Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) remain problematic due to high mortality rates and lack of effective treatments. Neutrophilic injury contributes to mortality in ALI/ARDS. Here, we developed and evaluated technology for rapid ARDS intervention, where intravenous salicylic acid-based polymer microparticles, i.e., Poly-Aspirin (Poly-A), interfere with neutrophils in blood, reducing lung neutrophil infiltration and injury in vivo in mouse models of ALI/ARDS. Importantly, Poly-A particles reduced multiple inflammatory cytokines in the airway and bacterial load in the bloodstream in a live bacteria lung infection model of ARDS, drastically improving survival. We observed that phagocytosis of the Poly-A microparticles, with salicylic acid in the polymer backbone, altered the neutrophil surface expression of adhesion molecules, potentially contributing to their added therapeutic benefits. Given the proven safety profile of the microparticle degradation products – salicylic acid and adipic acid – we anticipate the Poly-A particles represent a therapeutic strategy in ARDS with a rare opportunity for rapid clinical translation.
Keywords: Inflammation, Neutrophils, Lung Injury, Microparticles, Phagocytosis
Graphical Abstract
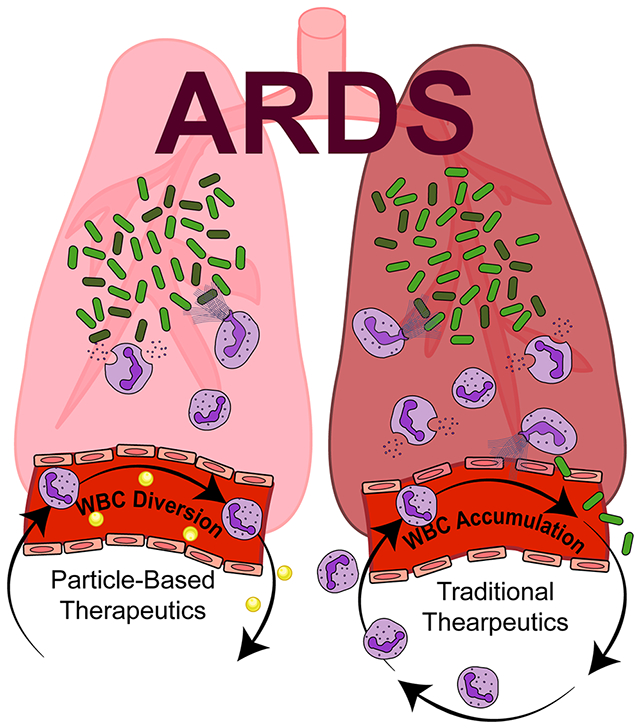
In this work, polymeric microparticles were fabricated from a salicylate-based polymer (Poly-A) and investigated as a therapeutic for acute inflammation of the lungs. This particle formulation performed exceptionally well in diverting neutrophils away from the lungs, ultimately reducing inflammatory-based damage. Furthermore, Poly-A particles were observed to change the neutrophil surface expression of inflammatory molecules.
Introduction
Acute lung injury (ALI) is a rapidly progressing inflammatory disease characterized by the disruption of the lung endothelial and epithelial barriers, leading to impaired lung function and mortality in most cases. Despite advances in treatment, ALI and its more severe form, acute respiratory distress syndrome (ARDS), still exhibit a mortality rate of ~40%, establishing the need for new treatment methods and therapeutics.[1, 2] Part of the difficulty with developing reliable therapeutics for ARDS lies in the various conditions that lead to ARDS, including pneumonia, systemic bacterial infection, severe burns, or viral infections.
Regardless of the primary cause, ARDS is characterized by an unrestrained innate inflammatory response, where a high count of neutrophils in the lung bronchoalveolar lavage fluid (BALF) in ARDS patients strongly correlates with disease severity and mortality.[3–7] As inflammatory stimuli are detected, circulating neutrophils migrate to the site of inflammation rapidly via cell adhesion molecules (CAMs), e.g., E-Selectin, P-Selectin, ICAM-1, and VCAM-1, expressed by endothelial cells (ECs) lining the lumen of blood vessels.[8–10] Under typical circumstances, this response is used to combat infections and other insults efficiently. However, if the innate immune response becomes unregulated due to the so-called “cytokine storm,” the massive influx of transmigrating neutrophils damages the lung endothelium and epithelium. The compromised lung barriers result in a “leaky” alveolar-capillary membrane through which fluids, proteins, and pathogens can migrate, exacerbating the disease.[4] Additionally, once present in the alveolar space, neutrophils release cytotoxic materials such as reactive oxygen species (ROS) and neutrophil elastase, both known to contribute to the severity of ARDS.[11–15]
While the immune system’s over-stimulation plays a significant role in ARDS, no therapeutics exist to treat the immune-mediated damage.[16] A potentially promising therapeutic approach is the use of agents that directly block immune cells (i.e., neutrophils) that are the source of the cytokines and cytotoxic agents in ARDS. Indeed, several studies have shown significant therapeutic benefits to depleting neutrophils in ARDS in mice via anti-Ly6G antibodies.[3, 17, 18] However, the antibody-based neutrophil depletion approach is unlikely for disease treatment given that human neutrophils lack a unique marker similar to Ly6G.[19] Here, we propose a particle-based approach to blocking neutrophils in ALI/ARDS based on our prior work finding that polystyrene (PS) microparticles interacted with neutrophils in the bloodstream and interfered with their vascular wall adhesion both in vitro and in vivo.[20, 21] We hypothesized that biodegradable polymeric particles could be deployed to prevent neutrophil lung accumulation in ALI/ARDS, mitigating inflammatory damage and facilitating disease resolution.
To test this hypothesis, we fabricated spheres (~1 μm diameter) of either a salicylate-based poly(anhydride-ester) polymer, termed PolyAspirin(“Poly-A”)[22] or the gold-standard poly(lactic-co-glycolic) (PLGA) polymer and evaluated their potential to treat inflammatory lung injury in mice with sterile (chemical) lung injury and mice with active bacterial infection-induced injury. While neutrophil involvement is similar for both models, the active bacterial infection substantially alters the functional requirements of a therapeutic to quell the inflammation, offering a complicated disease progression reminiscent of the human occurrence of ALI/ARDS. We found that intravenous (IV) treatment with Poly-A particles was optimal, significantly reducing lung neutrophil accumulation and lung injury in both endotoxin-based and bacterial murine models of ALI/ARDS. We demonstrated via in vitro assays with human blood that the salicylic acid in the particle backbone tempered neutrophil activation, likely contributing to disease resolution.
Results
Therapeutic Impact of Poly-A Particles in an LPS Model of Acute Lung Injury
The structure of the salicylate-based poly(anhydride-ester) polymer (Poly-A) used in this work is shown in Fig. S1, where “R” represents adipic acid linker that is generally recognized as safe (GRAS) by the FDA.[23] We achieved Poly-A spheres of ~ 1 μm diameter using the traditional single-emulsion fabrication process. We confirmed the particles have a smooth surface and undergo hydrolytic degradation to release salicylic acid for over two weeks (Fig. S1).[24] The Poly-A size choice is based on our prior work showing that particles in the 1-3 μm range have high margination to the vascular wall in vivo in a mouse model of vascular inflammation, as well as have maximum impact in preventing neutrophil adhesion in human blood flow in an in vitro model simulating the shear rate of the arterial blood flow.[20, 25]
We next tested our particles’ impact in a simple lung injury mouse model, where intratracheally-delivered lipopolysaccharide (LPS), or “endotoxins” found in the outer membrane of Gram-negative bacteria, induces mild epithelial injury and neutrophil infiltrates in the airways.[26] Mice (C57BL/6J) with lung injury were treated via IV injection with 2 x 108 per mouse of either Poly-A, polystyrene, or PLGA particles, with PLGA representing a standard, biodegradable particle control. All particles had similar size, surface charge, and surface chemistry (carboxyl groups) (Table S1). Based on Fig. S2 and as described in the supplemental methods, we chose to explore two times for particle IV injection: 2 and 4 hours post-LPS administration, as depicted in Fig. 1A–B. Treated mice were euthanized, and BALF was collected at 2 hr after particle injection.
Figure 1. Characterization of total BALF cells and neutrophils in the LPS ALI model in C57BL/6J mice with particle injections.
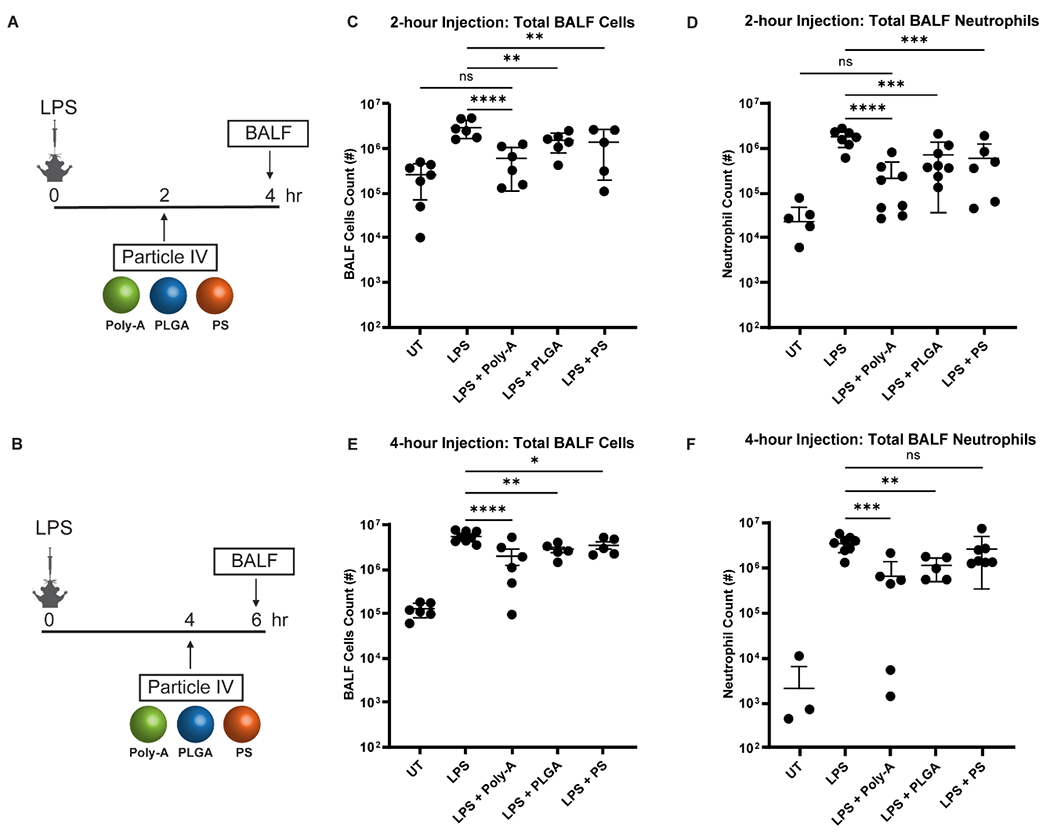
(A-B) Dosing/harvest schedule for the 2-hour and 4-hour injections. (C) Total BALF cells and (D) neutrophils for the 2-hour injection time experiments for untreated (UT) mice, LPS-only, LPS + Poly-A (PA) particles, LPS + Poly lactic-co-glycolic acid (PLGA) particles, and LPS + polystyrene (PS) particles. (E) Total BALF cells and (F) neutrophils for the 4-hour injection time experiments. All particles were injected at 2 x 108 per mouse. Statistical analysis was performed using GraphPad Prism Software using One-Way ANOVA with Fisher’s LSD test with a 95% confidence interval. Asterisks indicate p values of: * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001. n.s. indicates “not significant.” N ≥ 5 for all assays unless otherwise stated.
The particle IV injection, across all particle types, at 2 hours post-LPS treatment significantly reduced the total leukocytes in the BALF, directly linked to a reduction in the neutrophil count, as shown in Fig. 1C–D. However, the Poly-A particle treatment resulted in the most significant decrease in total neutrophils and BALF cells. See significance testing between each group in the supplement (Table S2). Specifically, the Poly-A particles reduced the BALF neutrophils by ~87% versus ~60% for the PLGA and polystyrene particle treatments. This significant neutrophil reduction led to 80% fewer total cells in the BALF for Poly-A versus only ~50% for the PLGA and polystyrene treatments. Notably, only the Poly-A particle treatment reduced the total BALF cells to a level statistically insignificant versus the untreated (UT) healthy mice for the 2-hour injection scheme (Fig. 1C), reflecting the Poly-A particles’ more considerable reduction of BALF neutrophils (Fig. 1D), which typically represent less than 2% immune cells present in the airways in healthy lung (Fig. S2E).
The Poly-A and PLGA particle IV injections at 4-hour post-LPS also significantly reduced the total BALF cells by ~50% (for PLGA) to ~64% (for Poly-A) and neutrophils by 68% (for PLGA) to 84% (for Poly-A) relative to LPS mice with no particle treatment (Fig 1E–F). The 4-hour post-LPS polystyrene particle treatment failed to significantly reduce total BALF neutrophils and only modestly decrease total BALF cells.
Analysis of the Poly-A and PLGA particle tissue distribution for the 2-hour injection shows that both particles were primarily trafficked to the liver and spleen with minimal particles found in the blood, lungs, heart, and kidneys by 30 minutes post-injection (Fig. S3). This distribution pattern is consistent with our prior work using non-PEGylated polystyrene particles of a similar size and numerous other studies.[21, 27, 28] We also evaluated Poly-A particles in BALB/cJ mice with lung injury and found result trends similar to those for C57BL6J mice (see Fig. S4) with the Poly-A treatment, resulting in a near-100% knockdown of neutrophil migration into the BALF in this model. This more dramatic Poly-A effect is expected in BALB/cJ mice due to more-rapid particle internalization than C57BL/6J mice, since the Poly-A particles’ efficacy relies on neutrophil-particle interactions.[21, 29, 30]
Impact of Poly-A Particles on Inflammation in P. aeruginosa Lung Infection Model
Next, we explored the impact of IV-administered particles in a Pseudomonas (P.) aeruginosa lung infection model that reproduces many of the histological and immunological features of ARDS in humans.[31] P. aeruginosa is the second-most common cause of pneumonia in hospitalized patients, often leading to lung injury with a 60–90% mortality rate in mechanically ventilated patients.[32] Thus, this bacterial infection model enables investigation of our particle therapeutics in treating inflammation associated with an active infection. Unlike the endotoxin ALI model, an over-active inflammatory response leads to vascular damage and systemic pathogen spread. We chose an IV injection time of 18 hours post-infection based on observed pattern of neutrophil lung influx in this model (Fig. S5), allowing neutrophil diversion before extensive accumulation in the lungs. The BALF was harvested 24 hours post-infection.
The 18-hour Poly-A injection significantly reduced the total BALF cells and neutrophils by ~50% relative to non-treated mice with P. aeruginosa infection. In contrast, PLGA and polystyrene particles did not significantly reduce the total BALF cells or neutrophils in the bacteria-induced ARDS (Fig 2B–C). Further, solubilized aspirin did not affect the total BALF cells or neutrophils in this model (as well as in the LPS-injury model – see Fig S6), emphasizing the particulate form is vital for therapeutic benefit.
Figure 2. Impact of Poly-A Particle Injection on P. aeruginosa lung infection in C57BL/6J mice.
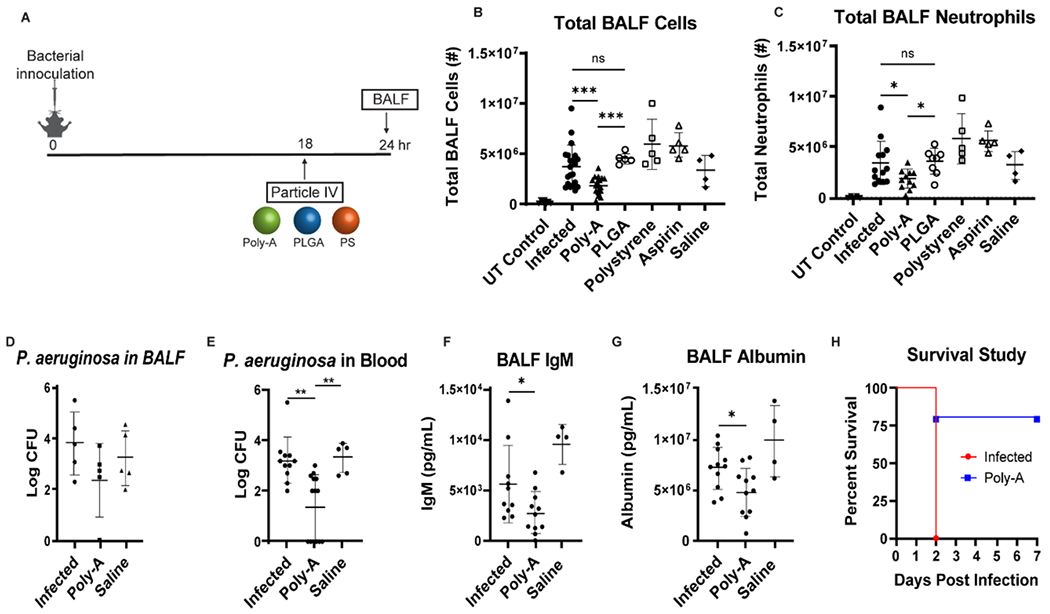
(A) Representation of the timeline of particle treatment and BALF sampling relative to the time of bacterial infection. (B) Total cells and (C) neutrophils in the BALF 24 hours post-infection after 18-hr injection of Poly-A, PLGA, polystyrene, or soluble aspirin compared to infected mice with no treatment and saline controls. UT control implies mice with no LPS or particle treatment. (D) Bacterial CFU in BALF collected at 24 hours post-infection for mice treated with Poly-A or saline at 18-hr post-infection. (E) Bacterial CFU in blood collected at 24 hours post-infection for mice treated with Poly-A or saline at 18-hr post-infection. (F) IgM and (G) albumin level in BALF collected at 24 hours post-infection for mice treated with Poly-A or saline at 18-hr post-infection. (H) Post-infection survival (N=5 for each group) for P. aeruginosa infected mice with and without 18-hour Poly-A injection. All mice received 2 x 108 particles for every particle type. Statistical analysis was performed using GraphPad Prism Software using One-Way ANOVA with Fisher’s LSD test with a 95% confidence interval. Asterisks indicate p values of: * = p < 0.05, ** = p < 0.01, *** = p < 0.001. N ≥ 5 for all assays unless otherwise stated.
Given that Poly-A particles were significantly more effective than PLGA and polystyrene particles, we focused the remainder of our studies on the former’s effects. Next, we assessed the bacterial colony-forming units (CFU) in the BALF and blood following the 18-hour Poly-A treatment. As shown in Fig. 2D, the Poly-A particles did not significantly reduce the bacterial CFU in the BALF. Conversely, Poly-A particles significantly reduced the bacterial CFU in the bloodstream by ~98% (Fig. 2E), indicating minimal damage to the endothelial and epithelial barriers that limit systemic bacterial dissemination. Indeed, the Poly-A particles reduced both the IgM (by ~51%) and albumin (by ~33%) levels in the BALF, which are large and lower molecular weight markers of lung endothelial and epithelial damage (Fig. 2 F–G), respectively.
Additionally, we performed survival studies for P. aeruginosa infected mice with or without an 18-hour Poly-A treatment. Crucially, we found that an 18-hour Poly-A particle injection resulted in a significant improvement in survival. Infected mice with no treatment died within 48 hours post-infection, while 80% of the mice receiving Poly-A injection lived out to one week (Fig. 2H). No bacterial CFU was detected in the blood or BALF samples of the surviving Poly-A treated mice, indicating recovery.
Next, we investigated whether the Poly-A particle treatment alters the BALF level of inflammatory markers typically predictive of human ARDS mortality.[16, 33–35] We used ELISAs to measure the levels of IL-10, KC (CXCL1), MCP1 (CCL2), MIP2 (CXCL2), TNF, and IL-6 in the BALF (Fig. 3). Poly-A particle injection significantly reduced the levels of KC, MCP1, TNF, and IL-6 by ~94%, ~81%, ~68%, and ~81%, respectively, as compared to infected controls. This result suggests that Poly-A particles’ administration and the corresponding reduction of neutrophils in the airways alleviates the lungs’ inflammatory response, both directly and indirectly, highlighting the Poly-A particles’ potential as an effective therapeutic for ARDS.
Figure 3. Cytokine and Protein Content in BALF of P. aeruginosa infected mice after 18-hour Poly-A injection, as measured by ELISA.
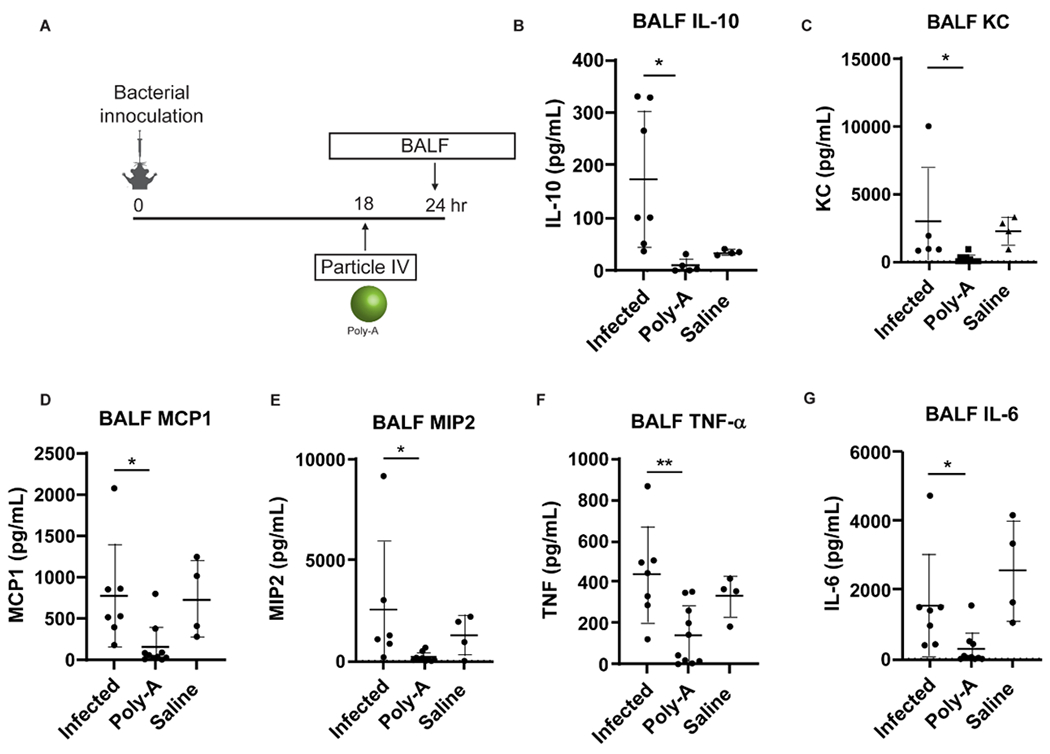
(A) Schematic of Poly-A particle treatment and BALF protein sampling relative to the timing of bacterial infection. The concentration of (B) IL-10, (C) KC, (D) MCP1, (E) MIP2, (F) TNF-a, and (G) IL-6 in the BALF of P. aeruginosa infected mice. Mice were injected with either 2 x 108 Poly-A particles or saline at 18 hours post-infection, and samples were collected 24 hours post-infection. Statistical analysis was performed using GraphPad Prism Software using One-Way ANOVA with Fisher’s LSD test with a 95% confidence interval. Asterisks indicate p values of: * = p < 0.05, ** = p < 0.01. N ≥ 5 for all assays unless otherwise stated.
Probing the Mechanism of Poly-A Particles’ Therapeutic Impact in ARDS
We next sought to probe the potential mechanistic underpinning for Poly-A’s therapeutic impact in ALI/ARDS. Given the similar size and surface characteristics (Table S1) of all particle types evaluated in the in vivo assays, we hypothesized that the Poly-A particle’s additional therapeutic benefit is due to added salicylic acid effects on neutrophils once internalized and rapidly degraded within the cells. Indeed, prior studies have shown the Poly-A polymer release active salicylic acid as it degrades.[22, 23, 36, 37]
Thus, we evaluated the surface-expression of crucial adhesion molecules associated with neutrophil activation in blood with or without Poly-A treatment and phagocytosis (Fig. 4A). We used human neutrophils as a well-developed protocol for their blood isolation exist and for the opportunity to gauge the potential clinical translation of Poly-A particles for human use. The particle uptake levels by neutrophils with or without prior LPS activation are shown in Fig. 4B. When exposed to LPS activation in whole human blood for 2 hr, we found that neutrophils exhibit a ~9-fold reduction in L-selectin expression (Fig. 4C; see Fig. S7 for the raw expression data). This L-selectin shedding by neutrophils is a known hallmark of inflammatory activation.[38–41] Conversely, when Poly-A particles were added to blood at 30 min after LPS treatment, we found that the L-selectin expression on particle-positive neutrophils is equal to that of cells in blood without LPS activation. That is, neutrophil uptake of Poly-A particles impairs the LPS-induced L-selectin shedding, suggesting that Poly-A particles have an anti-inflammatory impact on the cells.
Figure 4. Impact of Poly-A Particle Phagocytosis on surface protein expression by human neutrophils.
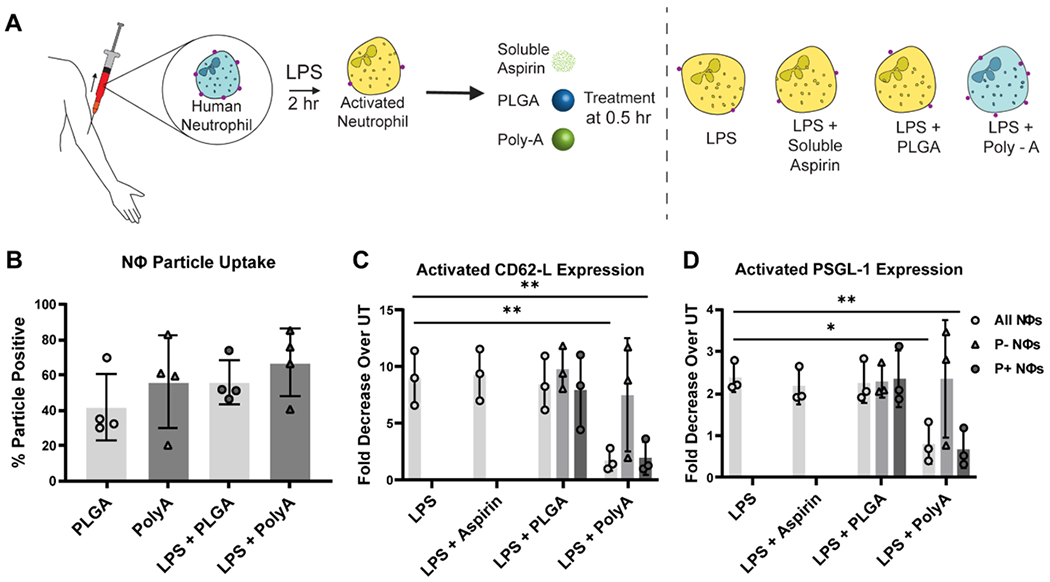
(A) Schematic depiction of L-selectin surface expression (red dots) by neutrophils in naïve or LPS-activated cells with or without particle treatment. (B) The fraction of neutrophils that internalized particles in whole blood. (C) Fold decrease in L-selectin (CD62-L) surface expression by neutrophils in whole blood exposed to LPS only, LPS + Aspirin, LPS + PLGA, and LPS + Poly-A particles relative to neutrophils in untreated (UT), non-LPS activated blood. (D) Fold decrease in PSGL-1. Each 100 μL blood sample received 2 x 106 particles for every particle type 30 min after LPS activation, and the protein expression was evaluated at 2 hr after LPS activation. Three human donors were used for this experiment, n=3. P- Nøs = Particle negative neutrophils; P+ Nøs = Particle positive neutrophils. Statistical analysis was performed using GraphPad Prism Software using One-Way ANOVA with Fisher’s LSD test with a 95% confidence interval. Asterisks indicate p values of: * = p < 0.05, ** = p < 0.01. N ≥ 3 for all assays unless otherwise stated.
Interestingly, neutrophils in the particle-treated blood that did not internalize Poly-A, ~ 20 to 40% of cells (Fig. 4B), exhibited the same L-selectin shedding as observed for cells in LPS-activated blood with no particle treatment (Fig 4C), suggesting phagocytosis as a prerequisite for the Poly-A anti-inflammatory effect. Conversely, PLGA particles did not impair the LPS-induced L-selectin shedding by neutrophils despite a similar PLGA uptake level as observed with Poly-A (Fig. 4C), suggesting that the salicylic acid backbone of the Poly-A particles is conferring this muted response to inflammatory stimulus. Again, the addition of soluble aspirin did not alter the LPS effect on the neutrophil L-selectin expression. PSGL-1 is another adhesion molecule reduced on neutrophils upon activation.[42] Accordingly, cells in LPS-activated blood experienced a ~2.5-fold decrease in PSGL-1. However, the addition of Poly-A particles to blood at 30 min after LPS-activation resulted in a slight increase in the PSGL-1 expression on cells that phagocytosed particles relative to untreated blood (Fig. 4D; see Fig. S7 for the raw expression data). Again, soluble aspirin or PLGA particle treatment did not protect neutrophils from the LPS-induced PSGL-1 neutrophil shedding. We speculate that when exposed to aspirin in solution, the neutrophils cannot accumulate a high enough concentration of salicylic acid internally to preserve L-selectin or PSGL-1 expression.
Validation of Mechanism for Poly-A Particles’ Therapeutic Impact In Vivo in ALI Mice
To determine if the in vitro Poly-A impact on neutrophils contributes to the particles’ therapeutic performance in vivo. We evaluated neutrophil counts, percentages, and protein expression in blood and the liver at 1-hour after particle treatment in ALI BALB/cJ mice. Based on our biodistribution data (Fig. S3), a majority of Poly-A positive neutrophils are expected to be in the liver at this time point. We find the neutrophil count in the blood in LPS-only mice was significantly higher than the count in untreated (UT) mice. Conversely, the neutrophil counts in blood for the LPS + particle treated mice were not significantly higher than the healthy control count, consistent with prior work showing particle treatment drives neutrophils out of blood in mice.[21] Compared to UT mice, LPS-only and LPS + Poly-A-treated mice had significantly greater neutrophil accumulation in the liver (Fig. 5C). PLGA particles were more likely to be phagocytosed than Poly-A particles, as shown by significantly higher uptake percentages in both compartments analyzed (Fig. S8). Despite this disparity, the overall particle positive neutrophil count/g liver tissue is the same for both particle types (Fig. 5D), suggesting that Poly-A particles drive higher neutrophil accumulation in the liver relative to PLGA (Fig. 5C). The liver, particularly hepatocytes, has a known role in clearance of apoptotic immune cells, which is a critical step towards inflammatory resolution in disease.[43–48] Thus, the larger liver neutrophil count observed with Poly-A particles matches their observed better therapeutic outcome in ALI/ARDS mice.
Figure 5. In vivo impact of Poly-A particles on neutrophil accumulation and surface protein expression 1-hour post-injection.
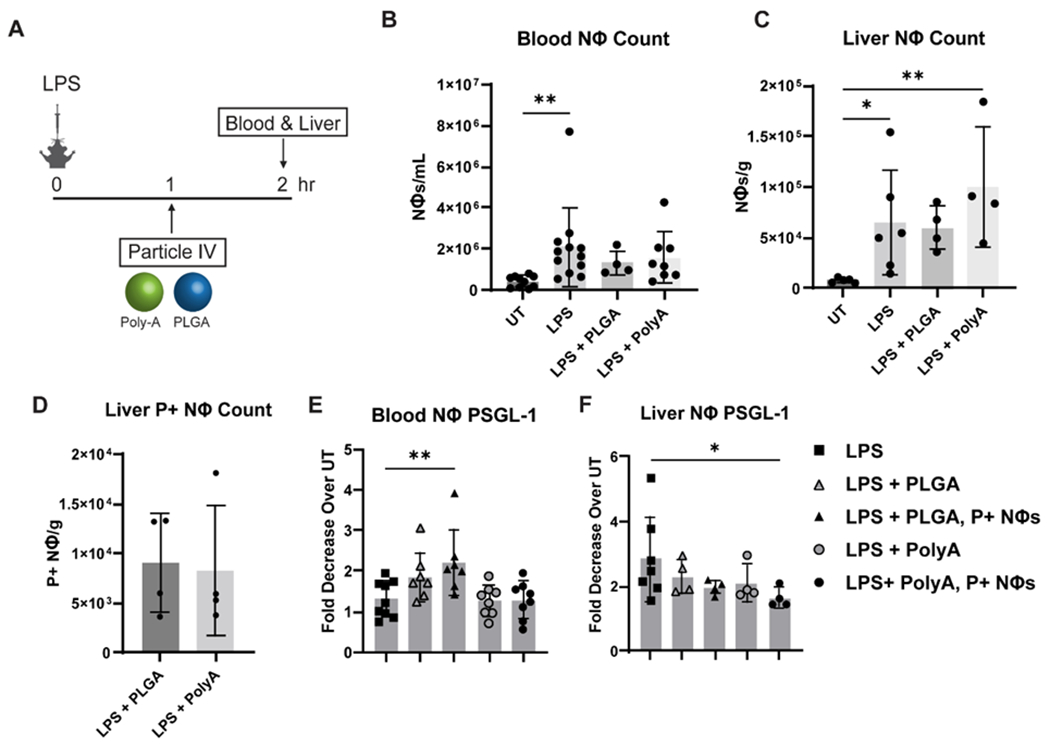
(A) Schematic showing experimental timing and set up. (B) Neutrophil concentration in blood 2-hours post LPS instillation and 1-hour post-injection. (C) Neutrophil accumulation in the liver post LPS instillations and particle injections. (D) Particle Positive neutrophil accumulation in the liver. Fold decrease in PSGL-1 expression compared to untreated neutrophils in the (E) blood and (F) neutrophils isolated from the liver. P+ Nøs = Particle positive neutrophils. Statistical analysis was performed using GraphPad Prism Software using One-Way ANOVA with Fisher’s LSD test with a 95% confidence interval. Asterisks indicate p values of: * = p < 0.05, ** = p < 0.01. N≥3 for this experiment.
Based on the human uptake data, both L-selectin and PSGL-1 expression are preserved post-Poly-A internalization. We found that this PSGL-1 preservation is present in neutrophils in vivo. In the liver, LPS-lung injury alone led to a 3-fold decrease in PSGL-1 expression, while Poly-A positive neutrophils in the liver exhibited only a 1.5-fold decrease (Fig 5F). In contrast to the in vitro assays, there was no change in L-selectin expression between treatment groups, including UT and LPS (Fig. S8), likely due to the additional processing required of mouse tissue samples, i.e., homogenization to obtain single cells, compared to the human blood samples.
Discussion
This study investigated the impact of intravenously-administered, Poly-A particles on inflammation in ALI/ARDS. We hypothesized that Poly-A particles would divert neutrophils from the inflammation site through physical interactions, allowing local tissue immunity to clear the primary source of injury. The Poly-A particle treatment resulted in a considerable reduction in BALF cells and neutrophils in mice with lung injury than the PLGA and polystyrene particles in both LPS- and P. aeruginosa-induced ALI/ARDS. The Poly-A particles were the only particle type to significantly reduce neutrophils infiltration into the lungs of mice infected with P. aeruginosa. Further, Poly-A particles significantly enhanced the survival of mice infected with P. aeruginosa and considerably reduced lung inflammatory cytokines and lung damage, as indicated by a reduction in IgM and albumin in the BALF.
We posit that Poly-A particles alleviate inflammation in ALI/ARDS via multiple mechanisms. For one, Poly-A particles prevent neutrophils from infiltrating the lung space and causing tissue damage. Per our previous studies, this particle-blocking of neutrophil migration is linked to particle-neutrophil collisions that lead to neutrophil phagocytosis of particles in the bloodstream and detour particle-laden cells from the inflammation site to the liver.[20, 21] It is known that neutrophils present in the airways in ARDS release cytokines, including TNFα, exaggerating the pro-inflammatory cycle by inducing airway immune cells to release additional cytokines and propagating lung damage.[49] Thus, the reduction in neutrophil lung infiltration, as we demonstrated, in itself is therapeutic, i.e., less cytokine secretion in the lungs directly (via physical removal of neutrophils) and indirectly (reduction of cytokines from neutrophils reduces cytokines from other cells) to significantly reduce lung damage as demonstrated via lower level of plasma proteins in the BALF of Poly-A treated mice (Fig. 2).[4, 7]
Additionally, Poly-A particles appear to exert added therapeutic benefits in ALI/ARDS relative to other particles. We suggest neutrophil activation is muted after uptake of Poly-A particles, as indicated by changes in crucial neutrophil cell adhesion molecules’ expression (Fig. 4). Several studies have shown that LPS stimulation induces rapid neutrophil shedding of L-selectin, making L-selectin shedding widely accepted as a sign of leukocyte activation in response to inflammation.[38–41, 50] Similarly, a few studies have reported that PSGL-1 expression is downregulated in human neutrophils in vivo in response to systemic inflammation caused by endotoxin infusion in healthy volunteers and in vitro by LPS in anticoagulated blood.[42] Thus, the observed preservation of L-selectin and PSGL-1 expression on neutrophils that phagocytosed Poly-A particles in LPS-activated human blood and PSGL-1 expression on neutrophils in vivo highlights an anti-inflammatory effect. Interestingly, the measurable impact of Poly-A on human neutrophils expressed L-selectin was not observed in mouse neutrophils in vivo (Fig. S8). Given that L-selectin expression can be readily perturbed, we suspect that the tissue dissociation process necessary to obtain single cells needed for flow cytometric analysis was enough to cause significant L-selectin shedding across all neutrophils in blood obtained from particle treated mice, limiting our ability to collect accurate measurements for in vivo assays.
A notable result is that soluble aspirin did not impact neutrophil lung localization and adhesion molecule expression both in vitro and in vivo, suggesting the importance of the Poly-A particle form. While others have reported aspirin sheds L-selectin expression on neutrophils, these studies were conducted with isolated human neutrophils and aspirin at non-physiologically relevant concentrations, up to 1000 μg/mL.[51, 52] Furthermore, aspirin itself was found not to be the culprit for L-selectin shedding.[53] Instead, nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase activation via aspirin at the plasma membrane induces superoxide anion production resulting in L-selectin downregulation.[53] Conversely, Poly-A particles are entirely phagocytosed by neutrophils into a phagosome. Unlike macrophages, neutrophil phagosomes experience significant alkalinization, pH of ~9, in the first 30 minutes after phagocytosis.[54] Given prior work demonstrating Poly-A polymer degradation is accelerated significantly in basic environments,[55] there is likely a burst of salicylic acid released within neutrophils immediately after Poly-A phagocytosis, leading to the observed modified inflammation response in neutrophils. We do not expect extracellular activity of Poly-A since Poly-A particles in solution in vitro did not release an appreciable amount of salicylic acid in the 2 hr period of the particle-blood assays. Indeed, we saw no change in neutrophils that did not uptake Poly-A in vitro.
Furthermore, given our previous work and biodistribution data showing that injected micron-sized particles are rapidly cleared from the circulation (~30 minutes) with very few particles moving into the lung tissue, we do not expect the non-targeted Poly-A particles to be significantly retained or extravasating and releasing salicylic acid into the lung tissue or the airspace, respectively.[21] Accordingly, we do not expect the Poly-A particles to directly impact lung interstitial and alveolar macrophages. Instead, this particle therapeutic aims to divert potentially inflammatory neutrophils away from the injured lung, while the salicylate backbone reduces downstream inflammation. Finally, it is possible that additional, yet to be determined, effects may be contributing to the therapeutic outcome.
An interesting finding in this study is that the use of intravenously-administered particles as a therapeutic for ALI/ARDS (and possibly other acute inflammatory conditions) depends heavily on the time of injection relative to the onset of inflammation, similar to other studies showing that the time of intervention in lung inflammation is crucial to treatment efficacy. For example, Kulkarni et al. found that depleting neutrophils before influenza infection significantly reduced survival in young mice, while depleting neutrophils six days after infection improved survival significantly for aged mice.[56] Given that the inflammatory response in human ARDS occurs on an extended timeline compared to mice, it is likely that the therapeutic window in humans is also much more expansive. Additionally, patient BALF assessment is a diagnostic method of ALI and lung injury severity in the clinics, suggesting an avenue for identifying patients for Poly-A particle therapeutic.[57] Thus, intervention via Poly-A particle injection would be possible at the point of clinical presentation of ARDS.[58, 59] Notably, mapping the Poly-A particle efficacy to the kinetics of neutrophil lung infiltration in both the LPS and P. aeruginosa ARDS model suggests these particles would be efficacious in ARDS regardless of the underlying cause, including in respiratory viral infections. Indeed, a few recent studies have reported elevated levels of neutrophils and neutrophil extracellular traps in the blood and lungs of COVID19 patients, which is linked to poor prognosis.[60–64]
As previously stated, the current standard of care for ARDS patients is mechanical ventilation for gas exchange.[16] However, in addition to ventilation not being curative, the process can further injure the lungs;[65] thus, the mortality rate of ARDS remains at 40% or higher.[1] The Poly-A microparticle-based neutrophil-blocking therapeutic approach for ARDS is promising in that it acts on a subset of cells involved in the triggering of cytokine storm in ARDS – neutrophils – directly. Conversely, prior single-molecule or cytokine blocking pharmaceutical approaches are likely plagued with vast redundancies in the inflammatory cascade,[4] with multiple adhesion molecules and cytokines involved in signaling neutrophil transmigration.[66] A significant concern for the neutrophil-blocking approach with Poly-A is the possibility of propagating the primary disease with reduced presence of neutrophils. Nevertheless, our results showed a positive outcome, i.e., significantly reduced lung injury and mortality, with the Poly-A particle treatment even with an active bacterial infection present in the lungs (Fig. 2).
However, this work is not without limitations. First, we did not fully optimize our Poly-A particle system to maximize therapeutic benefit, given that the focus of the current study is on developing the therapeutic strategy. Future work further iterating over various particle parameters (e.g., concentration, size, shape, and polymer chemistry) and dosing strategy (single versus multiple treatments) may shed more light on both the therapeutic mechanism and the optimal formulation for maximum effect, given the known impact of these parameters on particles interaction with immune cells.[20, 21, 67, 68] Further, we expect that the co-administration of Poly-A particles alongside drugs targeted to the primary cause of ARDS, e.g., antibiotics or antivirals, would result in an even greater therapeutic response. That is, the Poly-A particles could prevent neutrophil-associated lung damage while allowing antibiotics/antivirals to control infection.
The impact of Poly-A particles may also extend beyond neutrophils. For example, previous works have shown that COX-2 inhibitors such as aspirin decrease the formation of neutrophil-platelet aggregates in inflammation, which may decrease cytokine production by neutrophils.[69–71] To fully establish the therapeutic mechanism of Poly-A particles in ALI/ARDS, further ex vivo and in vivo experiments investigating the degradation characteristics of Poly-A are required. For one, it would be of interest in future work to track the level of salicylic acid present in the mouse blood, liver, and spleen over an extended period, expected to be released from Poly-A particles localized in the liver and spleen (Fig. S3) as they degrade over days to weeks (Fig. S1). Lastly, the bulk of the results here are based on murine models of ALI/ARDS, which though are useful first tools for developing potential therapies, lack in complexity relative to humans. All the same, our preliminary observation of an anti-inflammatory impact of Poly-A particles with human neutrophils and our prior work demonstrating a similar particle phagocytosis pattern in mouse and human neutrophils suggest the results obtained here are likely relevant in humans.[67]
Methods
Study Design
In this work, we hypothesized that the use of intravenously-injected, Poly-A particles would alleviate neutrophil influx and lung damage in ALI/ARDS, above and beyond the effect of polystyrene and PLGA particles. Specifically, we hypothesized that Poly-A particles would reduce neutrophil influx into the lungs in ALI/ARDS, reduce the inflammatory cytokines in the inflamed tissue, and assist in resolving the inflammation.
For all in vivo experiments, a minimum of N=5 animals was chosen for each experimental group. Unless otherwise stated, mice were wild-type C57BL/6J acquired from Jackson Laboratories, aged 4-6 weeks. More animals were included in some experiments/treatments due to experimental replicates and the need for contemporaneous controls.
The only criteria used for exclusion applied to control animals—for example, an LPS-treated mouse that did not exhibit an increase in BALF cells or neutrophils above untreated controls would be excluded under the assumption that the mouse did not receive a proper dose of LPS. Similarly, an untreated control mouse that exhibited a significantly increased number of BALF cells or neutrophils would be excluded under the assumption that the mouse had a pre-existing injury or condition of which we were unaware.
Blinding.
Briefly, one individual initiated the animal injury, and a second individual independently administered the particle treatment. Another investigator conducted the measurements of BALF cells and cytokine levels and was blinded to the intervention received by the different animal groups.
Study Approvals
Human blood from healthy donors was obtained via venipuncture per protocol approved by the University of Michigan Internal Review Board (IRB). Written informed consent was obtained from the individuals before the blood draw.
Animal studies were conducted following the National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan. All mice were housed under specific pathogen-free conditions and maintained in the University of Michigan in compliance with the University Committee on Use and Care of Animal (UCUCA) regulations.
Particle Preparation
Poly-A particles were prepared using a single emulsion solvent evaporation method. Briefly, 20 mg of Poly-A polymer was dissolved in 20 mL of dichloromethane. Then, 75 mL of 1% polyvinyl alcohol (PVA) solution was placed on a mixer at 4250 rpm. Poly-A solution was slowly injected into the PVA solution and allowed to mix for 2 hours. After mixing, the particle solution was allowed to settle for 45 minutes, and then 1 μm particles were isolated by centrifugation at 4000 rpm and washed 3x with DI water. Typically, this fabrication process yields approximately 10 mg dried particles. Particles were then lyophilized and stored at −40°C until use. Particles were suspended in deionized (DI) water, dried to a glass coverslip, and imaged via scanning electron microscopy (SEM) to determine particle size. Particles suspended in DI water were run on dynamic light scattering (DLS) to measure their surface charge. Histograms of the surface charge from DLS can be found in the supplement (Fig. S9). Finally, particle concentration was determined in solution via counting on a hemacytometer. Sonication was used to prevent particle agglomeration in solution.
For fluorescent particles, 50 mg of Poly-A was dissolved in 2 mL DMSO and combined with 22.8 mg N-(3-Dimethylaminopropyl)-N’-ethyl carbodiimide hydrochloride (EDC) dissolved in 1 mL DMSO. After 5 minutes of stirring, 13.7 mg of N-Hydroxysuccinimide (NHS) dissolved in 1 mL of DMSO was added to the Poly-A solution. The solution was rotated for 15 minutes, then 1.5 mg cy5.5 dissolved in 1 mL DMSO was added to the Poly-A solution and continued to rotate overnight. The resulting polymer was isolated with dialysis using a 3,500 molecular weight cut-off membrane in 1 liter of distilled water over two days, replacing the water every 8 hours. The isolated polymer was vacuum dried, and 2 mg of Poly-A conjugated to cy5.5 was combined with 18 mg unconjugated Poly-A for particle fabrication.
Particle Degradation
A total of 1 x 107 particles were suspended in 10mL of PBS without additives calcium and magnesium (PBS−/−) at pH 7.2 and placed under rotation at 37°C. Periodically, the particles were centrifuged, and the supernatant was removed and replaced. The supernatant was then added to a 96-well plate, and the fluorescence intensity was measured (ex = 315 nm and em = 408 nm for salicylic acid). The cumulative fluorescence intensity at each time point is plotted.
Particle Biodistribution
A total of 2 x 108 Cy5.5 conjugated particles were injected intravenously into C57BL/6J mice. Mice were euthanized 30 minutes post-particle injection via CO2 asphyxiation, and blood, lungs, livers, kidneys, and spleens were collected. Whole organs were scanned using an Odyssey CLx infrared imaging system (LI-COR). 100 μL of blood was plated in clear bottom 96-well plates and scanned as well. The percentage of total signal in each compartment was calculated and reported as % Injected Dose for each mouse.
LPS ALI Model
Male Balb/cJ or C57BL/6J mice were anesthetized briefly using isoflurane and given 50 uL of 0.4 mg/mL LPS orotracheally to induce inflammation. For the C57BL/6J mice, particles were injected either 2 hours or 4 hours post-instillation via a tail vein catheter at a clinically relevant dosage of 2 x 108 per mouse (~30 mg/kg). For the Balb/cJ mice, particles were injected at 1 hour post-LPS instillation. At 2 hours after particle injection, mice were euthanized via CO2 asphyxiation, and the lungs were lavaged 3x with PBS −/− to remove leukocytes present in the lungs for analysis. BALF cells were counted via hemocytometer in trypan blue, then samples were FC-blocked and stained with fluorescent antibodies for CD45, CD11b, and Ly6G. The white blood cells were fixed, and red blood cells were lysed with a 1-step fix/lyse (eBiosciences). Before flow cytometry analysis, the samples were washed with FACS buffer (2% FBS in PBS −/−, pH 7.4) at 500xG for 5 min. The samples were analyzed via flow cytometry to determine the percentage of neutrophils versus macrophages in the lungs. Neutrophils were differentiated from macrophages via positive expression of CD11b and Ly6G.
Bacterial ARDS Model
P. aeruginosa was grown overnight in Difco nutrient broth at 37°C under constant shaking. The concentration of bacteria in the broth was determined by measuring the absorbance at 600 nm, then plotting the OD on a standard curve generated by known CFU values. Mice were anesthetized with an intraperitoneal injection of ketamine/xylazine mixture and then given 30 μL of bacteria solution (15 μL in each nostril; 2 x 105 bacteria per mouse total) intranasally to induce lung infection. At 18 hours post-infection, mice were placed in a restrainer, and a catheter was inserted into the tail vein. Each mouse received 2 x 108 particles in 100 μL of injection volume or 200 μL of 3.75 mg/mL of aspirin in 10% DMSO in PBS−/−. The particle dosage is approximately equivalent to a dose of 30 mg/kg, and if degraded completely, is equivalent to a salicylic acid content of 0.75 mg aspirin. Twenty-four hours post-infection, mice were euthanized via CO2 overdose. After euthanasia, the chest cavity was exposed, and a cardiac puncture was used to collect blood from the mice. Additionally, the trachea was exposed and opened, and the lungs were lavaged with 3mL of PBS −/− to remove cells in the alveolar space.
BALF was centrifuged, and supernatants were saved at −80°C for ELISA to quantify inflammatory cytokines. The cell pellets were resuspended in 500 μL of RPMI media, then aliquots were diluted 1:1 with Turk Blood Diluting Fluid and counted via hemacytometer. Then, cytospin samples were prepared, and cells were stained to differentiate neutrophils from mononuclear cells. Blood and BAL were plated on auger plates and allowed to grow overnight at 37°C to determine CFUs. The blood was then centrifuged, and plasma was collected and stored at −80°C for ELISA analysis.
Human neutrophil phagocytosis assay and protein expression analysis
Heparinized whole blood was immediately aliquoted into 100 μL samples upon collection from human subjects. For LPS-treated samples, LPS was added to each sample at a final concentration of 1 μg/mL. All samples were incubated at 37°C for 30 min, then the respective aspirin or particle treatments were introduced. Soluble aspirin concentrations were determined based on the therapeutic plasma concentrations reported in the literature, 10 mg/dL. For particle-treated samples, each 100 μL sample received 2 x 106 particles. The samples were then incubated at 37°C for an additional 1.5 hrs. Post incubation, the samples were FC-blocked and stained with fluorescent antibodies for CD45, CD62-L, CD162, and CD11a. The white blood cells were fixed, and the red blood cells were lysed with a 1-step fix/lyse (eBiosciences). Before flow cytometry analysis, the samples were washed with FACS buffer (2% FBS in PBS −/−, pH 7.4) at 500xG for 5 min. Using an Attune flow cytometer, we isolated the neutrophil populations (CD45 positive and FSC/SSC) and measured mean fluorescent intensity (MFI). The MFI of each group was then divided by the untreated group, yielding fold change. Fold decrease was then calculated by taking the inverse of the normalized value.
In vivo mouse neutrophil tissue accumulation, harvesting, and protein expression measurements
Male Balb/cJ mice were anesthetized with isoflurane, and 20 mg LPS was orotracheally instilled. Particles were injected (2x108 particles/mouse) 1 -hour post LPS instillation. Mice were euthanized 1-hour post-injection via CO2 inhalation. Blood was collected via cardiac puncture, and one liver lobe was harvested. Blood samples were immediately placed on ice, FC blocked (TruStain FcX, BioLegend), stained, and lyse/fixed (eBioscience). Liver samples were rinsed with PBS −/− and placed into 5 mg/mL Type IV Collagenase in PBS −/−. Samples were chopped and incubated at 37°C for 1-hour total. Samples were mixed every 15 min to create a smooth mixture. Each sample was strained via a 70 μm strainer, and the resulting sample mass was weighed. Neutrophils were isolated using Lymphoprep (Cosmo Bio Usa Inc), FC blocked, stained, and fixed. Blood and liver samples were stained with CD45-BV711, CD11b-PE, Ly6G-BV605, PSGL1-BV421, and CD62L-PerCp (BioLegend). All samples were run on an Attune flow cytometer for analysis.
Statistical Analysis
All figures are plotted as scatter plots with standard deviation. Statistical analysis was performed using GraphPad Prism Software using One-Way ANOVA with Fisher’s LSD Test with a 95% confidence interval. Asterisks indicate p values of: * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001. n.s. indicates “not significant”.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation Graduate Research Fellowship Program (W.J.K., E.R.B.) and NIH R01 HL145709 (O.E.-A.).
Footnotes
Competing Interest Statement: A patent application based on the result described in this manuscript with the title of “Polymer Particle for Neutrophil Injury” was recently filed with the U.S. Patent Office (U.S. Provisional Application No: 62/870,879). O.E-A serves as a consultant for Asalyxa Bio, Inc.
Reference Cited
- 1.Bellani G, et al. , Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. Jama, 2016. 315(8): p. 788–800. [DOI] [PubMed] [Google Scholar]
- 2.Sinha P and Calfee CS, Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care, 2019. 25(1): p. 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham E, et al. , Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol, 2000. 279(6): p. L1137–45. [DOI] [PubMed] [Google Scholar]
- 4.Grommes J and Soehnlein O, Contribution of Neutrophils to Acute Lung Injury. Molecular Medicine, 2011. 17(3): p. 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthay MA, Eschenbacher WL, and Goetzl EJ, Elevated concentrations of leukotriene D4 in pulmonary edema fluid of patients with the adult respiratory distress syndrome. J Clin Immunol, 1984. 4(6): p. 479–83. [DOI] [PubMed] [Google Scholar]
- 6.Moraes TJ, Zurawska JH, and Downey GP, Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol, 2006. 13(1): p. 21–7. [DOI] [PubMed] [Google Scholar]
- 7.Williams AE and Chambers RC, The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol, 2014. 306(3): p. L217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aulakh GK, Neutrophils in the lung: “the first responders”. Cell and Tissue Research, 2018. 371(3): p. 577–588. [DOI] [PubMed] [Google Scholar]
- 9.Ley K, et al. , Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology, 2007. 7(9): p. 678–689. [DOI] [PubMed] [Google Scholar]
- 10.Sadik CD, Kim ND, and Luster AD, Neutrophils cascading their way to inflammation. Trends Immunol, 2011. 32(10): p. 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auten RL, Whorton MH, and Nicholas Mason S, Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed newborn rat lung. Am J Respir Cell Mol Biol, 2002. 26(4): p. 391–7. [DOI] [PubMed] [Google Scholar]
- 12.El Kebir D, et al. , Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res, 2008. 103(4): p. 352–9. [DOI] [PubMed] [Google Scholar]
- 13.Ginzberg HH, et al. , Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am J Physiol Gastrointest Liver Physiol, 2001. 281(3): p. G705–17. [DOI] [PubMed] [Google Scholar]
- 14.Lee CT, et al. , Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. The New England journal of medicine, 1981. 304(4): p. 192–196. [DOI] [PubMed] [Google Scholar]
- 15.Roper JM, et al. , In vivo exposure to hyperoxia induces DNA damage in a population of alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol, 2004. 286(5): p. L1045–54. [DOI] [PubMed] [Google Scholar]
- 16.Rawal G, Yadav S, and Kumar R, Acute Respiratory Distress Syndrome: An Update and Review. Journal of translational internal medicine, 2018. 6(2): p. 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chignard M and Balloy V, Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol, 2000. 279(6): p. L1083–90. [DOI] [PubMed] [Google Scholar]
- 18.Soehnlein O, et al. , Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur Respir J, 2008. 32(2): p. 405–12. [DOI] [PubMed] [Google Scholar]
- 19.Eruslanov EB, Singhal S, and Albelda SM, Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends Cancer, 2017. 3(2): p. 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley WJ, et al. , Model Particulate Drug Carriers Modulate Leukocyte Adhesion in Human Blood Flows. ACS Biomater Sci Eng, 2019. 5(12): p. 6530–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fromen CA, et al. , Neutrophil-Particle Interactions in Blood Circulation Drive Particle Clearance and Alter Neutrophil Responses in Acute Inflammation. ACS Nano, 2017. 11(11): p. 10797–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmeltzer RC, Schmalenberg KE, and Uhrich KE, Synthesis and Cytotoxicity of Salicylate-Based Poly(anhydride esters). Biomacromolecules, 2005. 6(1): p. 359–367. [DOI] [PubMed] [Google Scholar]
- 23.Rosario-Meléndez R, Ouimet MA, and Uhrich KE, Formulation of salicylate-based poly(anhydride-ester) microspheres for short- and long-term salicylic acid delivery. Polymer bulletin (Berlin, Germany), 2013. 70(1): p. 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-Giottonini KY, et al. , PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: effects of formulation parameters. RSC Advances, 2020. 10(8): p. 4218–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charoenphol P, et al. , Targeting therapeutics to the vascular wall in atherosclerosis–Carrier size matters. Atherosclerosis, 2011. 217(2): p. 364–370. [DOI] [PubMed] [Google Scholar]
- 26.Bastarache JA and Blackwell TS, Development of animal models for the acute respiratory distress syndrome. Dis Model Mech, 2009. 2(5-6): p. 218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferenz KB, et al. , Long-circulating poly(ethylene glycol)-coated poly(lactid-co-glycolid) microcapsules as potential carriers for intravenously administered drugs. J Microencapsul, 2013. 30(7): p. 632–42. [DOI] [PubMed] [Google Scholar]
- 28.Owens DE 3rd and Peppas NA, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm, 2006. 307(1): p. 93–102. [DOI] [PubMed] [Google Scholar]
- 29.Jones SW, et al. , Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J Clin Invest, 2013. 123(7): p. 3061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matute-Bello G, Frevert CW, and Martin TR, Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol, 2008. 295(3): p. L379–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu F.-s., et al. , Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. Journal of immunology (Baltimore, Md. : 1950), 2010. 185(2): p. 1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denis J-B, et al. , Multidrug-resistant Pseudomonas aeruginosa and mortality in mechanically ventilated ICU patients. American Journal of Infection Control, 2019. 47(9): p. 1059–1064. [DOI] [PubMed] [Google Scholar]
- 33.Butt Y, Kurdowska A, and Allen TC, Acute Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med, 2016. 140(4): p. 345–50. [DOI] [PubMed] [Google Scholar]
- 34.Kong SL, et al. , Elucidating the molecular physiopathology of acute respiratory distress syndrome in severe acute respiratory syndrome patients. Virus Res, 2009. 145(2): p. 260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockx B, et al. , Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol, 2009. 83(14): p. 7062–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell A, et al. , Development of a guided bone regeneration device using salicylic acid-poly(anhydride-ester) polymers and osteoconductive scaffolds. Journal of Biomedical Materials Research Part A, 2014. 102(3): p. 655–664. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell A, et al. , Use of salicylic acid polymers and bone morphogenetic protein-2 to promote bone regeneration in rabbit parietal bone defects. Journal of Bioactive and Compatible Polymers, 2015. 31(2): p. 140–151. [Google Scholar]
- 38.Borregaard N, et al. , Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leukoc Biol, 1994. 56(1): p. 80–7. [DOI] [PubMed] [Google Scholar]
- 39.Ivetic A, Hoskins Green HL, and Hart SJ, L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front Immunol, 2019. 10: p. 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soler-Rodriguez AM, et al. , Neutrophil activation by bacterial lipoprotein versus lipopolysaccharide: differential requirements for serum and CD14. J Immunol, 2000. 164(5): p. 2674–83. [DOI] [PubMed] [Google Scholar]
- 41.Walcheck B, et al. , Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature, 1996. 380(6576): p. 720–3. [DOI] [PubMed] [Google Scholar]
- 42.Marsik C, et al. , Endotoxin down-modulates P-selectin glycoprotein ligand-1 (PSGL-1, CD162) on neutrophils in humans. J Clin Immunol, 2004. 24(1): p. 62–5. [DOI] [PubMed] [Google Scholar]
- 43.Fox S, et al. , Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun, 2010. 2(3): p. 216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordenfelt P and Tapper H, Phagosome dynamics during phagocytosis by neutrophils. J Leukoc Biol, 2011. 90(2): p. 271–84. [DOI] [PubMed] [Google Scholar]
- 45.Fox S, et al. , Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. Journal of Innate Immunity, 2010. 2(3): p. 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordenfelt P and Tapper H, Phagosome dynamics during phagocytosis by neutrophils. Journal of Leukocyte Biology, 2011. 90(2): p. 271–284. [DOI] [PubMed] [Google Scholar]
- 47.Davies SP, Reynolds GM, and Stamataki Z, Clearance of Apoptotic Cells by Tissue Epithelia: A Putative Role for Hepatocytes in Liver Efferocytosis. Frontiers in Immunology, 2018. 9: p. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi J, et al. , Role of the liver in regulating numbers of circulating neutrophils. Blood, 2001. 98(4): p. 1226–1230. [DOI] [PubMed] [Google Scholar]
- 49.Potey PM, et al. , Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J Pathol, 2019. 247(5): p. 672–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada Y, et al. , Elevated serum L-selectin levels and abnormal regulation of L-selectin expression on leukocytes in atopic dermatitis: soluble L-selectin levels indicate disease severity. J Allergy Clin Immunol, 1999. 104(1): p. 163–8. [DOI] [PubMed] [Google Scholar]
- 51.Díaz-González F, et al. , Prevention of in vitro neutrophil-endothelial attachment through shedding of L-selectin by nonsteroidal antiinflammatory drugs. The Journal of Clinical Investigation, 1995. 95(4): p. 1756–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gómez-Gaviro MV, et al. , Down-regulation of L-selectin expression in neutrophils by nonsteroidal anti-inflammatory drugs: role of intracellular ATP concentration. Blood, 2000. 96(10): p. 3592–3600. [PubMed] [Google Scholar]
- 53.Domínguez-Luis M, et al. , Superoxide anion mediates the L-selectin down-regulation induced by non-steroidal anti-inflammatory drugs in human neutrophils. Biochemical Pharmacology, 2013. 85(2): p. 245–256. [DOI] [PubMed] [Google Scholar]
- 54.Levine AP, et al. , Alkalinity of neutrophil phagocytic vacuoles is modulated by HVCN1 and has consequences for myeloperoxidase activity. PloS one, 2015. 10(4): p. e0125906–e0125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erdmann L and Uhrich KE, Synthesis and degradation characteristics of salicylic acid-derived poly(anhydride-esters). Biomaterials, 2000. 21(19): p. 1941–1946. [DOI] [PubMed] [Google Scholar]
- 56.Kulkarni U, et al. , Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol, 2019. 12(2): p. 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Haro C, et al. , Patient-ventilator asynchronies during mechanical ventilation: current knowledge and research priorities. Intensive Care Medicine Experimental, 2019. 7(1): p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthay MA and Zimmerman GA, Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol, 2005. 33(4): p. 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matthay MA, et al. , Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med, 2003. 167(7): p. 1027–35. [DOI] [PubMed] [Google Scholar]
- 60.Huang C, et al. , Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020. 395(10223): p. 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagunas-Rangel FA, Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J Med Virol, 2020. 92(10): p. 1733–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D, et al. , Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA, 2020. 323(11): p. 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou F, et al. , Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 2020. 395(10229): p. 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuo Y, et al. , Neutrophil extracellular traps in COVID-19. JCI Insight, 2020. 5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ragaller M and Richter T, Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock, 2010. 3(1): p. 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doerschuk CM, Tasaka S, and Wang Q, CD11/CD18-dependent and -independent neutrophil emigration in the lungs: how do neutrophils know which route to take? Am J Respir Cell Mol Biol, 2000. 23(2): p. 133–6. [DOI] [PubMed] [Google Scholar]
- 67.Kelley WJ, et al. , PEGylation of model drug carriers enhances phagocytosis by primary human neutrophils. Acta biomaterialia, 2018. 79: p. 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Safari H, et al. , Neutrophils preferentially phagocytose elongated particles-An opportunity for selective targeting in acute inflammatory diseases. Science advances, 2020. 6(24): p. eaba1474–eaba1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura T, et al. , Suppressive effect of selective cyclooxygenase-2 inhibitor on cytokine release in human neutrophils. International immunopharmacology, 2003. 3(10-11): p. 1519–1528. [DOI] [PubMed] [Google Scholar]
- 70.Ortiz-Muñoz G, et al. , Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood, 2014. 124(17): p. 2625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Looney MR, et al. , Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. The Journal of Clinical Investigation, 2009. 119(11): p. 3450–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


