Abstract
Background:
Insomnia is common among people with HIV (PWH) and may be associated with increased risk of myocardial infarction (MI). This study examines the association between insomnia and MI by MI type among PWH.
Setting:
Longitudinal cohort study of PWH at five Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) sites.
Methods:
Clinical data and patient-reported measures and outcomes (PROs) from PWH in care between 2005–2018 were utilized in this study. Insomnia, measured at baseline, was defined as having difficulty falling or staying asleep with bothersome symptoms. CNICS centrally adjudicates MIs using expert reviewers, with distinction between type 1 (T1MI) and type 2 MIs (T2MI). Associations between insomnia and first incident MI by MI type were measured using separate Cox proportional hazard models adjusted for age, sex, race/ethnicity, traditional cardiovascular disease risk factors (hypertension, dyslipidemia, poor kidney function, diabetes, smoking), HIV markers (antiretroviral therapy, viral suppression, CD4 cell count), and stimulant use (cocaine/crack, methamphetamine).
Results:
Among 12,448 PWH, 48% reported insomnia. Over a median of 4.4 years of follow-up, 158 T1MIs and 109 T2MIs were identified; approximately half of T2MIs were attributed to sepsis or stimulant use. After adjustment for potential confounders, we found no association between insomnia and T1MI (HR=1.05, 95%CI:0.76–1.45) and a 65% increased risk of T2MI among PWH reporting insomnia compared to PWH without insomnia (HR=1.65, 95%CI:1.11–2.45).
Conclusions:
PWH reporting insomnia are at an increased risk of T2MI, but not T1MI, compared to PWH without insomnia, highlighting the importance of distinguishing MI types among PWH.
Keywords: HIV, insomnia, myocardial infarction, type 1 myocardial infarction, type 2 myocardial infarction
Introduction
People with HIV (PWH) have a higher rate of cardiovascular disease (CVD), including myocardial infarction (MI), than the general population.1,2 MIs are classified into types based on mechanism according to the Universal Definition of MI.3 Type 1 myocardial infarctions (T1MIs) occur from atherothrombotic coronary plaque rupture. Type 2 myocardial infarctions (T2MIs) occur secondary to oxygen supply-demand mismatch such as in cocaine-induced vasospasm or sepsis/bacteremia. T2MIs make up a much larger proportion of MIs among PWH (~49%) compared to the general population (<2–26%), despite the reduce relative risk of MI in PWH compared to the general population overtime.4,5
PWH also have a higher prevalence of insomnia and other sleep disturbances (~50–70%)6,7 compared to the general population (~10%),8 and insomnia appears to be associated with increasing duration and/or stage of HIV infection.9,10 Insomnia is a reported risk factor for CVD, including MI, in the general population.11,12 The underlying cause(s) of increased rates of insomnia amojng PWH are not fully understood but include sympathetic nervous system activation and elevated levels of cortisol and proinflammatory cytokines.11,13–15
Questions remain regarding the relationship between insomnia and MI among PWH, particularly given the differences in MI types among PWH.5 An association between insomnia and CVD among PWH was reported in a cohort of >3,000 veterans with HIV, but was based on a composite outcome including MI, stroke, and others, rather than adjudicated MIs distinguished by MI type and included virtually no women.16 Differentiating between T1MIs and T2MIs is important because their treatment and prevention methods differ, as do risk factors, patient demographics, and clinical characteristics.17 Therefore, we conducted this study to examine the relationship between insomnia and both T1MI and T2MI risk among PWH to better understand the mechanisms and risk factors for MIs among PWH.
Methods
This study was conducted among adult PWH receiving HIV care at five of the eight Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) sites between 2005–2018 (Johns Hopkins University; University of Alabama Birmingham; University of California San Diego; University of North Carolina Chapel Hill; and University of Washington). CNICS, a longitudinal, multisite clinical cohort of PWH, collects comprehensive clinical data from electronic medical records and other institutional data sources for both inpatient and outpatient visits including demographic characteristics, clinical codes, laboratory data, and patient-reported measures and outcomes (PROs).18 Sites were excluded if they did not have complete MI adjudication and/or did not have PRO data from the study period available.
Insomnia
CNICS participants complete an ~10 minute clinical assessment of PROs every ~6 months at the start of routine care appointments.19 The assessment includes the HIV Symptom Index, which measures sleep disturbance, including difficulty falling or staying asleep.20–22 For this analysis, insomnia was measured at baseline and was defined as having difficulty falling or staying asleep with symptoms that are bothersome (“bothers a little”, “bothers”, “bothers a lot” vs. “no symptom” or “does not bother”).
Myocardial Infarctions
CNICS has an established approach for high-quality MI adjudication,23 with MIs categorized by type based on the categories in the Universal Myocardial Infarction definition.24 Potential MIs are identified using a comprehensive set of MI diagnostic and procedure codes and elevated cardiac biomarker values, and deidentified packets of the primary data including ECGs, provider notes, etc. are centrally adjudicated by two expert physicians (more if discrepancies occur). The vast majority of MIs among PWH are type 1 or 2.
Covariates
Covariates of interest were measured at baseline and included demographic factors (age, sex, race/ethnicity), traditional CVD risk factors (hypertension, dyslipidemia, poor kidney function, diabetes, smoking), HIV markers (antiretroviral therapy use (ART), viral suppression, CD4 cell count), stimulant use (cocaine/crack, methamphetamines), and depressive symptoms. HIV viral suppression was defined as a viral load (VL) ≤400, hypertension as a diagnosis plus medication, dyslipidemia as a lipid abnormality requiring statin treatment, and poor kidney function as an estimated glomerular filtration rate (eGFR) <30. Diabetes was defined using a previously validated approach as any of the following: hemoglobin A1c ≥6.5%, clinical diagnosis of diabetes and prescription of a diabetes-related medication, or prescription of a diabetes-specific medication.25 Substance use (via modified ASSIST26), smoking, and depressive symptoms (via PHQ-927), were collected from PROs.
Statistical Analysis
Associations between insomnia and incident MI by MI type were evaluated using separate Cox proportional hazards regression analyses. We evaluated four iterations of model adjustment, each building upon the last. First, we examined the association between insomnia and MI adjusting for demographic factors only. Second, we additionally adjusted for traditional CVD risk factors. Third, we added adjustment for HIV markers. Lastly, we added adjustment for stimulant use. This adjustment scheme was used to understand how unmeasured or residual confounding may have affected our results.
We also conducted several sensitivity analyses including: (1) inclusion of PWH reporting “symptom, does not bother me” in the insomnia group, (2) a three-level categorization of insomnia (comparing [“bothers a little”] and [“bothers” or “bothers a lot”] to [“no symptom” or “does not bother”], (3) the association between persistent insomnia, defined as ≥2 consecutive PRO assessments indicating insomnia, and (4) different parameterizations of depressive symptoms as an additional adjustment factor. Depression was excluded from our main models out of concern that it could be a mediator rather than a confounder; the relationship between insomnia and depression is complex and not fully understood.28,29
Participants were censored at (1) the time of their first MI, (2) the time of last activity in CNICS, (3) time of death, or (4) date of administrative censoring (date of last data collection), whichever came first. The timescale for the models was time since baseline, which was considered as their first PRO assessment after MI adjudication began. Written informed consent is obtained for all CNICS participants. All analyses were conducted in Stata version 17 (StataCorp, College Station, TX).
Results
Overall, 12,448 PWH were eligible for inclusion in analyses. The mean age was 43 years, 16% were female, and insomnia was common: 57% reported any difficulty falling or staying asleep, while 48% reported bothersome symptoms. Overall, 267 incident MIs occurred over a median of 4.4 years (range: 0.3–13.4) of follow-up: 158 T1MIs and 109 T2MIs. The prevalence of insomnia among PWH with type 1 and 2 MIs was 49% and 61%, respectively (Table 1).
Table 1.
Demographic and clinical characteristics, by myocardial infarction status, of people with HIV from five CNICS sites across the US (N=12,448).
| N (%) or Mean (SD) | No MI | T1MI | T2MI | Overall |
|---|---|---|---|---|
| N | 12,181 (97.9%) | 158 (1.3%) | 109 (0.9%) | 12,448 |
| Age | 43 (11) | 51 (8) | 49 (11) | 43 (11) |
| Female | 1,905 (16%) | 13 (8%) | 23 (21%) | 1,941 (16%) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 5,677 (47%) | 87 (55%) | 46 (42%) | 5,810 (47%) |
| Non-Hispanic Black | 3,842 (32%) | 41 (26%) | 51 (47%) | 3,934 (32%) |
| Hispanic | 1,996 (16%) | 24 (15%) | 10 (9%) | 2,030 (16%) |
| Other/unknown | 666 (5%) | 6 (4%) | 2 (2%) | 674 (5%) |
| CD4 count (cells/mm3) | 541 (306) | 495 (316) | 398 (296) | 539 (307) |
| Currently taking ART | 11,518 (95%) | 150 (95%) | 103 (95%) | 11,771 (95%) |
| Undetectable VL (≤400 copies/ml) | 9,837 (81%) | 121 (77%) | 75 (69%) | 10,033 (81%) |
| Diabetes a | 962 (8%) | 35 (22%) | 23 (21%) | 1,020 (8%) |
| Hypertension b | 2,635 (22%) | 81 (51%) | 44 (40%) | 2,760 (22%) |
| Dyslipidemia c | 1,918 (16%) | 68 (43%) | 32 (29%) | 2,018 (16%) |
| CKD (eGFR, <30 mL/min/1.73 m2) | 108 (0.9%) | 9 (6%) | 12 (11%) | 129 (1%) |
| Current cigarette smoker | 4,456 (37%) | 77 (49%) | 51 (47%) | 4,584 (37%) |
| Insomnia – difficulty falling or staying asleep | ||||
| I do not have symptom | 5,309 (44) | 63 (40) | 36 (33) | 5,408 (43) |
| I have symptoms but it does not bother me | 1,084 (9) | 18 (11) | 7 (6) | 1,109 (9) |
| I have symptoms and it bothers me a little | 2,286 (19) | 27 (17) | 26 (24) | 2,339 (19) |
| I have symptoms and it bothers me | 1,643 (13) | 22 (14) | 23 (21) | 1,688 (14) |
| I have symptoms and it bothers me a lot | 1,859 (15) | 28 (18) | 17 (16) | 1,904 (15) |
Abbreviations: ART, antiretroviral therapy; CNICS; Centers for AIDS Research Network of Integrated Clinical Systems; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction; VL, viral load.
Diabetes is defined as any one of the following: hemoglobin A1c ≥ 6.5%, a clinical diagnosis of diabetes and prescription of a diabetes-related medication, or prescription of a diabetes-specific medication.
Hypertension is defined as a clinical diagnosis of hypertension and prescription of an antihypertensive medication.
Dyslipidemia is defined as a lipid abnormality requiring statin treatment.
After adjustment for demographic characteristics, the association between insomnia and T1MI was non-significant (Hazard Ratio (HR)=1.13, 95% Confidence Interval (CI):0.82–1.54), while insomnia was associated with an 89% increased hazard of T2MI (95%CI:1.28–2.78) (Figure 1). After additional adjustment for potential confounders, including CVD risk factors, HIV markers, and stimulant use, the association between insomnia and T1MI was null (HR=1.05, 95%CI:0.76–1.45) and the association with T2MI attenuated but remained signifigant (HR=1.65, 95%CI:1.11–2.45); stimulant use contributed minimally to this attenuation.
Figure 1.
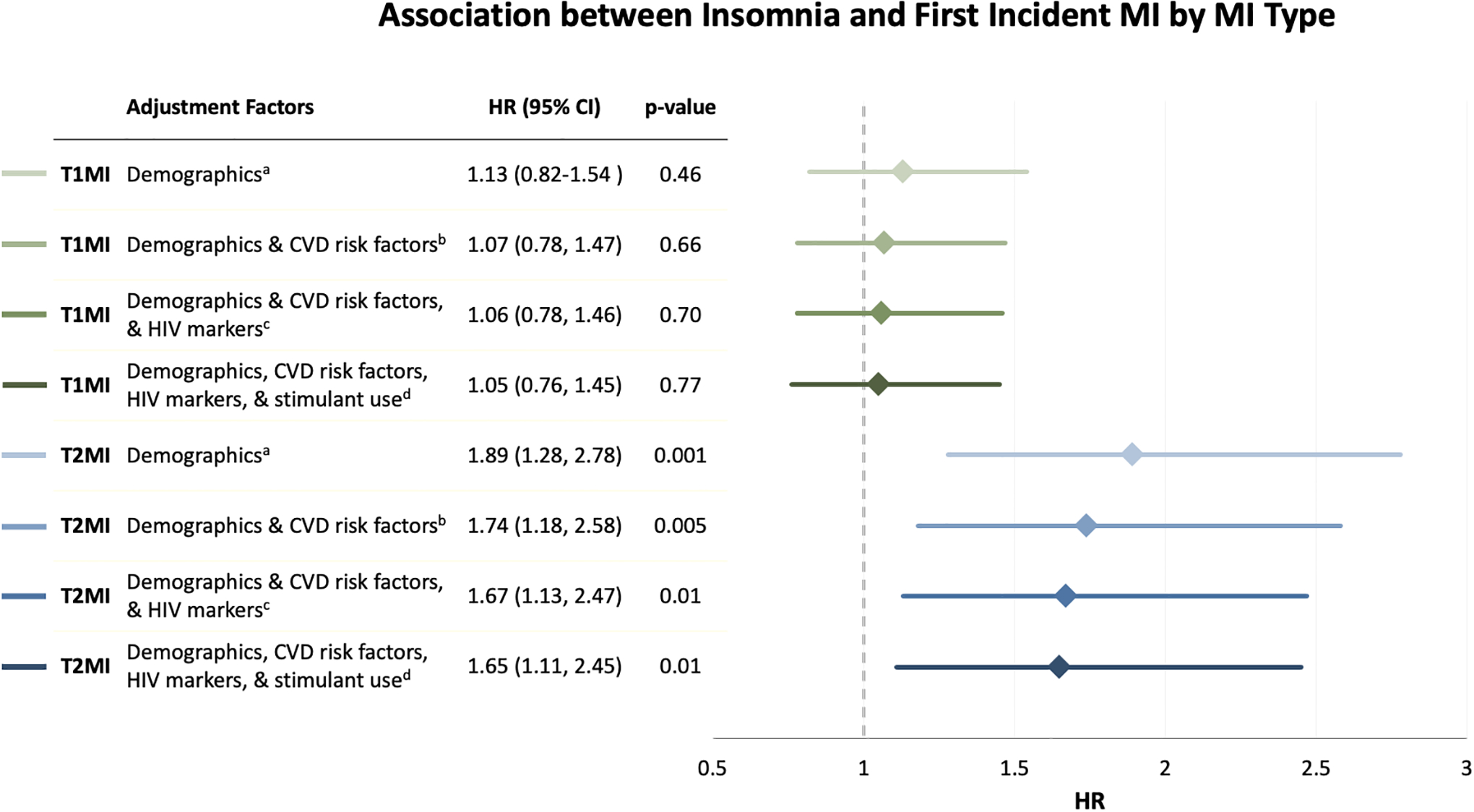
Association between insomnia and first incident myocardial infarction by myocardial infarction type among people with HIV.
Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction.
Insomnia is defined as having difficulty falling or staying asleep with bothersome symptoms.
a Cox model adjusted for age, sex, race/ethnicity.
b Cox model adjusted for age, sex, race/ethnicity, and traditional CVD risk factors (hypertension, dyslipidemia, poor kidney function, diabetes, and smoking).
c Cox model adjusted for age, sex, race/ethnicity, traditional CVD risk factors (hypertension, dyslipidemia, poor kidney function, diabetes, and smoking), and HIV markers (ART, viral suppression (VL≤400), and CD4 cell count).
d Cox model adjusted for age, sex, race/ethnicity, traditional CVD risk factors (hypertension, dyslipidemia, poor kidney function, diabetes, and smoking), HIV markers (ART, viral suppression (VL≤400), CD4 cellcount), and stimulant use (cocaine/crack and/or methamphetamines).
Including “symptom, does not bother me” in the definition of insomnia slightly attenuated the association between insomnia and T2MIs, while the HRs for T1MIs remained non-signifigant (see Table, Supplemental Digital Content 1). Using the three-level categorization of insomnia identified consistent associations between insomnia and T2MI, but not T1MI, although all T1MI estimates remained non-signifigant (see Table, Supplemental Digital Content 2). Using persistent insominia as the exposure (requiring ≥2 consecutive PROs indicating insomnia) did not change the association between insomnia and T1MI and strengthened the association between insomnia and T2MI (HR=1.98, 95%CI:1.14–3.44 in fully adjusted model), but widened confidence intervals due to loss of events. Results remained similar for both T1MI and T2MI after adjustment for depressive symptoms, both including and excluding sleep-related items (T1MI HR range:0.85–0.96; T2MI range:1.47–1.55) (see Table, Supplemental Digital Content 3, which shows estimates adjusted for depressive symptoms).
Compared to PWH with no MI or with a T1MI, PWH who experienced a T2MI had lower CD4 counts and were less likely to be virally suppressed, consistent with poorer control over their HIV (Table 1). Overall, the causes of T2MIs were heterogeneous, however >50% were attributed to sepsis (37%), hypertensive urgency/emergency (10%), and cocaine/other illicit drug use (9%) (see Figure, Supplemental Digital Content 5, which shows the breakdown of T2MIs by attributing factors).
Discussion
This is the first study to examine the association of insomnia with MI by type of MI in PWH. We demonstrated that PWH who reported insomnia were at an increased risk of T2MI, but not T1MI. Since insomnia is an established MI risk factor11,30,31 and is a reported CVD risk factor in HIV-infected veterans,16 these findings give important insight into this relationship and may guide future prevention and treatment strategies for CVD in PWH.
The relationship between insomnia and T2MIs, but not T1MIs, was not hypothesized. Understanding the mechanisms contributing to this relationship is complicated by the heterogeneous causes of T2MIs; the most common cause of T2MI in our cohort, sepsis/bacteremia, only comprised 37% of all T2MIs. To the best of our knowledge, no studies, even of the general population, have previously investigated the relationship of insomnia and MI by MI type, despite insomnia being a commonly cited risk factor for cardiovascular disease.11,12
Consistent with past estimates, approximately half of PWH in the CNICS cohort reported insomnia.6,7 The reason insomnia is so highly prevalent in PWH is not well understood, but proposed mechanisms include psychiatric morbidities32,33 and central nervous system damage from the cumulative neurotoxic effects of inflammation and neurotoxins produced by HIV.9,10 This correlates with cumulative VL being more strongly associated with T2MIs compared to T1MIs in PWH,34 despite the incidence of T1MIs in PWH also increasing with higher VL and lower CD4 counts.1 However, after adjusting for baseline VL and CD4 count, the relationship between insomnia and MI was only slightly attenuated, suggesting poor HIV control is not solely responsible for this relationship. Although some antiretroviral therapy side effects include insomnia (particularly efavirenz), these symptoms tend to improve.35,36
The role of depression in the association between insomnia and T2MI is complicated and not fully understood; adjusting for depressive symptoms may be inappropriate as it could be a mediator of the relationship between insomnia and T2MI, as depression has a complex and potentially bidirectional relationship with insomnia.28,29 After adjustment for depressive symptoms in sensitivity analyses, the relationship between insomnia and T2MI was attenuated (HR:1.65→1.47–1.55), with sleep-related items having the greatest impact on the point estimate. The accrual of additional T2MI events is necessary to elucidate the mechanisms between depressive symptoms and both insomnia and MI by MI type (i.e., formal mediation analysis).
A key strength of this study is the central adjudication protocol of MIs by type in CNICS.1 Additionally, the CNICS cohort represents a wide range of HIV disease in a large, ethnically and geographically diverse population of PWH. Our study also has several limitations. Since T2MIs do not have a distinct diagnostic code and the definition of T2MI is not fully consolidated (i.e., T2MI diagnosis relies on clinical judgment), incomplete ascertainment of T2MIs is possible, although we minimize this with multiple ascertainment approaches beyond diagnoses (e.g., cardiac biomarkers).37 Additionally, insomnia was from the baseline assessment for most analyses and we did not have data on obstructive sleep apnea. Lastly, residual confounding, from unmeasured or incompletely measured confounders, is a possibility in observational research. While we cannot eliminate the possibility of residual confounding, the association between insomnia and T2MI remained significant with the inclusion of important potential confounders.
Further studies are warranted to identify the insomnia-induced mechanisms of T2MI risk and whether it is a direct effect or an association through other factors contributing to the high rate of T2MIs in PWH. Additionally, understanding timing of insomnia impacts and whether, given the heterogeneous nature of T2MIs, insomnia is a risk factor for all or specific T2MIs is merited.
This study demonstrates that baseline insomnia, which is highly prevalent (~50–70%)6,7 in PWH, is associated with an increased risk of T2MI, but not T1MI. T2MIs are markedly increased in PWH relative to the general population (~50% vs <2–26%), however underlying reasons for this are not fully understood. Screening for insomnia in PWH may help to identify individuals with increased risk of T2MI. These findings underscore the importance of distinguishing the mechanisms and risk factors for MI types, especially in PWH who are at a considerably increased risk of T2MI.
Supplementary Material
Figure, Supplemental Digital Content 5. Identified causes of type 2 myocardial infarctions (N=109).
Acknowledgements
We would like to acknowledge all CNICS participants and study personnel for their essential contributions to this work.
This work was supported by several grants from the National Institutes of Health (CNICS NIAID grant R24 AI067039, CNICS MI supplement NIAID R24S AI067039, University of Washington Center for AIDS Research NIAID grant P30 AI027757, Third Coast Center for AIDS Research NIAID grant P30 AI117943, Johns Hopkins University Center for AIDS Research Administrative Core grant P30 AI094189, NHLBI grant R01 HL126538, NHLBI grant K01 HL137557, NIA grant R56 AG057262, and NIDA grant U01 DA036935). The work was also supported by a grant from the American Heart Association (16FTF31200010).
Footnotes
List of Supplemental Digital Content
Supplemental Digital Content 1. Table that shows estimates when “insomnia symptom present, does not bother me” is included in the definition of insomnia. DOCX
Supplemental Digital Content 2. Table that shows estimates using a three-category definition of insomnia. DOCX
Supplemental Digital Content 3. Table that shows estimates using requiring ≥2 PROs indicating insomnia. DOCX
Supplemental Digital Content 4. Table that shows estimates adjusted for depressive symptoms. DOCX
Supplemental Digital Content 5. Figure that shows the breakdown of T2MIs by attributing factors. PDF
Previous presentation: Aspects of these findings were presented at the Annual Conference on Retroviruses and Opportunistic Infections (CROI) in Boston, Massachusetts in March 2020. Title: Insomnia and Risk of Incident Myocardial Infarction Among People Living with HIV.
Conflicts of interest and source of funding: The authors report no potential conflicts of interest, including relevant financial interests, activities, relationships, and affiliations.
Contributor Information
Brandon R. Luu, Northern Ontario School of Medicine, Thunder Bay, ON, Canada.
Robin M. Nance, University of Washington, Seattle, WA, USA.
Joseph A. C. Delaney, University of Manitoba, Winnipeg, MB, Canada.
Stephanie A. Ruderman, University of Washington, Seattle, WA, USA.
Susan R. Heckbert, University of Washington, Seattle, WA, USA.
Matthew J. Budoff, University of California Los Angeles, Los Angeles, CA, USA.
William C. Mathews, University of California San Diego, San Diego, CA, USA.
Richard D. Moore, Johns Hopkins University, Baltimore, MD, USA.
Matthew J. Feinstein, Northwestern University, Chicago, IL, USA.
Greer A. Burkholder, University of Alabama at Birmingham, Birmingham, AL, USA.
Michael J. Mugavero, University of Alabama at Birmingham, Birmingham, AL, USA.
Joseph J. Eron, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Michael S. Saag, University of Alabama at Birmingham, Birmingham, AL, USA.
Mari M. Kitahata, University of Washington, Seattle, WA, USA.
Heidi M. Crane, University of Washington, Seattle, WA, USA.
Bridget M. Whitney, University of Washington, Seattle, WA, USA.
Data Statement
Statistical code is available upon request to rmnance@uw.edu. Data from CNICS may be shared with investigators with an approved concept proposal. Instructions for data access and concept proposal forms may be found at: https://www.uab.edu/cnics/submit-proposal.
References
- 1.Drozd DR, Kitahata MM, Althoff KN, et al. Increased Risk of Myocardial Infarction in HIV-Infected Individuals in North America Compared With the General Population. Journal of acquired immune deficiency syndromes (1999). 2017;75(5):568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. [DOI] [PubMed] [Google Scholar]
- 4.Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60(8):1278–1280. [DOI] [PubMed] [Google Scholar]
- 5.Crane HM, Paramsothy P, Drozd DR, et al. Types of Myocardial Infarction Among Human Immunodeficiency Virus-Infected Individuals in the United States. JAMA cardiology. 2017;2(3):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren J, Zhao M, Liu B, et al. Factors Associated With Sleep Quality in HIV. The Journal of the Association of Nurses in AIDS Care : JANAC. 2018;29(6):924–931. [DOI] [PubMed] [Google Scholar]
- 7.Taibi DM. Sleep disturbances in persons living with HIV. The Journal of the Association of Nurses in AIDS Care : JANAC. 2013;24(1 Suppl):S72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth T Insomnia: definition, prevalence, etiology, and consequences. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- 9.Gay CL, Zak RS, Lerdal A, Pullinger CR, Aouizerat BE, Lee KA. Cytokine polymorphisms and plasma levels are associated with sleep onset insomnia in adults living with HIV/AIDS. Brain, behavior, and immunity. 2015;47:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low Y, Goforth H, Preud’homme X, Edinger J, Krystal A. Insomnia in HIV-infected patients: pathophysiologic implications. AIDS reviews. 2014;16(1):3–13. [PubMed] [Google Scholar]
- 11.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124(19):2073–2081. [DOI] [PubMed] [Google Scholar]
- 12.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. European journal of preventive cardiology. 2014;21(1):57–64. [DOI] [PubMed] [Google Scholar]
- 13.Khan MS, Aouad R. The Effects of Insomnia and Sleep Loss on Cardiovascular Disease. Sleep medicine clinics. 2017;12(2):167–177. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Zhang X-W, Hou W-S, Tang Z-Y. Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. International journal of cardiology. 2014;176(3):1044–1047. [DOI] [PubMed] [Google Scholar]
- 15.Spiegelhalder K, Scholtes C, Riemann D. The association between insomnia and cardiovascular diseases. Nature and science of sleep. 2010;2:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polanka BM, Kundu S, So-Armah KA, et al. Insomnia as an Independent Predictor of Incident Cardiovascular Disease in HIV: Data From the Veterans Aging Cohort Study. Journal of acquired immune deficiency syndromes (1999). 2019;81(1):110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shroff GR. Acute Myocardial Infarction: What’s in a Name? Annals of Internal Medicine. 2015;162(6):448–449. [DOI] [PubMed] [Google Scholar]
- 18.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International journal of epidemiology. 2008;37(5):948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Current HIV research. 2007;5(1):109–118. [DOI] [PubMed] [Google Scholar]
- 20.Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. Journal of clinical epidemiology. 2001;54(12):S77–S90. [DOI] [PubMed] [Google Scholar]
- 21.Whalen CC, Antani M, Carey J, Landefeld CS. An index of symptoms for infection with human immunodeficiency virus: reliability and validity. Journal of clinical epidemiology. 1994;47(5):537–546. [DOI] [PubMed] [Google Scholar]
- 22.Wilson NL, Azuero A, Vance DE, et al. Identifying symptom patterns in people living with HIV disease. Journal of the Association of Nurses in AIDS Care. 2016;27(2):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crane H, Heckbert S, Drozd D, et al. Lessons learned from the design and implementation of myocardial infarction adjudication tailored for HIV clinical cohorts. American journal of epidemiology. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, et al. Third Universal Definition of Myocardial Infarction. Journal of the American College of Cardiology. 2012;60(16):1581–1598. [DOI] [PubMed] [Google Scholar]
- 25.Crane HM, Kadane JB, Crane PK, Kitahata MM. Diabetes case identification methods applied to electronic medical record systems: their use in HIV-infected patients. Current HIV research. 2006;4(1):97–106. [DOI] [PubMed] [Google Scholar]
- 26.Group WAW. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction (Abingdon, England). 2002;97(9):1183–1194. [DOI] [PubMed] [Google Scholar]
- 27.Crane PK, Gibbons LE, Willig JH, et al. Measuring depression levels in HIV-infected patients as part of routine clinical care using the nine-item Patient Health Questionnaire (PHQ-9). AIDS care. 2010;22(7):874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansson-Frojmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res. 2008;64(4):443–449. [DOI] [PubMed] [Google Scholar]
- 29.Khurshid KA. Comorbid Insomnia and Psychiatric Disorders: An Update. Innovations in clinical neuroscience. 2018;15(3–4):28–32. [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu C-Y, Chen Y-T, Chen M-H, et al. The Association Between Insomnia and Increased Future Cardiovascular Events: A Nationwide Population-Based Study. Psychosomatic medicine. 2015;77(7):743–751. [DOI] [PubMed] [Google Scholar]
- 31.Janszky I Insomnia is associated with risk of future cardiovascular events irrespective of comorbidities. Evidence-based medicine. 2016;21(3):107–107. [DOI] [PubMed] [Google Scholar]
- 32.Carney RM, Freedland KE, Jaffe AS. Insomnia and depression prior to myocardial infarction. Psychosomatic medicine. 1990;52(6):603–609. [DOI] [PubMed] [Google Scholar]
- 33.Insomnia Riemann D. and comorbid psychiatric disorders. Sleep medicine. 2007;8:S15–S20. [DOI] [PubMed] [Google Scholar]
- 34.Delaney JA, Nance RM, Whitney BM, et al. Cumulative Human Immunodeficiency Viremia, Antiretroviral Therapy, and Incident Myocardial Infarction. Epidemiology (Cambridge, Mass). 2019;30(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug safety. 2006;29(10):865–874. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral research. 2010;85(1):201–209. [DOI] [PubMed] [Google Scholar]
- 37.Sandoval Y, Smith SW, Thordsen SE, Apple FS. Supply/demand type 2 myocardial infarction: should we be paying more attention? Journal of the American College of Cardiology. 2014;63(20):2079–2087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 5. Identified causes of type 2 myocardial infarctions (N=109).
Data Availability Statement
Statistical code is available upon request to rmnance@uw.edu. Data from CNICS may be shared with investigators with an approved concept proposal. Instructions for data access and concept proposal forms may be found at: https://www.uab.edu/cnics/submit-proposal.


