Abstract
Background: Hidden blood loss (HBL) often occurs after joint replacement; however, the mechanism of HBL is not clear. We conducted a prospective study to analyze the correlation between high-level free fatty acids (FFA) and erythrocyte injury, and explore the pathologic mechanism of hidden blood loss (HBL) after total knee arthroplasty (TKA). Methods: Perioperative blood indexes were tested in 120 patients who underwent unilateral total knee replacement for end-stage knee osteoarthritis. The changes in FFA, reactive oxygen species (ROS), hemoglobin (Hb), and red blood cells (RBC) in the blood samples were detected. The activity of glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD) and hydrogen peroxide (H2O2) levels were measured. Morphologic changes of blood cells were analyzed under a microscope. Results: HBL occurred in all patients after TKA. The Hb and RBC decreased significantly 24 h after surgery (P <0.05), while FFA and ROS concentration were substantially elevated, and heteromorphic red blood cells appeared under the microscope. The hemoglobin content decreased to its lowest level at 48 h after the operation (P<0.01). With the increase of FFA and ROS levels, HBL appeared more obvious (P<0.01). GSH-PX activity, T-SOD activity, and H2O2 levels significantly decreased compared to preoperative tested samples (P<0.01). Microscopically, atypical erythrocytes increased significantly with cellular rupture and lysis identified. Conclusions: High levels of FFA in blood can induce oxidative stress and damage red blood cells, leading to the occurrence of HBL after surgery. Trial Registration: Chinese Clinical Trials Registry (the trial number: ChiCTR17010681, URL: http://www.chictr.org.cn).
Keywords: Hidden blood loss, free fatty acids, reactive oxygen species, total knee arthroplasty
Introduction
Hidden blood loss often occurs after traumatic fracture or surgery, which results directly in increased blood loss, and prolonged recovery time and hospital stay [1,2]. There are many hypotheses about hidden blood loss, but the pathologic mechanism is still not clear. As early as 1973, Pattison et al. detected anemia inconsistent with intraoperative bleeding after knee replacement, and attributed this to invisible blood loss [3]. Sehat et al. first proposed the concept of HBL in 2000 and suggested that the HBL accounted for about 50% of the total blood loss through the Gross equation. Their research pointed out that the average perioperative blood loss of patients with knee arthroplasty was about 1474 ml, while the HBL was as high as 735 ml [4].
During TKA, there are two noteworthy procedures. The first procedure is to use the alignment guide of the femur to drill a hole between the femoral condyles and enter the femur through the longitudinal channel of the femur. Another procedure is that with the trial components in place, a drill guide of the tibial component is used to expand the tibial hole by drilling a longitudinal channel through the tibial aperture. Both procedures can increase the pressure in the medullary cavity, where a large number of lipomicrons may enter the circulation under high pressure [5-7].
FFA is a catabolic product of lipomicrons, that is composed of oleic acid, palmitic acid, linoleic acid, and others, and can be combined with albumin to exist in the blood [8]. The concentration of free fatty acids in serum is related to lipid metabolism, sugar metabolism, and endocrine function. Studies have shown that high concentrations of FFA can induce oxidative stress and increase the production of ROS [9]. Active oxygen free radicals are directly or indirectly converted from oxygen and its derivatives. They can react with polyunsaturated fatty acids on the cell membrane to generate peroxides that have a toxic effect on the cells, resulting in protein oxidation, damage of DNA mutation, lipid oxidation, or cell foaming. Oxidative damage to lipids in the erythrocyte membrane can directly lead to damage of red blood cells [10-12]. In this study, we examined the perioperative blood data of knee replacement patients to study the relationship between FFA and HBL and explored the pathologic mechanism of HBL.
Patients and methods
Patients
This was a prospective study of 120 patients who received TKA in Jinling Hospital from April 2019 to December 2019. There were 55 males (45.8%) and 65 females (54.2%), with an average age of 69 years (range 58-78 years).
Inclusion criteria: ① All patients underwent aseptic knee osteoarthritis with clinical manifestations of knee pain, varus deformity and limitations to daily life, which were ineffective after conservative treatment and had no other basic diseases; ② Objective, actual, accurate, timely and complete perioperative medical records and the collected blood samples that met the measurement requirements; ③ Patients with informed consent to the trial scheme.
Exclusion criteria: ① Anemia and perioperative blood transfusion patients examined by preoperative blood tests; ② Patients with obesity, poor surgical tolerance, severe diabetes, or cardiopulmonary insufficiency; ③ Patients with hyperlipidemia, preoperative anemia or hematological diseases; ④ Patients with cerebral infarction or combined with varicose veins of lower limbs 3 months before surgery; ⑤ Patients with liver or kidney disease or dysfunction.
Materials
The prostheses used by all patients were EMINI MKII full-anatomical rotary platforms produced by LINK, Germany. Blood smear was prepared by Wright staining kit (Hongbokang pharmaceutical technology, China). Polarized light microscope (NIKON ECLIPSE 501, Japan) and hematology analyzer (SYSMEX XE-5000, Japan) were used. ROS release was detected with an ROS Detection Kit (Beyotime Institute of Biotechnology, China). The H2O2 concentration and GSH-Px and SOD activities were determined with commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, China). Centrifuge was from Hermle Universal Centrifuge (Z323, Germany).
Perioperative management of TKA
All operations were performed by the same chief physician and the perioperative management was performed by the same attending physician. Patient’s height, weight, body mass index, physical examination and vital signs were well recorded before the operation. All patients were routinely given antibiotics to prevent infection 0.5 h before surgery. During the operation, a tourniquet was used (the tourniquet pressure is subject to the disappearance of the dorsal foot artery fluctuations). In combination with epidural anaesthesia, the operation time was 45-62 minutes (53.6 minutes in average). Adequate intraoperative hemostasis and the tibial plateau and femur osteotomy were based on the principle of restoring force lines, balancing soft tissues and restoring the patella trajectory. After the operation, an elastic bandage was applied to bandage the affected limb with moderate pressure from the ankle to the middle thigh. 24 h after the operation, deep vein thrombosis was prevented by oral anticoagulant rivaroxaban (Bayer) and the use of a lower limb venous thrombotherapy device. During the perioperative period, homeostasis was maintained according to the fluid intake and outflow.
Evaluation of blood test
Blood samples were collected preoperatively and at 24 h, 48 h, 72 h and 120 h after surgery. Samples were placed in heparin anticoagulant tubes and mixed for backup. Appropriate blood samples were stained with Wright’s stain to prepare erythrocyte blood smears. Then the morphology of red blood cells was observed under a polarized light microscope. Other samples were submitted for inspection within two hours and the RBC, Hct, Hb content, and FFA concentration were measured by a fully automatic hematology analyzer system. The oxidative stress related indicators GSH-Px, H2O2 and T-SOD were measured by the kit-guided method: GSH-Px activity was measured by fluorescence assay, T-SOD activity was measured by xanthine oxidase assay, and H2O2 content change was measured by spectrophotometry. ROS release was detected with ROS Detection Kit.
Calculation of blood loss
Visible blood loss (VBL) mainly includes intraoperative blood loss (negative pressure aspirator retrieves blood, intraoperative gauze), and postoperative drainage volume (drainage tube drainage).
The patient’s estimated blood volume (EBV) can be calculated by using the formula:
EBV = k1 × height (m3) + k2 × weight (kg) + k3
Where k1 0.3669, k2 0.03219, k3 0.6041 for men; and k1 0.3561, k2 0.03308, k3 0.1833 for women.
Calculated blood loss (CBL) can be calculated by Gross formula: CBL= EBV (H0-H1)/Hav
Where, H0 is preoperative Hct, H1 is postoperative Hct, Hav = (H0-H1)/2 is mean value of hematocrit preoperative and postoperative.
As transfusion patients were excluded from our study, the CBL can be considered as total blood loss (TBL), so we can figure out: HBL= CBL-VBL.
Statistical analysis
All calculations and statistical analyses were performed using the SPSS version 19.0 software. Values were expressed as mean ± standard deviation (x ± SD) and analyzed by one-way analysis of variance followed by Dunnett’s t-test to compare differences in clinical indices. In all cases, P<0.05 was regarded as significant.
Results
Among the 120 patients involved in the study, 1 patient developed deep vein thrombosis in the lower extremity after surgery and 9 patients received blood transfusion during the perioperative period. Therefore, a total of 110 patients were brought into the result analysis including 54 males and 56 females. All patients had complete relevant examination data. The height of the patients was 168.3 ± 9.5 cm and the average weight was 67.5 ± 15.3 kg. The average Hct before operation was 35.2 ± 3.5%, while it was 27.4 ± 2.8% after operation. According to the intraoperative blood loss and postoperative drainage volume, the visible blood loss was 478 ± 126 mL, according to the Gross formula, the total blood loss was 1036 ± 128 ml, and the hidden blood loss was 565 ± 72 ml. Our findings suggest that HBL accounted for about 54.5% of total blood loss.
The average Hb value of the patients before surgery was 132.5 ± 11.7 g/L, and the RBC was (4.18 ± 0.45) × 1012/L. 24 h after surgery. The Hb and RBC were significantly reduced to 105.5 ± 8.3 g/L and (3.70 ± 0.38) × 1012/L. According to the decline of Hct combined with the Gross formula, HBL must have occurred. Hb and RBC continued to decline after surgery, and decreased to the lowest value after about 48 hours, dropping to 85.5 ± 7.5 g/L and (2.75 ± 0.30) × 1012/L, respectively. Gross formula results indicated that the hidden blood loss reached a peak value 72 h later. Hb and RBC gradually recovered and these indicators took a turn for the better 5 days after operation (Figure 1).
Figure 1.
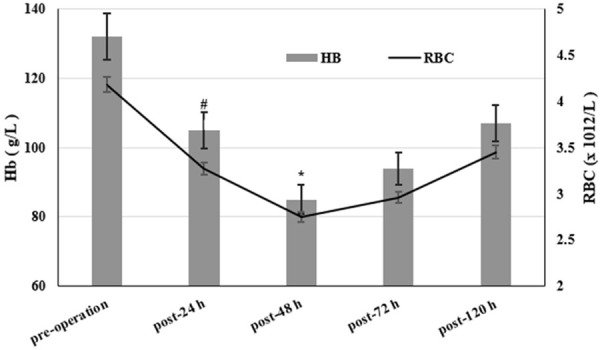
Hb and RBC changes before and after surgery. Hb and RBC decreased most significantly 48 h after operation. Post-24, 48, 72 and 120 h vs. Pre-operation. values are shown as mean ± SE; *P<0.01, #P<0.05.
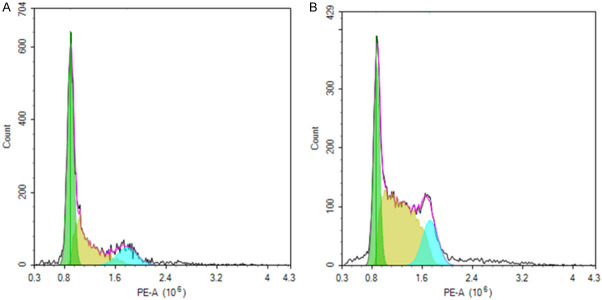
The concentration of FFA was 0.32 ± 0.11 mmol/L before surgery and increased to (0.95 ± 0.26) mmol/L 24 h after the operation, which was almost three times higher than that before surgery. The content of ROS also increased significantly. 48 h later, FFA concentration was as high as 1.28 ± 0.33 mmol/L and ROS test results indicated that it was also significantly increased. The alteration of HBL was also the most obvious (Figure 2). The levels of FFA gradually declined 72 h after surgery and tended to return to normal levels after 5 days. The above results suggested that FFA and ROS levels increased significantly 48 h after the operation, and the changes trended to synchronize with HBL, which indicated that FFA was closely related to HBL (Figure 3). Moreover, the increased ROS suggested that the pathogenesis mechanism of HBL may be associated with the oxidative stress response.
Figure 2.
ROS level changes pre-operation (A) and 48 h post-operation (B). The dyed cells were gathered to detect DCFH-DA fluorescence (488 nm-552 nm) using flow cytometry. Cell with fluorescence intensity at 102-103 nm were counted representing cells with high ROS release. The blue area represents the ROS level. The results showed that the ROS level at 48 hours after surgery was significantly higher than pre-operation. Meanwhile, HBL was the most obvious and FFA concentration reached its peak, suggesting that HBL is closely related to oxidative stress.
Figure 3.
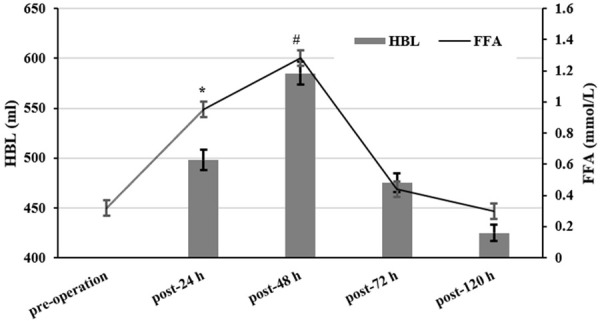
FFA and HBL changes before and after surgery. According to the variation tendency in the figure, HBL becomes more obvious with the increase of FFA concentration. Post-24, 48, 72 and 120 h vs. Pre-operation. Values are shown as mean ± SE; *P<0.01, #P<0.05.
Indicators related to the redox reaction showed that GSH-Px activity, T-SOD activity, and H2O2 content declined significantly 24 h after surgery. In addition, erythrocyte atypia was observed under the microscope, which was mainly characterized by crenated erythrocytes. This phenomenon continued to aggravate, and GSH-Px activity, T-SOD activity and H2O2 content decreased to a minimum 48 h later, and the erythrocyte atypia also increased significantly. From the microscopic viewpoint, there were many red blood cells with spindle and oblong shapes, pleomorphism, shrinkage, deformation, rupture, and breaking (Figure 4A-E). After 72 h, the situation began to improve, GSH-Px activity, T-SOD activity, and H2O2 content increased, while red blood cell aberrations decreased (Figure 5).
Figure 4.
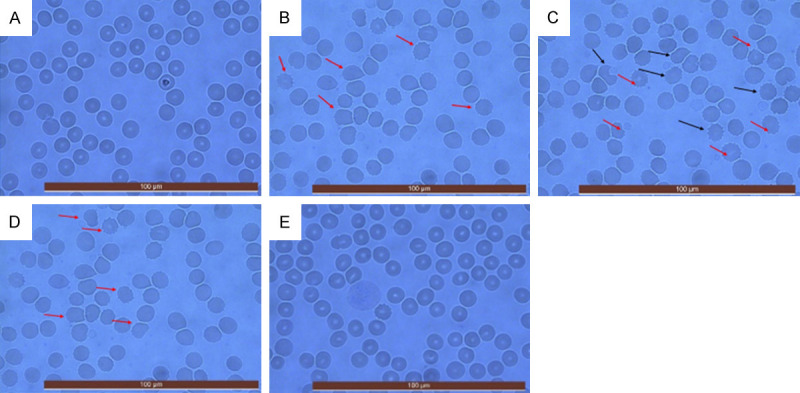
Morphologic changes of red blood cells under microscope during perioperative period. Samples were taken before and 24, 48, 72, and 120 h after surgery (A-E). Cell smears were prepared by Wright’s stain and observed under a polarized light microscope (400× magnification). A large number of atypical red blood cells can be found under the microscope at 24 h after operation (B). 48 h after the operation, the atypical erythrocytes increased, mainly manifested by polymorphic changes, shrinkage, deformation, and even breaking (C). 72 h later, the erythrocyte morphology improved, and the atypical cells decreased (D). However, the erythrocyte morphology was normal 5 days after the operation.
Figure 5.
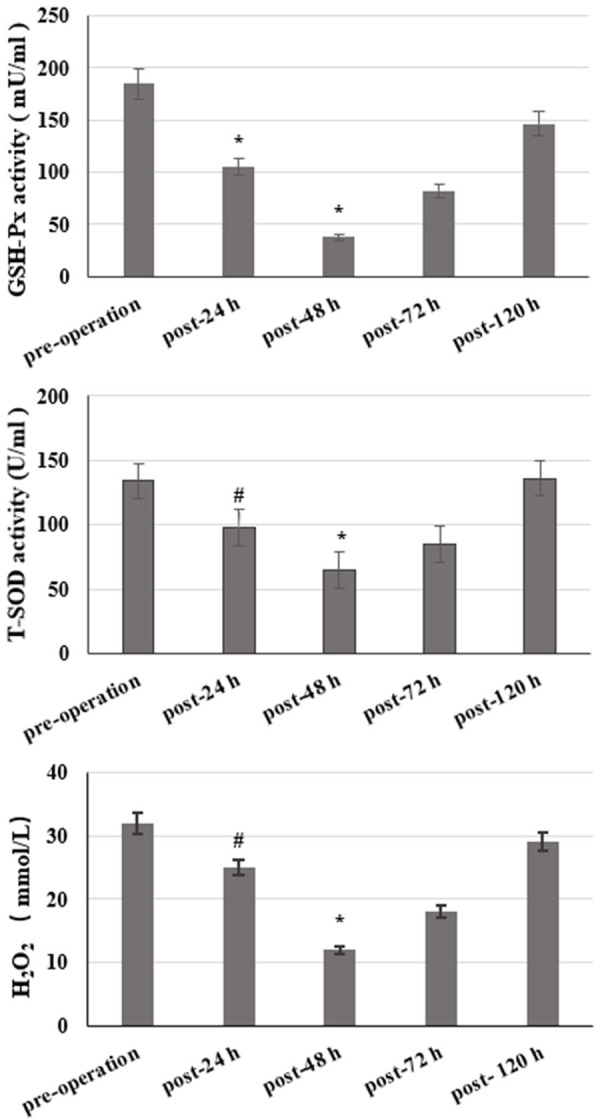
Changes of GSH-Px, T-SOD activity, and H2O2 levels during perioperative period. Post-24, 48, 72 and 120 h vs. Pre-operation. Values are shown as mean ± SE; *P<0.05, #P<0.01.
Discussion
In this study, we confirmed the existence of HBL by examining and analyzing the perioperative blood indexes of patients with knee arthroplasty. Results showed that the total perioperative blood loss was 1036 ml and was significantly lower than the earlier reported 1474 ml, suggesting that the improvement in perioperative management could reduce the total blood loss and visible blood loss. However, the proportion of HBL wass 54.5%, which is higher than the 50% reported previously, indicating that the control and management of HBL is crucial [4].
There are many hypotheses about HBL. Some studies had suggested that HBL may be due to partial hemolysis caused by blood transfusion. However, clinical studies had found that there was no difference in HBL between the transfusion group and non-transfusion group [13,14]. However, in order to eliminate interference, perioperative blood transfusion patients were not included in the study. It is reported that the use of tourniquet during TKA can increase the risk of HBL. The study divided the patients into the tourniquet group and non- tourniquet group, and the results showed that there was no significant difference in operation time between the groups, and the visible blood loss in the non-tourniquet group increased about 180 ml compared with the tourniquet group. Instead, the HBL was about 16% lower than that in the tourniquet group. However, there was still significant HBL in the non-tourniquet group that could not be explained [15,16]. In addition, the “third mesooecium” theory also partially explained the HBL. Boswell et al. believe that HBL occurs after TKA due to tissue blood oozing and fluid oozing into the surrounding tissue clearance (i.e., the third space) [17,18]. Therefore, after the surgery, we routinely use elastic bandages to bandage the affected limb from the ankle to the middle of the thigh and pressurize appropriately to reduce bleeding, and apply a negative pressure drainage ball to drain the hematocele or hydrops in the joint cavity. With these measures, it is difficult for the blood to penetrate into the surrounding tissue space, and the amount of HBL triggered in this manner is limited.
In the previous study, we successfully constructed the animal model of HBL by injecting linoleic acid and arachidonic acid into rats. Linoleic acid and arachidonic acid are important components of FFA. Our study found that when the blood linoleic acid or linolenic acid reached a certain concentration, it would induce an oxidative stress response, resulting in the destruction of red blood cells and HBL [19-21].
HBL often occurs after joint replacement and traumatic fracture surgery. The common feature is that a large amount of fat droplets leak out of the medullary cavity, and penetrate deep into the circulation, tissues and cells. Especially during joint replacement, the manipulation of the femur and tibia can increase the pressure of the medullary cavity and force the fat particles into the blood circulation [6,7,22,23]. The effects of its metabolites on tissue cells needs further study. Our results showed that the concentration of FFA in the blood increased significantly 48 h postoperatively, indicating that many small fat droplets were released into the bloodstream during the operation, and the RBC and Hb were significantly reduced. At the same time, HBL was also the most obvious, and a quantity of damaged red blood cells could be found under the microscope. 72 h after the operation, with the catabolic metabolism of fat particles, FFA concentration decreased significantly, and erythrocyte and hemoglobin level gradually increased. Meanwhile, the morphology of red blood cells began to improve under the microscope, and the number of erythrocytes with atypia decreased gradually. This indicates that FFA, a metabolite of small fat droplets, is closely related to the reduction and destruction of red blood cells after surgery.
Studies have pointed out that the FFA can help to induce the generation of ROS in endothelial and vascular smooth muscle cells [24]. High concentration of FFA stimulates the production of highly reactive molecular oxygen clusters and reactive nitrogen clusters, which triggers oxidative stress. A prolonged imbalance between the production of highly reactive molecules and anti-oxidant effects results in tissue damage. These active molecules can directly oxidize and damage DNA, proteins and lipids, and can also act as functional molecular signals, activating a variety of stress-sensitive signaling pathways in cells, which are closely related to insulin resistance and impaired β-cell function [25,26]. Studies have shown that high levels of FFA lead to a large amount of ROS production and oxidative stress, which can also activate stress-sensitive signaling pathways. Fatty acid synthase (FAS) genes activate Akt, and sterol regulatory element binding protein-1 under hypoxic conditions becomes up-regulated, thus FAS expression level is associated with hypoxia in the body. It is worth noting that Akt is also up-regulated by H2O2. Under hypoxic conditions, the level of FAS protein is significantly increased as well as the production of ROS in cells [27,28]. This suggests that the expression of FAS gene is actively controlled by hypoxia, which is also related to the amount of ROS in cells. During knee arthroplasty, the use of tourniquets can lead to poor blood supply and tissue hypoxia in the affected limb over a period of time, and local tissue swelling and reduced activity after operation also exacerbate blood and tissue hypoxia. As the concentration of fat droplets in blood and tissues increases after surgery, the FAS required for metabolism increases. Therefore, blood and tissue hypoxia will accelerate the catabolic metabolism of H2O2 in the blood. As a consequence, the level of H2O2 in the blood decreased significantly 24 h and 48 h after surgery. A high concentration of FFA will induce the production of ROS in vascular smooth muscle cells. The results of flow cytometry showed that ROS levels increased significantly after 48 hours, which may lead to an imbalance in the redox reaction. Free radicals can damage cells by acting on polyunsaturated fatty acids on cell membranes, exacerbating oxygen free radical reactions, and lipid peroxidation. The intermediates of lipid peroxidation can react with membrane proteins to polymerize and cross-link proteins. In addition, the carbonyl products of lipid peroxidation (such as malondialdehyde) can also attack the amino groups of membrane protein molecules, resulting in intramolecular and intermolecular cross-linking of proteins. On the other hand, free radicals can also covalently bind directly to enzymes or receptors on the membrane [29-31]. These oxidations damage the spatial configuration of enzymes, receptors, and ion channels embedded in the membrane system, destroying the integrity of the membrane and affecting the function of the membrane and antigen specificity, which eventually leads to the cell damage and lesions. Therefore, the oxidative stress induced by FFA can destroy red blood cells and result in erythropenia and HBL.
SOD is an antioxidant metal enzyme in body. It can catalyze the disproportionation of superoxide anion radicals to generate oxygen and H2O2, which plays a crucial role in the balance between oxidation and antioxidant activity in the body. So, SOD is closely related to the occurrence and development of many diseases [32]. The activity of total superoxide dismutase (T-SOD) can reflect the ability of the body to remove oxygen free radicals [33]. Glutathione peroxidase (GSH-Px) is an important peroxidase widely found in the body. The active center of GSH-Px is selenocysteine, and its activity can reflect the selenium level of the body. Selenium, as a component of the GSH-Px enzyme system, can catalyze the transformation of GSH into GSSG, and reduce toxic peroxides to non-toxic hydroxyl compounds so as to protect the structure and function of cell membranes from the interference and damage of oxides [34,35]. The decrease of NADPH was linearly related to the activity of GSH-Px. The physiologic function of GSH-Px in plasma is mainly to catalyze GSH, participate in the peroxidation reaction, and remove peroxides and hydroxyl radicals generated during cellular respiratory metabolism, thereby reducing the peroxidation of polyunsaturated fatty acids in the cell membrane [29,36]. Studies have shown that FFA can stimulate neutrophils to produce H2O2 and hypochlorous acid, which can oxidize and deplete SOD and GSH-Px on the surface of the cell membrane [37,38]. In our study, with the increase of FFA concentration in blood, the activities of T-SOD and GSH-Px decreased significantly, and the morphology of red blood cells also showed obvious cytomembrane destruction, suggesting that the lipid droplet metabolite FFA in the blood induced a series of oxidation reactions in the body. The imbalance of the redox reaction leads to a destruction and significant reduction of red blood cells. Accompanied by the metabolism of FFA and the disappearance of oxidative stress stimuli, the Hb and RBC increased by self-compensation, and the activities of T-SOD and GSH-Px returned to equilibrium.
Conclusions
In summary, a high level of FFA can induce oxidative stress in vivo and damage red blood cells, which eventually leads to HBL. This suggests that intraoperative clearance of fat droplets in the medullary cavity and the operative area, and the use of antioxidants postoperative may reduce the occurrence of HBL.
Disclosure of conflict of interest
None.
References
- 1.Smith GH, Tsang J, Molyneux SG, White TO. The hidden blood loss after hip fracture. Injury. 2011;42:133–135. doi: 10.1016/j.injury.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br. 2006;88:1053–1059. doi: 10.1302/0301-620X.88B8.17534. [DOI] [PubMed] [Google Scholar]
- 3.Pattison E, Protheroe K, Pringle RM, Kennedy AC, Dick WC. Reduction in haemoglobin after knee joint surgery. Ann Rheum Dis. 1973;32:582–584. doi: 10.1136/ard.32.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7:151–155. doi: 10.1016/s0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Ansari J, Pandit HG. Kinematically aligned total knee arthroplasty or mechanically aligned total knee arthroplasty. J Knee Surg. 2018;31:999–1006. doi: 10.1055/s-0038-1632378. [DOI] [PubMed] [Google Scholar]
- 6.Lu K, Xu M, Li W, Wang K, Wang D. A study on dynamic monitoring, components, and risk factors of embolism during total knee arthroplasty. Medicine (Baltimore) 2017;96:e9303. doi: 10.1097/MD.0000000000009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao N, Zhou L, Cong Y, Guo T, Fan W, Chang Z, Zhao J. Free fatty acids are responsible for the hidden blood loss in total hip and knee arthroplasty. Med Hypotheses. 2013;81:104–107. doi: 10.1016/j.mehy.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1:230–236. doi: 10.1159/000055816. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Su Y, Ju Y, Ma K, Li W, Li W. Astragalosides IV protected the renal tubular epithelial cells from free fatty acids-induced injury by reducing oxidative stress and apoptosis. Biomed Pharmacother. 2018;108:679–686. doi: 10.1016/j.biopha.2018.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava S, Singh D, Patel S, Singh MR. Role of enzymatic free radical scavengers in management of oxidative stress in autoimmune disorders. Int J Biol Macromol. 2017;101:502–517. doi: 10.1016/j.ijbiomac.2017.03.100. [DOI] [PubMed] [Google Scholar]
- 11.Kajaia T, Maskhulia L, Chelidze K, Akhalkatsi V, Mchedlidze T. Implication of relationship between oxidative stress and antioxidant status in blood serum. Georgian Med News. 2018;284:71–76. [PubMed] [Google Scholar]
- 12.Zhou J, Chen L, Liu Z, Sang L, Li Y, Yuan D. Changes in erythrocyte polyunsaturated fatty acids and plasma eicosanoids level in patients with asthma. Lipids Health Dis. 2018;17:206. doi: 10.1186/s12944-018-0853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huerfano E, Huerfano M, Shanaghan KA, Gonzalez Della Valle A. Topical tranexamic acid in revision total knee arthroplasty reduces transfusion rates and may be associated with earlier recovery. J Arthroplasty. 2019;34:S249–S255. doi: 10.1016/j.arth.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Hu KZ, Sun HY, Sui C. Effects of five treatment regimens on blood loss and blood transfusion in total knee arthroplasty: a preliminary study in China. Int J Clin Pharmacol Ther. 2017;55:433–441. doi: 10.5414/CP202813. [DOI] [PubMed] [Google Scholar]
- 15.Tetro AM, Rudan JF. The effects of a pneumatic tourniquet on blood loss in total knee arthroplasty. Canadian Journal of Surgery. 2001;44:33. [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Wen Y, Wu H, Qian Q, Lin X, Zhao H. The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop. 2009;33:1263–1268. doi: 10.1007/s00264-008-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(Suppl):I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 18.Boswell CA, Ferl GZ, Mundo EE, Bumbaca D, Schweiger MG, Theil FP, Fielder PJ, Khawli LA. Effects of anti-VEGF on predicted antibody biodistribution: roles of vascular volume, interstitial volume, and blood flow. PLoS One. 2011;6:e17874. doi: 10.1371/journal.pone.0017874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan T, Cong Y, Meng J, Qian H, Ye W, Sun WS, Zhao JN, Bao NR. Arachidonic acid causes hidden blood loss-like red blood cell damage through oxidative stress reactions. J Surg Res. 2017;211:14–20. doi: 10.1016/j.jss.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 20.Yuan T, Fan WB, Cong Y, Xu HD, Li CJ, Meng J, Bao NR, Zhao JN. Linoleic acid induces red blood cells and hemoglobin damage via oxidative mechanism. Int J Clin Exp Pathol. 2015;8:5044–5052. [PMC free article] [PubMed] [Google Scholar]
- 21.Qian H, Yuan T, Tong J, Sun WS, Jin J, Chen WX, Meng J, Bao N, Zhao J. Antioxidants attenuate oxidative stress-induced hidden blood loss in rats. Turk J Haematol. 2017;34:334–339. doi: 10.4274/tjh.2016.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53:335–347. doi: 10.1016/j.cnur.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Lin Y, Wang S, Zhang L, Guo L. GLP-1 Inhibits high-glucose-induced oxidative injury of vascular endothelial cells. Sci Rep. 2017;7:8008. doi: 10.1038/s41598-017-06712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemes R, Koltai E, Taylor AW, Suzuki K, Gyori F, Radak Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants (Basel) 2018;7:85. doi: 10.3390/antiox7070085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newsholme P, Keane KN, Carlessi R, Cruzat V. Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction. Am J Physiol Cell Physiol. 2019;317:C420–C433. doi: 10.1152/ajpcell.00141.2019. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Tang L, Xin G, Li S, Ma L, Xu Y, Zhuang M, Xiong Q, Wei Z, Xing Z, Niu H, Huang W. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic Biol Med. 2019;130:48–58. doi: 10.1016/j.freeradbiomed.2018.10.419. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Chen Y, Xiong Z, Yu S, Zhou B, Ling Y, Zheng Z, Shi G, Wu Y, Qian X. Ginsenoside Rb1 inhibits free fatty acids-induced oxidative stress and inflammation in 3T3-L1 adipocytes. Mol Med Rep. 2017;16:9165–9172. doi: 10.3892/mmr.2017.7710. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Baker RD, Bhatia T, Zhu L, Baker SS. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol Life Sci. 2016;73:1969–1987. doi: 10.1007/s00018-016-2161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gęgotek A, Skrzydlewska E. Biological effect of protein modifications by lipid peroxidation products. Chem Phys Lipids. 2019;221:46–52. doi: 10.1016/j.chemphyslip.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Deng Y, Chen Y, Qu G, Feng Z, Shang J, Zheng J, He N. Disordered metabolism and repair mechanism: mitochondria influenced by cationic and neutral nanoparticles. J Biomed Nanotechnol. 2019;15:2428–2438. doi: 10.1166/jbn.2019.2864. [DOI] [PubMed] [Google Scholar]
- 31.Gentile F, Arcaro A, Pizzimenti S, Daga M, Cetrangolo GP, Dianzani C, Lepore A, Graf M, Ames PRJ, Barrera G. DNA damage by lipid peroxidation products: implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017;4:103–137. doi: 10.3934/genet.2017.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanjeev S, Bidanchi RM, Murthy MK, Gurusubramanian G, Roy VK. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res Int. 2019;26:20631–20653. doi: 10.1007/s11356-019-05420-7. [DOI] [PubMed] [Google Scholar]
- 33.Munne-Bosch S, Pinto-Marijuan M. Free radicals, oxidative stress and antioxidants. Encycl Appl Plant Sci. 2016;2:16–19. [Google Scholar]
- 34.Ekoue DN, He C, Diamond AM, Bonini MG. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim Biophys Acta Bioenerg. 2017;1858:628–632. doi: 10.1016/j.bbabio.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu YH, Huang QH, Wu X, Wu JZ, Liang JL, Lin GS, Xu LQ, Lai XP, Su ZR, Chen JN. Polydatin protects against acetaminophen-induced hepatotoxicity in mice via anti-oxidative and anti-apoptotic activities. Food Funct. 2018;9:5891–5902. doi: 10.1039/c8fo01078a. [DOI] [PubMed] [Google Scholar]
- 36.Qi X, Qin Z, Tang J, Han P, Xing Q, Wang K, Yu J, Zhou G, Tang M, Wang W, Zhang W. Omega-3 polyunsaturated fatty acids ameliorates testicular ischemia-reperfusion injury through the induction of Nrf2 and inhibition of NF-κB in rats. Exp Mol Pathol. 2017;103:44–50. doi: 10.1016/j.yexmp.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]