Abstract
Ethionamide, 250 mg every 12 h for a total of nine doses, was administered to 40 adult volunteers (10 men with AIDS, 10 healthy men, 10 women with AIDS, and 10 healthy women). Blood was obtained for drug assay prior to administration of the first dose, 2 h after the last dose, and at the completion of standardized bronchoscopy and bronchoalveolar lavage, which were performed 4 h after the last dose. Ethionamide was measured in epithelial lining fluid (ELF) and alveolar cells (AC) using a new mass spectrometric method. The presence of AIDS or gender was without significant effect on the concentrations of ethionamide in plasma, AC, or ELF. Plasma concentrations (mean ± standard deviation [SD]) were 0.97 ± 0.65 and 0.65 ± 0.35 μg/ml at 2 and 4 h after the last dose, respectively, and both values were significantly greater than the concentration of ethionamide in AC (0.38 ± 0.47 μg/ml) (P < 0.05). The concentration of ethionamide was significantly greater in ELF (5.63 ± 3.8 μg/ml) than in AC or plasma at 2 and 4 h and was approximately 10 to 20 times the reported MIC for ethionamide-susceptible strains of Mycobacterium tuberculosis. For all 40 subjects, the ELF/plasma concentration ratios (mean ± SD) at 2 and 4 h were 8.7 ± 11.7 and 9.7 ± 5.6, respectively. We conclude that the absorption of orally administered ethionamide, as measured in this study, was not affected by gender or the presence of AIDS. Ethionamide concentrations were significantly greater in ELF than in plasma or AC, suggesting that substantial antimycobacterial activity resides in this compartment.
Ethionamide is a second-line, orally administered drug that is used for the treatment of tuberculosis. It is often combined with other antituberculous agents for the treatment of multiple-drug-resistant organisms. The usual dose is 250 to 500 mg two to four times per day (1, 21). The daily dose is limited by gastrointestinal toxicity. The elimination half-life in humans is approximately 2 to 3 h (18, 19, 22). Peak plasma concentrations occur at approximately 2 h postdosing and have been reported to be between 0.6 and 1.9 μg/ml following an oral dose of 250 mg (11, 18) and 2.2 μg/ml following an oral dose of 500 mg (22). Several authors in the United States have reported that the absorption of antimycobacterial agents, including ethionamide, is impaired in patients with AIDS (10, 23, 26; S. E. Berning, G. A. Huitt, M. D. Iseman, and C. A. Peloquin, Letter, N. Engl. J. Med. 327:1817–1818, 1992; C. A. Peloquin, A. A. MacPhee, and S. E. Berning, Letter, N. Engl. J. Med. 329:1122–1123, 1993). Malabsorption of antituberculous drugs has not been demonstrated in other studies of patients with AIDS (6, 9, 16, 28). The effects of gender and AIDS on the steady-state plasma and pulmonary kinetics of ethionamide have not been reported.
Ethionamide is active against tubercle bacilli that are growing within human macrophages (24). The in vivo penetration of ethionamide into pulmonary macrophages and epithelial lining fluid (ELF) in humans has not been reported.
We (7–9) and others (2–4) have developed techniques for the measurement, in vivo, of the concentration of drugs in pulmonary ELF and alveolar cells (AC). The purpose of this study was to compare the steady-state plasma and intrapulmonary ethionamide concentrations in healthy volunteers and men and women with AIDS.
MATERIALS AND METHODS
Study design and subjects.
The investigation was prospective and nonblinded. After giving informed consent, subjects underwent a medical history, physical examination, purified protein derivative skin test, and baseline laboratory testing including complete blood count, platelet count, blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and total bilirubin. Subjects were required to be 18 years or older and within 10% of acceptable weight for their height according to the Metropolitan Life height-weight tables (20). If female, they were required to be nonlactating and not pregnant. Subjects were excluded who had a history of asthma requiring daily therapy; tuberculosis or a positive purified protein derivative skin test (greater than 10-mm induration for healthy subjects and greater than 5 mm for subjects with AIDS); intolerance to ethionamide or lidocaine; presence of clinically significant organ dysfunction; requirement for chronic medications other than self-prescribed vitamins, birth control pills, or thyroid replacement therapy (healthy subjects only); abnormal serum creatinine; or other screening laboratory values outside the normal range (greater than twice the normal value for subjects with AIDS). Patients with AIDS were required to meet the revised Centers for Disease Control and Prevention criteria for the diagnosis of AIDS (5) and to have (a) fewer than four soft stools per day without hematochezia, (b) no abdominal pain or cramping, (c) no nausea or vomiting, and (d) a negative chest X ray within 2 weeks of enrollment. If an X ray had not been done, it was performed as part of the study. Forty subjects were assigned to one of four comparative groups: healthy men, healthy women, men with AIDS, and women with AIDS.
Ethionamide was administered orally in a dose of 250 mg every 12 h for a total of nine doses. The first and last doses of study medication were administered under direct supervision in the General Clinical Research Center at the University of California, San Francisco. Subjects were observed for 30 min after the first dose for adverse effects. Subsequent doses were taken according to verbal and written instructions and documented in a written diary by the subjects.
Bronchoscopy and BAL.
Standardized bronchoscopy and bronchoalveolar lavage (BAL) (7–9) were performed 4 h after the administration of the last dose. The subjects' blood pressure, heart rate, and respiratory rate were recorded prior to and at the completion of bronchoscopy and as clinically indicated following the procedure. Fingertip oximetry was monitored, and all subjects received nasal oxygen throughout the procedure.
Subjects were prepared with a 4% topical lidocaine gargle followed by 4% topical lidocaine spray. Pledgets soaked with 4% topical lidocaine were then applied to each side of the posterior pharynx followed by the application of topical 1% lidocaine more distally. No systemic sedation was used. A fiber optic bronchoscope (Pentax FB-19H) was inserted in the right middle lobe. The average duration of the bronchoscopy was 4 min.
A total of four 50-ml aliquots of normal saline were instilled into the right middle lobe, and each was immediately aspirated into a trap. The specimens were kept on ice until they were frozen. The first aspirate was discarded. The second, third, and fourth aspirates were pooled (pooled BAL). The volume of the pooled BAL was measured and recorded. Measured aliquots of the pooled BAL were sent to the clinical laboratory for cell count and differential. A known volume of the pooled BAL was immediately spun at 400 × g for 5 min in a refrigerated centrifuge. The supernatant and the cells were separated and frozen at −70°C until assay. A small aliquot of the supernatant was frozen separately for urea assay.
Specimen handling.
Blood samples were kept on ice until centrifuged. The plasma was separated and then frozen until assay. The cells from the BAL were resuspended in 0.8% phosphate-buffered saline, pH 7.4, in water, to a 10-fold concentration of the lavage fluid, which was centrifuged to produce the cell pellet. The cell suspension was sonicated for 2 min using a Model 550 Sonic Dismembrator (Fisher Scientific, Santa Clara, Calif.). Samples were prepared by a deproteinization step using acetonitrile which contained internal standard.
Ethionamide assay.
Ethionamide was measured in plasma, BAL fluid, and AC by a new column chromatographic-mass spectrometric technique (9a). Briefly, a mobile phase containing 90% acetonitrile and 0.06% trifluoroacetic acid was run through a Hypersil silica column (4.6 mm [inside diameter] by 50 mm; particle size, 5 μm) at a flow rate of 1.0 ml/min, utilizing a Shimadzu LC-10 AD pump (Shimadzu, Columbia, Md.). Extracts from samples were injected onto the system with a Waters Intelligent Sample Processor 717 Plus (Waters, Milford, Mass.). The retention time for ethionamide was 2.62 min. The retention times for clemastine fumarate, promethazine HCl, and propranolol (used as internal standards) were approximately 1.21, 2.14, and 2.22 min, respectively, with a total run time of 3.2 min. Peak detection for plasma was carried out on a liquid chromatography/mass spectrum/mass spectrum API III (Perkin-Elmer, Foster City, Calif.). The assay was designed specifically to detect ethionamide (9a); interfering peaks from ethionamide sulfoxide, the main metabolite, or other metabolites (15, 17, 18, 22) were not observed. The following mass spectrometry conditions were used: (a) the multiple reaction monitor scanning mode was set at 167 to 139 for ethionamide and 244 to 215 for clemastine fumarate; (b) atmospheric pressure chemical positive ionization was used; (c) the sample inlet used a heated nebulizer at 450°C; (d) the discharge current was set to +3 μA; (e) the gas curtain flow rate was 1.2 liter/min (N2 = 99.999%); (f) the nebulizer pressure was 80 lb/in2; and (g) the collision-activated dissociation gas mixture was 9.99% nitrogen–90.01% argon (set at 250 × 1012 molecules/cm2). BAL and cell pellets and some plasma specimens were analyzed on a Micromass Quattro LC (Micromass Co., Beverly, Mass.). The following mass spectrometry conditions were used: (a) the multiple reaction monitor scanning mode was set at 166.77 to 106.79 for ethionamide, 260.18 to 115.95 for propranolol, and 285.7 to 86.04 for promethazine HCl; (b) electrospray-positive ionization was used; (c) the sample inlet used a heated nebulizer; (d) the sample cone was set to 35 V; (e) collision was set to 25.0 eV for ethionamide, 15 eV for propanolol, and 28 eV for promethazine HCl. A Macintosh Quadra 800 computer was used for peak integration and analysis.
The detection limits for ethionamide were 0.05 μg/ml for plasma and 0.005 μg/ml for BAL supernatants and AC suspensions. The mean (± standard deviation [SD]) coefficients of variation and ranges of the assay for intra-day and interday determinations together for plasma, BAL supernatants, and AC were (9.66 ± 2.67%) (range, 5.0 to 12.47%), (7.13 ± 2.67%) (range, 1.83 to 10.18%), and (7.73 ± 3.94%) (range, 3.40 to 13.64%), respectively. The mean (± SD) recoveries and ranges of the assay for intra-day and interday determinations together, in plasma, BAL supernatants, and AC were (101.3 ± 4.06%) (range, 92 to 104.2%), (106.6 ± 6.72%) (range, 100 to 118.8%), and (102.9 ± 7.41%) (range, 94 to 110%), respectively. The accuracy ranges for all determinations in plasma, BAL supernatants, and AC were −8.0 to 5.0, 0 to 18.8, and −6.0 to 10%, respectively.
Quantitation of volume of ELF and concentration of antibiotics in ELF and AC.
The amount of ELF recovered was calculated by the urea dilution method (25). The concentration of urea in serum was analyzed by the clinical laboratory at University of California, San Francisco, using a coupled urease-glutamate dehydrogenase enzymatic method (27) modified by Boehringer Mannheim Corporation (Indianapolis, Ind.). Measurements were made at a fixed time interval permitting automated analysis with a BM 747 analyzer (Boehringer Mannheim). Urea was measured in BAL supernatant utilizing a modified enzymatic assay (blood urea nitrogen kit UV-66; Sigma, St. Louis, Mo.) as previously reported (7, 8, 25). Controls were included with every run, and if not within 10% of the known value, the standard curve, controls, and specimen assays were repeated.
The volume of ELF in BAL fluid was derived from the relationship VELF = VBAL × ureaBAL/ureaSER, where VELF is volume of ELF sampled by the BAL, VBAL is volume of aspirated BAL fluid, ureaBAL is concentration of urea in BAL fluid, and ureaSER is concentration of urea in serum.
The concentration of antibiotic in the ELF (ABXELF) was derived from the relationship ABXELF = ABXBAL × VBAL/VELF, where ABXBAL is the measured concentration of antibiotic in BAL.
The volume of AC collected in the pellet suspension was determined from the cell count in the BAL fluid. Cells were counted in a hemocytometer with a lower detection limit of 1.0 × 106/liter. The number of cells in 1.0 ml of pellet suspension was calculated to be equal to the number of cells per liter of BAL fluid divided by 100. Because of cell loss during centrifugation, the actual number of cells recovered may be lower than the number counted and the antibiotic concentration may be approximately 20% more than calculated (29). Differential cell counting was performed after spinning the specimen in a cytocentrifuge. The volume of AC in the pellet suspension was determined using a mean macrophage cell volume of 2.42 μl/106 cells (4).
The concentration of antibiotic in AC, ABXAC, was calculated from the relationship ABXAC = ABXPELLET/VAC, where ABXPELLET is the antibiotic concentration in the 1-ml cell suspension and VAC is the volume of AC in the 1-ml cell suspension.
Statistical analysis.
PROPHET software, version 6.0 (Division of Research Resources, National Institutes of Health, Bethesda, Md., and Abtech Corporation, Charlottesville, Va.), was used to compute descriptive statistics and sample sizes and to perform the linear regression analyses. Analysis of variance using a two-factor factorial model was used to assess the effects of gender and AIDS status on subject physical characteristics, clinical laboratory values, drug dosage, drug concentrations, AC recovery, ELF recovery, and AC/plasma and ELF/plasma concentration ratios. The ethionamide concentrations in ELF and AC were compared for subjects with and without AIDS using one-way analysis of variance. The two-sample equal-variance t test (two sided) was used to compare AC and ELF recovery and drug concentrations in plasma, AC, and ELF between the groups of women with AIDS who were smokers and nonsmokers. The equality of variances of the smoking and nonsmoking groups was calculated using the F test (Levene's test). The two-sample Mann-Whitney rank sum test (two sided) was used to compare the daily doses in men and women and CD4 counts in men and women and to compare the serum creatinine determinations between healthy subjects and men or women with AIDS. The Shapiro-Wilk test was used to evaluate the normality of the distributions of the data sets prior to comparison. A P value of <0.05 was regarded as significant.
RESULTS
Ten men with AIDS, 10 men without AIDS, 10 women with AIDS, and 10 women without AIDS were enrolled. Because a fixed oral dose (250 mg every 12 h) of ethionamide was used, the daily weight-corrected dose was 17.2% greater for the 20 women (7.7 ± 1.5 mg/kg of body weight) than for the 20 men (6.6 ± 1.1 mg/kg) (P = 0.007). The age (mean ± SD) of the 40 volunteers was 37.6 ± 8.3 years. The subjects with AIDS (men and women) were older than the subjects without AIDS (42.6 ± 5.6 versus 32.7 ± 7.7 years) when the two groups were compared (P ≤ 0.0001).
The CD4 counts (mean ± SD) for the 10 men and 10 women with AIDS were 256 ± 135 and 371 ± 283, respectively, and were not significantly different (P > 0.05). All of the serum creatinine determinations were within normal limits; however, the values were significantly greater (P < 0.05) for the men (0.95 ± 0.28 mg/dl) than for the women (0.87 ± 0.15 mg/dl), irrespective of AIDS status. Four of the 10 female subjects with AIDS were cigarette smokers; the remainder of the subjects were nonsmokers.
All 40 subjects recruited for the study underwent and successfully completed the bronchoscopy and BAL. There were no major adverse events, and all of the subjects returned to their normal duties. Two subjects experienced mild, self-limited chest discomfort, and the temperature was transiently elevated in two subjects. Mild, self-limited postbronchoscopy cough occurred in two subjects, and six subjects complained of lightheadedness.
The number (mean ± SD) of AC recovered from BAL fluid in all 40 subjects was 2.0 × 108 ± 1.7 × 108 cells/liter; AC recovery was not affected by AIDS status but was greater in women than in men (P = 0.01) (Table 1). Cell recovery was not significantly different when smoking and nonsmoking women with AIDS were compared (P > 0.05). The majority of the cells in both groups were in the monocyte/macrophage class (Table 1). The volume (mean ± SD) of ELF recovered from the 40 subjects was 1.0 ± 0.5 ml, was not significantly affected by gender or AIDS status (P > 0.05), and was not affected by smoking in the women with AIDS (P > 0.05).
TABLE 1.
Recovery of cells from BAL in 40 subjects according to gender and AIDS stratification
| Statistic or cell type | Value for group (n = 10):
|
|||
|---|---|---|---|---|
| Men with AIDS | Men without AIDS | Women with AIDS | Women without AIDS | |
| No. of cells/liter | ||||
| Meana | 1.8 × 108 | 1.1 × 108 | 3.7 × 108 | 1.3 × 108 |
| SD | 1.7 × 108 | 5.6 × 107 | 2.1 × 108 | 6.6 × 107 |
| Minimum | 5.1 × 107 | 3.2 × 107 | 1.1 × 108 | 3.5 × 107 |
| Maximum | 6.3 × 108 | 1.9 × 108 | 6.5 × 108 | 2.4 × 108 |
| % Cell typeb | ||||
| PMNs | 2.1 ± 2.8 | 1.8 ± 0.9 | 1.6 ± 1.3 | 1.5 ± 1.4 |
| Lymphocytes | 14.9 ± 10.4 | 11.4 ± 8.9 | 13.6 ± 16.3 | 15.2 ± 11.7 |
| Monocytes/ macrophages | 78.2 ± 16.2 | 81.2 ± 14.1 | 84.5 ± 17.2 | 81.1 ± 13.4 |
| Eosinophils | 1.3 ± 1.9 | 0.4 ± 0.5 | 0.3 ± 0.7 | 0.2 ± 0.4 |
| Degenerated cells | 3.5 ± 6.8 | 5.6 ± 8.0 | 0 | 2.0 ± 6.3 |
All women > all men (P = 0.01); no effect of AIDS status alone or interaction of gender and AIDS status on cell recovery (P > 0.05).
Gender, AIDS status, or interaction between gender and AIDS had no significant effect on percentages of polymorphonuclear leukocytes (PMNs), lymphocytes, monocytes, or eosinophils in BAL fluid (for all, P > 0.05).
There was no significant effect of gender or AIDS status on the concentrations of ethionamide in plasma (Table 2). Plasma concentrations (mean ± SD) of ethionamide were greater at 2 h (0.97 ± 0.65 μg/ml) than at 4 h (0.65 ± 0.35 μg/ml) following the last dose when the two time periods were compared for all 40 subjects (P = 0.01). There was a weak but significant inverse correlation (R = 0.39, P = 0.01) between the weights of the subjects and the concentrations of ethionamide at 2 h and no correlation at 4 h (R = 0.27, P = 0.08).
TABLE 2.
Ethionamide concentration in plasma, ELF, and AC
| Compartment | Value for group (n = 10)a:
|
|||
|---|---|---|---|---|
| Men with AIDS | Men without AIDS | Women with AIDS | Women without AIDS | |
| Plasma | ||||
| 2 h | 1.0 ± 0.7 | 1.1 ± 0.5 | 0.9 ± 1.0 | 0.9 ± 0.4 |
| 4 h | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.5 | 0.6 ± 0.2 |
| ELF | ||||
| Mean ± SDb | 4.6 ± 3.2 | 5.5 ± 3.3 | 6.2 ± 4.6 | 6.2 ± 4.2 |
| Range | 1.8–12.0 | 1.3–13.5 | 1.9–17.4 | 2.1–16.7 |
| AC | ||||
| Mean ± SD | 0.5 ± 0.6 | 0.5 ± 0.6 | 0.3 ± 0.2 | 0.2 ± 0.4 |
| Range | 0–1.4 | 0–1.6 | 0–0.6 | 0–1.0 |
Data are given as means ± 1 SD in micrograms per milliliter. Range data are minimum to maximum values. There was no significant effect of the presence of AIDS or gender or an interaction between AIDS and gender (P > 0.05) on ethionamide concentrations in plasma, ELF, or AC (P > 0.05).
Within each group, concentrations in ELF were significantly greater than concentrations in plasma at 2 or 4 h or in AC (P < 0.05).
When smoking women with AIDS were compared to nonsmoking women with AIDS, plasma drug concentrations (mean ± SD) at 2 h (1.34 ± 1.51 and 0.64 ± 0.43 μg/ml, respectively) and at 4 h (0.79 ± 0.80 and 0.54 ± 0.24 μg/ml, respectively) were not significantly different (P > 0.05). CD4 counts in the human immunodeficiency virus-positive subjects (n = 20) were not correlated with the concentrations of ethionamide in plasma at 2 h (R = −0.05, P = 0.8) or 4 h (R = −0.19, P = 0.4) or in AC (R = −0.16, P = 0.5) or ELF (R = 0.01, P = 1.0).
There was no significant effect of gender or AIDS status on the concentrations of ethionamide in AC (Table 2). For all subjects (n = 40), the concentration (mean ± SD) of ethionamide in AC was 0.38 ± 0.47 μg/ml. When the male and female data were grouped to create a larger sample size (n = 20 in each group), the difference in AC concentrations between subjects with (0.24 ± 0.44 μg/ml) and without (0.39 ± 0.51 μg/ml) AIDS was still not significant (P = 0.7). AC concentrations (0.38 ± 0.47 μg/ml) were significantly less than plasma concentrations at 2 h (0.97 ± 0.6 μg/ml) and 4 h (0.65 ± 0.3 μg/ml). There was no correlation between the weights of the subjects and the concentrations of ethionamide in AC (R = 0.08, P = 0.6). The AC/plasma drug concentration ratios were 0.67 ± 1.5 and 0.53 ± 0.6 when the 2-h and 4-h plasma concentrations, respectively, were used for the calculations. AC drug concentrations (mean ± SD) in smoking (0.35 ± 0.2 μg/ml) and nonsmoking (0.26 ± 0.3 μg/ml) women with AIDS were not significantly different (P > 0.05).
There was no significant effect of gender or AIDS status on the concentrations of ethionamide in ELF (Table 2). For all subjects (n = 40), the concentration (mean ± SD) of ethionamide in ELF was 5.63 ± 3.8 μg/ml. When the male and female data were grouped to create a larger sample size (n = 20 in each group), the ELF ethionamide concentrations (mean ± SD) in subjects with (5.43 ± 4.0 μg/ml) and without (5.84 ± 8.7 μg/ml) AIDS were not significantly different (P > 0.05). For all 40 subjects, the ELF/plasma ratios (mean ± SD) at 2 and 4 h were 8.7 ± 11.7 and 9.7 ± 5.6, respectively. There was no correlation between the weights of the subjects and the concentrations of ethionamide in ELF (R = −0.27, P = 0.09). ELF ethionamide concentrations (mean ± SD) in smoking (8.32 ± 6.8 μg/ml) and nonsmoking (4.82 ± 2.3 μg/ml) women with AIDS were not significantly different (P > 0.05).
DISCUSSION
MICs for sensitive strains of M. tuberculosis, tested with the BACTEC method, have been reported to be in the range of 0.25 to 0.50 μg/ml (24) and 0.3 to 1.2 μg/ml (14). For clinical purposes, the recommended breakpoints for susceptible, moderately susceptible, moderately resistant, and resistant strains are <1.25, 2.5, 5.0, and >5.0 μg/ml, respectively (12).
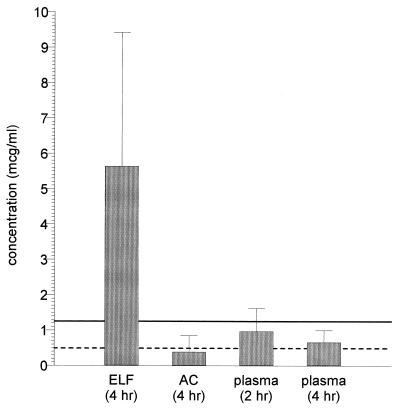
We have demonstrated that the plasma ethionamide concentrations were not affected by gender or by the presence of AIDS, as defined in our subjects. With all subjects combined (Fig. 1), plasma drug concentrations at 2 h were significantly greater than the concentrations at 4 h, consistent with previous reports that have described the kinetics of ethionamide in plasma (11, 22). It is noteworthy that 31 (77.5%), 38 (95%), and 38 (95%) of the 40 subjects had 2-h plasma, 4-h plasma, and AC drug concentrations below the MIC (1.25 μg/ml) that has been recommended as the laboratory breakpoint for susceptible strains of M. tuberculosis (13). Moreover, 9 (22.5%), 17 (42.5%), and 27 (67.5%) of the 40 subjects had 2-h plasma, 4-h plasma, and AC concentrations, respectively, below the reported MIC (0.5 μg/ml) for wild strains of M. tuberculosis considered to be susceptible to ethionamide (24). A greater dose size of ethionamide, e.g., 500 rather than the 250 mg used in this study, would have resulted in greater plasma, AC, and ELF concentrations and more of the subjects would have had plasma and AC drug concentrations that exceeded the published MICs of ethionamide for M. tuberculosis. This study also did not address the fluctuations in AC and ELF drug concentrations that may have occurred at other time points during the 12-h dosing interval, and such concentrations may have been greater than or less than those observed at the 4-h sampling time. Further investigation in this area is warranted. Maximum plasma concentrations of 2.24 ± 0.82 μg/ml have been reported with a 500-mg dose (22). The dose of ethionamide used in this study (250 mg every 12 h) was intentionally at the low end of the dose that is recommended for clinical purposes (0.5 to 1.0 g/day) in order to avoid toxicity in volunteer subjects.
FIG. 1.
Comparison of plasma, ELF, and AC ethionamide concentrations in 40 subjects. The suggested breakpoint (solid line) comes from reference 12. The reported MIC (dashed line) comes from reference 24.
All of the ELF ethionamide concentrations were greater than 1.25 μg/ml. The high drug concentrations in ELF relative to plasma and AC were similar to those that we have described with pyrazinamide (9). ELF/plasma concentration ratios were approximately 9.7 to 1 at the time that the bronchoscopy was performed (4 h following the last dose). This finding would indicate that, as with pyrazinamide, considerable antituberculous activity resides in the ELF. For example, for organisms for which MICs are 0.25 to 0.5 μg/ml, inhibitory ratios of 10 or 20 to 1 would be present in ELF. High inhibitory and killing ratios are viewed as desirable in the treatment of infectious diseases. The clinical significance of plasma, AC, and ELF ethionamide concentrations in the treatment of tuberculosis is uncertain and requires further investigation. Although the sulfoxide metabolite of ethionamide may have antituberculous activity (18), its contribution to the clinical outcome in the treatment of tuberculosis has not been studied and is unknown.
AC concentrations were 39 and 54% of the plasma concentrations at 2 and 4 h, respectively, suggesting exclusion or rapid removal of the drug from this compartment. The physiological basis for the differential penetration of ethionamide into ELF and its exclusion from AC is unknown. We were unable to demonstrate a difference in plasma, AC, or ELF drug concentrations between smoking and nonsmoking women with AIDS. The study was not designed to detect interactions between ethionamide and the many other drugs taken by our AIDS patients.
ACKNOWLEDGMENTS
This work was carried out with funds provided by the NIH, grant AI36054, and with funds provided by NIH grant MO1RR00079 (General Clinical Research Center) at the University of California, San Francisco.
We acknowledge Charles L. Daley for assistance, Margareta Andersson for performing the assays, and Eve Benton for manuscript preparation.
REFERENCES
- 1.Alford R H, Wallace R J., Jr . Antimycobacterial agents. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone Inc.; 1995. pp. 389–400. [Google Scholar]
- 2.Baldwin D R, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agents Chemother. 1992;36:1176–1180. doi: 10.1128/aac.36.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin D R, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother. 1992;36:1171–1175. doi: 10.1128/aac.36.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin D R, Wise R, Andrews J M, Ashby J P, Honeybourne D. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J. 1990;3:886–890. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269:729–730. [PubMed] [Google Scholar]
- 6.Choudhri S H, Hawken M, Gathua S, Minyiri G O, Watkins W, Sahai J, Sitar D S, Aoki F Y, Long R. Pharmacokinetics of antimycobacterial drugs in patients with tuberculosis, AIDS, and diarrhea. Clin Infect Dis. 1997;25:104–111. doi: 10.1086/514513. [DOI] [PubMed] [Google Scholar]
- 7.Conte J E J, Golden J, Duncan S, McKenna E, Lin E, Zurlinden E. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob Agents Chemother. 1996;40:1617–1622. doi: 10.1128/aac.40.7.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte J E Jr, Golden J A, Duncan S, McKenna E, Zurlinden E. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob Agents Chemother. 1995;39:334–338. doi: 10.1128/aac.39.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte J E, Jr, Golden J A, Duncan S, McKenna E, Zurlinden E. Intrapulmonary concentrations of pyrazinamide. Antimicrob Agents Chemother. 1999;43:1329–1333. doi: 10.1128/aac.43.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Conte, J. E., Jr., E. Zurlinden, and E. Lin. Column liquid chromatographic-mass spectrometric method for the determination of ethionamide in human plasma, bronchoalveolar lavage fluid, and alveolar cells. J. Chromatogr., in press. [DOI] [PubMed]
- 10.Gordon S M, Horsburgh C R, Jr, Peloquin C A, Havlik J A, Jr, Metchock B, Heifets L, McGowan J E, Jr, Thompson S E., III Low serum levels of oral antimycobacterial agents in patients with disseminated Mycobacterium avium complex disease. J Infect Dis. 1993;168:1559–1562. doi: 10.1093/infdis/168.6.1559. [DOI] [PubMed] [Google Scholar]
- 11.Gronroos J A, Toivanen A. Blood ethionamide levels after administration of enteric-coated and uncoated tablets. Curr Ther Res. 1964;6:105–114. [PubMed] [Google Scholar]
- 12.Heifets L. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am Rev Respir Dis. 1988;137:1217–1222. doi: 10.1164/ajrccm/137.5.1217. [DOI] [PubMed] [Google Scholar]
- 13.Heifets L. Drug susceptibility in the management of chemotherapy of tuberculosis. In: Heifets L, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 89–121. [Google Scholar]
- 14.Heifets L B, Lindholm-Levy P J, Flory M. Comparison of bacteriostatic and bactericidal activity of isoniazid and ethionamide against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1991;143:268–270. doi: 10.1164/ajrccm/143.2.268. [DOI] [PubMed] [Google Scholar]
- 15.Holdiness M R. Chromatographic analysis of antituberculosis drugs in biological samples. J Chromatogr. 1985;340:321–359. doi: 10.1016/0378-4347(85)80201-2. [DOI] [PubMed] [Google Scholar]
- 16.Jaruratanasirikul S. The pharmacokinetics of oral rifampicin in AIDS patients. J Med Assoc Thail. 1998;81:25–28. [PubMed] [Google Scholar]
- 17.Jenner P J, Ellard G A. High-performance liquid chromatographic determination of ethionamide and prothionamide in body fluids. J Chromatogr. 1981;225:245–251. doi: 10.1016/s0378-4347(00)80269-8. [DOI] [PubMed] [Google Scholar]
- 18.Jenner P J, Ellard G A, Gruer P J, Aber V R. A comparison of the blood levels and urinary excretion of ethionamide and prothionamide in man. J Antimicrob Chemother. 1984;13:267–277. doi: 10.1093/jac/13.3.267. [DOI] [PubMed] [Google Scholar]
- 19.Jenner P J, Smith S E. Plasma levels of ethionamide and prothionamide in a volunteer following intravenous and oral dosages. Lepr Rev. 1987;58:31–37. doi: 10.5935/0305-7518.19870004. [DOI] [PubMed] [Google Scholar]
- 20.Metropolitan Life. Metropolitan height and weight tables. Stat Bull. 1983;64:2–9. [PubMed] [Google Scholar]
- 21.Peloquin C A. Pharmacology of the antimycobacterial drugs. Med Clin N Am. 1993;77:1253–1262. doi: 10.1016/s0025-7125(16)30191-2. [DOI] [PubMed] [Google Scholar]
- 22.Peloquin C A, James G T, McCarthy E, Goble M. Pharmacokinetic evaluation of ethionamide suppositories. Pharmacotherapy. 1991;11:359–363. [PubMed] [Google Scholar]
- 23.Peloquin C A, Nitta A T, Burman W J, Brudney K F, Miranda-Massari J R, McGuinness M E, Berning S E, Gerena G T. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother. 1996;30:919–925. doi: 10.1177/106002809603000901. [DOI] [PubMed] [Google Scholar]
- 24.Rastogi N, Labrousse V, Goh K S. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol. 1996;33:167–175. doi: 10.1007/s002849900095. [DOI] [PubMed] [Google Scholar]
- 25.Rennard S I, Basset G, Lecossier D, O'Donnell K M, Pinkston P, Martin P G, Crystal R G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 26.Sahai J, Gallicano K, Swick L, Tailor S, Garber G, Seguin I, Oliveras L, Walker S, Rachlis A, Cameron D W. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann Intern Med. 1997;127:289–293. doi: 10.7326/0003-4819-127-4-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Talke H S G E. Enzymatische harnstoffbestimmung im blut und serum im optischem test nach warburg. Klin Wochenschr. 1965;43:174. doi: 10.1007/BF01484513. [DOI] [PubMed] [Google Scholar]
- 28.Taylor B, Smith P J. Does AIDS impair the absorption of antituberculosis agents? Int J Tuberc Lung Dis. 1998;2:670–675. [PubMed] [Google Scholar]
- 29.Willcox M, Kervitsky A, Watters L C, King T E J. Quantification of cells recovered by bronchoalveolar lavage. Comparison of cytocentrifuge preparations with the filter method. Am Rev Respir Dis. 1988;138:74–80. doi: 10.1164/ajrccm/138.1.74. [DOI] [PubMed] [Google Scholar]