Abstract
Hybrid immunity has been accepted as the most robust immunity to fight against SARS-CoV-2. The hybrid immunity against the virus is produced in individuals who have contracted the disease and received the COVID-19 vaccine. This happens due to the cumulative effect of natural and acquired (vaccine) immunity, which provides higher antibody responses compared to natural and vaccine-produced immunity alone. Scientists have noted that it provides about 25 to 100 times higher antibody responses than natural and vaccine-produced immunity alone. Here, we have tried to illustrate the molecular basis of hybrid immunity against various SARS-CoV-2 variants.
We have described hybrid immunity under different headings, which are as follows: an overview of hybrid immunity; a comparison between herd immunity and hybrid immunity against SARS-CoV-2; hybrid immunity in different countries; hybrid immunity and different SARS-CoV-2 variants; the molecular basis of hybrid immunity; and hybrid immunity in Indian scenario.
India’s large population has recovered from SARS-CoV-2, and data shows that over 1000 million of the population received at least one dose of the vaccine. Besides, many infected individuals who have recovered also received at least one dose of the vaccine leading to hybrid immunity with a less severe third wave compared to the first and second waves. Based on the available data, we hypothesize that people's hybrid immunity could be a major cause of the less severe third wave.
Keywords: Hybrid immunity, SARS-CoV-2, Infection, COVID-19 vaccine, Third wave in India
1. Introduction
The continuous emergence of the SARS-CoV-2 variants is a significant issue worldwide. It has been noted that each significant variant has created a surge in their particular country of origin and subsequently transmitted worldwide. However, scientists have noted that the transmitted variants have circulated globally, increasing the number of infections [1], [2], [3], [4], [5].
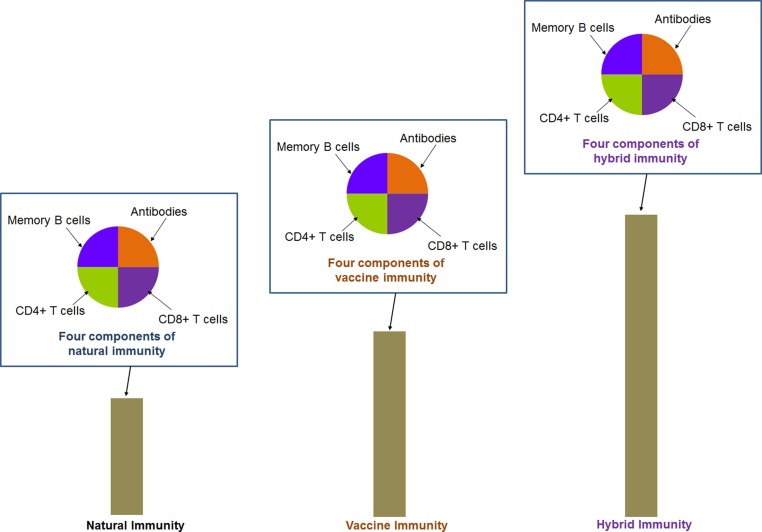
Therefore, immunity is a significant way to fight against SARS-CoV-2 and its variants. Protective immunity can guard the individual against SARS-CoV-2, developed from the immune memory. For the protective immunity against SARS-CoV-2, natural immunity and vaccine-mediated immunity are the main ways to provide the desired protection [6], [7]. The B and T cells (CD4+ T cells and CD8+ T cells) are crucial for the adaptive immune system. It has been noted that there are three major components of the immune system B, CD4+ T, and CD8+ T cells. It is well known that the B cells are involved in antibody production. Similarly, CD4+ T are noteworthy cells in the adaptive immune system that recognize antigens and process them. They use antigenic receptors of T cells to identify and recognize antigenic peptides produced through phagosomes or endosomes exhibited on the host cell surface bound to MHC molecules [8]. Together, CD8+ T cells are important for clearing the viral infection [9]. Le Bert et al. have identified CD8+ T cells in SARS-CoV-2 infected patients, and they have noted CD8+ memory T cells from people who recovered from SARS-CoV/ SARS-CoV-2. Furthermore, they have observed that active T cells (CD8+) might produce either TNF/IFNγ or both in response to infection [10]. Scientists have reported that the components of natural immunity, especially immune memory components (antibodies, memory B cells, CD4+ T, and CD8+ T cells), were noted against SARS-CoV-2 in infected individuals (Fig. 1 ) for over 8 months [11]. However, a gradual decline in the immune memory was reported within a year. Therefore, it appears that immune memory against SARS-CoV-2 was partially stable [11], [12]. Researchers found robust hybrid immunity against SARS-CoV-2 (Fig. 1a). Hybrid immunity has been noted in individuals infected with SARS-CoV-2 and who received COVID-19 vaccines. Therefore, it is the combination of natural infection of SARS-CoV-2 along with a single dose of COVID-19 vaccination. Previously, it was termed “super” immunity [13].
Fig. 1.
A conceptual diagram of the components of hybrid immunity. The schematic diagram illustrates the robustness of hybrid immunity, which is much stronger than natural immunity and vaccine-generated immunity. It has been noted that hybrid immunity is produced when natural immunity is associated with vaccine immunity, and this immunity shows several folds higher antibody responses than other immunity. The components of immunity are observed as antibodies, memory B cells, CD4+ T, and CD8+ T cells. The four components of immunity (antibodies, memory B cells, CD4+T, and CD8+ T cells.) have been shown through pie charts for natural immunity, vaccine-generated immunity, and hybrid immunity. The pie chart and the bar diagram were generated on a conceptual basis to provide a graphical view, and no specific data were used for these cases.
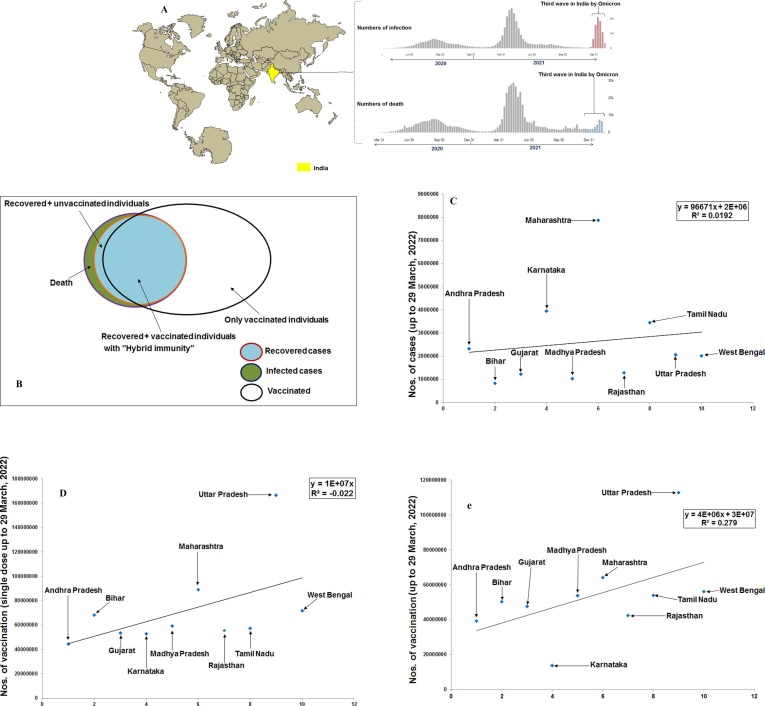
India was worst affected by the first and second waves of COVID-19. The first case of COVID-19 was reported on 22 January 2020 in Kerala state of south India [14]. Simultaneously, the infection and death cases increased immensely afterward, and the first wave was noted from March to October 2020 (Fig. 2 a) [15], [16]. Similarly, the second wave impacted badly from March to June 2021(Fig. 2b) [17], [18], [19], leading to an emergency health situation across India [20].
Fig. 2.
The hybrid immunity in the Indian scenario. The figure depicts the statistical models of India’s total infection cases, the number of individuals vaccinated with a single-dose vaccine, and the number of individuals vaccinated with the double-dose vaccine. The statistical models show the statewide distribution of 10 states with the highest numbers of infection cases and vaccinated individuals. (a) The comparison of India’s s first wave, second and third wave. The figure illustrates the number of infected and death cases during these three waves. (b) A schematic Venn diagram depicts the cases of hybrid immunity among the individuals and the cases of infected individuals, recovered individuals, and vaccinated individuals from India’s perspective. The schematic Venn diagram was generated on a conceptual basis to provide a graphical view, and no specific data were used for these cases. However, we have tried to collect the data for each case for India till today (March 29 or 30, 2022), and we found that the total number of infected individuals is 4,30,23,215; the total number of death cases is 5,21,101; the total recover cases are 4, 25,02,114. We found vaccinated individuals number (single dose, 29 March 2022) to be 98,48,10,951. However, we were not able to calculate the accurate number of individuals with hybrid immunity. Therefore, we have generated a schematic Venn diagram. All data was collected from the Ministry of Health and Family Welfare, GOI (https://www.mohfw.gov.in/). Here, the total infected individuals contain two populations: the total death cases and the total recovered individuals. Among the total recovered individuals, some groups are vaccinated with hybrid immunity. In India, all recovered individuals might not be vaccinated (at least one dose) due to its huge population. (c) A statistical model depicts the statewide distribution of 10 Indian states with the highest infected cases. The model shows the state wise infection cases up to 29 March 2022. (d) A statistical model depicts the statewide distribution of 10 Indian states with the highest number of single-dose COVID-19 vaccination till 29 March 2022. (e) A statistical model illustrates the statewide distribution of 10 Indian states with the highest number of double doses of COVID-19 vaccination till 29 March 2022.
This article describes hybrid immunity using different headings such as an overview of hybrid immunity, a comparison between herd immunity and hybrid immunity against SARS-CoV-2, hybrid immunity in different countries, hybrid immunity and different SARS-CoV-2 variants, the molecular basis of hybrid immunity and hybrid immunity in Indian scenario. We have also discussed a less severe third wave compared to the first and second waves. We have tried to understand the less severe third wave and its cause. Finally, we illustrated that hybrid immunity in Indian individuals might be one of the major causes of the less severe third wave.
2. Overview of hybrid immunity
At present, researchers reported that hybrid immunity is the vigor immunity against the SARS-CoV-2 that can provide the strongest protection against the virus. Hybrid immunity includes components like antibodies, memory B cells, CD4+ T, and CD8+ T cells [21]. Several findings have reported that the first dose of Moderna mRNA vaccine or Pfizer mRNA vaccine resulted in high antibody titers, as well as elevated neutralization activity against SARS-CoV-2 infections in people who were infected previously before vaccination [22], [23], [24], [25]. Levi et al. noted that exponential augment of the antibodies were noted in the individuals who received the SARS-CoV-2 vaccine after the infection of the virus. The study was conducted on 124 health care persons vaccinated with the BioNTech/ Pfizer vaccine [25].
Stamatatos et al. have noted that hybrid immunity provides about 25 to 100 times higher antibody responses than natural and vaccine-produced immunity [26]. After that, several other research groups have confirmed this enhanced neutralization of these neutralizing antibodies (nAbs) [27], [28]. Stamatatos et al. tried to understand the role of nAbs in protection against B.1.351. The researchers have found that individuals with previous infection (non- B.1.351 strain of SARS-CoV-2) and one dosage of mRNA vaccine showed higher protection compared to non-infected individuals [26]. The individuals showed 100 times higher protection against SARS-CoV-2 variants. At the same time, the individuals showed 25 times higher protection against the B.1.351 strain compared to only vaccination individuals. In this case, the B.1.351 S-glycoprotein was not involved in the infection of the individuals and the vaccination. The study was conducted with pre-infected (PID) individuals [27].
Andreano et al. evaluated the memory B cells of five naïve individuals and the memory B cells of convalescent individuals (five) vaccinated with the mRNA vaccine (BNT162b2). They have evaluated antibody response at the single-cell level and the character of the B cell. They have found that hybrid immunity can protect against variants of SARS-CoV-2 through the improvement of B cells and antibodies [28]. These experiments provide strong evidence that, after infection, primary vaccination will generate antibodies with augmented strength and span, and this will be able to control the emerging variants of SARS-CoV-2 in a better way.
3. Comparison between herd immunity and hybrid immunity against SARS-CoV-2
Herd immunity can be achieved if enough people in a particular population are vaccinated or infected and recovered. On the other hand, people who have not recovered from the disease or have not been vaccinated are also protected from the disease due to herd immunity. The protective antibodies developed can fight against future infection. However, herd immunity against the SARS-CoV-2 is now questionable. Scientists have illustrated why COVID-19 herd immunity is probably impossible [29]. Scientists have listed several reasons for the herd immunity against COVID-19, which are now a big question for debate. Several researchers stated that attaining herd immunity through vaccines is unlikely for this virus. They found several challenges to fight against this virus through herd immunity [30]. Anderson et al. noted several challenges in generating herd immunity through mass vaccination against SARS-CoV-2. The researchers pointed out the challenges like the priorities for vaccine distribution, less clarity about mass vaccination, and the main priority group individuals due to uncertain duration of protection against the virus. Another challenge is that it is not clear to every country is the amount of vaccine required year by year to produce herd immunity against the virus. Another concern is if countries are unable to attain high vaccine coverage and what will happen in that situation. All these things are quite challenging equally to the governments of each country and need quick actions if a population has to be protected through herd immunity [30]. With mass vaccination, hybrid immunity can be a viable, effective option to fight against SARS-CoV-2.
4. Hybrid immunity in different countries
A number of scientists have studied hybrid immunity using infected and vaccinated individuals from different countries (Table 1 ). A study from Qatar showed two cohorts with or without infected individuals using Moderna (mRNA-1273) and (Pfizer-BioNTech) BNT162b2 vaccines. The study found a lower risk in the case of those individuals who are infected after receiving the vaccination [31]. Cavanaugh et al. performed a study in the USA with individuals aged ≥ 18 years among Kentucky residents and found that full vaccination provides additional protection against reinfection. It was also found that the antibody titers were higher in those individuals who were previously infected and received the first mRNA vaccine dose [32]. In the USA, Kim et al. found from a cohort study that infection by the virus shows robust protection against variants reinfection, especially the Delta variant [33]. In Italy, Callegaro et al. described that the individuals who received a single mRNA vaccine dose and were previously infected with SARS-CoV-2 showed adequate immunity [34]. Furthermore, Levi et al. found that the individuals with augmented antibodies were those who recovered from SARS-CoV-2 infection and received one-dose mRNA vaccination. In this case, the human subjects showed elevated antibody titer [25]. Another study from Italy also reported that the individuals who obtained adequate reactivate immune memory received one-dose mRNA vaccination and SARS-CoV-2 infection [35].
Table 1.
Hybrid immunity reported by several researchers in different countries.
| Sl. No. | Hybrid immunity studied by different researchers | Hybrid immunity studied in a different country | Remark | Reference |
|---|---|---|---|---|
| 1 | Abu-Raddad et al. 2021 | Qatar | Infected individuals after receiving the vaccination show a lower risk of further infection. | [31] |
| 2 | Cavanaugh et al. 2021 | USA | COVID- 19 vaccination reduced the chance of re-infection by the SARS-CoV-2 virus | [32] |
| 3 | Kim et al. 2021 | USA | The SARS-CoV-2 infection has shown highly protective against the re-infection of the Delta variant | [33] |
| 4 | Callegaro et al. 2021 | Italy | Individuals having a single mRNA vaccine dose and also previously infected by SARS-CoV-2 showed adequate immunity | [34] |
| 5 | Levi et al. 2021 | Italy | An exponential increase of the antibodies was recorded in the individuals who received the COVID-19 vaccine after the infection made by virus | [25] |
| 6 | Mazzoni et al. 2021 | Italy | Individuals recovered from COVID-19 having the first dose of mRNA vaccine is sufficient to reactivate immunological memory to SARS-CoV-2 | [35] |
5. Hybrid immunity and different SARS-CoV-2 variants
Several variants have been generated from time to time, such as B.1.1.7 (Alpha), P.1 (Gamma), B.1.351 (Beta), and B.1.617.2 (Delta). These variants and subsequent infections reduce antibody recognition, but the phenomenon is unclear. It was also noted that the case of reinfection increases due to the variants. In most cases, a reduction of vaccine-generated immunity and natural immunity was noted, supporting the nAb escape occurrence in infected individuals. Another important point noted by scientists includes partial vaccine escape as observed with a significant drop in immunity (about 75% to 11%) after vaccination with AstraZeneca (ChAdOx1 nCoV-19) [36].
Likewise, vaccine efficacy of the Pfizer/BioNTech (BNT162b2) was also found to drop significantly (about 95% to 75%) against the Beta variant (B.1.351), and protection against severe disease remained at 97% [37].
However, Andreano et al. stated that B.1.1.248 (Gamma) and B.1.351 (Beta) variants escaped about 70% of nAbs. They performed a comparative study at a single-cell level and found the sensitivity of the nAbs response against the S-glycoprotein of the Wuhan strain and the VOCs. In this direction, researchers have analyzed S-glycoprotein and the nAb responses from individuals who were immunized with the mRNA vaccine (BNT162b2). Antibodies with increased potency were noted in the case of infected individuals or vaccinated individuals [38]. In a recent study, Walls et al. showed that human subjects immunized after SARS-CoV-2 infection showed three times higher serum-neutralizing efficiency [39] but were infected with the Delta variant of SARS-CoV-2. This study has shown commendable results for hybrid immunity. Bates et al. have also noted the superior immune responses from the recovered COVID-19 individuals who are also vaccinated [40]. They point out that hybrid immunity is responsible for the enhanced immune responses in the above cases.
6. Molecular basis of hybrid immunity
Recently, it has been noted that high-quality and diverse memory B cells are required to produce hybrid immunity. In fact, hybrid immunity produces various types of nAbs to fight against the variants [41]. It was observed that diverse memory B cells and T cells are necessary to generate hybrid immunity. Therefore, B cells and T cells function collectively to generate a broad range of antibodies against various variants. In hybrid immunity, there is a 5-to 10-times increase of memory B cells compared to vaccination alone or natural infection [41], [42].
7. Hybrid immunity in the Indian scenario
At the latest, 4,30,23,215 infected cases were recorded in India (Fig. 2b). Among them, 4,25,02,114 individuals were reported as recovered [17], [43]. Meanwhile, India vaccinated the vast population quickly, and over 1000 million people have received at least one required dose of the vaccine [single dose vaccinated individuals numbered (till 29 March 2022) is 98,48,10,951] (1000 million equal 100 crores as India is promoting the success story of more than 100 crores vaccination). Therefore, it is over 1000 million of the total population that has been vaccinated through the COVID-19 vaccination program (Fig. 2b)
Therefore, a large number of people with recovered infections also received at least one dose of the COVID-19 vaccine and developed hybrid immunity among them. Thiagarajan also reported that hybrid immunity is a dominant factor among the Indian population [44]. Conversely, Shenoy et al. have also reported hybrid immunity from India. For the patients who had received the COVID-19 vaccine previously, a single dose of vaccine produces hybrid immunity, and the immunity can respond even against autoimmune rheumatic diseases. However, the study did not analyze T cell immunogenicity [45].
Researchers observed a less severe India’s third wave through the Omicron variant. Due to hybrid immunity, India’s third wave was less severe than the first and second waves (Fig. 2b). However, the report of Shenoy et al. is the first report of hybrid immunity from India. No other study has reported the role of hybrid immunity from India in the current pandemic. To develop an overall view of hybrid immunity in India, we have developed different statistical models. First, we have developed a statistical model to illustrate the infected individuals from the 10 highest infected states (Fig. 2c). At the same time, we have also depicted a statistical model using individuals who received a single dose of COVID-19 vaccine from 10 Indian states with the highest number of vaccinations (Fig. 2d). Similarly, we also developed another statistical model using individuals who received two COVID-19 vaccine doses from 10 Indian states (two doses) with the highest number of vaccinations (Fig. 2e). This report can provide a general observation of infected individuals, single-dose vaccinated individuals, and double doses vaccinated individuals with hybrid immunity in India. However, we found that India still has a large population size that has neither infected nor vaccinated.
8. Conclusion
Presently, it is a big question whether hybrid immunity can be reproducible, which can be used to enhance immunity and fight against the pandemic.
The combination of two kinds of vaccines can provide stronger immunity; however, whether it can generate a similar kind of hybrid immunity needs to be answered. More research is required to understand this phenomenon. If hybrid immunity can be developed, it can be applied to elderly people, comorbid individuals, and immunocompromised individuals to protect them against the virus. Therefore, scientists should unfold more about the hybrid immunity to fight against the SARS-CoV-2 virus and other diseases.
Funding
There is not any financial support for this study.
Ethical approval
Not required.
CRediT authorship contribution statement
Manojit Bhattacharya: Validation, Data curation. Ashish Ranjan Sharma: Validation, Writing – review & editing. Kuldeep Dhama: Validation, Formal analysis. Govindasamy Agoramoorthy: Writing – review & editing. Chiranjib Chakraborty: Conceptualization, Data curation, Writing – original draft, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Otto S.P., Day T., Arino J., Colijn C., Dushoff J., Li M., Mechai S., Van Domselaar G., Wu J., Earn D.J. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr. Biol. 2021;31(14):R918–R929. doi: 10.1016/j.cub.2021.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell F., Archer B., Laurenson-Schafer H., Jinnai Y., Konings F., Batra N., Pavlin B., Vandemaele K., Van Kerkhove M.D., Jombart T. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021;26(24):2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geidelberg L., Hinsley W.R., Laydon D.J., Dabrera G., O’Toole Á. Assessing transmissibility of SARS-CoV-2 lineage B. 1.1. 7 in England. Nature. 2021;593(7858):266–269. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty C., Sharma A.R., Bhattacharya M., Agoramoorthy G., Lee S.-S. Evolution, mode of transmission, and mutational landscape of newly emerging SARS-CoV-2 variants. Mbio. 2021;12(4):e01140–21. doi: 10.1128/mBio.01140-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty C., Bhattacharya M., Sharma A.R. Present variants of concern and variants of interest of severe acute respiratory syndrome coronavirus 2: their significant mutations in S-glycoprotein, infectivity, re-infectivity, immune escape and vaccines activity. Rev. Med. Virol. 2021:e2270. [Google Scholar]
- 6.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tubo N.J., Jenkins M.K. CD4+ T Cells: guardians of the phagosome. Clin. Microbiol. Rev. 2014;27(2):200–213. doi: 10.1128/CMR.00097-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M.E., Varga S.M. The CD8 T cell response to respiratory virus infections. Front. Immunol. 2018:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 11.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen K.W., Linderman S.L., Moodie Z., Czartoski J., Lai L., Mantus G., Norwood C., Nyhoff L.E., Edara V.V., Floyd K. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Reports Medicine. 2021;2(7):100354. doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlov M. Waning COVID super-immunity raises questions about Omicron. Nature. 2021 doi: 10.1038/d41586-021-03674-1. [DOI] [PubMed] [Google Scholar]
- 14.Andrews M., Areekal B., Rajesh K., Krishnan J., Suryakala R., Krishnan B., Muraly C., Santhosh P. First confirmed case of COVID-19 infection in India: A case report. Indian J. Med. Res. 2020;151(5):490. doi: 10.4103/ijmr.IJMR_2131_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamrakar V., Srivastava A., Parmar M.C., Shukla S.K., Shabnam S., Boro B., Saha A., Debbarma B., Saikia N. District level correlates of COVID-19 pandemic in India. PlosOne. 2020 doi: 10.1371/journal.pone.0257533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain V.K., Iyengar K.P., Vaishya R. Differences between First wave and Second wave of COVID-19 in India. Diab. Metabolic Syndrome. 2021;15(3):1047–1048. doi: 10.1016/j.dsx.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborty C., Sharma A.R., Bhattacharya M., Agoramoorthy G., Lee S.-S. The current second wave and COVID-19 vaccination status in India. Brain Behav. Immun. 2021;96:1–4. doi: 10.1016/j.bbi.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kar S.K., Ransing R., Arafat S.Y., Menon V. Second wave of COVID-19 pandemic in India: Barriers to effective governmental response. EClinicalMedicine. 2021;36:36:100915. doi: 10.1016/j.eclinm.2021.100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asrani P., Eapen M.S., Hassan M.I., Sohal S.S. Implications of the second wave of COVID-19 in India. Lancet Respirat. Med. 2021;9(9):e93–e94. doi: 10.1016/S2213-2600(21)00312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S. Second wave of COVID-19: emergency situation in India. J. Travel Med. 2021;28(7):taab082. doi: 10.1093/jtm/taab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crotty S. Hybrid immunity. Science. 2021;372(6549):1392–1393. [Google Scholar]
- 22.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadat S., Tehrani Z.R., Logue J., Newman M., Frieman M.B., Harris A.D., Sajadi M.M. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325(14):1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., Van Eyk J.E., Braun J.G. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27(6):981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levi R., Azzolini E., Pozzi C., Ubaldi L., Lagioia M., Mantovani A., Rescigno M. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic COVID-19. J. Clin. Investig. 2021;131(12):e149154. doi: 10.1172/JCI149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamatatos L., Czartoski J., Wan Y.H., Homad L.J., Rubin V., Glantz H., Neradilek M., Seydoux E., Jennewein M.F., MacCamy A.J., Feng J. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M., Dysinger S. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 2021;6(58):p.eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreano E., Paciello I., Piccini G., Manganaro N., Pileri P., Hyseni I., Leonardi M., Pantano E., Abbiento V., Benincasa L., Giglioli G. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021;600(7889):530–535. doi: 10.1038/s41586-021-04117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature. 2021:520–522. doi: 10.1038/d41586-021-00728-2. [DOI] [PubMed] [Google Scholar]
- 30.Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. The Lancet. 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., Yassine H.M., Benslimane F.M., Al Khatib H.A., Tang P., Hasan M.R., Coyle P., Al Kanaani Z. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326(19):1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanaugh A.M., Spicer K.B., Thoroughman D., Glick C., Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination—Kentucky, May–June 2021. Morb. Mortal. Wkly Rep. 2021;70(32):1081. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim P., Gordon S.M., Sheehan M.M., Rothberg M.B. Duration of SARS-CoV-2 natural immunity and protection against the Delta variant: a retrospective cohort study. Clin. Infect. Dis. 2021:ciab999. doi: 10.1093/cid/ciab999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callegaro A., Borleri D., Farina C., Napolitano G., Valenti D., Rizzi M., Maggiolo F. Antibody response to SARS-CoV-2 vaccination is extremely vivacious in subjects with previous SARS-CoV-2 infection. J. Med. Virol. 2021;93(7):4612–4615. doi: 10.1002/jmv.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzoni A., Di Lauria N., Maggi L., Salvati L., Vanni A., Capone M., Lamacchia G., Mantengoli E., Spinicci M., Zammarchi L. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J. Clin. Investig. 2021;131(12):e149150. doi: 10.1172/JCI149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B. 1.351 variant. N. Engl. J. Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B. 1.1. 7 and B. 1.351 Variants. N. Engl. J. Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreano E., Paciello I., Piccini G., Manganaro N., Pileri P., Hyseni I., Leonardi M., Pantano E., Abbiento V., Benincasa L. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021;600(7889):530–535. doi: 10.1038/s41586-021-04117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates T.A., Leier H.C., Lyski Z.L., McBride S.K., Coulter F.J., Weinstein J.B., Goodman J.R., Lu Z., Siegel S.A., Sullivan P. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nat. Commun. 2021;12(1):1–7. doi: 10.1038/s41467-021-25479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 2021;6(58):eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatatos L., Czartoski J., Wan Y.-H., Homad L.J., Rubin V., Glantz H., Neradilek M., Seydoux E., Jennewein M.F., MacCamy A.J. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakraborty C., Sharma A.R., Bhattacharya M., Agoramoorthy G., Lee S.-S. Asian-origin approved COVID-19 vaccines and current status of COVID-19 vaccination program in Asia: a critical analysis. Vaccines. 2021;9(6):600. doi: 10.3390/vaccines9060600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiagarajan K. Covid-19: The significance of India’s emerging “hybrid immunity”. bmj. 2021;375 doi: 10.1136/bmj.n3047. 375:n3047. [DOI] [PubMed] [Google Scholar]
- 45.Shenoy P., Ahmed S., Paul A., Cherian S., Umesh R., Shenoy V., Vijayan A., Babu S., Nivin S., Thambi A. Hybrid immunity versus vaccine-induced immunity against SARS-CoV-2 in patients with autoimmune rheumatic diseases. Lancet Rheumatol. 2022;4(2):e80–e82. doi: 10.1016/S2665-9913(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]