Abstract
Clinical and laboratory studies have shown that environmental exposure to cadmium produces damage to several organs, including bones, lungs, and kidneys. The involvement of cadmium in central nervous system (CNS) disorders has also been widely reported, but the precise pathophysiological mechanism is not yet fully understood. Children who were exposed to cadmium during pregnancy are known to suffer from developmental delays, learning difficulties, attention deficit hyperactivity disorder (ADHD), and other cognitive and neurobehavioral deficits. Results from numerous studies suggest that dysfunction of the blood-brain barrier (BBB) structures is an important step in the neurotoxicity of cadmium. A rat-specific BBB marker protein, the endothelial barrier antigen (EBA), has been previously isolated and classified by Sternberger and others. The mouse IgG1 clone, anti-endothelial barrier antigen (anti-EBA), detects a protein triplet (23.5kDa, 25 kDa, and 30kDa) localized to the luminal surface of central and peripheral nervous system (CNS and PNS) vascular endothelial cells with selective permeability barrier functions. This marker has been widely used for characterizing BBB alterations under demyelinating, inflammatory, and other CNS pathologies. Many studies have been published using the rat model system for studying the neurotoxic effect of acute and chronic exposure to cadmium.
We applied the indirect immunofluorescent techniques using the anti-EBA antibody in conjunction with the Olympus cellSens computerized image analysis to detect and quantify the surface areas of BBB-competent microvessel profiles in paraformaldehyde-fixed, paraffin-embedded brains of term-delivered young rats after intraperitoneal injection of a single dose of cadmium chloride. We detected a statistically significant reduction in EBA-positive microvessel surface areas in the forebrain (t = 5.86, df = 1789, p-value < 0.001) and cerebellum (t=73.40, df=1337, p < 0.001) of cadmium-treated rats compared to the normal controls. Thus, this study supports the hypothesis that the EBA is a sensitive and measurable indicator for quantitative assessment of the impact of cadmium exposure in the developing rat brain.
Keywords: blood–brain barrier, endothelial barrier antigen, automated digital image analysis, cavalieri principle, stereology, cadmium neurotoxicity, rat
Introduction
Cadmium is a heavy metal typically found in association with zinc, lead, and copper ores as natural components of the earth’s crust, and its harmful effects are well known worldwide. Cadmium is known to cause damage to many organs and tissues, including the testes, kidneys, bones, lungs, and brain [1-10]. The metal is found in at least 1,014 of the 1,669 most hazardous sites identified and designated as National Priorities List (NPL) by the Environmental Protection Agency (EPA), indicating the public health significance of cadmium as an environmental pollutant [11-13]. As further evidence of its hazardous potential, the International Agency for Research on Cancer (IARC) has classified Cd as a Group 1 carcinogen in humans [11]. Cadmium typically contaminates the drinking water, air, and soil via a wide range of anthropogenic activities such as industrial production of rechargeable batteries, smelting, electroplating, phosphate fertilizers, and high-volume waste disposal by incineration. Through these activities, the metal readily enters the human food chain, rendering it a worldwide food and environmental contaminant [14-18]. A review of the literature indicates that tobacco leaves accumulate significant amounts of cadmium from the soil and are directly associated with exacerbation of respiratory pathologies, including chronic obstructive pulmonary disease (COPD) and emphysema, especially in heavy cigarette smokers [11,19-23]. Reports have also been published showing that cadmium can be released into the air, soil, and water through natural processes such as soil and rock erosion, wildfires, and volcanic eruptions [15,18,24,25]. Occupational and non-occupational exposure potential to Cd is very high, and studies have shown that maternal exposure during pregnancy results in children suffering from abnormalities, including growth retardation, learning disorders, neurobehavioral and other cognitive disabilities postnatally [12,26-31,32-38]. According to the Agency for Toxic Substances and Disease Registry [11], dietary ingestion constitutes the highest source of cadmium exposure for non-smoking adults and children in the United States. Among United States residents, the daily ingestion of cadmium in non-smoking adult males and females has been estimated at 0.35 and 0.30 μg Cd/kg/day, respectively [11]. The adverse impact of cadmium is further worsened by its slow elimination and long biological half-life in the body [39]. The mechanism by which Cd produces damage to the brain has been a subject of extensive investigations but is not yet completely elucidated. It has been hypothesized that Cd neurotoxicity is due to its key role in oxidative stress-induced morphological and functional perturbation at the cellular level, including blood-brain barrier (BBB) structures [7,40-47].
The electron microscopic studies by Reese and Karnovsky [48] and by Brightman et al. [49] have shown that the mammalian blood-brain barrier (BBB) location is at the brain microvascular endothelial cells (BECs) [50]. Until recently, it was impossible to selectively identify or observe the individual cells of the BBB due to the lack of a specific marker for the functionally and morphologically unique microvessels [47,51-55]. During postnatal development of the vertebrate mammalian brain, including rats and humans, in response to brain-derived factors, the BECs acquire barrier characteristics (barrier genesis), including tight junction-associated proteins phenotype [56-63]. The identification and localization of markers within single cells and in tissues by immunohistochemistry with specific antibodies have greatly facilitated in-depth studies of the underlying molecular mechanisms of diseases, including BBB alterations under pathological conditions [5,40,56-58,63-71]. A microvascular endothelial marker, specific to rat BBB, the endothelial barrier antigen (EBA), has been identified and classified by Sternberger et al. [72] based on the lymphocyte fusion technique of Köhler and Milstein [73], using rat whole brain homogenate as immunogen. The monoclonal antibody, anti-endothelial barrier antigen (anti-EBA), reacts exclusively with neurovascular endothelia with permeability barrier functions. It is unreactive with non-endothelial cells in or outside of the nervous system. Additionally, fenestrated microvessels of peripheral body organs such as the heart, liver, and kidney, and microvessels of the brain circumventricular organs (CVOs), do not display immunoreactivity with anti-EBA. Previous studies have utilized EBA expression as a valid model for evaluating BBB alterations under normal and pathological conditions [74-80]. In a previous study using Sprague-Dawley rats, Ghabriel et al. [43] reported a decrease in EBA immunoreactivity and extensive extravasation into the brain parenchyma of endogenous albumin and intravenously administered horseradish peroxidase (HRP) after a single parenteral injection of anti-EBA monoclonal antibody. This observation suggests an inverse correlation between BBB disruption and the brain's microvascular EBA content. More recently, Pelz et al. [81] demonstrated a similar inverse correlation between EBA reactivity and BBB disruption in focal cerebral ischemia induced by an embolic stroke model in Wistar rats. In an earlier study utilizing Lewis rats with induced experimental allergic encephalomyelitis (EAE), a rodent analogue of human multiple sclerosis (MS), Sternberger et al. [82] detected a complete loss of immunoreactivity with anti-EBA in brain microvessels surrounded by inflammatory cells within the brain lesions. In the same study, it was also reported that BECs in rats that had recovered from EAE reacted with the antibody, while microvessels in brain areas with persistent inflammation remained EBA-negative, suggesting that EBA reappeared during recovery from EAE). The data from several studies have shown that in the developing rat and human brain, the immature, nascent BBB is potentially more sensitive to the effects of exogenously or environmentally induced pathogenic agents, including cadmium-induced neurotoxicity [40,83]. Despite many reports on BBB changes using EBA as an indicator [43,81-82], the impact of cadmium exposure on possible alterations in EBA as an indicator has not been widely studied. The results from previous work by other investigators have revealed that prenatal, newborn, and adult rats are valid model systems for studying the neurotoxicity of parenterally or orally administered cadmium in the setting of both acute and chronic exposures to the metal [84-88].
The goal of the present study was to evaluate alterations in the postnatal expression of the EBA as a rat-specific BBB indicator by objectively and accurately determining the surface areas of EBA-positive microvessels in the forebrain and cerebellum in young rats. We applied the indirect immunofluorescence protocols with anti-EBA and an Olympus cellSens automated digital imaging software suite to determine the surface areas of BBB-competent microvessels within standardized microscopic fields, following a short duration exposure to cadmium by intraperitoneal injection of neonate rats.
Materials and methods
Animals and brain tissue preparation
Two equal groups of 40-day-old healthy male Sprague-Dawley rats weighing 150-200 g (Charles River Laboratories Inc., USA) were utilized for this study. Male rats were used based on the data from previous studies showing that exposure of female rats to cadmium caused suppression of the immune system, including altered responses of the spleen cells [89]. Furthermore, we used animal cohorts matched for age, sex, and strain to ensure reliable between-group comparisons, given the previously published data suggesting a differential expression of the EBA within different rat brain regions [75, 89-91]. The present study was performed under stringent adherence to the Institutional Animal Care and Use Committee (IACUC) and the Institutional Review Board (IRB) regulations of the Alabama State University and the Alabama College of Osteopathic Medicine (ACOM), respectively. Both the experimental and control animals, 10 animals per group, were housed two per cage and allowed free access to standard rat chow and drinking water throughout the experiment. After 10 days of adaptation to the laboratory environment, a group of 10 animals was parenterally (intraperitoneally, IP) injected with a single dose of cadmium chloride (Sigma-Aldrich, St. Louis, Missouri, USA) at a dose of 4 mg/kg body weight dissolved in isotonic saline [75]. This cadmium dose was chosen based on previous studies by other authors on the toxic effects of short-term exposure to cadmium in the brain and peripheral organs of young rats [13,84,92]. The second group of 10 rats received IP isotonic saline injection each and was used as the normal control group. Three days post-injection, all rats were sacrificed by exposure to increasing concentration of carbon dioxide followed by cervical dislocation. After removal, each brain was transferred into a Petri dish with a unique identification code and placed on wet ice at 4°C. The cadmium administration and all subsequent dissection steps were carried out in a laminar flow hood. The brain was kept wet throughout the dissection with an intermittent application of a jet of cold 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) to facilitate dissection and preserve tissue antigen. The forebrain was rapidly detached from the midbrain and cerebellum by making a transverse incision at a level corresponding to the rostral border of the superior colliculus, as previously described [75]. Next, the cerebellum was separated from the midbrain by cutting transversely through the brainstem at the level corresponding to the caudal border of the inferior colliculus. Finally, the cerebellum was detached from the brainstem by gently retracting it upward and cutting transversely through the rostral medulla. The isolated forebrain and cerebellum from each rat were then cut in a coronal plane into blocks and immersion-fixed overnight at 4°C in 120 ml specimen bottles precoded with the rat numbers containing 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). The forebrain and cerebellum were each cut into four and two coronal slices, respectively (Figure 1). To ensure consistency of between-group comparison of similar brain regions, every second and third block from each forebrain starting from the rostral end and two blocks from the cerebellum of experimental and control rats were placed in cassettes with original rat identification codes and processed to paraffin embedding overnight in a vacuum infiltration tissue processor (Tissue-Tek, Sakura Finetek, Torrance, CA, USA). The blocks were embedded in a coronal plane and sectioned serially at 8 μm using a rotary microtome (Microm M355S, ThermoFisher, USA). Every fifth section from the paraffin-embedded blocks was mounted on Superfrost glass slides (Fisher Scientific, Pittsburgh, PA, USA) labelled with the original identification codes and immunofluorescently stained with anti-EBA for the computerized image analysis as described below. To confirm tissue specificity of an anti-EBA monoclonal antibody, small pieces (~ 3cm3) were resected from the kidney and processed to paraffin embedding as above, and thin sections (8 μm) were cut. In some cases, some brains were sectioned in a para-sagittal plane, revealing the fourth ventricular cavity, ependymal epithelial lining, and the choroid plexus to examine the vascular endothelium-specificity of EBA. Additionally, thin sections from the brain and peripheral organs were stained with monoclonal antibody CD105 (endoglin) as a general vascular endothelial marker for distribution comparison with anti-EBA as a rat BBB-specific vascular endothelial marker.
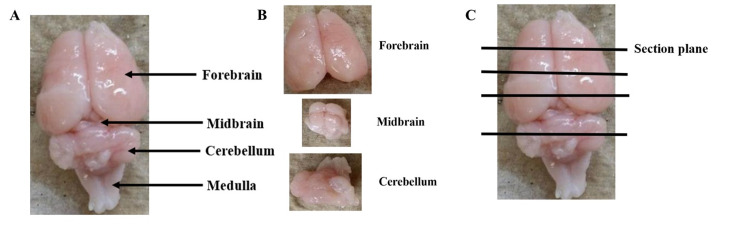
Figure 1. Isolation and sectioning of rat brain regions.
Representative rat brain isolation and sectioning orientation, (A) Whole-brain dorsal view. (B) Detached forebrain, midbrain, and cerebellum. (C) Coronal plane orientation for tissue block preparation and microtome thin (8 µm) sectioning.
Preparation for immunofluorescent detection of EBA-positive microvessels
To visualize the EBA immunoreactivity in normal control and cadmium-treated paraffin-embedded brains, we used affinity-purified mouse anti-rat BBB monoclonal antibody (IgM, clone SMI-71, Biolegend, Inc., San Diego, USA). The indirect immunofluorescent detection was performed as follows: the sections were dewaxed for 5 minutes in each of three changes of xylene followed by rehydration for 5 minutes in each of two changes of descending grades of absolute, 95%, 70% and 50% ethanol; immersed for 5 minutes in each of two changes of deionized water and two changes of PBS; pretreated with trypsin antigen retrieval solution (ab970 - ABCAM Inc., Cambridge, MA, USA) for 10 minutes in an Isotemp incubator (Thermo Fisher Scientific, Waltham, MA USA) maintained at 37°C for optimal EBA detection; non-specific binding was blocked by treating sections for 1 hour in a moist slide chamber at room temperature with animal-free diluent and blocker reagent (Cat# SP-5035-100; Vector Laboratories, Inc. Burlingame, CA, USA); excess blocking solution was gently tapped off followed by overnight incubation (4°C) with affinity-purified mouse anti-rat EBA monoclonal antibody SMI-71 (IgM) (cat #836804; Biolegend, San Diego, USA) diluted 1:1000 in animal-free diluent; washed for 5 minutes in each of two changes of PBS (pH 7.4); incubated at room temperature for 1 hour with donkey anti-mouse fluorescein isothiocyanate (FITC)-conjugate (Jackson ImmunoResearch Laboratories, USA) at a dilution of 1:400 using animal-free diluent; finally, the immunostained slides were washed in two changes of PBS as above. Labeled sections were mounted in a VectaShield vibrance antifade mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) as nuclear counterstain (cat #H-1800; Vector Laboratories Inc., Burlingame, CA, USA). Negative control tissue sections were treated with the same immunohistochemical reagents under identical conditions, but the primary antibodies were skipped. EBA-positive microvessels appeared as green, fluorescent profiles, while the nuclei appeared as blue structures under the Olympus BX63 epifluorescence microscope.
Preparation for heat-activated epitope recovery and immunofluorescent detection of CD105 (endoglin)
The detection of CD105 in paraffin-embedded kidney and brain sections was carried out as follows: sections were dewaxed as described above and rehydrated to PBS (pH 7.4) and tap water; the sections were subjected to 3 minutes heat-mediated antigen retrieval under constant boiling temperature and pressure in a pressure cooker containing 2 liter 100 Mm Citrate buffer (pH, 6.0) (catalogue #ab93678, ABCAM, Cambridge, Massachusetts, USA); cooled under gentle running tap water; permeabilized by washing for 5 minutes in each of two changes of 0.01%Triton X-100 (Fisher Scientific, Pittsburgh, PA, USA ) in PBS (pH 7.4); washed in two changes of PBS (pH 7.4); non-specific binding blocked by incubation in animal-free blocking/diluent reagent (Vector Laboratories, Burlingame, CA, USA) for 1 hour at room temperature to reduce non-specific binding by secondary antibody; incubated overnight (4 °C) in rabbit anti-CD105 monoclonal antibody (ABCAM, Cambridge, Massachusetts, USA) (1:50 dilution in animal-free diluent and blocker); washed in 2 changes of PBS (pH 7.4) and incubated in goat anti-rabbit FITC-conjugated secondary antibody diluted 1:400 in animal-free diluent for 1 hour at room temperature; washed twice in PBS and mounted in Vectashield vibrance medium with diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) as nuclear counterstain.
Negative control brain sections were treated with the same immunohistochemical reagents under identical conditions, but the primary antibody was omitted. Some rat brains were sectioned in a sagittal paramedian plane revealing portions of the pons, cerebellum, ventricular ependymal lining, and the choroid plexus were also stained with anti-EBA to serve as internal controls and for comparison with the anti-CD105 distribution. In addition, paraffin-embedded sections from the kidney, known to possess both fenestrated and continuous capillaries lacking a permeability barrier function, were immunostained with the anti-CD105 for comparison with anti-EBA specificity.
Quantitative computerized image analysis
Overall, two blocks from the forebrain and two blocks from the cerebellum were used per experimental and control rat, and five fields of view (FOVs) from each of eight thin sections were analyzed per block for this study. Before surface area measurement, the original rat identification codes on the slides were covered with tape, and each slide was randomly assigned a number to eliminate observer bias in the assessment. From a random point within each tissue section, every second FOV in equidistant series of fields was examined. All FOVs were evaluated per slide and each slide was assessed thrice by the same observer. Computerized image analysis was carried out as follows: the immunofluorescently labeled sections were examined, and images were acquired with an Olympus BX63 fluorescence microscope equipped with a DP80 CCD color camera and cellSenseTM Dimension software program (Olympus, cellSens Dimension 1.17 Build 21199). The cellSens Dimension software suite incorporates a Count and Measure module that allows for global thresholding to segment immunofluorescently labeled profiles of interest from the surrounding background and measure surface areas accurately and reproducibly within the standardized region of interest (ROI). The software can also determine the number of immunofluorescently labeled profiles within the defined ROI. We used the entire field of view (FOV) in a ×40 objective lens (ROI) for the evaluation, which was equivalent to an area of 90610.43 mµ2 (0.1 mm2) that was automatically generated by the software on the computer screen. The mean surface areas of EBA-positive microvessels were measured in each field for all slides from both the cadmium-treated and control rat brains. The grand means (Mean ± SE) were computed for both groups of rats for statistical analysis.
Before surface area determination, each image was separated into its red, green, and blue (RGB) component channels, followed by the selection of the red channel and utilizing the red phase to highlight the individual immunoreactive microvessels. The global thresholding was set by using the manual threshold mode to carefully adjust the minimum and maximum intensity distribution histogram on a sliding scale until the best quality image was obtained for contrast and intensity while also ensuring that all immunoreactive microvessels were highlighted in red against a clear, black background. We determined that highlighting the EBA-reactive microvessels using the red phase instead of the green phase allowed for more precise detection and differentiation of the profiles of interest and a more complete segmentation of the background and its green endogenous autofluorescence from the brain tissue components. The same global threshold was applied for analysis of all fields subsequently examined to ensure consistency and precision of analysis (Figures 2C-2F).
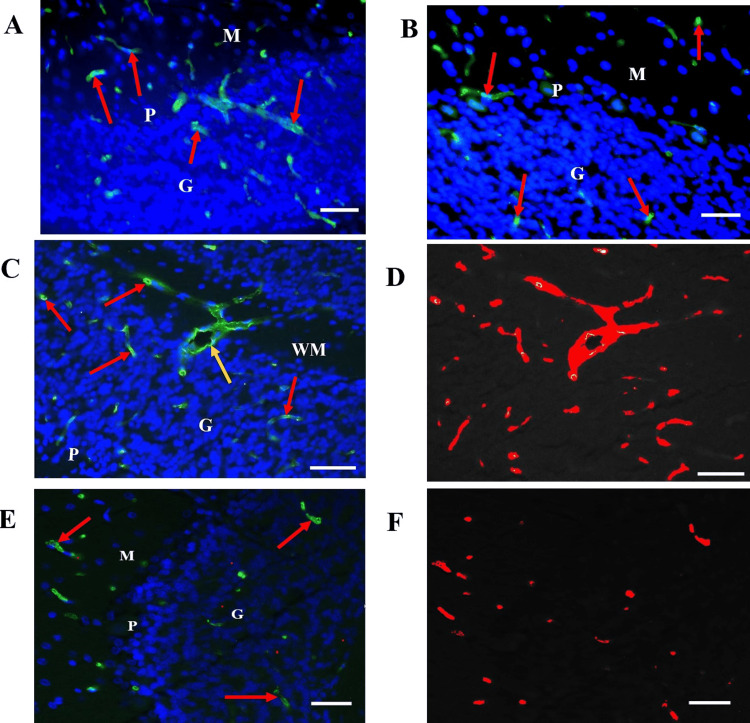
Figure 2. EBA-positive microvessels are reduced in the cerebellum of cadmium-treated rats.
(A) EBA immunoreactivity in a control rat cerebellum showing strong labeling of microvessels by anti-EBA (red arrows). (B) Cadmium-exposed rat cerebellum showing decreased EBA-positive microvessels (red arrows). (C) to (F) Microvessel surface area measurement in normal control (C) and cadmium-treated (E) rat cerebellum showing EBA-immunopositive venules (yellow arrows) and capillaries (red arrows) and their collaterals. (D) & (F), global thresholding and segmentation of EBA-positive microvessels for surface area measurement. WM: cerebellar white matter; G: Granule cell layer; P: Purkinje cell layer. Scale bars, 20 µm.
The class measurement tool was used to automatically generate the number and mean surface areas of the individual EBA-positive microvessel structures within the ROI. Finally, the data generated were exported to an excel spreadsheet, and grand mean surface areas (μm2) of the EBA-positive microvessels in all microscopic fields were computed for the control and cadmium-exposed forebrain and cerebellum. These data were presented as Mean ± SE values.
Statistical analysis comparing profile grand mean surface areas of the cadmium-treated brains and normal controls was performed by Welch’s two‐sample t‐test with a confidence interval set at 95%.
Results
Histological observations and endothelial barrier antigen immunoreactivity
In hematoxylin- and eosin-stained histological sections, the principal light microscopic observations were dark-staining of the neuronal and glial cell nuclei and ill-defined cell bodies that appeared to be degenerated in cadmium-treated forebrains compared to normal control brains (Figure 3). Similar sections from the cerebellum of cadmium-exposed rats exhibited hyperchromatic glial cell nuclei and irregularly shaped, Purkinje neurons, which appeared to be shrunken with strongly basophilic and homogeneous nuclei compared to the normal control cerebellum (Figure 4).
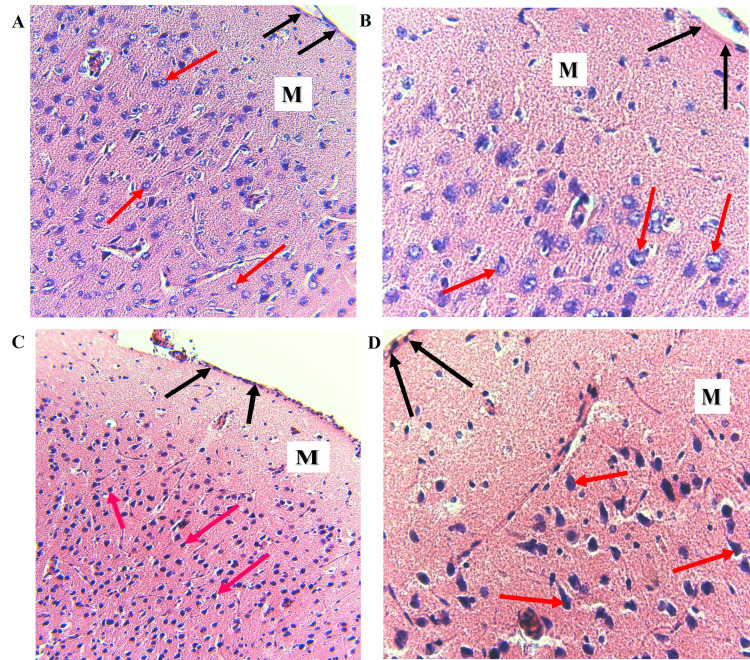
Figure 3. Cadmium-treated rats display evidence of neuronal and glial damage.
(A) Normal forebrain, H & E. Uniformly eosinophilic, neuropil, clearly defined neuronal and glial cell bodies, open face neuronal nuclei with prominent nucleoli and visible cytoplasm (red arrows), (B) High magnification of A, showing clearly defined neuronal and glial cell bodies and nuclei (red arrows). (C) Cadmium-treated Forebrain. Dark-staining neuronal and glial nuclei with ill-defined cell bodies (red arrows). (D) High magnification of C showing dark-staining neuronal and glial nuclei and ill-defined, with dark, acidophilic cytoplasm (red arrows). M: Molecular layer. The leptomeningeal layer is indicated by black arrows. H & E: (A) and (C) (x200), (B) and (D) (x400).
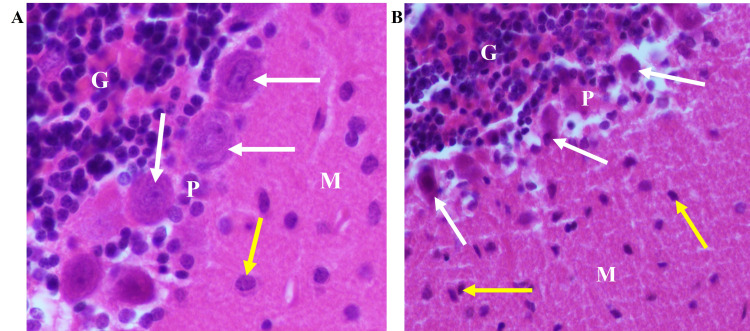
Figure 4. Cadmium-treated rats display abnormalities in the cerebellar cortex.
(A) Normal control rat cerebellum. Well preserved, normal-appearing Purkinje neuronal (white arrows) and glial (yellow arrows) cell bodies, nuclei, and cytoplasm. (B) Cadmium-treated rat cerebellum showing hyperchromatic glial nuclei (yellow arrows) and shrunken Purkinje neurons with hyperchromatic nuclei (white arrows). M: cerebellar molecular layer; P: Purkinje cell layer; G: granule cell layer. H & E (x400).
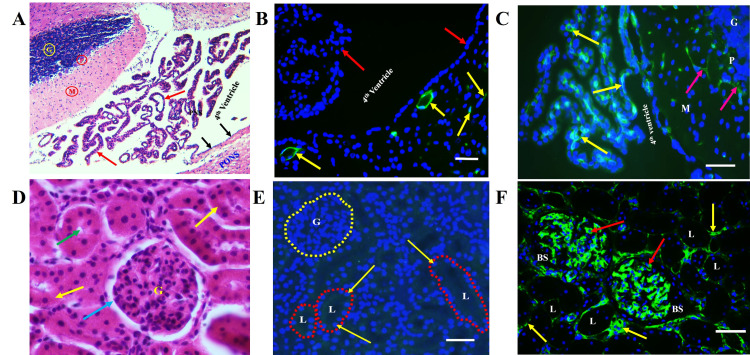
We detected immunoreactivity in microvessels in the forebrain and cerebellum of experimental and control rats (Figures 5B, 6A, 6B). By contrast, the EBA immunoreactivity was undetectable in negative control slides in which the primary antibody was skipped, or in sections from the kidney and area of the choroid plexus of the 4th ventricle, the histological organization is shown for reference (Figures 5A, 5B, 5D, 5E). A reduction in the number of EBA-positive microvessels was observed in cadmium-treated forebrain and cerebellum compared to the normal control animals (Figures 5, 6A). The anti-CD105 monoclonal antibody was detected in vascular endothelial in the sections from the kidney, brain, and choroid plexus (Figures 5C, 5F).
Figure 5. EBA is specific to brain regions.
(A) Section showing the histological organization of normal rat cerebellum, adjacent fourth ventricle and choroid plexus (red arrows), and pons. The ependymal lining of the fourth ventricle is shown by black arrows; part of the cerebellum is shown in the top left corner. M: Molecular layer; P: Purkinje cell layer; G: Granule cell layer. H & E (x100). (B) Normal rat brain section stained with anti-EBA. Note positive staining of brain capillaries and venules (yellow arrows), but ventricular ependymal lining and choroid plexus epithelial cells (red arrows) are unreactive. (C) Anti-CD105 is reactive with the brain (red arrows) and choroid plexus (yellow) microvessels. (D) Kidney histological section from normal control rat. G: Glomerulus; renal tubular epithelial cells (yellow arrows); renal tubule lumen (green arrow); Bowman space (blue arrow). H & E (x400). (E) No EBA-immunoreactivity is detected in capillaries within the glomeruli (area G, surrounded by a yellow circle) or in peritubular areas (surrounded by red circles). G: Glomerulus; L: renal tubule lumen. Tubular epithelial cell nuclei are indicated by yellow arrows. (F) Section from a paraffin-embedded rat kidney showing strong labeling of the renal glomerular (red arrows) and peritubular (yellow arrows) microvasculature by anti-CD105. L: renal tubule lumen; BS: Bowman’s space. Scale bar, 20 µm
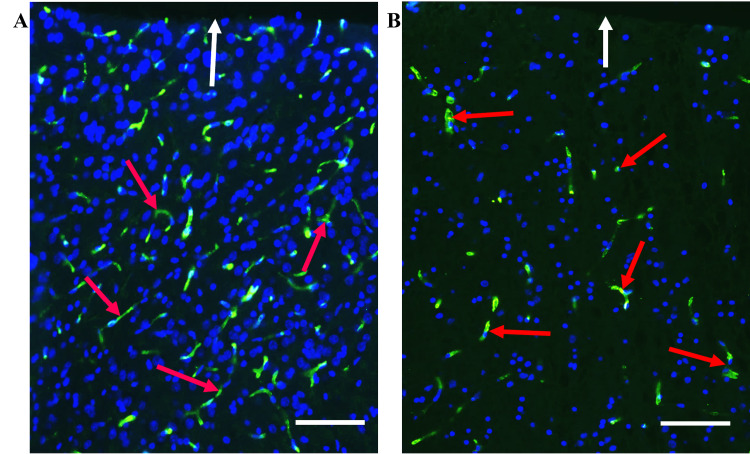
Figure 6. EBA-positive microvessels are reduced in the cerebral cortex of cadmium-treated rats.
(A) EBA immunoreactivity in a control rat cerebral cortex showing labeling of microvessels of various sizes by anti-EBA antibody (red arrows). (B) EBA immunoreactivity in a cadmium-treated rat cerebral cortex (red arrows). The leptomeningeal layer is indicated by white arrows. Scale bars, 50 µm.
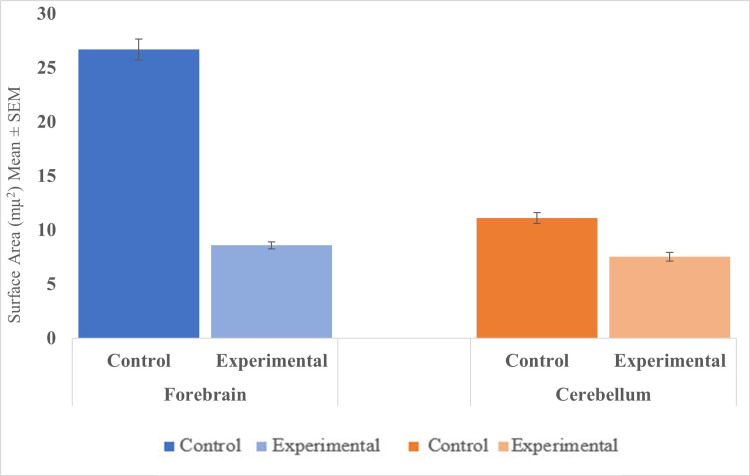
In normal control brains, the surface areas for EBA-positive microvessel profiles were 11.11 ± 0.5 µm2 (Mean ± S.E) and 26.68 ± 0.98 µm2 (Mean ± S.E) in the cerebellum and forebrain, respectively. In the cerebellum and forebrain of rats exposed to cadmium, the EBA-positive microvessels surface areas were 7.51 ± 0.4 µm2 (Mean ± S.E) and 8.58 ± 0.33 µm2 (Mean ± S.E), respectively. We found a statistically significant reduction in EBA-positive microvessel areas in the forebrain (t = 5.86, df = 1789, p-value < 0.001) and cerebellum (t=73.40, df=1337, p < 0.001) of cadmium-treated brain compared to the normal controls (Tables 1, 2, Figure 7).
Table 1. Mean surface areas of EBA-positive microvessels in the forebrain. N: Total number of EBA-positive microvessel profiles.
Welch’s t-test: t = 5.86, df = 1789, p-value < 0.001
| Group | N | Mean surface area | SE |
| Control | 1515 | 26.68 | 0.98 |
| Experimental | 1274 | 8.58 | 0.33 |
Table 2. Mean surface areas of EBA-positive microvessels in the cerebellum. N: total number of microvessel profiles.
Welch’s t-test: t=73.40, df=1337, p-value < 0.001
| Group | N | Mean surface area | SE |
| Control | 1100 | 11.11 | 0.5 |
| Experimental | 551 | 7.51 | 0.4 |
Figure 7. Surface areas of EBA-positive microvessels in the forebrain and cerebellum (mµ2) (Mean ± SE). Forebrain: p < 0.001; Cerebellum: p < 0.001 .
Discussion
The purpose of the present study was to apply the computerized image analysis using the Olympus cellSens software to determine changes in EBA-immunoreactive microvessels in the rat forebrain and cerebellum more objectively and accurately from standardized microscopic fields following intraperitoneal injection of cadmium. In this study, capillaries and venules and their collaterals of varying lengths were labeled by the ant-EBA in all brain sections examined (Figures 2, 6). There were no apparent within-group differences in the caudal-rostral spatial distribution of the EBA-immunoreactive vasculature. An interesting observation from this study is the marked difference in the density of EBA-positive microvessels in the forebrain compared to the cerebellum in control rats (26.68 mµ2 and 11.11 mµ2, respectively). The specific functions of EBA in the CNS or the significance of these density differences are unclear but may reflect differences in metabolic needs between the two CNS regions or differential EBA expression accompanying ongoing angiogenesis and maturation of the nascent BBB-competent normal rat microvessels. Another noteworthy observation from this study is that in cadmium-exposed rats, density decreased to 7.51 mµ2 and 8.58 mµ2 in the forebrain, respectively. This represents a 32.4% decrease in the cerebellum compared to 67.8% in the forebrain of EBA-positive microvessels. These differences are significant (Figure 7) and appear to correlate with the histological observations (Figures 3, 4). The mechanisms underlying these cadmium-associated decreases in EBA-positive microvessels are currently unknown and warrant future investigation. A review of the literature indicates that most clinical manifestations of cadmium neurotoxicity are related to lesions in the cerebral neocortex, such as ADHD, dyslexia, mental retardation, neurobehavioral, and other cognitive disabilities [30,33,93]. The higher percentage change in the forebrain EBA-positive microvessels appears to correlate with the manifestation of cerebral symptoms commonly seen in cadmium neurotoxicity, although cadmium-related histopathological changes have been reported not only in the forebrain but also in the cerebellum and other brain regions in the rat [84,94] and swine [95] models of cadmium neurotoxicity. To further confirm that the measured cadmium-induced microvascular differences are due to alteration in EBA expression and not due to a general reduction in vascular endothelial density, we employed an antibody directed against endoglin (CD105) as a general endothelial marker. Endoglin is a 180 kDa transmembrane protein and is a used marker for studying active angiogenesis such as in brain development, tumors, or demyelinating CNS pathologies [96-97]. Immunofluorescent staining demonstrated that the EBA and CD105 are very different epitopes. While the anti-EBA is negative in peripheral tissues, the anti-CD105 strongly reacted with blood vessels at these anatomical locations (Figures 5E, 5F). Moreover, the anti-EBA immunoreactivity was restricted to microvessels within the brain neuropil and was completely negative in fenestrated endothelial cells in the kidney and choroid plexus (Figures 5B, 5E). By contrast, anti-CD105 strongly labeled endothelial cells within the brain and choroid plexus, apparently with equal efficiency (Figures 5C, 5F). Taken together, the above findings, along with quantitative changes reported, here suggest a true reflection of alterations in microvessel content of EBA and could not have been due to general changes in vasculature density. It also demonstrates that CD105 and EBA are two very different epitopes. Results from our current study suggest that EBA immunoreactivity provides a specific and accurately measurable indicator of cadmium toxicity on the developing, nascent BBB in the rat CNS. Many previous studies have documented the sensitivity of EBA-immunoreactivity for observational, qualitative evaluation of BBB involvement in infectious and non-infectious CNS diseases. In an investigation using Wistar rats that were injected with Clostridium perfringens prototoxin, Zhu et al. [79] found an inverse correlation between EBA immunoreactivity and BBB disruption as shown by increased permeability to endogenous albumin detected immunohistochemically both at the light and electron microscopic levels. Many authors have previously employed subjective and semi-quantitative methods to evaluate temporal-spatial dispositions of EBA immunoreactivity under normal and pathological conditions [75,77,80,82].
The introduction of a variety of computerized image analysis protocols has facilitated the performance of unbiased, accurate, and efficient quantitation of profiles in biological structures [98-99]. These methods have been extended to the evaluation of EBA under normal and pathological situations. For example, Sibbons and others [100] applied Cavalieri’s stereological principle of template point counting on fresh brain slices together with EBA immunohistochemistry on thick vibratome brain sections to determine age-related changes in total surface area of BBB-competent microvessels per unit volume of brain tissue in normal rat neocortex. A study by Jin et al. [101] utilized an Image-Proplus 5.0 medical imaging analysis software was used to measure the integrated optical density (IOD) as an indicator of EBA-immunoreactivity in the rat's parietal cortex following targeted irradiation. Another study by El-Salhy et al. [102] employed the Olympus cellSens like that described in our present study to compute the total number of endocrine cells per unit area of tissue in ileal biopsies from patients with irritable bowel syndrome. The authors used manual counting by mouse-pointing and clicking on profiles of interest and drawing the areas containing those profiles on the computer screen. More recently, Youssef et al. [103] utilized the Olympus cellSense Dimension Software with manual delineation of profiles of interest like the study by El-Salhy et al. [102], to determine the area of the dentate gyrus and granule cell layer in thick sections from the developing mouse brain after exposure to stress. By contrast, we captured all profiles of interest by setting a global threshold and applying this to all fields to automatically generate the numbers and surface areas of the BBB-competent microvessels rapidly and unbiasedly in each FOV. Other investigators have applied similar quantitative computer-assisted image analysis to determine the number and areal fraction of EBA-immunopositive microvessel profiles within defined microscopic fields in a rat model of traumatic brain injury [78]. Taken together, these studies indicate that the EBA is a sensitive and reliable indicator for evaluating BBB involvement in the pathogenesis of rat models of CNS disorders.
Despite the use of gestational, newborn, and adult rats as valid model systems for studying the neurotoxic effects of cadmium [104-109], the possible alterations in EBA in the developing rat brain as a result of cadmium exposure have not been widely studied. Rodents, including rats and mice, have been extensively used in studies to understand the pathophysiologic mechanisms of environmentally induced CNS pathologies such as cadmium intoxication and other disorders in humans. To reliably extrapolate findings from such studies, it is important to recognize the prenatal and postnatal species differences in brain development. In terrestrial vertebrates, including rats and humans, CNS development begins with the formation of the neuroectoderm-derived neural tube, which then progresses through three primary and five secondary brain vesicles that ultimately differentiate into forebrain, midbrain, cerebellum, medulla oblongata, and spinal cord. These stages occur at different rates in rats and humans. Details of the comparative developmental neuroanatomy and the identification of key landmarks when the developing rat and human brains appear to be more susceptible to the impact of pathogenic agents, including cadmium exposure, have been well described in the literature. A review of these studies indicates that most of the key developmental processes, including changes in brain volume, weight, gross structure, formation of synaptic contacts, gliovascular unit (BBB), myelination, and neurotransmission activities, continue through the postnatal periods in both rat and man, though at different time scales. These studies also indicate that the rat brain at postnatal day 10 is developmentally equivalent to the human brain milestone of three months (first trimester) intrauterine life. Further, five weeks postnatal rat brain is developmentally estimated to correspond to a human brain at age 2-4 years (under-five-year-old). At these time points, the rat and human CNS and its BBB gliovascular structures are immature compared to similar adult structures. It is conceivable, therefore, that both rat and human BBB at these early stages are more susceptible to cadmium exposure in both acute, subacute, and chronic settings compared to adult brains [110,111].
Here we have investigated a single timepoint evaluation of the impact of cadmium exposure during the neonatal period. To address this limitation, additional longitudinal studies are underway whereby we will expose the developing rat brain from gestation through birth to post-weaning, collecting tissue at each time point post-partum. This will allow us to more closely mimic human exposure to the metal for downstream analysis of postnatal EBA recovery or otherwise from the impact of chronic cadmium exposure in utero.
Conclusions
The results from the present study suggest that EBA could serve as a potential tool for accurately and reproducibly analyzing BBB alterations following exposure to cadmium in the rat model system. The cellSens computer-assisted quantitative methodology described in this study could serve as a foundational reference for future mechanistic investigations of the pathophysiologic mechanisms of cadmium neurotoxicity in this model of developing, in situ rat BBB model system.
Acknowledgments
This study was financially supported by the Alabama College of Osteopathic Medicine. The authors are grateful to Mrs. Krissy Travers, Ms. Ann Stulginski, Ms. Casey Cornell, and Ms. Caitlin Patterson for superlative technical assistance.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Alabama State University at Montgomery IACUC Issued protocol number 022618
References
- 1.Selenium modifies associations between multiple metals and neurologic symptoms in Gulf states residents. Werder EJ, Engel LS, Curry MD, Sandler DP. Environ Epidemiol. 2020;4:0. doi: 10.1097/EE9.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effects of paternal cadmium exposure on the sperm quality of male rats and the neurobehavioral system of their offspring. Zhao X, Cheng Z, Zhu YI, Li S, Zhang L, Luo Y. Exp Ther Med. 2015;10:2356–2360. doi: 10.3892/etm.2015.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadmium: toxic effects on the reproductive system and the embryo. Thompson J, Bannigan J. Reprod Toxicol. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Effects of cadmium exposure on testis apoptosis in the marine teleost Gobius niger. Migliarini B, Campisi AM, Maradonna F, Truzzi C, Annibaldi A, Scarponi G, Carnevali O. Gen Comp Endocrinol. 2005;142:241–247. doi: 10.1016/j.ygcen.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Cadmium exposure negatively affects the microarchitecture of trabecular bone and decreases the density of a subset of sympathetic nerve fibers innervating the developing rat femur. Graniel-Amador MA, Torres-Rodríguez HF, Jiménez-Andrade JM, Hernández-Rodríguez J, Arteaga-Silva M, Montes S. https://www.researchgate.net/publication/346491522_Cadmium_exposure_negatively_affects_the_microarchitecture_of_trabecular_bone_and_decreases_the_density_of_a_subset_of_sympathetic_nerve_fibers_innervating_the_developing_rat_femur. Biometals. 2021;34:87–96. doi: 10.1007/s10534-020-00265-x. [DOI] [PubMed] [Google Scholar]
- 6.Bone resorption and environmental exposure to cadmium in children: a cross - sectional study. Sughis M, Penders J, Haufroid V, Nemery B, Nawrot TS. Environ Health. 2011;10:104. doi: 10.1186/1476-069X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Shukla A, Shukla GS, Srimal RC. Hum Exp Toxicol. 1996;15:400–405. doi: 10.1177/096032719601500507. [DOI] [PubMed] [Google Scholar]
- 8.Heavy metals and adult neurogenesis. Wang H, Matsushita MT. https://doi.org/10.1016/j.cotox.2021.03.006. Curr Opin Toxicol. 2021;26:14–21. doi: 10.1016/j.cotox.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The impact of chronic co-exposure to different heavy metals on small fibers of peripheral nerves. A study of metal industry workers. Koszewicz M, Markowska K, Waliszewska-Prosol M, et al. https://doi.org/10.1186/s12995-021-00302-6. J Occup Med Toxicol. 2021;16:12. doi: 10.1186/s12995-021-00302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The relationship between occupationally exposed arsenic, cadmium and lead and brain bioelectrical activity-a visual and brainstem auditory evoked potentials study. Waliszewska-Prosół M, Ejma M, Gać P, et al. Brain Sci. 2021;11 doi: 10.3390/brainsci11030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faroon O, Ashizawa A, Wright S, et al. Agency for Toxic Substances and Disease Registry (ATSDR) Vol. 2012. Atlanta, GA, USA: Public Health Service U.S. Department of Health and Human Services; 2012. Toxicological profile for cadmium; pp. 1–487. [PubMed] [Google Scholar]
- 12.Cadmium exposure and cognitive abilities and behavior at 10 years of age: a prospective cohort study. Gustin K, Tofail F, Vahter M, Kippler M. Environ Int. 2018;113:259–268. doi: 10.1016/j.envint.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Effects of cadmium on Bcl-2/Bax expression ratio in rat cortex brain and hippocampus. Mahdavi S, Khodarahmi P, Roodbari NH. Hum Exp Toxicol. 2018;37:321–328. doi: 10.1177/0960327117703687. [DOI] [PubMed] [Google Scholar]
- 14.Cadmium toxicity and treatment: an update. Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. Caspian J Intern Med. 2017;8:135–145. doi: 10.22088/cjim.8.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadmium and zinc in polluted mining soils and uptake by plants. Casado M, Anawar A, Garcia-Sanchez A, et al. Int J Environ Poll. 2008;33:146–159. [Google Scholar]
- 16.Chronic exposure to lead and cadmium in residents living near a zinc smelter. Jo H, Kim G, Chang J, Lee K, Lee C, Lee B. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drinking water exposure to cadmium, an environmental contaminant, results in the exacerbation of autoimmune disease in the murine model. Leffel EK, Wolf C, Poklis A and White Jr KL. Toxicology. 2003;188:233–250. doi: 10.1016/s0300-483x(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 18.Soil contamination with cadmium, consequences and remediation using organic amendments. Khan MA, Khan S, Khan A, Alam M. Sci Total Environ. 2017;601-602:1591–1605. doi: 10.1016/j.scitotenv.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Disease-related responses induced by cadmium in an in vitro human airway tissue model. Xiong R, Wu Q, Trbojevich R, et al. Toxicol Lett. 2019;303:16–27. doi: 10.1016/j.toxlet.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Cadmium exposure in living organisms: a short review. Zhang H, Reynolds M. Sci Total Environ. 2019;678:761–767. doi: 10.1016/j.scitotenv.2019.04.395. [DOI] [PubMed] [Google Scholar]
- 21.Cadmium in tobacco smokers: a neglected link to lung disease? Ganguly K, Levänen B, Palmberg L, Åkesson A, Lindén A. Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0122-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serum heavy metals and obstructive lung disease: results from the National Health and Nutrition Examination Survey. Rokadia HK, Agarwal S. Chest. 2013;143:388–397. doi: 10.1378/chest.12-0595. [DOI] [PubMed] [Google Scholar]
- 23.Extracellular cadmium in the bronchoalveolar space of long-term tobacco smokers with and without COPD and its association with inflammation. Sundblad BM, Ji J, Levänen B, et al. Int J Chron Obstruct Pulmon Dis. 2016;11:1005–1013. doi: 10.2147/COPD.S105234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The influence of various organic amendments on the bioavailability and plant uptake of cadmium present in mine-degraded soil. Khan MA, Ding X, Khan S, Brusseau ML, Khan A, Nawab J. Sci Total Environ. 2018;636:810–817. doi: 10.1016/j.scitotenv.2018.04.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Impact of cadmium pollution on food safety and human health. Suhani I, Sahab S, Srivastava V, et al. https://doi.org/10.1016/j.cotox.2021.04.004 Current Opinion. 2021;27:1–7. [Google Scholar]
- 26.Neurobehavioural effects of occupational exposure to cadmium: a cross sectional epidemiological study. Viaene MK, Masschelein R, Leenders J, De Groof M, Swerts LJ, Roels HA. Occup Environ Med. 2000;57:19–27. doi: 10.1136/oem.57.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadmium and exposure to stress increase aggressive behavior. Terçariol SG, Almeida AA and Godinho AF. Environmental Pharmacology. 2011;32:40–45. doi: 10.1016/j.etap.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Cadmium neurotoxicity. Méndez-Armenta M and Ríos C. Envir Toxico Pharma. 2007;3:350–358. doi: 10.1016/j.etap.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Effects of prenatal exposure to cadmium on neurodevelopment of infants in Shandong, China. Wang Y, Chen L, Gao Y, et al. Environ Pollut. 2016;211:67–73. doi: 10.1016/j.envpol.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 30.Heavy metals' effect on susceptibility to attention-deficit/hyperactivity disorder: implication of lead, cadmium, and antimony. Lee MJ, Chou MC, Chou WJ, Huang CW, Kuo HC, Lee SY, Wang LJ. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15061221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadmium exposure impairs cognition and olfactory memory in male C57BL/6 mice. Wang H, Zhang L, Abel GM, Storm DR, Xia Z. https://doi.org/10.1093/toxsci/kfx202. Toxicol Sci. 2018;161:87–102. doi: 10.1093/toxsci/kfx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Effect of cadmium pollution on neuromorphology and function of brain in mice offspring. Kaoud HA and Mekawy MM. http://www.sciencepub.net/nature/ns0904/05_4933ns0904_28_35.pdf Natu Sci. 2011;9:28–35. [Google Scholar]
- 33.Effects of low levels of cadmium and lead on cognitive functioning in children. Thatcher RW, Lester ML, McAlaster R, Horst R. Arch Environ Health. 1982;37:159–166. doi: 10.1080/00039896.1982.10667557. [DOI] [PubMed] [Google Scholar]
- 34.The effects of cadmium toxicity. Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadmium exposure and neurodevelopmental outcomes in U.S. children. Ciesielski T, Weuve J, Bellinger DC, Schwartz J, Lanphear B, Wright RO. https://doi.org/10.1289/ehp.1104152. Environ Health Perspect. 2012;120:758–763. doi: 10.1289/ehp.1104152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Karri V, Schuhmacher M, Kumar V. Environ Toxicol Pharmacol. 2016;48:203–213. doi: 10.1016/j.etap.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Early-life cadmium exposure and child development in 5-year-old girls and boys: a cohort study in rural Bangladesh. Kippler M, Tofail F, Hamadani JD, Gardner RM, Grantham-McGregor SM, Bottai M, Vahter M. Environ Health Perspect. 2012;120:1462–1468. doi: 10.1289/ehp.1104431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Effect of chronic administration of cadmium on anxiety-like, depression-like and memory deficits in male and female rats: possible involvement of oxidative stress mechanism. Lamtai M, Chaibat J, Chaibat J, et al. J Behav Br Sci. 2018;8:240–268. [Google Scholar]
- 39.Cadmium: a possible etiological factor in peripheral polyneuropathy. Viaene MK, Roels HA, Leenders J, De Groof M, Swerts LJ, Lison D, Masschelein R. https://pubmed.ncbi.nlm.nih.gov/10091854/ Neurotoxicology. 1999;20:7–16. [PubMed] [Google Scholar]
- 40.The blood-brain barrier in health and disease: important unanswered questions. Profaci CP, Munji RN, Pulido RS, Daneman R. J Exp Med. 2020;217 doi: 10.1084/jem.20190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Back to nucleus: combating with cadmium toxicity using Nrf2 signaling pathway as a promising therapeutic target. Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian S. Biol Trace Elem Res. 2020;197:52–62. doi: 10.1007/s12011-019-01980-4. [DOI] [PubMed] [Google Scholar]
- 42.Cadmium-induced oxidative stress: focus on the central nervous system. Branca JJ, Fiorillo C, Carrino D, et al. Antioxidants (Basel) 2020;9:6. doi: 10.3390/antiox9060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Immunological targeting of the endothelial barrier antigen (EBA) in vivo leads to opening of the blood-brain barrier. Ghabriel MN, Zhu C, Hermanis G, Allt G. Brain Res. 2000;878:127–135. doi: 10.1016/s0006-8993(00)02721-9. [DOI] [PubMed] [Google Scholar]
- 44.Cadmium-induced neurotoxicity: still much ado. Branca JJ, Morucci G, Pacini A. Neural Regen Res. 2018;13:1879–1882. doi: 10.4103/1673-5374.239434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Effects of cadmium on ZO-1 tight junction integrity of the blood brain barrier. Branca JJ, Maresca M, Morucci G, et al. Int J Mol Sci. 2019;20:23. doi: 10.3390/ijms20236010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Front Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blood-brain barrier: from physiology to disease and back. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. https://doi.org/10.1152/physrev.00050.2017. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fine structural localization of a blood-brain barrier to exogenous peroxidase. Reese TS, Karnovsky MJ. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The blood-brain barrier to proteins under normal and pathological conditions. Brightman MW, Klatzo I, Olsson Y, et al. J Neuro Sci. 1970;10:215–239. doi: 10.1016/0022-510x(70)90151-6. [DOI] [PubMed] [Google Scholar]
- 50.Development of the blood-brain barrier: a historical point of view. Ribatti D, Nico B, Crivellato E, Artico M. Anat Rec B New Anat. 2006;289:3–8. doi: 10.1002/ar.b.20087. [DOI] [PubMed] [Google Scholar]
- 51.The blood-brain barrier. Daneman R, Prat A. Cold Spring Harb Perspect Biol. 2015;7:0. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Kadry H, Noorani B, Cucullo L. Fluids Barriers CNS. 2020;17:69. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. Liu WY, Wang ZB, Zhang LC, Wei X, Li L. CNS Neurosci Ther. 2012;18:609–615. doi: 10.1111/j.1755-5949.2012.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Tissue Barriers. 2016;4:0. doi: 10.1080/21688370.2016.1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tight junctions of the blood-brain barrier: development, composition, and regulation. Wolburg H, Lippoldt A (2002. Vasc Pharma. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 56.Development and cell biology of the blood-brain barrier. Langen UH, Ayloo S, Gu C. Annu Rev Cell Dev Biol. 2019;35:591–613. doi: 10.1146/annurev-cellbio-100617-062608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Development and function of the blood-brain barrier in the context of metabolic control. Haddad-Tóvolli R, Dragano NR, Ramalho AF, Velloso LA. https://doi.org/10.3389/fnins.2017.00224. Front Neurosci. 2017;11:224. doi: 10.3389/fnins.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail-chick transplantation chimeras. Stewart PA, Wiley MJ. 1981. Devel Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 59.Development of blood-brain barrier tight junctions in the rat cortex. Kniesel U, Risau W and Wolburg H. Bra Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- 60.The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Villabona-Rueda A, Erice C, Pardo CA, Stins MF. https://doi.org/10.3389/fncel.2019.00405. Front Cell Neurosci. 2019;13:405. doi: 10.3389/fncel.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Development, maintenance and disruption of the blood-brain barrier. Obermeier B, Daneman R, Ransohoff RM. https://doi.org/10.1038/nm.3407. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Immunolocalization of tight junction proteins in the adult and developing human brain. Virgintino D, Errede M, Robertson D, et al. Histochem Cell Biol. 2004;122:51–59. doi: 10.1007/s00418-004-0665-1. [DOI] [PubMed] [Google Scholar]
- 63.Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Semin Cell Dev Biol. 2015;38:16–25. doi: 10.1016/j.semcdb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 64.The blood-brain barrier/neurovascular unit in health and disease. Hawkins BT, Davis TP. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 65.Albert Coons: harnessing the power of antibody. Arthur G. Lancet. 2016;4:181–182. doi: 10.1016/S2213-2600(16)00020-5. [DOI] [PubMed] [Google Scholar]
- 66.The blood-brain barrier in systemic infection and inflammation. Galea I. Cell Mol Immunol. 2021;18:2489–2501. doi: 10.1038/s41423-021-00757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Zheng PP, Romme E, van der Spek PJ, Dirven CM, Willemsen R, Kros JM. Ann Neurol. 2010;68:835–844. doi: 10.1002/ana.22318. [DOI] [PubMed] [Google Scholar]
- 68.The cell biology of the blood-brain barrier. Rubin LL, Staddon JM. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 69.Bacterial vivisection: how fluorescence-based imaging techniques shed a light on the inner workings of bacteria. Cambré A, Aertsen A. https://doi.org/10.1128/MMBR.00008-20. Microbiol Mol Biol Rev. 2020;84 doi: 10.1128/MMBR.00008-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.The blood-brain barrier in health and disease. Daneman R. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 71.Tight junction proteins at the blood-brain barrier: far more than claudin-5. Berndt P, Winkler L, Cording J, et al. Cell Mol Life Sci. 2019;76:1987–2002. doi: 10.1007/s00018-019-03030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blood-brain barrier protein recognized by monoclonal antibody. Sternberger NH, Sternberger LA. https://doi.org/10.1073/pnas.84.22.8169. Proc Natl Acad Sci USA. 1987;84:8169–8173. doi: 10.1073/pnas.84.22.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Continuous cultures of fused cells secreting antibody of predefined specificity. Köhler G, Milstein C. https://doi.org/10.1038/256495a0. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 74.Loss of endothelial barrier antigen immunoreactivity in rat retinal microvessels is correlated with Clostridium perfringens type D epsilon toxin-induced damage to the blood-retinal barrier. Mander KA, Finnie JW. J Comp Pathol. 2018;158:51–55. doi: 10.1016/j.jcpa.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Immunocytochemical study of a vascular barrier antigen in the developing rat brain. Ibiwoye Ibiwoye, MO Sibbons PD, Howard CV, van Velzen D. J Comp Path. 1994;111:43–53. doi: 10.1016/s0021-9975(05)80110-0. [DOI] [PubMed] [Google Scholar]
- 76.Association of acute, high-dose cadmium exposure with alterations in vascular endothelial barrier antigen expression and astrocyte morphology in the developing rat central nervous system. Ibiwoye MO, Matthews Q, Travers K, Foster JD. J Comp Pathol. 2019;172:37–47. doi: 10.1016/j.jcpa.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Immunocytochemical expression of the endothelial barrier antigen (EBA) during brain angiogenesis. Rosenstein JM, Krum JM, Sternberger LA, Pulley MT, Sternberger NH. Developmental Brain Research. 1992;66:47–54. doi: 10.1016/0165-3806(92)90138-m. [DOI] [PubMed] [Google Scholar]
- 78.Quantitative analysis of microvascular alterations in traumatic brain injury by endothelial barrier antigen immunohistochemistry. Lin B, Ginsberg MD, Zhao W, Alonso OF, Belayev L, Busto R. J Neurotrauma. 2001;18:389–397. doi: 10.1089/089771501750170958. [DOI] [PubMed] [Google Scholar]
- 79.Clostridium perfringens prototoxin-induced alteration of endothelial barrier antigen (EBA) immunoreactivity at the blood-brain barrier (BBB) Zhu C, Ghabriel MN, Blumbergs PC, et al. Exp Neurol. 2001;169:72–82. doi: 10.1006/exnr.2001.7652. [DOI] [PubMed] [Google Scholar]
- 80.Expression of endothelial barrier antigen immunoreactivity in blood vessels following compression trauma to rat spinal cord: temporal evolution and relation to the degree of the impact. Perdiki M, Farooque M, Holtz A, Li GL, Olsson Y. Acta Neuropathol. 1998;96:8–12. doi: 10.1007/s004010050854. [DOI] [PubMed] [Google Scholar]
- 81.Endothelial barrier antigen-immunoreactivity is conversely associated with blood-brain barrier dysfunction after embolic stroke in rats. Pelz J, Härtig W, Weise C, et al. Eur J Histochem. 2013;57:0. doi: 10.4081/ejh.2013.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cell surface endothelial proteins altered in experimental allergic encephalomyelitis. Sternberger NH, Sternberger LA, Kies MW, Shear CR. Journal of Neuroimmunology. 1989;21:241–248. doi: 10.1016/0165-5728(89)90180-x. [DOI] [PubMed] [Google Scholar]
- 83.Barrier mechanisms in the developing brain. Saunders NR, Liddelow SA, Dziegielewska KM. https://doi.org/10.3389/fphar.2012.00046. Front Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Protective effects of Fragaria ananassa methanolic extract in a rat model of cadmium chloride-induced neurotoxicity. Elkhadragy MF, Kassab RB, Metwally D, et al. https://doi.org/10.1042/BSR20180861. Biosci Rep. 2018;38 doi: 10.1042/BSR20180861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Andjelkovic M, Buha Djordjevic A, Antonijevic E, et al. Int J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prenatal exposure to cadmium during organogenesis impairs memory in young rats. Gumilar F, Bras C, Aggio P, et al. https://doi.org/10.1177/1091581819850579. Int J Toxicol. 2019;38:312–318. doi: 10.1177/1091581819850579. [DOI] [PubMed] [Google Scholar]
- 87.Characterization of developmental neurotoxicity of As, Cd, and Pb mixture: synergistic action of metal mixture in glial and neuronal functions. Rai A, Maurya SK, Khare P, et al. Toxico Sci. 2010;118:586–560. doi: 10.1093/toxsci/kfq266. [DOI] [PubMed] [Google Scholar]
- 88.The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Abdel Moneim AE, Bauomy AA, Diab MM, Shata MT, Al-Olayan EM, El-Khadragy MF. Biol Trace Elem Res. 2014;160:392–399. doi: 10.1007/s12011-014-0066-9. [DOI] [PubMed] [Google Scholar]
- 89.Immunomodulatory effects of estradiol and cadmium in adult female rats. Pillet S, D'Elia M, Bernier J, Bouquegneau JM, Fournier M, Cyr DG. Toxicol Sci. 2006;92:423–432. doi: 10.1093/toxsci/kfl005. [DOI] [PubMed] [Google Scholar]
- 90.Heterogeneity in the rat brain vasculature revealed by quantitative confocal analysis of endothelial barrier antigen and P-glycoprotein expression. Saubaméa B, Cochois-Guégan V, Cisternino S, Scherrmann JM. J Cereb Blood Flow Metab. 2012;32:81–92. doi: 10.1038/jcbfm.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Differential expression of an endothelial barrier antigen between the CNS and the PNS. Lawrenson JG, Ghabriel MN, Reid AR, et al. https://www.semanticscholar.org/paper/Differential-expression-of-an-endothelial-barrier-Lawrenson-Ghabriel/303375373eb7c06bb2df72c69b3e13a15c3d3c94. J Anat. 1995;186:217–221. [PMC free article] [PubMed] [Google Scholar]
- 92.The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Dkhil MA, Al-Quraishy S, Diab MM, Othman MS, Aref AM, Abdel Moneim AE. Food Chem Toxicol. 2014;74:98–106. doi: 10.1016/j.fct.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 93.The brain anatomy of attention-deficit/hyperactivity disorder in young adults - a magnetic resonance imaging study. Gehricke JG, Kruggel F, Thampipop T, Alejo SD, Tatos E, Fallon J, Muftuler LT. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0175433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cadmium sulfide-induced toxicity in the cortex and cerebellum: In vitro and in vivo studies. Varmazyari A, Taghizadehghalehjoughi A, Sevim C, et al. Toxicol Rep. 2020;7:637–648. doi: 10.1016/j.toxrep.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cadmium exposure promotes activation of cerebrum and cerebellum ferroptosis and necrosis in swine. Luan P, Sun Y, Zhu Y, Qiao S, Hu G, Liu Q, Zhang Z. Ecotoxicol Environ Saf. 2021;224:112650. doi: 10.1016/j.ecoenv.2021.112650. [DOI] [PubMed] [Google Scholar]
- 96.Increased blood vessel density and endothelial cell proliferation in multiple sclerosis cerebral white matter. Holley JE, Newcombe J, Whatmore JL, Gutowski NJ. Neurosci Lett. 2010;470:65–70. doi: 10.1016/j.neulet.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 97.Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Farshad Nassiri, Michael D Cusimano, Bernd W Scheithauer, et al. https://ar.iiarjournals.org/content/31/6/2283.long. Anti Res. 2011;6:2283–2290. [PubMed] [Google Scholar]
- 98.The efficiency of systematic sampling in stereology and its prediction. Gundersen HJ, Jensen EB. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 99.Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Gundersen HJ, Bendtsen TF, Korbo L, et al. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 100.A quantitative immunocytochemical analysis of total surface area of blood-brain barrier in developing rat brain. Sibbons PD, Aylward GL, Howard CV, et al. Comparative Haematology International. 1996;6:214–220. [Google Scholar]
- 101.The expression of endothelial barrier antigen (EBA) and S100B in the rat parietal cortex following brain irradiation. Jin X, Chen C, Liang B, Zhang H, Zhang Z. Brai Res. 2014;1558:84–89. doi: 10.1016/j.brainres.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 102.Endocrine cells in the ileum of patients with irritable bowel syndrome. El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. World J Gastroenterol. 2014;20:2383–2391. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Youssef M, Atsak P, Cardenas J, Kosmidis S, Leonardo ED, Dranovsky A. Sci Rep. 2019;9:4120. doi: 10.1038/s41598-019-40868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cadmium-induced neurotoxic effects on rat basal forebrain cholinergic system through thyroid hormones disruption. Sola E, Moyano P, Flores A, et al. Environ Toxicol Pharmacol. 2022;90:103791. doi: 10.1016/j.etap.2021.103791. [DOI] [PubMed] [Google Scholar]
- 105.Enhanced zinc intake protects against oxidative stress and its consequences in the brain: a study in an in vivo rat model of cadmium exposure. Brzóska MM, Kozłowska M, Rogalska J, Gałażyn-Sidorczuk M, Roszczenko A, Smereczański NM. Nutrients. 2021;13 doi: 10.3390/nu13020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Developmental neurotoxicity of cadmium on enzyme activities of crucial offspring rat brain regions. Stolakis V, Tsakiris S, Kalafatakis K, et al. Biometals. 2013;26:1013–1021. doi: 10.1007/s10534-013-9678-3. [DOI] [PubMed] [Google Scholar]
- 107.Neurochemical changes in newborn rat's brain after gestational cadmium and lead exposure. Antonio MT, I Corpas I and Leret ML. Toxi Let. 1999;104:1–9. doi: 10.1016/s0378-4274(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 108.Neurotoxic effects of gestational administration of low-dose lead acetate. Antonio MT, Martínez S, Leret ML, et al. Journal of Applied Toxicology. 1998;16(5):431–436. doi: 10.1002/(SICI)1099-1263(199609)16:5<431::AID-JAT372>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 109.Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: modulation by zinc, calcium and L-cysteine co-administration. Carageorgiou H, Tzotzes V, Sideris A, Zarros A, Tsakiris S. Basic Clin Pharmacol Toxicol. 2005;97:320–324. doi: 10.1111/j.1742-7843.2005.pto_174.x. [DOI] [PubMed] [Google Scholar]
- 110.The laboratory rat: relating its age with human's. Sengupta P. https://pubmed.ncbi.nlm.nih.gov/23930179/ Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 111.Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Prog Neurobiol. 2013;106-107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]