Abstract
Background:
To examine longitudinal neurocognitive decline among Latino, non-Latino Black, and non-Latino White people with HIV (PWH) and factors that might explain ethnic/racial disparities in neurocognitive decline.
Methods:
499 PWH (13.8% Latino, 42.7% Black, 43.5% White; baseline age: M=43.5) from the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study completed neurocognitive, neuromedical, and laboratory assessments every 6–12 months with up to five years of follow-up. Longitudinal neurocognitive change was determined via published regression-based norms. Survival analyses investigated the relationship between ethnicity/race and neurocognitive change, and baseline and time-dependent variables that might explain ethnic/racial disparities in neurocognitive decline, including socio-demographic, HIV-disease, medical, psychiatric, and substance use characteristics.
Results:
In Cox proportional hazard models, hazard ratios for neurocognitive decline were increased for Latino compared to White PWH (HR=2.25, 95% CI=1.35–3.73, p=0.002), and Latino compared to Black PWH (HR=1.86, 95% CI=1.14–3.04, p=0.013), with no significant differences between Black and White PWH (p=0.40). Comorbidities, including cardiometabolic factors and more severe neurocognitive comorbidity classification, accounted for 33.6% of the excess hazard for Latino compared to White PWH, decreasing the hazard ratio associated with Latino ethnicity (HR=1.83, 95% CI=1.06–3.16, p=0.03), but did not fully account for elevated risk of decline.
Conclusion:
Latino PWH may be at higher risk of early neurocognitive decline compared to Black and White PWH. Comorbidities accounted for some, but not all, of this increased risk among Latino PWH. Future research examining institutional, sociocultural, and biomedical factors, including structural discrimination and age-related biomarkers, may further explain the observed disparities.
Keywords: Hispanic Americans, African Americans, cognitive disorders, health status disparities
Introduction
With the advent of antiretroviral therapy (ART), increasing numbers of people with HIV (PWH) with reliable access to ART are reaching older age1. Despite treatment advances, HIV continues to be a major public health concern, especially among Latino/a/x/Hispanic (hereafter referred to as Latino) and non-Latino Black/African American (hereafter referred to as Black) communities in the United States. Latino and Black people have three and eight times the rate of HIV infection compared to non-Latino Whites (hereafter referred to as White)2. Latino and Black PWH also face systematic disadvantage at multiple points in the HIV disease treatment cascade compared to White PWH3, and show higher rates of morbidity and mortality4–6.
HIV-associated neurocognitive impairment (NCI) occurs in 20–50% of PWH7, is more prevalent among older PWH8, and is associated with compromised everyday functioning and early mortality9,10. Cross-sectional studies show elevated risk for NCI among some Latino11–14 and Black PWH15 compared to White PWH, and one longitudinal study of PWH identified Latino ethnicity as a predictor of neurocognitive decline16. Yet, the underlying reasons for these disparities remain unknown. Latino and Black PWH experience unequal access to HIV care, and tend to present with worse HIV disease burden and more medical comorbidities3,4,6, which may increase risk for neurocognitive decline. In a recent cross-sectional study, worse historical HIV disease burden (i.e., nadir CD4) partially explained neurocognitive disparities between Latino and White PWH14, but much of the difference remained unexplained. Yet, no studies to date have investigated ethnic/racial disparities in longitudinal neurocognitive decline among Latino and Black PWH. We aimed to examine: (1) longitudinal neurocognitive decline among Latino, Black, and White PWH; and (2) factors that might explain any observed disparities. We hypothesized that Latino and Black PWH may be at higher risk for neurocognitive decline than White PWH, and increased HIV disease burden and comorbidities would partially explain any ethnic/racial disparities observed.
Methods
Participants were enrolled in the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study, a NIH-funded longitudinal study of PWH at six academic medical centers across the U.S. located in San Diego, California; St. Louis, Missouri; New York, New York; Galveston, Texas; Baltimore, Maryland; and Seattle, Washington. Study visits took place every 6–12 months between 2002 and 2015, and included comprehensive, standardized neurocognitive, neuromedical, and psychiatric assessments, a blood draw, and lumbar puncture, which have been described elsewhere17. Participants were fluent in English, as ascertained by self-report and determined by study staff. Inclusion criteria for the current analyses were: (1) self-identification as Latino/a or Hispanic, non-Latino Black or African American, or non-Latino White; and (2) having neurocognitive data available over 3–7 study visits. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Neurocognitive assessment and change over time.
All participants completed a comprehensive neurocognitive test battery in English assessing seven domains: verbal fluency, attention/working memory, processing speed, learning, memory, executive function, and complex motor skills, which have been described previously18. To examine neurocognitive change over time, z scores were generated for each domain based on published normative data, and changes were averaged to create a summary regression-based change score (sRCS)19. These scores control for the effect of repeated testing (normal test–retest variability, practice effects, and statistical artifacts) and baseline neurocognitive function on test performance in the normative sample, and included demographic adjustments for age, education, sex, and ethnicity/race (non-Hispanic White compared to other ethnic/racial groups)19.
Consistent with previous analyses16, neurocognitive change was ascertained for each participant at each follow-up visit relative to baseline performance based on the distribution of the normative sample, with the top 5% of the sRCS classified as “improve,” the middle 90% as “stable,” and the bottom 5% as “decline.” Overall change status for each participant over the study period was determined as: “improve” (if a participant had at least one “improve” status and no “decline” status); “decline” (if a participant had at least one “decline” status and no “improve” status), and (c) “stable” (if a participant had “stable” status across all visits). Thus, participants classified as experiencing “decline” showed a notable neurocognitive decline at some point over follow-up and never showed improvement. Three participants out of an original sample of 502 had both “improve” and “decline” across study follow-up and were excluded.
Socio-demographics.
Demographic information was obtained via self-report and followed NIH guidelines, which note: Latino or Hispanic refers to a person of Mexican, Puerto Rican, Cuban, South or Central American, or other Spanish-speaking culture of origin/descent, regardless of race20. Latino or Hispanic participants were asked to indicate their country of origin/descent. A distinct variable, country of birth, was assessed by self-report, and dichotomized by those born in and outside of the 50 U.S. states. If a participant identified as both Latino and Black (e.g., Afro-Latino), or Latino and White, they were classified as Latino for current analyses.
Socioeconomic status (SES) was characterized by the Two Factor Hollingshead Index, a weighted average of years of education and current or longest held occupation 21. Hollingshead Index scores range from 11 to 69 with lower scores indicating higher SES. Reading level, considered a proxy for educational quality among English-speakers in the U.S., was assessed with the Wide Range Achievement Test (WRAT)22.
Neuromedical and laboratory.
Participants completed a structured neuromedical examination, including collection of blood, urine, and cerebrospinal fluid (CSF). HIV infection was determined via ELISA with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin, hepatitis C virus antibody, and CD4 T cells assays were performed. HIV RNA levels were measured in plasma and CSF by reverse transcriptase PCR (Roche Amplicor, v. 1.5, lower limit of quantitation 50 copies/mL). A central nervous system (CNS) Penetration Effectiveness (CPE) score was included as an estimate of the degree of CNS penetration of current ART regimen23,24. The Veterans Aging Cohort Study (VACS) Index, a composite marker of HIV disease severity including age, traditional HIV biomarkers, and comorbidity biomarkers, was calculated as described previously25. Higher VACS Index scores indicate worse health and the top tertile of scores was classified as “High VACS Index.”
Medical comorbidities assessed included hepatitis C virus (HCV), markers of cardiometabolic health, and neurocognitive comorbidity status. Cardiometabolic markers included body mass index (BMI), systolic and diastolic blood pressure, levels of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides, and self-report of diabetes, hypertension, and hyperlipidemia. Non-fasting levels of plasma total cholesterol, HDL, LDL, and triglycerides were collected, processed, and assayed by standard certified laboratory methods. Other biomarkers examined included: aspartate aminotransferase (AST) (a marker of liver damage)26; serum total protein (composite measure of albumin and globulin protein in blood); and hematocrit (percentage of oxygen-rich red blood cells in blood).
Neurocognitive comorbidity status was ascertained as described previously27, and based on self-reported history of non-HIV developmental, cerebrovascular, psychiatric, substance use, and neuromedical conditions that may confer risk for NCI. These neuromedical and neuropsychiatric comorbidities are common with PWH were categorized as incidental (normal/mild), contributing (moderate), or confounding (severe)17.
Psychiatric and substance use.
Current (last 30 days) and past histories of major depressive and substance use disorders were assessed using the Composite International Diagnostic Interview (CIDI)28. History of injection drug use was ascertained by self-report, and recent substance use was identified via urine toxicology on the day of testing.
Statistical Analysis
Baseline socio-demographic, HIV disease, medical comorbidity, psychiatric, and substance use characteristics were compared across Latino, Black, and White PWH using ANOVAs for continuous variables, and Chi-Square or Fisher’s exact tests for categorical variables. For variables which showed significant differences at p < 0.05 across ethnic/racial groups, pairwise comparisons were conducted with Tukey’s Honest Significant Difference (HSD) tests for continuous variables and Chi-Square or Fisher’s exact tests for categorical variables.
To examine differences in neurocognitive change status by ethnic/racial group (Aim 1), we used a Chi-Square test comparing Latino, Black, and White PWH by neurocognitive change group (decline, stable, improve). We examined time to neurocognitive decline (versus “no decline”) across Latino, Black, and White PWH with Kaplan-Meier survival curves and log-rank tests. To examine factors that might explain racial/ethnic differences in neurocognitive decline (Aim 2), we ran two Cox proportional hazard models. We examined hazard ratios for time to neurocognitive decline by ethnicity/race (Model 1). In addition to ethnicity/race, we included other relevant baseline and time-dependent predictors; predictors collected at multiple study visits were modelled as time-dependent (Model 2). To select these predictors, we first examined ethnic/racial group differences in all socio-demographic, HIV disease, medical, psychiatric, and substance use factors in Table 1. Predictors that differed by ethnic/racial group at p < 0.20 were entered into a stepwise regression model predicting time to neurocognitive decline, and we used Akaike Information Criterion (AIC) with backward elimination to reduce model predictors and select a final optimized model (Model 2). To quantify the contribution of predictors to ethnic/racial differences in neurocognitive decline in our final models, we calculated reductions in hazard ratios tied to race/ethnicity from Model 1 to Model 2, consistent with prior published studies of racial cognitive health disparities29. We did not consider baseline neurocognitive performance nor study site as predictors, given our cognitive change scores account for baseline performance, and study site was highly confounded with the distribution of ethnic/racial groups in the U.S.
Table 1.
Sample characteristics at baseline (N=499) by ethnic/racial group
| Characteristic | Latino | Blacka | Whitea | Group differences | Group Comparison |
|---|---|---|---|---|---|
| (n=69) | (n=213) | (n=217) | (p value) | ||
|
| |||||
| No. of visits | 5.5 (1.7) | 5.9 (1.6) | 5.6 (1.6) | 0.10 | – |
| Years of study follow-up | 2.7 (1.2) | 2.8 (1.0) | 2.8 (1.2) | 0.75 | – |
|
| |||||
| Socio-demographic | |||||
|
| |||||
| Age (years) | 42.4 (7.5) | 43.8 (7.7) | 43.6 (8.9) | 0.45 | |
| Sex (% men) | 79.7% | 68.1% | 90.3% | <0.001 | W>L, B |
| Education (years) | 12.6 (2.4) | 12.5 (2.5) | 13.7 (2.6) | <0.001 | W>L, B |
| Hollingshead (SES)d | 42.1 (10.7) | 44.8 (11.0) | 36.9 (11.8) | <0.001 | B,L>W |
| Reading level (WRAT) | 93.4 (12.7) | 85.5 (14.9) | 102.1 (11.3) | <0.001 | W>L>B |
|
| |||||
| HIV Disease | |||||
|
| |||||
| AIDS diagnosis | 69.6% | 61.0% | 53.9% | 0.06 | – |
| Duration of infection (years) | 11.0 [5, 16] | 10.7 [5, 14] | 10.1 [3, 15] | 0.50 | – |
| Current CD4b | 431 [274, 632] | 419 [264, 610] | 508 [337, 695] | 0.005 | W>B |
| Nadir CD4 b | 70.0 [21, 311] | 157 [26, 295] | 197 [73, 320] | 0.03 | W>L |
| On ART | 76.8% | 69.0% | 68.7% | 0.42 | – |
| % Detectable Plasma (on ART) | 55.8% | 42.5% | 33.1% | 0.01 | L>W |
| % Detectable CSF (on ART) | 18.0% | 17.3% | 8.9% | 0.08 | – |
| ART Regimen CPE | 8.06 (1.76) | 8.27 (2.16) | 8.03 (2.00) | 0.59 | – |
| Duration of all ART (months)b | 63.5 (47.1) | 53.4 (48.1) | 64.9 (57.8) | 0.20 | – |
| High VACS Index | 23.5% | 36.4% | 19.4% | <0.001 | B>W |
|
| |||||
| Medical | |||||
|
| |||||
| Hepatitis C virus | 14.5% | 43.2% | 19.8% | <0.001 | B>W, L |
| Body Mass Index (kg/m2) | 26.6 | 26.5 | 26.0 | 0.51 | – |
| Systolic Blood Pressure (mm Hg) | 124.4 | 124.0 | 124.6 | 0.93 | – |
| Diastolic Blood Pressure (mm Hg) | 76.8 | 79.0 | 76.7 | 0.06 | – |
| Total Cholesterol (mg/dL) | 183.7 | 173.3 | 181.4 | 0.06 | – |
| HDL (mg/dL) | 40.8 | 51.5 | 39.8 | <0.001 | B>L,W |
| LDL (mg/dL) | 99.2 | 89.7 | 100.8 | 0.004 | W,L>B |
| Triglycerides (mg/dL) | 228.7 | 164.1 | 238.8 | <0.001 | W,L>B |
| Diabetes | 7.3% | 12.7% | 7.4% | 0.14 | – |
| Hypertension | 18.8% | 18.8% | 15.7% | 0.65 | – |
| Hyperlipidemia | 7.3% | 5.6% | 14.3% | 0.008 | W>B,L |
| ASTc | 36.9 (21.4) | 42.1 (30.1) | 36.1 (28.9) | 0.08 | – |
| Serum total protein | 8.0 (0.8) | 8.3 (0.8) | 7.5 (0.7) | <0.001 | B>L>W |
| Hematocrit | 41.1 (4.66) | 40.2 (3.9) | 42.3 (4.4) | <0.001 | W>B |
| Neurocognitive Comorbidity Status (%) | 0.015 | B>W | |||
| Incidental (normal/mild) | 52.2% | 52.6% | 67.7% | ||
| Contributing (moderate) | 33.3% | 32.4% | 23.0% | ||
| Confounding (severe) | 14.5% | 15.0% | 9.22% | ||
|
| |||||
| Psychiatric and Substance Use | |||||
|
| |||||
| Current Major Depressive Disorder | 20.3% | 10.8% | 15.2% | 0.12 | – |
| Lifetime Major Depressive Disorder | 50.7% | 45.5% | 59.5% | 0.02 | W>B |
| Current Any Substance Use Disorder | 7.3% | 9.4% | 6.0% | 0.41 | – |
| Lifetime Alcohol Use Disorder | 49.3% | 54.0% | 56.7% | 0.56 | – |
| Lifetime Cannabis Use Disorder | 21.7% | 31.5% | 29.5% | 0.29 | – |
| Lifetime Meth Use Disorder | 15.9% | 1.9% | 28.6% | <0.001 | W>L>B |
| Lifetime Cocaine Use Disorder | 37.7% | 58.7% | 25.4% | <0.001 | B>L,W |
| Lifetime Opioid Use Disorder | 13.0% | 25.4% | 8.3% | <.0.001 | B>L,W |
| Injection drug use ever | 26.1% | 29.1% | 27.6% | 0.89 | – |
| Urine toxicology (% positive) | 11.8% | 30.5% | 6.0% | <0.001 | B>W,L |
Abbreviations: B, black; W, white; L, Latino; IQR, interquartile range; SES, socioeconomic status; WRAT, Wide Range Achievement Test; ART, antiretroviral therapy; CSF, cerebrospinal fluid; CPE, central nervous system penetration effectiveness score; AST, Aspartate aminotransferase; VACS, Veterans Aging Cohort Study Index Score; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Notes: Values shown as Mean (SD), Median [IQR], or %
non-Latino
square-root transformed
log10 transformation prior to comparison analysis
Lower scores indicate higher socioeconomic status (SES)
Results
Participants were 499 PWH (69 Latino, 213 Black, 217 White) with an average baseline age of 43.5 (range = 18–68), 79.4% were men, and 63.1% were gay or bisexual. Among Latinos, 43.5% were Mexican, 24.6% Puerto Rican, 7.2% South or Central American, 4.3% Cuban, and 20.3% of “other Spanish culture/origin,” and 85.5% of Latinos were born within the 50 U.S. states.
Sample characteristics by ethnicity/race
Table 1 shows baseline socio-demographic, HIV disease, medical, psychiatric and substance use characteristics by ethnic/racial group. White PWH were more likely to be men, had more years of education, and had higher SES than Latino and Black PWH. Reading level was highest among White, then Latino, followed by Black PWH. Regarding HIV disease characteristics, White PWH had higher current CD4 counts than Black PWH, and higher Nadir CD4 than Latino PWH. Among those on ART, Latino PWH had higher rates of detectable plasma HIV RNA than White PWH. Black PWH had higher VACS Index (indicative of worse health) than White PWH. Regarding medical comorbidities, Black PWH had higher rates of HCV, higher HDL cholesterol, lower LDL cholesterol, lower triglycerides, and higher serum total protein compared to Latino and White PWH. White PWH had higher rates of hyperlipidemia compared to Black and Latino PWH. Black PWH had lower hematocrit and worse neurocognitive comorbidity status (i.e. more serious comorbid non-HIV conditions, which confer increased risk for NCI) than White PWH. Regarding psychiatric and substance use characteristics, White PWH had higher rates of lifetime major depressive disorder than Black PWH, and higher lifetime methamphetamine use disorder than Latino and Black PWH. Black PWH had higher lifetime cocaine and opioid use disorder, and were more likely to have positive drug urine toxicology on the day of testing compared to White and Latino PWH.
Neurocognitive Change by ethnicity/race
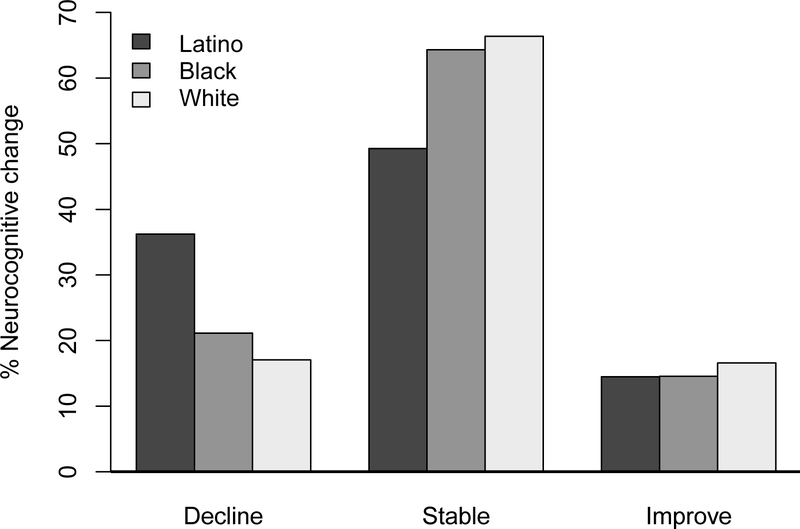
Over study follow-up, 107 participants (21.4%) declined, 315 (63.1%) remained stable, and 77 (15.4%) improved in neurocognitive status. Figure 1 shows the proportion of participants by ethnicity/race who declined, remained stable, or improved. The overall model showed significant group differences (χ2 = 11.84, df = 4, p = 0.019). In follow-up analyses, Latino PWH were at higher risk of neurocognitive decline (versus remaining stable or improving) compared to White (χ2 = 10.24, df = 1, p = 0.001) and Black (χ2 = 5.59, df = 1, p = 0.018) PWH, but the latter two groups did not differ (χ2 = 0.91, df = 1, p = 0.34). The proportion of participants who improved did not differ by ethnic/racial group (ps > 0.68).
Figure 1.
Neurocognitive change (decline, stable, improve) by ethnicity/race. The overall model showed significant ethnic/racial differences in the proportion of participants who declined, remained stable, or improved neurocognitively (χ2 = 11.84, df = 4, p = 0.019). Latino PWH were at higher risk of neurocognitive decline compared to White (χ2 = 10.24, df = 1, p = 0.001) and Black (χ2 = 5.59, df = 1, p = 0.018) PWH, while Black and White PWH did not differ (χ2 = 0.91, df = 1, p = 0.34).
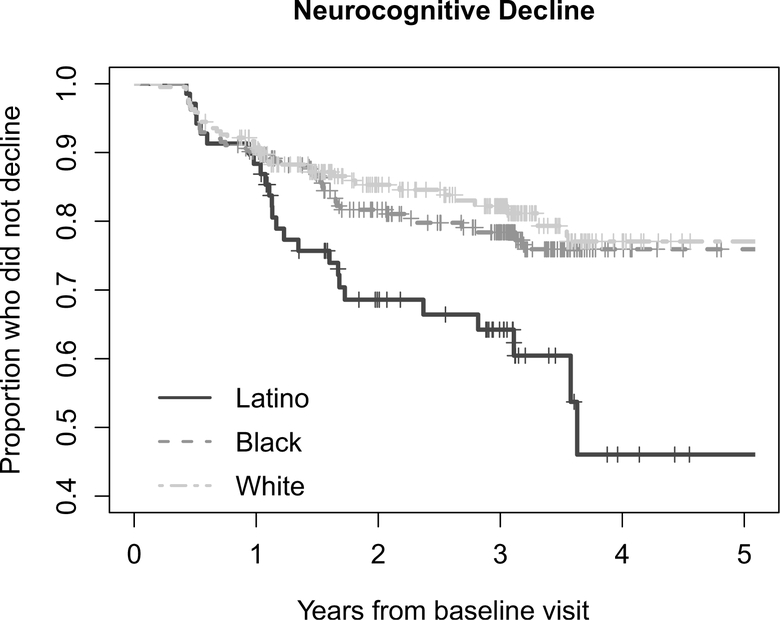
Figure 2 shows the pattern of decline by ethnicity/race over years of follow-up in a Kaplan-Meier survival analysis (p = 0.005). In pairwise log-rank tests, Latino PWH declined faster and had a higher proportion of neurocognitive decliners compared to White (HR = 2.24, 95% CI 1.24–4.05, p = 0.001) and Black (HR = 1.86, 95% CI 1.07–3.25, p = 0.01) PWH. Black and White PWH showed similar trajectories of neurocognitive decline over study follow-up (HR = 1.21, 95% CI 0.78–1.87, p = 0.40).
Figure 2.
Neurocognitive decline over study follow-up by ethnicity/race. In a Kaplan-Meier survival analysis, the pattern of decline over years of follow-up differed significantly by ethnicity/race (p = 0.005). In pairwise log-rank tests, Latino PWH declined faster and had a higher proportion of neurocognitive decliners compared to White (HR = 2.24, 95% CI 1.24–4.05, p = 0.001) and Black (HR = 1.86, 95% CI 1.07–3.25, p = 0.01) PWH. Black and White PWH showed similar trajectories of neurocognitive decline over study follow-up (HR = 1.21, 95% CI 0.78–1.87, p = 0.40).
Examination of factors driving ethnic/racial differences in neurocognitive decline
Multivariable survival analyses examined whether baseline and time-dependent predictors might explain ethnic/racial differences in neurocognitive decline between Latino and White PWH, and Latino and Black PWH (Table 2). In unadjusted analyses (Table 2, Model 1A), hazard ratios for neurocognitive decline were elevated for Latino compared to White PWH (HR = 2.25, 95% CI 1.35–3.73, p = 0.002). All socio-demographic, HIV disease, medical, psychiatric, and substance use factors in Table 1 that differed across racial/ethnic groups at p < .0.20 were included in a stepwise regression model predicting neurocognitive decline among Latino and White PWH. Backwards AIC selected baseline (neurocognitive comorbidity status), and time-dependent (hematocrit, total cholesterol, and triglycerides) factors as optimal predictors, which were added to the final Cox model (Table 2, Model 2A). Lower hematocrit (HR = 1.08, p = 0.006), confounding neurocognitive comorbidity status (HR = 3.16, p = 0.004; HR = 3.38, p < 0.001), and lower total cholesterol (HR=1.01, p=0.04) were associated with earlier time to neurocognitive decline. With inclusion of these predictors, the excess hazard for early neurocognitive decline among Latino PWH decreased by 33.6%, but the hazard ratio associated with Latino ethnicity remained significant (HR = 1.83, 95% CI 1.06–3.16, p = 0.03).
Table 2.
Cox proportional hazard models for time to neurocognitive decline comparing Latino and White people with HIV (Model A) and Latinos and Black people with HIV (Model B)
| Model A | Predictor | Reference | Risk | HR (95% CI) | P Value |
|
| |||||
| Model 1A | |||||
|
| |||||
| Ethnicity/race | White | Latino | 2.25 [1.35, 3.73] | 0.002 | |
|
| |||||
| Model 2A | |||||
|
| |||||
| Ethnicity/race | White | Latino | 1.83 [1.06, 3.16] | 0.03 | |
| Hematocritc | 1 unita | Lower | 1.08 [1.02, 1.14] | 0.006 | |
| Comorbidity | Contributing | Confounding | 3.16 [1.44, 6.93] | 0.004 | |
| Comorbidity | Incidental | Confounding | 3.38 [1.69, 6.73] | <0.001 | |
| Total Cholesterolc | 1 unita | Lower | 1.01 [1.00, 1.02] | 0.04 | |
| Triglyceridesc | 1 unitb | Higher | 1.00 [1.00, 1.03] | 0.07 | |
|
| |||||
| Model B | Predictor | Reference | Risk | HR (95% CI) | P Value |
|
| |||||
| Model 1B | |||||
|
| |||||
| Ethnicity/race | Black | Latino | 1.86 [1.14, 3.04] | 0.013 | |
|
| |||||
| Model 2B | |||||
|
| |||||
| Ethnicity/race | Black | Latino | 2.39 [1.34, 4.19] | 0.002 | |
| Nadir CD4 | 1 sqrtb | Higher | 1.04 [1.00, 1.07] | 0.06 | |
| Hematocritc | 1 unita | Lower | 1.08 [1.02, 1.14] | 0.009 | |
| Diabetesc | No | Yes | 1.69 [0.85, 3.34] | 0.13 | |
| Urine toxicologyc | Negative | Positive | 1.61 [0.91, 2.87] | 0.10 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; sqrt, square root transformation; CD4, cluster of differentiation 4; Comorbidity, Neurocognitive Comorbidity Status; LT Meth SUD, lifetime methamphetamine substance use disorder
Notes:
Higher
Lower
Variable modelled in a time-dependent manner
In unadjusted analyses (Table 2, Model 1B), hazard ratios for neurocognitive decline were elevated for Latino compared to Black PWH (HR = 1.86, 95% CI 1.14–3.04, p = 0.013). All factors in Table 1 that differed across racial/ethnic groups at p < .0.20 were included in a stepwise regression model predicting neurocognitive decline among Latino and Black PWH. Backwards AIC selected baseline (nadir CD4) and time-dependent (hematocrit, urine drug toxicology, and self-reported diabetes) factors as optimal predictors, which were added to the final Cox model (Table 2, Model 2B). Only lower hematocrit (HR = 1.08, p = 0.009) was significantly associated with earlier time to neurocognitive decline. With inclusion of these predictors, the elevated risk for early neurocognitive decline among Latino compared to Black PWH did not decrease, and remained significant (HR = 2.39, 95% CI 1.34–4.19, p = 0.002).
Discussion
Present findings suggest that Latino PWH have a higher risk of early neurocognitive decline compared to White and Black PWH. Medical comorbidities, such as markers of cardiometabolic health, explained a portion of these disparities among Latino compared to White PWH, but much of the elevated risk for neurocognitive decline among Latinos remained unexplained after considering a host of HIV disease, psychiatric, and substance use characteristics, and a limited number of available socio-demographic factors. Many variables that differed by ethnicity/race at baseline were not significant predictors of neurocognitive decline. Our analyses used published, regression-based norms that allowed us to detect neurocognitive change in individual participants, while controlling for baseline neurocognitive performance, demographics, and effects of repeated testing.
Study results are consistent with previous cross-sectional findings of higher rates of NCI among some Latino groups compared to Whites11,13,14. Our results add to this literature by demonstrating an ethnic/racial disparity in longitudinal neurocognitive decline among Latino PWH. In a previous longitudinal analysis of the CHARTER cohort, Latino ethnicity was a significant predictor of neurocognitive decline16, but this prior study did not include separate non-Latino White and Black comparison groups, did not examine factors that might partially explain neurocognitive disparities, and only included a small number of cardiometabolic factors, which are important predictors of neurocognitive decline in Latinos30. The present study extends prior research by showing that risk of neurocognitive decline is elevated in Latino PWH compared both to White and Black PWH. This study is the first to directly investigate differences in HIV-associated neurocognitive outcomes between Latino and Black PWH. Our findings showing increased risk of neurocognitive decline among Latino compared to Black PWH, and no significant differences between Black and White PWH are somewhat unexpected, given that both Latino and Black PWH face structural disadvantage in the HIV care, and present with worse HIV disease burden. One prior cross-sectional study found higher rates of HIV-associated NCI among Black compared to White PWH15, yet it assessed neurocognition via the International HIV Dementia Scale, which is a brief measure with no normative adjustments and limited sensitivity for detecting mild forms of HIV-associated NCI31. Further, these two studies’ findings are not necessarily inconsistent, ethnic/racial disparities observed cross-sectionally may not extend to differences in longitudinal neurocognitive decline.
We were unable to identify a set of factors that fully explained the increased risk for neurocognitive decline among Latino PWH compared to White and Black PWH after considering several characteristics traditionally considered relevant to HIV-associated NCI. Our models selected by the AIC method for maximal predictive accuracy found that many variables that differed by ethnic/racial group at baseline were not independent predictors of time to neurocognitive decline. Risk for neurocognitive decline among Latino PWH remained elevated in our final models, suggesting that unassessed variables may play an important role in ethnic/racial differences in HIV-associated neurocognitive trajectories. Interestingly, after inclusion of relevant predictors in our final models, elevated risk for neurocognitive decline was not reduced among Latino compared to Black PWH, but was reduced considerably when comparing Latino and White PWH. Significant predictors in this latter model (i.e., hematocrit [lower percentage of oxygen-carrying red blood cells], confounding neurocognitive comorbidity status [presence of more severe non-HIV risks for neurocognitive impairment], and total cholesterol) were markers of comorbid conditions in HIV, rather than traditional HIV disease indicators. This finding highlights the import of medical comorbidities, in particular cardiometabolic factors, in neurocognitive disparities between Latino and White PWH.
Several intersecting structural/institutional, sociocultural, and biomedical variables may be important to consider in future ethnic/racial neurocognitive disparities research. Our current assessment of sociocultural constructs was limited to SES and reading level. Furthermore, the psychometrics of the measures we used to assess these constructs have yet to be established among Latinos. Sociocultural variables (e.g. acculturation, language use, health behaviors) and environmental factors (e.g. exposure to air pollution and other neurotoxicants, limited access to quality healthcare) not assessed in our study may contribute to the observed differences in HIV-associated NCI among Latino PWH13,32,33. Furthermore, structural racism is known to be a root cause of racial/ethnic health inequities34, and experiences of overlapping discrimination due to ethnicity/race, immigration, sexual orientation, and HIV status may be compounded among Latino PWH in the U.S.35. Growing research shows combined discrimination predicts worse health outcomes among Latino PWH36, and that exposure to trauma and stress are particularly detrimental to neurocognition in PWH37.
Neighborhood and institutional-level factors have also been shown to contribute to higher rates of chronic diseases such as diabetes38 that are more common among Latino compared to White PWH4. Our study ascertained diabetes only by self-report, which may have underestimated actual rates of disease among Latino and Black PWH due to disparities in healthcare access, quality, and literacy4,39. Some of our measurements of other cardiometabolic risk indicators (i.e. non-fasting plasma measures of cholesterol, triglycerides) were notable predictors in our models comparing Latinos and Whites. Assessment of cardiometabolic and vascular risk factors via standard criteria40 is crucial to future research on neurocognitive decline in aging PWH, given their association with HIV-associated cognitive impairment40 and late-life dementias among Latinos41. Furthermore, given reports of accelerated neurocognitive aging in HIV42, including biomarkers linked to Alzheimer’s disease and HIV-related genetic factors associated with neurocognitive impairment43 may be relevant to future work examining ethnic/racial neurocognitive disparities in PWH.
Strengths of this study include a large, geographically and demographically diverse cohort of PWH. Our Latino sample reported varied countries of origin. Neurocognitive assessment of seven domains at each study visit was robust, and neurocognitive change over time was determined via regression-based norms that adjust for expected change based on an individual’s baseline performance. Assessment of HIV disease characteristics, and psychiatric and substance use comorbidities were detailed, standardized across sites and visits, and were modeled in a time-dependent manner.
Our study has several limitations. Participants were assessed from 2002–2015, and findings may not generalize to contemporary cohorts of PWH who acquired HIV after ART became widely available in the U.S. Of note, however, Latino and Black PWH continue to face systematic disadvantage in accessing ART3. Our findings may also be influenced by selection bias, such that participants who were able to participate were healthier than those who were not, thus underestimating physical and cognitive difficulties among PWH. The lack of an HIV-negative comparison group precludes us from fully ascribing these disparities to HIV-associated underlying mechanisms. Sample sizes for neurocognitive decliners across the three ethnic/racial groups were modest, and the sample size of Latinos was considerably smaller than the White and Black groups. Further, there were fewer normative neurocognitive adjustments for Latinos compared to White and Black groups. While previous work indicates that country of origin/descent may be an important factor in investigations of neurocognitive difference among Latinos14, our sample sizes of Latino sub-groups, as well as of Afro-Latinos, were too small to assess their impact of neurocognitive trajectories longitudinally. Further, study criteria limited the Latino cohort to fluent English speakers. Future studies with larger samples of Latino PWH that better reflect the sociocultural, linguistic, geographic, and racial diversity of this ethnic group are needed.
The present study is the first to examine longitudinal neurocognitive disparities among Latino, Black, and White PWH, and represents an important step towards identifying factors that might be driving these inequities. Our findings suggest that management of cardiometabolic health among Latino PWH may be one critical pathway to reduce the elevated risk of neurocognitive decline observed in this population. Ongoing work by our lab incorporating factors such educational opportunity, socioeconomic disadvantage, and dementia-related biomarkers may shed further light onto these health disparities. In the ART era, HIV-associated neurocognitive deficits continue to contribute to challenges with medication adherence, employment, mental health, and social functioning10. Investigation and mitigation of factors that increase risk for early neurocognitive decline among ethnically and racially diverse PWH is an important task, and may help diminish neurocognitive disparities.
Acknowledgements
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; Washington University, St. Louis; and is headquartered at the University of California, San Diego and includes: Directors: Robert K. Heaton, Ph.D., Scott L. Letendre, M.D.; Center Manager: Donald Franklin, Jr.; Coordinating Center: Brookie Best, Pharm.D., Debra Cookson, M.P.H, Clint Cushman, Matthew Dawson, Ronald J. Ellis, M.D., Ph.D., Christine Fennema Notestine, Ph.D., Sara Gianella Weibel, M.D., Igor Grant, M.D., Thomas D. Marcotte, Ph.D. Jennifer Marquie-Beck, M.P.H., Florin Vaida, Ph.D.; Johns Hopkins University Site: Ned Sacktor, M.D. (P.I.), Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.); University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Sher Storey, PA-C.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Mengesha Teshome, M.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Conflicts of Interest and Source of Funding: The CNS HIV Anti-Retroviral Therapy Effects Research was supported by NIH awards N01 MH22005, HHSN271201000036C, HHSN271201000030C and R01 MH107345. Other support include the following NIH grants: T32-DA031098 (C.W.-M.W., M.A.H.), T32 AA013525 (L.K.), K23MH105297 (M.J.M.), and P30AG059299 (M.J.M). The authors declare no conflicts of interest.
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. HIV Surveillance Report, 2018 (Updated). 2020.
- 3.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clinical infectious diseases. 2013;57(8):1164–1171. [DOI] [PubMed] [Google Scholar]
- 4.Buchacz K, Baker R, Palella F, et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antiviral Therapy. 2013;18(1):65–75. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings MK, Masters HL. Comorbidities and challenges affecting African Americans with HIV infection. Journal of the National Medical Association. 2008;100(12):1477–1481. [DOI] [PubMed] [Google Scholar]
- 6.Chen NE, Gallant JE, Page KR. A systematic review of HIV/AIDS survival and delayed diagnosis among Hispanics in the United States. Journal of Immigrant and Minority Health. 2012;14(1):65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iudicello JE, Hussain M, Watson C, et al. HIV-associated neurocognitive disorders. Oxford Handbook of Adult Cognitive Disorders. 2019:29–60. [Google Scholar]
- 8.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychology review. 2009;19(2):186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casaletto KB, Weber E, Iudicello JE, Woods SP. Real-world impact of HIV-associated neurocognitive impairment. In: Changes in the Brain. Springer; 2017:211–245. [Google Scholar]
- 11.Rivera-Mindt M, Miranda C, Arentoft A, et al. Aging and HIV/AIDS: neurocognitive implications for older HIV-positive Latina/o adults. Behavioral Medicine. 2014;40(3):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojna V, Skolasky RL, Hechavarría R, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. Journal of neurovirology. 2006;12(5):356–364. [DOI] [PubMed] [Google Scholar]
- 13.Rivera-Mindt M, Byrd D, Ryan EL, et al. Characterization and sociocultural predictors of neuropsychological test performance in HIV+ Hispanic individuals. Cultural Diversity and Ethnic Minority Psychology. 2008;14(4):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquine MJ, Heaton A, Johnson N, et al. Differences in neurocognitive impairment Among HIV-infected latinos in the United States. Journal of the International Neuropsychological Society. 2018;24(2):163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross S, Önen N, Gase A, Overton ET, Ances BM. Identifying risk factors for HIV-associated neurocognitive disorders using the international HIV dementia scale. Journal of Neuroimmune Pharmacology. 2013;8(5):1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Franklin DR Jr, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clinical Infectious Diseases. 2015;60(3):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cysique LA, Franklin D Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. Journal of clinical and experimental neuropsychology. 2011;33(5):505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budget OoMa. Revisions to the standards for the classification of federal data on race and ethnicity. Federal Register. 1997;62(210):58782–58790. [Google Scholar]
- 21.Hollingshead AB. Four factor index of social status. Unpublished Manuscript, Yale University, New Haven, CT: 1975. [Google Scholar]
- 22.Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 (WRAT4). Lutz, FL: Psychological Assessment Resources. 2006. [Google Scholar]
- 23.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Archives of neurology. 2008;65(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letendre S Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Topics in antiviral medicine. 2011;19(4):137. [PMC free article] [PubMed] [Google Scholar]
- 25.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clinical Infectious Diseases. 2012;54(7):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price JC, Seaberg EC, Badri S, Witt MD, D’Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. Journal of Infectious Diseases. 2012;205(6):1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittchen H-U, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. The British Journal of Psychiatry. 1991;159(5):645–653. [DOI] [PubMed] [Google Scholar]
- 29.Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. Bmj. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. Journal of the American Geriatrics Society. 2007;55(5):758–762. [DOI] [PubMed] [Google Scholar]
- 31.Zipursky AR, Gogolishvili D, Rueda S, et al. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS (London, England). 2013;27(15):2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arentoft A, Byrd D, Monzones J, et al. Socioeconomic status and neuropsychological functioning: Associations in an ethnically diverse HIV+ cohort. The Clinical neuropsychologist. 2015;29(2):232–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda C, Arce Rentería M, Fuentes A, et al. The relative utility of three English language dominance measures in predicting the neuropsychological performance of HIV+ bilingual Latino/a adults. The Clinical neuropsychologist. 2016;30(2):185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet. 2017;389(10077):1453–1463. [DOI] [PubMed] [Google Scholar]
- 35.Reisen CA, Brooks KD, Zea MC, Poppen PJ, Bianchi FT. Can additive measures add to an intersectional understanding? Experiences of gay and ethnic discrimination among HIV-positive Latino gay men. Cultural Diversity and Ethnic Minority Psychology. 2013;19(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogart LM, Landrine H, Galvan FH, Wagner GJ, Klein DJ. Perceived discrimination and physical health among HIV-positive Black and Latino men who have sex with men. AIDS and Behavior. 2013;17(4):1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson CW-M, Sundermann EE, Hussain MA, et al. Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychology. 2019;38(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Class M, Jurkowski J. The limits of self-management: community and health care system barriers among Latinos with diabetes. Journal of Human Behavior in the Social Environment. 2010;20(6):808–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burkholder GA, Tamhane AR, Safford MM, et al. Racial disparities in the prevalence and control of hypertension among a cohort of HIV-infected patients in the southeastern United States. PLoS One. 2018;13(3):e0194940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu B, Pasipanodya E, Montoya JL, et al. Metabolic Syndrome and Neurocognitive Deficits in HIV infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2019;81(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissberger GH, Gollan TH, Bondi MW, et al. Neuropsychological deficit profiles, vascular risk factors, and neuropathological findings in Hispanic older adults with autopsy-confirmed Alzheimer’s disease. Journal of Alzheimer’s Disease. 2019;67(1):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheppard DP, Iudicello JE, Morgan EE, et al. Accelerated and accentuated neurocognitive aging in HIV infection. Journal of neurovirology. 2017;23(3):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulgan T, Samuels DC, Bush W, et al. Mitochondrial DNA haplogroups and neurocognitive impairment during HIV infection. Clinical Infectious Diseases. 2015;61(9):1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]