5-Aza-2CdR were replaced each 24 h. Control cells were treated with same amount of saline solution and PBS. The experiments were assayed in triplicate.
MTT assay
Cell viability was assessed by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT, Invitrogen, Carlsband, CA, USA). Briefly, cells were seeded in 96-well plates (n=8 wells/group) at a density of 1 × 104 cells/well, allowed to adhere for 24 h and after the experimental period the MTT test was performed. Cells were first incubated for 3 h (37°C and 5% CO2) in culture medium (200 µL/well) containing MTT (0.3 mg/mL). Wells containing only the culture medium without cells were incubated with MTT in parallel with the experiment samples to normalize the absorbance values obtained. Then, solubilization of the formazan crystals with 100% ethanol was performed and then the absorbance reading at a 570 nm wavelength was performed by a microspectrophotometer (ASYS UVM340, Biochrom Ltd., Cambridge, England) with the aid of ScanPlus software. Results were expressed as a percentage of cell viability, considering the Control group as 100% that were treated with the corresponding volume of serum culture medium.
DNA isolation and sodium bisulfite treatment
Total DNA was purified from cells using the Quick-DNA Universal kit (Zymo Research, USA) following the manufacturer’s protocol. After DNA quantification and purity verification using Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA), 800 ng of genomic DNA were treated with sodium bisulfite using the Epitect® Bisulfite Kit (Qiagen, Germany), following the manufacturer’s instructions. The converted DNA was stored at 4°C until Methylation-Sensitive High-Resolution Melting (MS-HRM) analysis.
MS-HRM analysis
Real-time PCR followed by MS-HRM analysis was performed on the Light Cycler 480 II thermal cycler (Roche, Mannheim, Germany). Primer sets were designed in accordance with guidelines proposed by Wojdacz and Dobrovic [50] (Table 1). Reactions ran in duplicate per sample and each reaction consisted of: 1 μL bisulfite converted DNA, 1x LightCycler®480 HRM Master Mix+4 mM MgCl2 (Roche, Mannheim, Germany), 250 nM of each primer, in one 10 μL volume end. Amplification parameters were: 95°C for 10 minutes (initial DNA denaturation), followed by 45 cycles: 95°C for 10 seconds (denaturation), 20 seconds at 55°C (MGMT); 20 seconds at 58°C (MLH1) (annealing temperature) and 72°C for 20 seconds (extension). MS-HRM analyses were performed with manufacturer-recommended ramping temperature and fluorescence acquisition adjustments: 1 minute at 95°C, maintained at 70°C for 1 minute (to allow re-annealing of all PCR product), acquisition step from 70°C to 95°C, rising 0.2°C/s with 25 acquisitions per °C. Fully methylated (100%) and fully unmethylated (0%) DNA (Qiagen EpiTect PCR Control DNA Set) were used as controls to estimate the DNA methylation level of each sample. From these, an intermediate 1:1 dilution (50% methylated) was prepared, resulting in 0, 50 and 100% methylated DNA standard melting curves of a serial dilution. To compensate for the initial fluorescence variation, MS-HRM data were normalized (Light Cycler 480 II analysis software). The melting curve of the product from each sample was compared to the standard melting curves (0, 50 and 100% methylated). As a result, the samples were classified in methylation ranges 0-50% and 51-100%. Assays were run in triplicate.
Table 1.
Methylation-sensitive high-resolution melting (MS-HRM) primer data
| Gene | Primer 5’-3’ | Genome position | CpG | Amplicon size | Annealing |
|---|---|---|---|---|---|
| MGMT | F-GCGTTTCGGATATGTTGGGATAGT | chr10:131,155,459-131,155,631 | 18 | 173 bp | 55 |
| R-CCTACAAAACCACTCGAAACTACCA | |||||
| MLH1* | F-AGTTTTTAAAAACGAATTAATAGGAAGAG | chr3:37,034,741-37,034,821 | 5 | 81 bp | 58°C |
| R-ACTACCCGCTACCTAAAAAAATATAC |
Ref [51].
Quantitative real-time PCR
Reverse transcription followed by qPCR was utilized on the expression of MGMT and MLH1 genes. The total RNA was purified by Trizol® reagent (Invitrogen, Carlsbad, CA, USA); 1 μg of total highly purified RNA was treated with DNAse (Invitrogen, Carlsbad, CA, USA); 500 ng of RNA were used for cDNA synthesis using SuperScript III First-Strand Synthesis Systems for RT-PCR (Invitrogen, Carlsbad, CA, USA), according to manufacturer’s recommendation. The sequences of the primers used are in Table 2. qPCR was carried out in 10 μL total, containing 1 μL cDNA, 5 μL SYBR Green (LightCycler® 480, Roche Applied Science), 250 nM (MGMT); 100 nM (MLH1) each primer, 3 μL (MGMT); 2 μL (MLH1) of cDNA and nuclease-free H2O. The SYBR Green amplification conditions consisted in an initial 10 min denaturation at 95°C, followed by 45 cycles of 15 s at 95°C (denaturation), 20 s at 62°C (MGMT); 20 s at 60°C (MLH1) (annealing temperature), and 20 s at 72°C (extension). Relative levels of gene expression were performed using the cycle threshold (Ct) method in reference to GAPDH. Assays were run in triplicate. Relative gene expression was determined using 2-ΔΔCT method [53]. The graphical representation of results was created using the R package version 3.6.1 (2019).
Table 2.
Reverse transcription real time polymerase chain reaction (RT-qPCR) primer sequences
Statistical analysis
Data are displayed as mean ± standard deviation. Statistical analysis of data was performed by one-way ANOVA followed by Dunnett’s post-hoc test. Statistical analysis was performed using the R package version 3.6.1 (2019) using a 5% level of significance.
Results
Low dose 5-aza-2CdR sensitizes SCC-9 (IC50 value) and SCC-15 cells reducing cell viability by effect of cisplatin, but not in SCC-25 cells
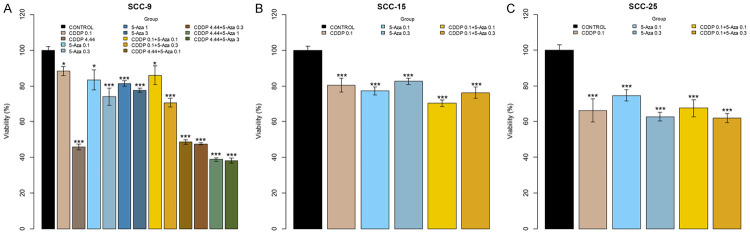
MTT assay results showed that different treatments with CDDP and 5-aza-2CdR affected the mitochondrial metabolic activity of the 3 tumor lineages studied - SCC-9, SCC-15 and SCC-25 - in different ways (Figure 1). All treatments in both lineages significantly decreased the number of viable cells when compared to controls. In the SCC-9 lineage (Figure 1A), the groups with isolated treatments of cisplatin, mainly in the 4.44 µM concentration, decreased the number of living cells in 50%. Additionally, when combined with the lower concentration 5-Aza groups (5-Aza 0.1 and 5-Aza 0.3) the decreased ratio is maintained. However, the combination with the higher concentration 5-Aza groups (5-Aza 1 and 5-Aza 3) led to additional reduction of about 20% of viable cells when compared to the other groups with reference to control, within the same significance level. The decrease in the percentage of viable cells was proportional among the low concentration isolated 5-Aza groups and the combined groups in the presence of 0.1 µM cisplatin (P<0.05 and P<0.001) in reference to control at a dose-response 5-Aza relationship.
Figure 1.
The treatments with cisplatin and 5-Aza-2CdR affect the cell viability of the lineages SCC-9, SCC-15, and SCC-25 differently. A. The SCC-9 cells were challenged with two cisplatin concentrations (0.1 and 4.44 µM) and four 5-Aza-2CdR concentrations (0.1, 0.3, 1 and 3 µM). The six groups combined demonstrate the influence of cisplatin concentrations associated with 5-aza-2CdR. B, C. The viability of SCC-15 and SCC-25 cells was assessed only at 0.1 µM concentration of cisplatin and two 5-Aza-2CdR concentrations (0.1 and 0.3 µM), with two combinations between the drugs. No treatment cells were used (control). Cellular viability was assessed by MTT reduction assay and results were expressed as percentage of cell viability, considering the Control group as 100% and presented as mean ± SD of three independent experiments (*P<0.05; ***P<0.001).
The isolated treatments on SCC-15 cells (CDDP 0.1, 5-Aza 0.1 and 5-Aza 0.3, Figure 1B) maintained an approximately 20% proportion of viability decrease (P<0.001) and on the combined groups (CDDP 0.1+5-Aza 0.1 and CDDP 0.1+5-Aza 0.3) an additional 10% decrease in relation to control occurred. However, only the CDDP 0.1+5-Aza 0.1 group had the cytotoxic effect of cisplatin potentiated more significantly (P<0.001).
Cisplatin concentration on SCC-25 cells (Figure 1C) decreased about 35% of the viability and when combined with 0.1 µM 5-Aza the same results were observed in reference to controls. The isolated and combined groups with 5-Aza showed a dose-response relationship but maintaining viability between 60 and 70%. SCC-25 was the cell lineage with the greatest decrease in viability relative to cisplatin exposure at 0.1 µM (P<0.001).
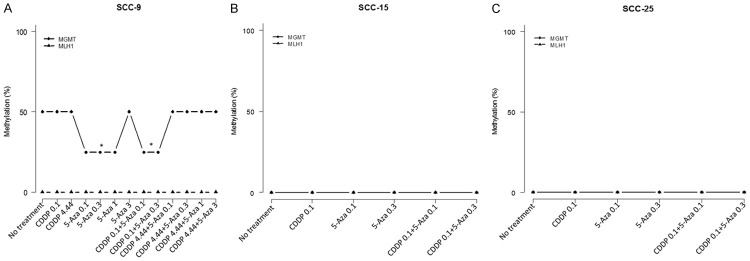
Expression and methylation profile of MLH1 and MGMT genes are differently affected by treatment with 5-aza-2CdR
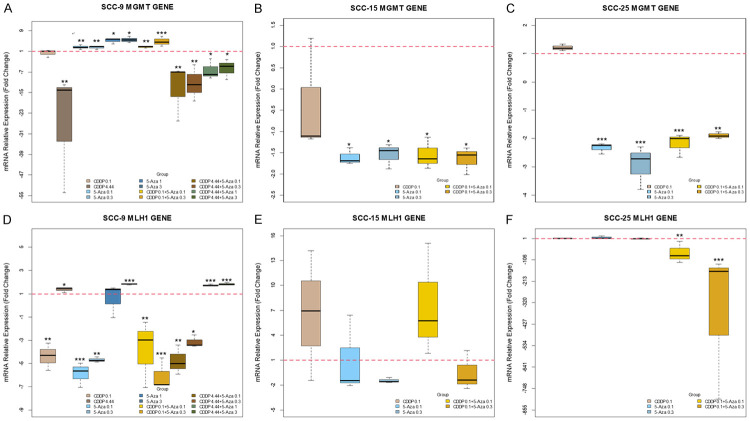
The effect of 5-aza-2CdR on demethylation of MGMT and MLH1 genes was evaluated by real-time PCR followed by HRM analysis in SCC-9, SCC-15 and SCC-25 cells (Figure 2). MGMT gene was partially methylated (50% methylation) in SCC-9 cells (Control group, Figure 2A) and totally demethylated in SCC-15 and SCC-25 cells. MLH1 gene is usually demethylated in all cells studied (Figure 2A-C). All treatments with 5-aza-2CdR alone increased MGMT expression in SCC-9 cells besides demethylating around 25% in the applied concentrations, except for the 3 µM dose that not demethylated, but increased the expression (Figure 3A). We observed significant downregulation of MLH1 gene when cells were submitted to the lowest 5-aza-2CdR concentrations (0.1 µM and 0.3 µM); however, the 3 µM dose increased expression 1.82-fold (P<0.001; Figure 3D).
Figure 2.
Methylation pattern of the MGMT and MLH1 genes in SCC-9, SCC-15, and SCC-25 cells. A. MS-HRM analysis showed MGMT gene is partially (50%) methylated in SCC-9 cells. The effect of 5-Aza-2CdR at 0.1 μM, 0.3 and 1 μM after 72 h of treatment promoted significant demethylation (25%) of the MGMT gene in SCC-9 (P<0.05). However, no effect of 5-Aza-2CdR at 3 μM concentration. No effect of cisplatin (0.1 and 4.44 μM) and combined groups with cisplatin 0.1 μM in methylation profile. No effect in methylation pattern also in the association of cisplatin at 4.44 μM with the 5-Aza-2CdR groups. MLH1 gene was demethylated in SCC-9. B, C. Both genes (MGMT and MLH1) were originally demethylated in SCC-15 and SCC-25 cells.
Figure 3.
The box plot showed the effect of cisplatin and 5-aza-2CdR on gene expression. A-C. Effect of isolated and combined of drugs on MGMT expression after 72 h treatment. D-F. Effect of isolated and combined of drugs on MLH1 expression after 72 h treatment. A, D. SCC-9 cells treated with two cisplatin concentrations (0.1 and 4.44 µM), four 5-Aza-2CdR concentrations (0.1, 0.3, 1 and 3 µM) and six groups combined. B, E. SCC-15 cells were treated only at 0.1 µM concentration of cisplatin and two 5-Aza-2CdR concentrations (0.1 and 0.3 µM), with two combinations between the drugs concentrations. C, F. SCC-25 cells with five groups treated like SCC-15. The relative gene expression was determined using 2-ΔΔCT method. Box plots represent medians and interquartile ranges of relative mRNA expression. The statistical analysis (ANOVA/Dunnett’s) considered the following ΔCt values (*P<0.05; **P<0.01; ***P<0.001).
MGMT gene expression in SCC-15 cells significantly decreased (P<0.05) by approximately 1.5-fold in all groups with the two concentrations of 5-aza-2CdR (Figure 3B). Regardless of treatment, MLH1 gene expression remained significantly unchanged, although the fold values changed (Figure 3E).
Analyzing SCC-25 cells after treatment, MGMT gene was significantly downregulated ≅2.5-fold in groups that received only treatment with 5-aza-2CdR (Figure 3C). MLH1 expression was not altered with the isolated treatments (Figure 3F).
Cisplatin IC50 value ensures methylation profile of MGMT in SCC-9 cells decreasing the expression
As expected, cisplatin does not change MGMT methylation status or expression in SCC-9, even at the 0.1 µM concentration (Figures 2A, 3A). However, in the IC50 cisplatin value (4.44 µM) in these cells even though there was no change in the DNA methylation, this concentration decreased the gene expression. On the other hand, the combined groups of 5-aza-2CdR with 0.1 µM cisplatin partially demethylates SCC-9 and significantly increased MGMT expression, mainly in the CDDP 0.1+5-Aza 0.3 group (P<0.001). Curiously, the combined groups with 4.44 µM cisplatin did not change the methylation profile in SCC-9 cells even in the presence of different 5-Aza concentrations, although the expression remains low. Surprisingly, the group isolated 5-Aza 3 was unable to demethylate the MGMT gene but increased its expression.
Combination of low doses of cisplatin and 5-aza-2CdR is not useful for cells in relation to MLH1 gene expression profile
In SCC-9 cells, MLH1 gene had significantly decreased expression when cells were submitted to low 0.1 µM CDDP concentration; however, expression increased at a 4.44 µM dose (Figure 3D). We observed significant MLH1 upregulation of 1.74-fold and 1.83-fold at high concentration 5-Aza combined with 4.44 µM cisplatin groups, CDDP 4.44+5-Aza 1 and CDDP 4.44+5-Aza 3, respectively, after 72 h treatment.
The combined treatment with 0.1 µM cisplatin+0.1 µM 5-Aza-2CdR upregulated the MLH1 expression at 7.55-fold in SCC-15 cells, but it was not significant (Figure 3E).
Curiously, when Cisplatin and 5-aza-2CdR were combined in SCC-25 treatment, the expression level decreased significantly: 72-fold for CDDP 0.1+5-Aza 0.1 (P<0.01) and 363.49-fold for CDDP 0.1+5-Aza 0.3 (P<0.001) (Figure 3F).
Discussion
The unusual histological composition of the tongue (rich lymphatic network and highly muscular structure) contributes to its susceptibility to metastatic invasion and tumor recurrence [54] and to poor prognoses, also associated with late diagnoses [55], resulting in lower treatment efficiency for squamous cell carcinoma [56,57].
Surgery, radiotherapy, and the combination of radiotherapy and chemotherapy are among the main treatments [58-60]. Cisplatin is an effective but highly cytotoxic radiosensitizer chemotherapy drug [61]. Given this tumor’s highly heterogeneous profile, studies found that patients develop some resistance to DNA-damaging anticancer agents like cisplatin, either intrinsically or acquired [21,62].
In an attempt to bypass the resistance mechanism associated with epigenetic events, studies have combined chemotherapeutic drugs and the demethylating agent 5-aza-2CdR [63-66] to establish alternative therapy methods to many types of solid tumors.
Numerous studies have related DNA methylation profile with DNA repair pathways, showing that this system is among the most studied mechanisms of resistance to chemotherapeutic drugs [67-69]. The nucleotide excision repair (NER) pathway and homologous recombination (HR) are the systems responsible for the most DNA damage [70,71]. In addition to NER and HR, studies have revealed the role of the mismatch repair (MMR) pathway (MLH1, MLH3, MSH2, MSH3, MSH6, PMS1 and PMS2) in signaling apoptosis to cell death and O-6-methylguanine-DNA methyltransferase (MGMT) gene in CDDP resistance by repairs of alkyl adducts at the O6-position of guanine, but still controversial [37,70,72]. In this study, we made three novel observations about the drug association therapy between cisplatin and the demethylating agent 5-aza-2CdR that may help develop new OSCC treatments: MLH1 gene is demethylated in the 3 OSCC lineages and low doses of the drugs studied decrease the expression profile; MGMT gene is demethylated in the SCC-15 and SCC-25 lineages and treatments with 5-aza-2CdR decrease expression; and cisplatin IC50 value ensures the partial methylation of MGMT in the SCC-9 cells and decrease expression.
Beyrouthy et al. described that low doses of 5-aza-2CdR sensitize cell and a pretreatment with this demethylating agent increases CDDP cytotoxicity in pluripotent embryonal carcinomas (ECs) in 3 days [66]. From the results of our study, by proposing to associate these drugs for 72 hours, we speculate no potentiation action and no cell sensitization to the action of cisplatin occurred. The effect in SCC-9 that reduces viability is attributed only to CDDP at IC50 value. The lineages responded in different ways when considering cellular viability (Figure 1). While SCC-15 and SCC-25 presented decreased cell viability to both low concentrations of 5-aza-2CdR-0.1 and 0.3 µM, the SCC-9 cells only had viability reduction in the high concentration of the drug (0.3, 1 and 3 µM). Thus, for SCC-9, 5-aza-2CdR acts in a time dependent manner [42]. These findings are supported, since the use of demethylating agents in high concentrations is associated with immediate cytotoxic effects such as DNA damage, apoptosis and cell cycle arrest [73,74], whereas low concentrations become more specific for tumor cell populations [75]. SCC-15 and SCC-25 cells are more sensitive to treatment with CDDP at the lowest concentration (0.1 µM), whereas SCC-9 are more resistant to cisplatin when compared. Even studies show that for the SCC-9 strain the IC50 value found (4.79 µM) for cisplatin treatment, that is, the concentration that reduces cell viability by 50% is higher than for the other strains (SCC-15 IC50=3 µM and SCC-25 IC50=2.41 µM) [76,77], which brings our IC50 value found closer for SCC-9 cells.
The MGMT gene promoter is often seen to be hypermethylated both in normal surrounding oral mucosa and in squamous cell oral carcinoma itself [32]. In the study published by Zuo et al., in 94 cases of squamous cell carcinoma of the head and neck, about 18% had hypermethylation in the promoter of this gene and 20% had loss of expression [78]. Interestingly, we found that this gene is partially methylated in SCC-9 (heterogeneous methylation) (Figure 2A), which means that 50% of the cells were hypermethylated and with low expression (Figure 3A) in the analyzed region, as already described by Amaral et al. [42], and 100% demethylated in SCC-15 and 25 (Figure 2B, 2C), which causes a high expression in these cells (Figure 3B, 3C). The fact that the MGMT gene is partially or totally demethylated and overexpressed, as has been discussed, can culminate in increased resistance by removing cisplatin (alkylating agent). Our data show that the CDDP IC50 value maintains the methylation profile and low MGMT expression whether combined with 5-aza-2CdR or not in SCC-9 cells and all treatments with only 5-aza-2CdR effectively decrease MGMT gene expression in SCC-15 and 25 cells.
Both SCC-9, 15 and 25 cells have the MLH1 gene 100% demethylated, but the combination therapy with low concentration to decrease the expression is not useful because the low levels or inactivation of MLH1 give rise to cisplatin resistance [27,79,80]. The IC50 dose of cisplatin, on the other hand, that was present potentiated action by 5-aza-CdR 1 and 3 µM to increase expression in SCC-9.
This study has some limitations. The decrease in expression does not represent a direct effect of 5-aza-2CdR on methylation of MGMT or MLH1 genes in SCC-15 and 25 cells, since they unmethylated before treatment in those genes. Besides that, the study cannot clarify the precise causal relationship between MGMT demethylation and the increase in gene expression as the 5-aza-2CdR is a global demethylating agent. However, we can infer that non-target genes are being affected by the action of 5-aza-2CdR and possibly, some of these genes are modulating the expression of MGMT in these cells. Recently, Liu et al. showed that the Trps1 gene modulates the expression of MGMT in cisplatin-resistant lung cancer cells [81].
In conclusion, even though 5-aza-2CdR does not sensitize tongue squamous cell carcinoma for the treatment with cisplatin DNA methylation has an important role in the resistance mechanism induced by this drug. Further investigations into the signaling pathway are needed. The prospects are to explore this mechanism and provide new insights about the changes in genetics and epigenetics into oral cancer research and identify a useful molecular target for efficiently treating this disease.
Acknowledgements
This research project was partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Proc. 140439/2020-0). The authors thank Espaço da Escrita-Pró-Reitoria de Pesquisa-UNICAMP.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol. 2009;45:361–85. doi: 10.1016/j.oraloncology.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Johnson NW, Jayasekara P, Amarasinghe AA. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontol 2000. 2011;57:19–37. doi: 10.1111/j.1600-0757.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 5.Ong TK, Murphy C, Smith AB, Kanatas AN, Mitchell DA. Survival after surgery for oral cancer: a 30-year experience. Br J Oral Maxillofac Surg. 2017;55:911–6. doi: 10.1016/j.bjoms.2017.08.362. [DOI] [PubMed] [Google Scholar]
- 6.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (I) Oral Oncol. 2010;46:630–5. doi: 10.1016/j.oraloncology.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–62. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 8.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–41. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascolo M, Siano M, Ilardi G, Russo D, Merolla F, De Rosa G, Staibano S. Epigenetic Disregulation in oral cancer. Int J Mol Sci. 2012;13:2331–53. doi: 10.3390/ijms13022331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailoor D, Manjunatha B. Role of genetic and epigenetic factors in oral squamous cell carcinoma - a structured review. Recent Pat Biomark. 2015;5:14–9. [Google Scholar]
- 11.Irimie A, Ciocan C, Gulei D, Mehterov N, Atanasov A, Dudea D, Berindan-Neagoe I. Current insights into oral cancer epigenetics. Int J Mol Sci. 2018;19:670. doi: 10.3390/ijms19030670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–71. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–4. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 16.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77:887–94. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilari D, Guancial E, Kim ES. Role of copper transporters in platinum resistance. World J Clin Oncol. 2016;7:106–13. doi: 10.5306/wjco.v7.i1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen DW, Ma J, Okabe M, Zhang G, Xia D, Gottesman MM. Elevated expression of TMEM205, a hypothetical membrane protein, is associated with cisplatin resistance. J Cell Physiol. 2010;225:822–8. doi: 10.1002/jcp.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikuta K, Takemura K, Sasaki K, Kihara M, Nishimura M, Ueda N, Naito S, Lee E, Shimizu E, Yamauchi A. Expression of multidrug resistance proteins and accumulation of cisplatin in human non-small cell lung cancer cells. Biol Pharm Bull. 2005;28:707–12. doi: 10.1248/bpb.28.707. [DOI] [PubMed] [Google Scholar]
- 20.Yamasaki M, Makino T, Masuzawa T, Kurokawa Y, Miyata H, Takiguchi S, Nakajima K, Fujiwara Y, Matsuura N, Mori M, Doki Y. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br J Cancer. 2011;104:707–13. doi: 10.1038/sj.bjc.6606071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2011;31:1869–83. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 22.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–21. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skowron M, Melnikova M, van Roermund J, Romano A, Albers P, Thomale J, Schulz W, Niegisch G, Hoffmann M. Multifaceted mechanisms of cisplatin resistance in long-term treated urothelial carcinoma cell lines. Int J Mol Sci. 2018;19:590. doi: 10.3390/ijms19020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehmé A, Christen RD, Howell SB. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- 25.Fink D, Zheng H, Nebel S, Norris PS, Aebi S, Lin TP, Nehmé A, Christen RD, Haas M, MacLeod CL, Howell SB. In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res. 1997;57:1841–5. [PubMed] [Google Scholar]
- 26.Mojas N, Lopes M, Jiricny J. Mismatch repair-dependent processing of methylation damage gives rise to persistent single-stranded gaps in newly replicated DNA. Genes Dev. 2007;21:3342–55. doi: 10.1101/gad.455407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adachi M, Ijichi K, Hasegawa Y, Nakamura H, Ogawa T, Kanematsu N. Human MLH1 status can potentially predict cisplatin sensitivity but not microsatellite instability in head and neck squamous cell carcinoma cells. Exp Ther Med. 2010;1:93–6. doi: 10.3892/etm_00000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian J, Geng Y, Lv D, Li P, Cordova M, Liao Y, Tian X, Zhang X, Zhang Q, Zou K, Zhang Y, Zhang X, Li Y, Zhang J, Ma Z, Shao Y, Song L, Owen GI, Li T, Liu R, Liu Q, Zou L, Zhang Z, Li Z. Using plasma cell-free DNA to monitor the chemoradiotherapy course of cervical cancer. Int J Cancer. 2019;145:2547–57. doi: 10.1002/ijc.32295. [DOI] [PubMed] [Google Scholar]
- 29.Cooper JS, Pajak TF, Forastiere A, Jacobs J, Fu KK, Ang KK, Laramore GE, Al-Sarraf M. Precisely defining high-risk operable head and neck tumors based on rtog #85-03 and #88-24: targets for postoperative radiochemotherapy? Head Neck. 1998;20:588–94. doi: 10.1002/(sici)1097-0347(199810)20:7<588::aid-hed2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Koutsimpelas D, Pongsapich W, Heinrich U, Mann S, Mann WJ, Brieger J. Promoter methylation of MGMT, MLH1 and RASSF1A tumor suppressor genes in head and neck squamous cell carcinoma: pharmacological genome demethylation reduces proliferation of head and neck squamous carcinoma cells. Oncol Rep. 2012;27:1135–41. doi: 10.3892/or.2012.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moutinho C, Garcia-del-Muro X, Guino E, Vidal A, Puertas S, Muñoz C, Piulats J, Villanueva A, Esteller M. Abstract 432: loss of MGMT promoter methylation and resistance to cisplatin in non-seminomatous germ cell tumors. Cancer Res. 2016;76:432. [Google Scholar]
- 32.Kato K, Hara A, Kuno T, Mori H, Yamashita T, Toida M, Shibata T. Aberrant promoter hypermethylation of p16 and MGMT genes in oral squamous cell carcinomas and the surrounding normal mucosa. J Cancer Res Clin Oncol. 2006;132:735–43. doi: 10.1007/s00432-006-0122-8. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S, Salehi F, Scheithauer BW, Rotondo F, Syro LV, Kovacs K. Role of MGMT in tumor development, progression, diagnosis, treatment and prognosis. Anticancer Res. 2009;29:3759–68. [PubMed] [Google Scholar]
- 34.Chen R, Zheng Y, Zhuo L, Wang S. Association between MGMT promoter methylation and risk of breast and gynecologic cancers: a systematic review and meta-analysis. Sci Rep. 2017;7:12783. doi: 10.1038/s41598-017-13208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR. Correlation of O 6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008;26:4189–99. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 36.Fan CH, Liu WL, Cao H, Wen C, Chen L, Jiang G. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4:e876. doi: 10.1038/cddis.2013.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SH, Kuo CC, Li CF, Cheung CH, Tsou TC, Chiang HC, Yang YN, Chang SL, Lin LC, Pan HY, Chang KY, Chang JY. O(6)-methylguanine DNA methyltransferase repairs platinum-DNA adducts following cisplatin treatment and predicts prognoses of nasopharyngeal carcinoma. Int J Cancer. 2015;137:1291–305. doi: 10.1002/ijc.29486. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Zhang M, Gan H, Wang H, Lee JH, Fang D, Kitange GJ, He L, Hu Z, Parney IF, Meyer FB, Giannini C, Sarkaria JN, Zhang Z. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat Commun. 2018;9:2949. doi: 10.1038/s41467-018-05373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamska A, Elaskalani O, Emmanouilidi A, Kim M, Abdol Razak NB, Metharom P, Falasca M. Molecular and cellular mechanisms of chemoresistance in pancreatic cancer. Adv Biol Regul. 2018;68:77–87. doi: 10.1016/j.jbior.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. DNA methylation inhibitor 5-Aza-2’-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008;28:752–71. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seelan RS, Mukhopadhyay P, Pisano MM, Greene RM. Effects of 5-Aza-2’-deoxycytidine (decitabine) on gene expression. Drug Metab Rev. 2018;50:193–207. doi: 10.1080/03602532.2018.1437446. [DOI] [PubMed] [Google Scholar]
- 42.do Amaral GCLS, Planello AC, Borgato G, de Lima DG, Guimarães GN, Marques MR, de Souza AP. 5-Aza-CdR promotes partial MGMT demethylation and modifies expression of different genes in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127:425–32. doi: 10.1016/j.oooo.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Veigl ML, Kasturi L, Olechnowicz J, Ma A, Lutterbaugh JD, Periyasamy S, Li GM, Drummond J, Modrich PL, Sedwick WD, Markowitz SD. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci U S A. 1998;95:8698–702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–5. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shang D, Ito N, Kamoto T, Ogawa O. Demethylating Agent 5-Aza-2’-deoxycytidine enhances susceptibility of renal cell carcinoma to paclitaxel. Urology. 2007;69:1007–12. doi: 10.1016/j.urology.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Tawbi HA, Beumer JH, Tarhini AA, Moschos S, Buch SC, Egorin MJ, Lin Y, Christner S, Kirkwood JM. Safety and efficacy of decitabine in combination with temozolomide in metastatic melanoma: a phase I/II study and pharmacokinetic analysis. Ann Oncol. 2013;24:1112–9. doi: 10.1093/annonc/mds591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viet CT, Dang D, Achdjian S, Ye Y, Katz SG, Schmidt BL. Decitabine rescues cisplatin resistance in head and neck squamous cell carcinoma. PLoS One. 2014;9:e112880. doi: 10.1371/journal.pone.0112880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu X, Zhang Y, Wang X, Chen M, Wang Y, Nie J, Meng Y, Han W. Low dose decitabine combined with taxol and platinum chemotherapy to treat refractory/recurrent ovarian cancer: an open-label, single-arm, phase I/II study. Curr Protein Pept Sci. 2015;16:329–36. doi: 10.2174/138920371604150429155740. [DOI] [PubMed] [Google Scholar]
- 49.Young CS, Clarke KM, Kettyle LM, Thompson A, Mills KI. Decitabine-Vorinostat combination treatment in acute myeloid leukemia activates pathways with potential for novel triple therapy. Oncotarget. 2017;8:51429–46. doi: 10.18632/oncotarget.18009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wojdacz TK, Dobrovic A. Methylation-sensitive high resolution melting (MS-HRM): a new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007;35:e41. doi: 10.1093/nar/gkm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Do H, Wong NC, Murone C, John T, Solomon B, Mitchell PL, Dobrovic A. A critical re-assessment of DNA repair gene promoter methylation in non-small cell lung carcinoma. Sci Rep. 2015;4:4186. doi: 10.1038/srep04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uno M, Oba-Shinjo SM, Camargo AA, Moura RP, de Aguiar PH, Cabrera HN, Begnami M, Rosemberg S, Teixeira MJ, Marie SK. Correlation of MGMT promoter methylation status with gene and protein expression levels in glioblastoma. Clinics (Sao Paulo) 2011;66:1747–55. doi: 10.1590/S1807-59322011001000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 54.Lim SY, Kim SA, Ahn SG, Kim HK, Kim SG, Hwang HK, Kim BO, Lee SH, Kim JD, Yoon JH. Metastatic tumours to the jaws and oral soft tissues: a retrospective analysis of 41 Korean patients. Int J Oral Maxillofac Surg. 2006;35:412–5. doi: 10.1016/j.ijom.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Varela-Centelles P, Seoane J, García-Pola MJ, Seoane-Romero JM, García Martín JM. Diagnostic delay in symptomatic oral cancer. Cham: Springer International Publishing. 2019:95–108. [Google Scholar]
- 56.Kelner N, Vartanian JG, Pinto CA, Coutinho-Camillo CM, Kowalski LP. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br J Oral Maxillofac Surg. 2014;52:590–7. doi: 10.1016/j.bjoms.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Liu JC, Sopka DS, Mehra R, Lango MN, Fundakowski C, Ridge JA, Galloway TJ. Early oral tongue cancer initially managed with surgery alone: treatment of recurrence. World J Otorhinolaryngol Head Neck Surg. 2016;2:193–7. doi: 10.1016/j.wjorl.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–6. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 59.Mazeron JJ, Ardiet JM, Haie-Méder C, Kovács G, Levendag P, Peiffert D, Polo A, Rovirosa A, Strnad V. GEC-ESTRO recommendations for brachytherapy for head and neck squamous cell carcinomas. Radiother Oncol. 2009;91:150–6. doi: 10.1016/j.radonc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Rapidis AD, Gullane P, Langdon JD, Lefebvre JL, Scully C, Shah JP. Major advances in the knowledge and understanding of the epidemiology, aetiopathogenesis, diagnosis, management and prognosis of oral cancer. Oral Oncol. 2009;45:299–300. doi: 10.1016/j.oraloncology.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 62.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 63.Coronel J, Cetina L, Pacheco I, Trejo-Becerril C, González-Fierro A, de la Cruz-Hernandez E, Perez-Cardenas E, Taja-Chayeb L, Arias-Bofill D, Candelaria M, Vidal S, Dueñas-González A. A double-blind, placebo-controlled, randomized phase III trial of chemotherapy plus epigenetic therapy with hydralazine valproate for advanced cervical cancer. Preliminary results. Med Oncol. 2011;28(Suppl 1):S540–6. doi: 10.1007/s12032-010-9700-3. [DOI] [PubMed] [Google Scholar]
- 64.Chang X, Monitto CL, Demokan S, Kim MS, Chang SS, Zhong X, Califano JA, Sidransky D. Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Cancer Res. 2010;70:2870–9. doi: 10.1158/0008-5472.CAN-09-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Appleton K, Mackay HJ, Judson I, Plumb JA, McCormick C, Strathdee G, Lee C, Barrett S, Reade S, Jadayel D, Tang A, Bellenger K, Mackay L, Setanoians A, Schätzlein A, Twelves C, Kaye SB, Brown R. Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. J. Clin. Oncol. 2007;25:4603–9. doi: 10.1200/JCO.2007.10.8688. [DOI] [PubMed] [Google Scholar]
- 66.Beyrouthy MJ, Garner KM, Hever MP, Freemantle SJ, Eastman A, Dmitrovsky E, Spinella MJ. High DNA methyltransferase 3B expression mediates 5-Aza-deoxycytidine hypersensitivity in testicular germ cell tumors. Cancer Res. 2009;69:9360–6. doi: 10.1158/0008-5472.CAN-09-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–7. [PubMed] [Google Scholar]
- 68.Ha PK, Califano JA. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol. 2006;7:77–82. doi: 10.1016/S1470-2045(05)70540-4. [DOI] [PubMed] [Google Scholar]
- 69.Hema K, Smitha T, Sheethal H, Mirnalini SA. Epigenetics in oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2017;21:252. doi: 10.4103/jomfp.JOMFP_150_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rocha CRR, Silva MM, Quinet A, Cabral-Neto JB, Menck CFM. DNA repair pathways and cisplatin resistance: an intimate relationship. Clinics (Sao Paulo) 2018;73:e478s. doi: 10.6061/clinics/2018/e478s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12:587–98. doi: 10.1038/nrc3342. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Chen H, Peng Z, Banerjee S, Li W, Zhao Z, Sun J, Lv J, Huang H, Bai R, Lin K, Li Z. A novel MLH1 initiation codon mutation (c.3G>T) in a large Chinese lynch syndrome family with different onset age and mRNA expression Level. Biomed Res Int. 2018;2018:1460835. doi: 10.1155/2018/1460835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abele R, Clavel M, Dodion P, Bruntsch U, Gundersen S, Smyth J, Renard J, Van Glabbeke M, Pinedo HM. The EORTC early clinical trials cooperative group experience with 5-aza-2’-deoxycytidine (NSC 127716) in patients with colo-rectal, head and neck, renal carcinomas and malignant melanomas. Eur J Cancer Clin Oncol. 1987;23:1921–4. doi: 10.1016/0277-5379(87)90060-5. [DOI] [PubMed] [Google Scholar]
- 74.Momparler RL, Bouffard DY, Momparler LF, Dionne J, Belanger K, Ayoub J. Pilot phase l-ll study on 5-aza-2’-deoxycytidine (decitabine) in patients with metastatic lung cancer. Anticancer Drugs. 1997;8:358–68. doi: 10.1097/00001813-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, Shin JJ, Harbom KM, Beaty R, Pappou E, Harris J, Yen RW, Ahuja N, Brock MV, Stearns V, Feller-Kopman D, Yarmus LB, Lin YC, Welm AL, Issa JP, Minn I, Matsui W, Jang YY, Sharkis SJ, Baylin SB, Zahnow CA. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21:430–46. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elias ST, Borges GA, Rêgo DF, E Silva LF, Avelino S, DE Matos Neto JN, Simeoni LA, Guerra EN. Combined paclitaxel, cisplatin and fluorouracil therapy enhances ionizing radiation effects, inhibits migration and induces G0/G1 cell cycle arrest and apoptosis in oral carcinoma cell lines. Oncol Lett. 2015;10:1721–7. doi: 10.3892/ol.2015.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kotowski U, Heiduschka G, Brunner M, Erovic BM, Martinek H, Thurnher D. Arsenic trioxide enhances the cytotoxic effect of cisplatin in head and neck squamous cell carcinoma cell lines. Oncol Lett. 2012;3:1326–30. doi: 10.3892/ol.2012.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zuo C, Ai L, Ratliff P, Suen JY, Hanna E, Brent TP, Fan CY. O6-methylguanine-DNA methyltransferase gene: epigenetic silencing and prognostic value in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:967–75. [PubMed] [Google Scholar]
- 79.Sawant A, Kothandapani A, Zhitkovich A, Sobol RW, Patrick SM. Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair (Amst) 2015;35:126–36. doi: 10.1016/j.dnarep.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avdievich E, Reiss C, Scherer SJ, Zhang Y, Maier SM, Jin B, Hou H, Rosenwald A, Riedmiller H, Kucherlapati R, Cohen PE, Edelmann W, Kneitz B. Distinct effects of the recurrent Mlh1G67R mutation on MMR functions, cancer, and meiosis. Proc Natl Acad Sci U S A. 2008;105:4247–52. doi: 10.1073/pnas.0800276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu H, Liao Y, Tang M, Wu T, Tan D, Zhang S, Wang H. Trps1 is associated with the multidrug resistance of lung cancer cell by regulating MGMT gene expression. Cancer Med. 2018;7:1921–32. doi: 10.1002/cam4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]