Abstract
Background:
Adolescents living with HIV have elevated mental distress and suboptimal ART adherence.
Setting:
Two urban clinics in Kigali, Rwanda.
Methods:
A 2-arm individual randomized controlled trial compared Trauma Informed Cognitive Behavioral Therapy enhanced to address HIV (TI-CBTe) to usual care (time-matched, long-standing, unstructured support groups) with 356 12–21 year-old (M=16.78) Rwandans living with HIV. TI-CBTe included six group-based 2-hour sessions led by trained and supervised 21–25 year old Rwandans living with HIV. Participants reported their ART adherence, depression/anxiety, and PTSD symptoms at baseline, 6-, 12-, and 18-months.
Results:
ART adherence was relatively high at baseline, and youth reported elevated rates of depression/anxiety and trauma symptoms. There was no differential treatment effects on adherence, but depression/anxiety improved over time. Youth with lower depression/anxiety at baseline appeared to benefit more from TI-CBTe than usual care, whereas females with high baseline distress appeared to benefit more from usual care. Youth were less likely to score in high PTSD symptom categories at follow-up, with no differential treatment effects.
Conclusions:
TI-CBTe did not outperform usual care on ART adherence, possibly reflecting relatively high adherence at baseline, simplified medication regimens over time, a strong comparison condition, or because youth assigned to TI-CBTe returned to their support groups following the intervention. TI-CBTe was more effective for youth with lower depression/anxiety symptoms, whereas youth with high distress benefitted more from the support groups. TI-CBTe was feasible and acceptable, and young adults living with HIV were able to deliver a mental health intervention with fidelity. The powerful nature of the comparison group, ongoing support groups, points to the potential value of locally-crafted interventions in low resource settings.
Keywords: ART adherence, adolescents, sub-Saharan Africa, mental health, task-shifting
Introduction
Successes during the HIV/AIDS epidemic include reducing incident infections,1 new technologies to prevent transmission,2 increased treatment coverage,3 and full lifespans for people living with HIV who achieve viral suppression. Yet, 37.9 million people live with HIV worldwide, with 54% in sub-Saharan Africa,1 revealing stark disparities in who is benefiting from scientific advances.4,5 Among 10 – 19 years-olds living with HIV, 85% reside in sub-Saharan Africa,6 and AIDS is the primary cause of adolescent mortality in several African countries.5 Compared to adults, adolescents show worse outcomes at each stage of the HIV care continuum.7 Achieving an end to HIV/AIDS will require concentrated attention on adolescent prevention and treatment.
Rwanda is a leader in reducing new HIV transmissions, linking individuals to care, and achieving viral suppression.3 HIV incidence reached its peak in the mid-1990s in Rwanda. With the activation of public health measures to combat transmissions, initiate no-cost HIV testing and counseling, and provide free access to antiretroviral therapy (ART),8 Rwanda saw one of the most dramatic declines in incident infections worldwide.9 However, the decline accelerated after a national scale-up of ART in 2004 resulting in a stable incidence of 3%.10 Yet, like other sub-Saharan countries, Rwandan adolescents continue to acquire HIV faster and their adherence to ART is lower than adults.8 Targeted strategies are needed to improve ART adherence among Rwandan adolescents.
Addressing the Rwandan HIV epidemic requires recognition of the historical context of HIV/AIDS and specifically the legacy of the 1994 genocide. In a matter of 100 days, one million Tutsis and moderate Hutus were brutally murdered, and 350,000 women and girls were raped.11 Rape was wielded as a weapon of war during the genocide, and people living with HIV continue to experience high rates of traumatic stress and depression.12 Intergenerational transmission of trauma has been well-established from research on the Armenian genocide 13, Holocaust,14 and American Indian boarding schools15. As the first born generation post-genocide, Rwandan youth in this study are at significant risk for mental health impairment that includes trauma and depression, especially where they are the product of rape and HIV transmission.16,17
Thus, interventions to improve ART adherence must attend to Rwandans’ historical trauma and mental distress. This study evaluated a locally tailored trauma-informed cognitive behavioral intervention enhanced to improve ART adherence and reduce depression and trauma among Rwandan adolescents living with HIV. The adapted intervention18 was guided by a theoretical framework emphasizing individual (depression, trauma), social (peer and romantic relationships), and structural (stigma, gender-based violence, caregiver support) factors that impact ART adherence in the Rwandan context. Youth living with HIV report high rates of mental health difficulties,19–24 gender-based violence,25,26 stigma,3 and low social support.27 Depressive symptoms contribute to lower ART adherence and quality of life in patients living with HIV.28,29 For example, among adults, a 2011 meta-analysis of 95 studies by Gonzalez et al., demonstrated consistent links between depression (clinical and non-clinical levels) and non-adherence to HIV treatment.30
Despite these well-established barriers to adherence, few evidence-based interventions promote ART while addressing mental distress for adolescents in sub-Saharan Africa. Cognitive behavioral therapy has demonstrated effectiveness in improving depression and ART adherence in adults 31 and was adapted with feasibility to the South African context. 32 Trauma-informed cognitive behavioral therapy interventions are similarly feasible, acceptable, and effective in sub-Saharan Africa33–38 and may improve ART adherence.39 They have demonstrated positive effects on traumatic stress and depression in Ugandan adolescent survivors of war,33 grief and trauma in child orphans in Tanzania40 and Zambia,41 and PTSD in South Africa.35 Individuals are taught relaxation techniques and healthy strategies to manage distress through psychosocial education, cognitive restructuring, and mastery of trauma. In this study, “mastery of trauma” occurred by working through stress related to living with HIV rather than a specific traumatic experience narrative. New content about medication adherence, gender stereotypes, and adolescent experiences of HIV stigma were added to contextualize and apply key principles. Content was delivered via groups, because they are an effective strategy to minimize stigma, challenge negative peer norms, and provide social support when discussing sensitive topics.42
The dearth of trained mental health professionals in sub-Saharan Africa43,44 underscores a need for alternative intervention delivery approaches (e.g., community health workers, task shifting) and capacity development in low resource settings.45–47 Evidence supports training peer leaders to deliver health interventions,48 while providing high-quality preparation and ongoing coaching and technical assistance.49–51 Treatment fidelity can be maintained with timely feedback and continuous monitoring.52 We trained Rwandan young adults living with HIV (Youth Leaders; YL) to deliver the intervention to their younger peers and employed a cascading supervision model including local psychologists and an expert trainer.
This study reports findings from a 2-arm individually randomized controlled trial to improve ART adherence in Rwandan youth. The study addressed multiple gaps in the literature by (a) adapting evidence-based intervention strategies for Rwandan youth; (b) training YL to deliver the intervention and increasing local capacity to increase sustainability; (c) evaluating the impact of the intervention on ART adherence and two well-established determinants of poor adherence, depression and trauma; and (d) examining outcomes over 18-months. We expected participants in the intervention to report fewer trauma and depression symptoms, and better ART adherence compared to youth in usual care at all follow-up time points. We expected the strongest effects at 6-months post-baseline with tapering by 12-months. We predicted slight improvement from 12- to 18-months following a 12-month booster session, but we did not anticipate effects would reach the 6-month level.
Methods
Overview of Procedures
The Kigali Imbereheza Project (KIP) is a 2-arm individually randomized controlled trial comparing Trauma Informed Cognitive Behavioral Therapy–Enhanced (TI-CBTe) to usual care (i.e., time-matched unstructured support groups) at two urban clinics in Kigali, Rwanda, Women’s Equity in Access to Care and Treatment (WE-ACTx) and the Central Hospital of Kigali (CHUK). WE-ACTx is an international community-based initiative established in 2003 that provides no-cost, highly-integrated medical and psychosocial services to people living with HIV (we-actx.org/about-us/). The Rwandan Ministry of Health (RMH) ran the Therapy, Research and AIDS Care clinic at CHUK. This study grew out of a collaboration and request by the RMH to address low viral suppression among children and adolescents living with HIV.53
Data collection started January 2014 and ended December 2017. KIP staff, who were also employed at the clinics, recruited youth during clinic activities broadly, including appointments and support group meetings, and obtained guardian consent and child assent. Study materials and surveys were culturally adapted, translated into Kinyarwanda, and back-translated for accuracy. Youth completed a 2-hour audio computer-assisted self-interview within one month before the first intervention session, and at 6-, 12-, and 18-months post-baseline. Randomization occurred immediately before session one to minimize dropout due to group assignment. Youth participated in a 2-hour booster summarizing the main content of the TI-CBTe program after the 12-month follow-up assessment.
Both TI-CBTe and usual care consisted of six 2-hour sessions delivered simultaneously over two months (e.g., weekly, bi-weekly, or monthly) depending on clinic space and schedule. Participants who received TI-CBTe returned to their long-standing support group at the completion of the intervention. This study presents findings on adolescent reports of depression, trauma, and ART adherence at 6-, 12-, and 18-months post-intervention. We explored viral load outcomes, but significant missing data prevented a comprehensive test of treatment effects. Study procedures were approved by all relevant ethics committees.
TI-CBTe
TI-CBTe targets psychosocial factors for coping with stress related to living with HIV. Sessions cover basic HIV education, the effects of stress on the body, trauma associated with having HIV and managing a chronic stigmatized illness, problem-solving strategies, relaxation exercises and links between HIV and ART non-adherence, gender-based violence, and traditional gender roles and inequities. Each session follows a similar format starting with an icebreaker, followed by a re-cap of the previous session, then new material, and finally a relaxation exercise to end on a positive note and provide a strategy to manage stress outside of sessions.
In session 1, we emphasize stress as a normal part of life and how it affects thoughts, feelings, and bodies. Session 2 introduces coping with stress, especially in relation to living with HIV. Participants reflect on personal stressors and their coping strategies noting what has been helpful (listen to music, talk to a friend) and/or unhelpful (drink alcohol, contemplate suicide). In session 3, participants learn to identify and understand the connection between thoughts-feelings-behaviors. Session 4 focuses on gender roles and expectations, defined as “the ways our culture, community and family teaches about being a boy then a man and a girl then a woman.” Adapted from the Stepping Stones intervention,54 males and females split into same-gender groups and create lists of how males vs. females are expected to act in the Rwandan context. Youth are encouraged to consider how expectations change if someone is living with HIV. After returning to the large group and sharing their lists, participants read prepared statements out loud representing different individual’s (teachers, parents, neighbor, religious leader) views on gender expectations (e.g., “Boys living with HIV should not be allowed in my classroom,” “As my daughter, I love you with our without HIV”). Group members share how the comments make them feel and think, and possibly behave. To address gender-based violence, data and definitions of psychological, physical, economic, and sexual gender-based violence are presented. Common myths are dispelled and participants reflect on how each type of violence impacts health, particularly for women living with HIV. Session 5 teaches healthy and helpful coping and how to change unhealthy strategies into helpful ones. All sessions employ interactive activities and role-plays to facilitate learning and effective problem solving, large and small group discussions, break-outs, and games to practice new skills to encourage real life applications.
Caregivers were invited to participate in two, mixed sex, group-based 2-hour sessions led by research staff psychologists. Session 1 focused improving HIV and AIDS knowledge, including modes of transmission, dispelling myths, and the effects of HIV on the body. Facilitators reviewed the facts about antiretroviral therapy (ART), potential side effects, and the importance of adherence to achieve viral suppression. Caregivers engaged in small group discussions about why young people do not adhere to ART with special attention to the impact of stigma, embarrassment, caregiver attitudes, lack of family and peer support, and feelings of depression and hopelessness. Session 1 ended with homework to discuss the barriers and facilitators to ART adherence with their child. In session 2, following discussion of the homework assignment, facilitators returned to the need for ART adherence to maximize health and well-being. Role-plays were used to practice managing youth resistance to adherence. Both sessions emphasized strategies to strengthen youth adherence and caregivers practiced relaxation techniques.
Usual Care
Usual care comprised pre-established long-standing mixed gender peer support groups led by young adults living with HIV but not trained to deliver TI-CBTe. Support groups were matched in time to TI-CBTe and included same age peers. There was no caregiver component in usual care condition.
YL Training and Supervision
Young adults were nominated by clinic staff to serve as YL and then interviewed by the study investigators for subsequent selection. Inclusion criteria were Rwandan nationality, 21–24 years-old, biologically confirmed HIV, self-reported ART adherence ≥ 80%, able to commit to the study timeline, including training, completion of ≥ 2 years of secondary school or equivalent skills, demonstrated responsibility for health care appointments, good communication skills, and willingness to work. Of the 18 YL who met inclusion criteria, 14 completed the TI-CBTe training; 43% were female and 22 years-old on average (SD=1.14).
YL were trained over three weeks, 5–6 hours daily, by an expert in TI-CBT with 10 years of experience supporting mental health care in Rwanda. During week 1, YL learned the basic concepts of TI-CBT, the importance of ART adherence and maintaining viral suppression, links between HIV and traumatic stress and depression, and coping strategies. In week 2, YL reviewed and practiced the curriculum, alternating between leading the session and being a participant. At the end of week 2, the expert trainer selected four YL to deliver the intervention, two at each clinic, and four YL to observe sessions for fidelity. During week 3, the four YL designated to deliver the intervention practiced and received feedback from the expert trainer, local supervisor, and other YL. They reached competency when they: (1) demonstrated knowledge and comfort with the material, (2) followed the intervention manual, (3) delivered the session easily, (4) worked collaboratively with their co-facilitator, and (5) could field questions from mock participants. All YL received two refresher trainings annually.
We implemented a cascading supervision model with coaching and feedback to improve post-training efficiency55 and monitor treatment fidelity. YL participated in twice weekly supervision with locally trained psychologists, who then received weekly supervision from the expert trainer. The first meeting addressed prior session activities, fidelity ratings, and observer notes. In the second meeting, YL practiced delivering the upcoming session and received feedback and additional training. The expert trainer and study investigators observed sessions during in-person visits to monitor fidelity.
YL observers completed a checklist after each session to assess facilitator adherence (the activity was delivered (yes/no)), and competence (how well the facilitator conducted each activity on a scale from 0=not very well to 4=excellent) for each activity. Adherence and competence scores were calculated for each session across activities. Adherence was high for all sessions (93% to 99%) and the average competence rating was “very well” (M=3.57–3.69, SD=0.33–0.40).
Participants
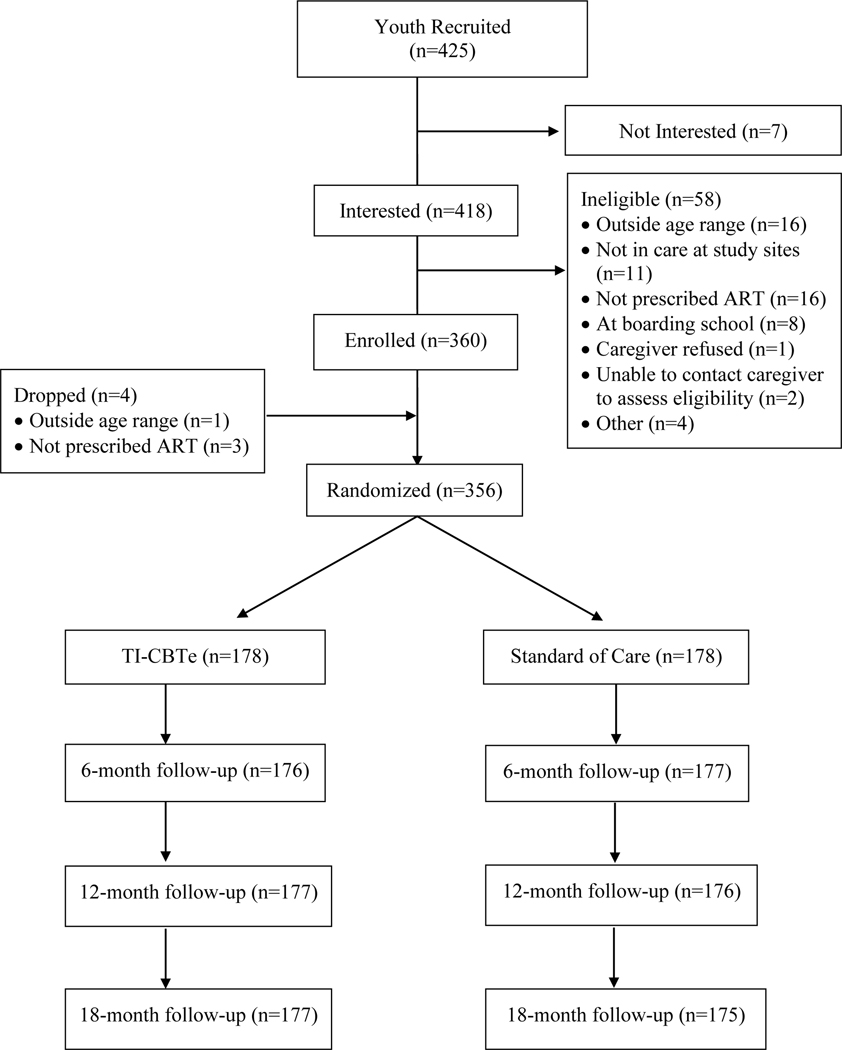
Figure 1 presents a CONSORT map of participant recruitment, randomization, and retention. Eligible youth were 14–21 years-old, were confirmed to be living with HIV, knew their status, and were prescribed ART at either WFH or CHUK clinic.
Figure 1.
Consort Map.
Measures
Demographics. Interviewers collected demographic information from youth participants using a paper form. Questions assessed age, gender, birthdate, family structure and support (e.g., who lives in home and supports youth, whether or not parents were alive and how they died), address relocation (e.g., number of times youth moved and reason for moving), schooling (currently in school, current grade or highest grade completed), and employment. For income level, youth reported on the Ubudehe category (i.e., government classifications based on economic status of households) of their family by selecting one of six categories: 1) First category: abject poverty (beggars); 2) Second category: very poor, homeless but work; 3) Third category: poor, some land, poor diets; 4) Fourth category: resourceful poor, some land, access to education and healthcare; 5) Fifth category: rich, large land, employ others; and 6) Sixth category: money rich, money in banks. Youth also answered questions about whether or not they had certain household amenities in a working condition (e.g., electricity, radio, television, landline phone, cell phone, refrigerator, computer, car).
Depression/Anxiety.
Youth completed an adapted version of the Youth Self-Report56 for Rwanda57 on recent symptoms of depression/anxiety on a scale from 0=not at all true to 2=often true (e.g., “Now or during the last 6 months, I worried a lot.”). Higher scores indicate more symptoms. The total score (range 0–46) was used in the analysis and internal reliability was strong (.90).
Trauma.
Youth completed the adapted abbreviated UCLA-PTSD Reaction Index (RI).57,58 They indicated if they experienced a traumatic event (yes/no) from a list and reported on 12 related symptoms on a scale from 0=never to 2=often (e.g., “In the last 30 days, I have dreams about what happened or other bad dreams.”) Higher scores indicate more trauma symptoms. Youth who indicated no events were assigned a zero on the symptom scale. Internal reliability was acceptable (.87).
Medication Adherence.
Youth reported their ART adherence on 3 items.59 (1) “In the last 30 days, on how many days did you miss at least one dose of your HIV medicines?” (2) “In the last 30 days, how good a job did you do at taking your HIV medicines in the way you were supposed to?” Responses ranged from 1=very poor to 6=excellent. (3) “In the last 30 days, how often did you take your HIV medicines in the way you were supposed to?” Youth indicated 1=0% or Never; 2 < 25% Rarely; 3 25–75% Sometimes; 4 75–94% Usually; 5 > 95% Almost always; 6 100% Always. Following Wilson et al.59, we transformed each item to a 0–100 scale and averaged the three scores. Internal reliability was moderate (.56). Published data with adults use an ≥ 80% adherence score as the threshold for “good” adherence60. However, other research does not provide a specific cut-point for “good” adherence noting that viral load suppression is linearly related to adherence.61
Viral Load.
Viral load data were available for 207 out of 356 participants at baseline and at least once after baseline. Data were abstracted from medical records. A viral load score present 0–12 months prior to baseline (M=5.9 months prior, SD=3.7) was coded as Time 1, and a score available 3–13 months after baseline (M= 7.8 months, SD=3.3) was coded as Time 2. Viral load was dichotomized as undetectable (<200copies/ml) or detectable (>200 copies/ml) for analyses.62
Cross-Talk.
At follow-up, participants were asked “In the last six months, did you talk to members of your youth support group about what you learned, saw or did in the KIP program?” The percentage of youth who reported yes were 34%, 23%, and 16% reported yes at 6-, 12-, and 18-months, respectively. Usual care participants were asked “In the last six months, did anyone from your youth support group talk to you about what they learned, saw or did in the KIP program?”; 30%, 26%, and 21% reported yes at 6-, 12-, and 18-months, respectively.
Data Analytic Plan
We used hierarchical linear modeling (HLM)63 to examine intervention effects on the three outcomes, depression/anxiety symptoms, PTSD symptoms, and ART adherence, over time. Self-reported adherence and depression/anxiety symptoms were analyzed as continuous variables using PROC MIXED in SAS. Depression/anxiety scores were positively skewed at all time points, requiring a square-root transformation that yielded distributions sufficiently close to normal. Models for continuous variables were fit using a compound symmetry covariance matrix for repeated measures and fixed effects for intercepts and slopes. PTSD symptoms were highly skewed with a spike at zero, and thus, they were transformed into a categorical variable using approximate quartile cutoff points: 1 = 0 symptoms/no trauma, 2 = 1–3 symptoms, 3 = 4–7 symptoms, and 4 = 8+ symptoms. The resulting categorical variables were analyzed using a proportional-odds cumulative logit model implemented with PROC GENMOD in SAS. For viral load, we employed logistic regression with PROC SURVEYLOGISTIC to predict the likelihood of having more than 200 copies/ml at follow-up. Number of months post-baseline was included as a predictor to account for the different timeframes of viral load scores.
For all outcomes, each model was tested with the effects of linear time, arm (TI-CBTe=1 versus usual care=0), baseline symptoms, gender, and all two-way, three-way, and four-way interactions among time, baseline symptoms, gender, and arm. Standard errors were adjusted for clustering. Models were reduced step-wise, removing non-significant terms based on effect size. Each reduced model was tested for site and site by intervention effects to account for any known (e.g., income) or unknown site-level differences. Models were estimated using restricted maximum likelihood estimation (REML) to accommodate missing data under the assumption they were missing-at-random. All analyses were conducted by intention to treat. To assess for potential impact of cross-talk contamination, all final models were re-run to compare contaminated control participants (i.e., youth in the control group who reported that they heard about the intervention from another participant) to pure control (i.e., youth who reported not hearing about the intervention from another participant).
Results
Participants
Participants (N=356) were 16.8 years-old on average (SD=2.2) and 51% female. Over 97% reported being poor, 23% were orphans, and 85% attended school (Table 1). Youth reported 75% ART adherence and 82% experienced at least one lifetime traumatic event. Groups did not differ on outcome variables at baseline, but the usual care condition had more orphans, X2(1, N= 355) = 5.5, p=.02 and one site served a slightly higher resourced population, t(348)=.37, p= .048. Seventy-six percent of youth attended all 6 KIP sessions. Missingness on all outcome variables was unassociated with treatment arm.
Table 1.
Descriptive Statistics at Baseline
| Total (n= 356) | Usual Care (n=178) | TI-CBTe (n=178) | |
|---|---|---|---|
| Variable | Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) |
|
|
|
|
|
| Female | 51% (183) | 51% (91) | 52% (92) |
| Age | 16.78 (2.16) | 16.81 (2.15) | 16.75 (2.18) |
| Age<18 | 64% (227) | 61% (109) | 66% (118) |
| Income Level | |||
| Abject poverty | 14% (49) | 13% (22) | 15% (27) |
| Very poor/homeless | 36% (128) | 35% (60) | 38% (68) |
| Poor/some land | 46% (165) | 50% (87) | 44% (78) |
| Resourceful poor | 1% (3) | 1% (1) | 1% (2) |
| Rich/large land | 1% (2) | 0% (0) | 1% (2) |
| Money rich | 1% (3) | 2% (3) | 0% (0) |
| Currently in School | 85% (301) | 86% (153) | 83% (148) |
| Highest Grade Completed | |||
| Primary | 38% (135) | 40% (71) | 36% (64) |
| Secondary | 61% (218) | 59% (105) | 64% (113) |
| University | 1% (2) | 1% (1) | 1% (1) |
| Orphana | 23% (83) | 29% (51) | 18% (32) |
| Traumatic Experiences, lifetime | 2.43 (2.11) | 2.44 (2.15) | 2.42 (2.08) |
| Traumatic Experiences, Past 6 months | 1.38 (1.74) | 1.33 (1.76) | 1.43 (1.72) |
| Depression/Anxiety Symptoms | 12.67 (9.15) | 12.43 (8.36) | 12.91 (9.90) |
| PTSD Symptomsb | 6.41 (5.18) | 6.18 (4.92) | 6.66 (5.43) |
| PTSD Symptoms, categorical | |||
| 0 | 26% (92) | 24% (42) | 28% (50) |
| 1–3 | 20% (72) | 20% (36) | 20% (36) |
| 4–7 | 26% (94) | 31% (55) | 22% (39) |
| 8+ | 27% (98) | 25% (45) | 30% (53) |
| Viral load (% above 200 copies/ml)c | 28% (83) | 25% (38) | 32% (45) |
Notes: TI-CBTe is Trauma Informed Cognitive Behavioral Therapy Enhanced
Difference between intervention and usual care arms is significant at p<.05 level.
Calculated only for participants who reported at least one lifetime traumatic event, n=293.
Viral load data total n=29; usual care n=154; intervention n=139.
ART Adherence
Analyses revealed no main effects of the intervention on youth self-reported ART adherence and little change over time (Table 2). While scores remained between 75% to 78% at all time points in both conditions, is noteworthy that in applying the > 80% cutoff for “good” adherence, only 35–36% of the youth in the intervention and 32–40% of youth in the control arms across the four time points qualified as “good” adherers. A significant interaction between site and intervention emerged (B=4.40, p=.02). WE-ACT participants in both arms reported improved adherence from baseline to 6-months, but the change was statistically significant for usual care participants only, t(68)= −2.21, p=.03. Adherence scores for CHUK youth were consistent from baseline to 6-months for both conditions. Adherence decreased slightly at 12- and 18-months at both sites, but treatment arms did not differ.
Table 2.
Model Predicting Wilson Adherence Scores Over Time
| Effect | B | SE B | F | p |
|---|---|---|---|---|
|
| ||||
| Intercept | 76.80 | 1.08 | 70.84 | <.01 |
| Time | −.57 | .34 | 2.89 | .09 |
| Intervention | 2.70 | 1.40 | .27 | .60 |
| ref= usual care | ||||
| Baseline adherence (Z-scored) | 4.72 | .63 | 121.60 | <.01 |
| Gender | −1.07 | .96 | 1.24 | .27 |
| ref=female | ||||
| Site | 2.74 | 1.36 | .32 | .57 |
| ref=CHUK | ||||
| Intervention * Site | −4.40 | 1.92 | 5.24 | .02 |
| Baseline adherence*Gender | 2.03 | 1.04 | 3.77 | .05 |
Viral load results should be considered exploratory given the high rate of missing data (i.e., 42%; n=149/356) and variable timing of viral load testing. At baseline, more youth randomized to TI-CBTe had detectable viral loads than usual care participants (> 200 copies/ml) (32% vs. 28%; p=.65) ), and this persisted at follow-up (33% vs. 23%; p=.12) although the differences were not statistically significant. Logistic regression models revealed a significant interaction between treatment arm and data collection months from baseline (B= −.15, p=.02). For youth whose viral load was collected closer to baseline, those in TI-CBTe were more likely to have a detectable viral load (>200/ml). For youth whose viral load was collected further from baseline, all participants had similar viral loads. Youth in TI-CBT were more likely to have detectable viral loads at follow-up than usual care participants after controlling for baseline viral load (above/below 200), gender, months after baseline, and the interaction between months after baseline and intervention (B=1.52, p<.01).
Depression/Anxiety Symptoms
Depression/anxiety symptoms decreased in all youth from baseline to 6-months and again at 12-months (B= 2.62, p<.01), and this was maintained at 18-months. HLM analyses revealed a significant three-way interaction including time, baseline depression/anxiety scores, and intervention arm (B=.17, p<.01) (Table 3). TI-CBTe youth reported significantly greater decreases in depression/anxiety over time compared to usual care youth, but only for participants with lower symptoms at baseline. Using a median split, usual care youth with fewer symptoms at baseline revealed no significant symptom reduction at 6-, 12-, or 18-months. For youth with higher baseline depression/anxiety, both arms showed a significant reduction in symptoms from baseline to 6-months. At 12- and 18-months, however, the usual care group reported slightly fewer symptoms than the intervention group.
Table 3.
Model Predicting Depression/Anxiety Symptoms Over Time
| Effect | B | SE B | F | p |
|---|---|---|---|---|
|
| ||||
| Intercept | 2.62 | .08 | 32.02 | <.01 |
| Time | −.14 | .04 | 14.34 | <.01 |
| Intervention | .13 | .12 | .83 | .36 |
| ref= usual care group | ||||
| Baseline YSR (square root and Z-scored) | .70 | .08 | 295.38 | <.01 |
| Gender | .13 | .10 | 1.27 | .26 |
| ref=female | ||||
| Time*Intervention | .06 | .06 | 1.09 | .30 |
| Time*baseline YSR | −.18 | .04 | 10.38 | <.01 |
| Intervention*Gender | −.44 | .15 | 8.15 | <.01 |
| Baseline YSR *Intervention | .12 | .10 | 1.43 | .23 |
| Baseline YSR*Gender | .18 | .08 | 5.08 | .02 |
| Time* baselineYSR * Intervention | .17 | .06 | 7.68 | <.01 |
Two-way interactions yielded significant effects for gender x baseline depression/anxiety (B=.18, p=.02,) and gender x arm (B=−.44, p<.01). Males in both arms who reported more depression/anxiety at baseline endorsed fewer symptoms at 6-months and the improvement was sustained at 18-months. For males in usual care who reported less depression/anxiety at baseline, there was no reduction in symptoms at any follow-up time point. However, for males in TI-CBTe who reported lower levels of depression/anxiety at baseline, there was a decrease in symptoms from baseline to 12-months and their symptoms were significantly lower than for males in usual care at 12- and 18-months.
By contrast, all females with higher depression/anxiety at baseline showed improvement at 6-months regardless of treatment arm, but usual care females had lower symptoms than TI-CBTe females at 12- and 18-months. Among females with less depression/anxiety at baseline, the usual care group did not change over time, but females in TI-CBTe improved at 6-months and the change was maintained at 12- and 18-months.
PTSD Symptoms
All participants reported fewer symptoms at follow-up. There was no significant effect of intervention or any interactions other than time; participants were less likely to score in higher symptom categories over time.
Session Attendance
All youth, prior to randomization, participated in the clinic support groups that consisted of the “control condition.” After randomization, youth assigned to TI-CBTe stopped attending the support groups and instead attended the TI-CBTe groups. About 93% (n=166) of youth assigned to TI-CBTe attended all 5 (17%) or 6 intervention sessions (76%). Youth assigned to the usual care groups continued to attend them consistent with clinic programming (e.g., if support groups were cancelled, the groups did not meet). Attendance at support groups was tracked only if the groups occurred at the same time as the TI-CBTe groups. In this case, 72% of youth attended at least one support group session, 24% attended two support group sessions, and 3.4% attended six at exactly the same time as the TI-CBTe groups. However, because the support groups met after the 6-session TI-CBTe intervention ended, it is highly likely that the usual care support group participants received at least as many sessions over time as the intervention group and probably more.
Cross-Talk Contamination
For all outcomes, analyses comparing the intervention to participants who indicated no cross-talk (i.e., pure control) were nearly identical to the intent-to-treat analyses. Similarly, findings that compared the intervention group to the contaminated control (i.e., participants who reported hearing about the intervention from another person) mirrored those in the intent-to-treat analyses. There were no significant differences when comparing the contaminated control to the pure control.
Discussion
This study evaluated TI-CBTe tailored for Rwandan youth and enhanced to address ART adherence and trauma related to living with HIV. ART adherence was relatively high at baseline, 75–78% in both arms, and these rates persisted over time. TI-CBTe did not outperform usual care on ART adherence, and findings for depression/anxiety and trauma were nuanced. TI-CBTe was more effective for youth with lower depression/anxiety symptoms, and improvement at 12-months was retained at 18-months. By contrast, youth with high distress and females especially, benefitted more from the usual care support groups, while males who received TI-CBTe appeared to benefit more than females and more than males in usual care. All youth were less likely to score high in PTSD symptom categories at follow-up, with no differential treatment effects.
We hypothesized that intervention youth would acquire new skills to improve ART adherence, but findings did not bear this out. We did not restrict the study to youth with poor adherence, and it is possible that the relatively high baseline adherence left little room for improvement, compromising the ability to detect effects. Relatively high baseline adherence (75–78%) is consistent with data underscoring Rwanda’s success managing the epidemic. Moreover, the advent of new ART formulations during the study period significantly simplified medication regimens from several pills twice daily to one pill daily, addressing a well-known barrier to adherence. Future research should evaluate whether the intervention works for adolescents with poor ART adherence. Unexpectedly, the effects of ART adherence differed by site. The improvement in adherence from baseline to 6-months by WE-ACTx youth in both arms compared to youth from CHUK may reflect the extensive wrap around programming available at WE-ACTx but not CHUK. For example, it is possible that the addition of TI-CBTe to WE-ACTx’s other services (e.g., nutrition supplements, yoga and music education) may explain site differences.
We expected intervention youth to report less distress over time and fewer symptoms than the usual care group, but findings revealed a more complex pattern. Youth with high distress and females especially, benefitted more from the usual care support groups, while the intervention worked better for males and females who entered the study with lower levels of depression/anxiety. It is possible that the strength of the “usual care” condition made it a powerful comparison. The support groups, organized by age, had been in existence for years before the study and youth had developed strong and enduring relationships within groups. By contrast, the intervention assembled unfamiliar youth of diverse ages for six weeks who for ethical reasons returned to their support groups after the intervention. The improvement in mental health among youth in both conditions may reflect the non-specific effects of participation in a research project or exposure to intervention participants returning to the support groups. That youth with higher distress and girls benefitted more from the usual care condition may reflect the strength of the peer relationships, trust, and comfort that existed in the ongoing support groups. Compared to males, females perceive greater support from peers64 and use social support as a coping strategy.65 Individuals with high distress may be particularly sensitive to long-term supportive relationships,66 whereas the TI-CBTe was only 6-sessions and group participants were unfamiliar.
Males seemed to benefit more than females in the TI-CBTe arm. The treatment effects favoring males is consistent with research demonstrating the advantages of co-ed groups for males and disadvantages for females.67 Females participate less in groups with males present, a pattern especially pronounced in Rwanda where cultural norms dictate female deference. It is possible that females in the intervention, particularly those with higher levels of distress, were less likely to participate in activities and shied away from expressing themselves – factors known to predict better outcomes.68 It is also possible that females were more negatively impacted by missing the support groups. Future research should consider single sex intervention groups to ameliorate these concerns.
A key study limitation was the high ART adherence at baseline, potentially mitigating intervention effects, further confounded by improved ART regimens. The significant amount of missing variable time frames for viral load data required reliance on self-reported adherence well-known to be subject to biases. The question of cross-talk between the intervention and control conditions made it difficult to evaluate the nature of potential contamination, yet there was no evidence that cross-talk contamination altered study outcomes.
Still, TI-CBTe proved feasible and acceptable in Rwanda as evidenced by strong youth recruitment and enrollment, high session attendance, and favorable evaluations of youth leaders by participants,53 and evidence that young adults living with HIV can be trained to deliver a complex mental health intervention with fidelity.69 Although a LMIC, Rwanda is unique in its care of youth living with HIV. The clinic, WE-ACTx, for example, provides nutrition supplements for families, music education, yoga, additional support groups for adolescent mothers, and mental health screening and services by trained psychologists. By contrast, most LMICs lack resources and professionally trained individuals to deliver weekly support groups indefinitely for adolescents living with HIV. A 6-week program that improves mental health and/or ART adherence may be more feasible and acceptable in LMICs. Task shifting can bolster in-country capacity to deliver mental health interventions. Findings demonstrated that the additional resources dedicated to implementing TI-CBTe, a new and tailored intervention for the Rwandan context did not improve youth outcomes in important ways. The powerful nature of the comparison group highlights the potential value of locally-crafted interventions in LMICs. Evaluating the effects of TI-CBT against a typical standard of care in LMICs could shed light on this issue.
Acknowledgements
We thank the Kigali Imbereheza Project staff who carried out the project, our collaborators and clinics, the youth leaders who learned to deliver the intervention with great skill, and the caregivers and young people living with HIV who graciously shared their stories with us and trusted us with their time.
Source of Funding: This study was funded by the National Institute of Child Health and Human Development (R01HD074977).
Footnotes
Conflicts of Interest None of the authors have a conflict of interest.
ClinicalTrials.gov registration #: NCT02464423
References
- 1.UNAIDS. Global HIV & AIDS statistics - 2019 fact sheet. 2019. Accessed November 21, 2019.
- 2.Ward H, Garnett GP, Mayer KH, et al. Maximizing the impact of HIV prevention technologies in sub-Saharan Africa. J Int AIDS Soc. 2019;22(Suppl 4):e25319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Communities at the Center: Defending rights, breaking barriers, reaching people with HIV services. 2019. Accessed November 21, 2019.
- 4.UNAIDS. Miles to go - closing gaps, breaking barriers, righting injustices. Geneva: 2018. [Google Scholar]
- 5.UNAIDS. Ending the AIDS epidemic for adolescents, with adolescents. Geneva: 2016. [Google Scholar]
- 6.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: Current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66:S144–S153. [DOI] [PubMed] [Google Scholar]
- 7.Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults--seven African countries, 2004–2013. MMWR, 2014;63(47):1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 8.International Center for AIDS Care and Treatment Program. Rwanda population-based HIV impact assessment: RPHIA 2018–2019. 2019. Accessed November 26, 2019.
- 9.Centers for Disease Control and Prevention. Survey results put Rwanda on track to achieve HIV epidemic control 2019. Accessed November 26, 2019.
- 10.Nsanzimana S, Remera E, Kanters S, et al. Household survey of HIV incidence in Rwanda: a national observational cohort study. The Lancet HIV. 2017;4(10):e457–e464. [DOI] [PubMed] [Google Scholar]
- 11.Zraly M, Rubin SE, Mukamana D. Motherhood and resilience among Rwandan genocide-rape survivors. Ethos. 2013;41:411–439. [Google Scholar]
- 12.Heim L, Schaal S. Rates and predictors of mental stress in Rwanda: investigating the impact of gender, persecution, readiness to reconcile and religiosity via a structural equation model. Int J Ment Health Syst. 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangassarian SL. 100 Years of Trauma: the Armenian Genocide and Intergenerational Cultural Trauma. J Aggress Maltreat Trauma. 2016;25(4):371–381. [Google Scholar]
- 14.Danieli Y, Norris FH, Lindert J, et al. The Danieli Inventory of Multigenerational Legacies of Trauma, Part I: Survivors’ posttrauma adaptational styles in their children’s eyes. J Psychiatr Res. 2015;68:167–175. [DOI] [PubMed] [Google Scholar]
- 15.Bombay A, Matheson K, Anisman H. The intergenerational effects of Indian Residential Schools: Implications for the concept of historical trauma. Transcultural Psychiatry. 2013;51(3):320–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denov M, Woolner L, Bahati JP, et al. The Intergenerational Legacy of Genocidal Rape: The Realities and Perspectives of Children Born of the Rwandan Genocide. Journal of Interpersonal Violence. 2020;35(17–18):3286–3307. [DOI] [PubMed] [Google Scholar]
- 17.Shrira A, Mollov B, Mudahogora C. Complex PTSD and intergenerational transmission of distress and resilience among Tutsi genocide survivors and their offspring: A preliminary report. Psychiatry Res. 2019;271:121–123. [DOI] [PubMed] [Google Scholar]
- 18.Aarons G, Hurlburt M, Horwitz S. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health. 2011;38(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dow DE, Turner EL, Shayo AM, et al. Evaluating mental health difficulties and associated outcomes among HIV-positive adolescents in Tanzania. AIDS Care. 2016;28(7):825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamau J, Kuria W, Mathai M, et al. Psychiatric morbidity among HIVinfected children and adolescents in a resource-poor Kenyan urban community. AIDS Care. 2012;24(7):836–842. [DOI] [PubMed] [Google Scholar]
- 21.Memiah P, Shumba C, Etienne-Mesubi M, et al. The effect of depressive symptoms and CD4 count on adherence to highly active antiretroviral therapy in sub-Saharan Africa. Journal of the International Association of Providers of AIDS Care. 2014;13(4):346–352. [DOI] [PubMed] [Google Scholar]
- 22.Nakimuli-Mpungu E, Mutamba B, Othengo M, et al. Psychological distress and adherence to highly active anti-retroviral therapy (HAART) in Uganda: a pilot study. Afr Health Sci. 2009;9 Suppl 1:S2–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Smith Fawzi MC, Ng L, Kanyanganzi F, et al. Mental Health and Antiretroviral Adherence Among Youth Living With HIV in Rwanda. Pediatrics. 2016;138(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whetten K, Shirey K, Pence BW, et al. Trauma history and depression predict incomplete adherence to antiretroviral therapies in a low income country. PLoS One. 2013;8(10):e74771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abramsky T, Watts CH, Garcia-Moreno C, et al. What factors are associated with recent intimate partner violence? findings from the WHO multi-country study on women’s health and domestic violence. BMC Public Health. 2011;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jewkes R, Morrell R. Sexuality and the limits of agency among South African teenage women: theorising femininities and their connections to HIV risk practices. Social science & medicine (1982). 2012;74(11):1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavhu W, Berwick J, Chirawu P, et al. Enhancing Psychosocial Support for HIV Positive Adolescents in Harare, Zimbabwe. PLoS One. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asrat B, Schneider M, Ambaw F,et al. Effectiveness of psychological treatments for depressive symptoms among people living with HIV/AIDS in low- and middle-income countries: A systematic review and meta-analysis. J Affect Disord. 2020;270:174–187. [DOI] [PubMed] [Google Scholar]
- 29.Uthman OA, Magidson JF, Safren SA, et al. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez JS, Batchelder AW, Psaros C,et al. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen LS, Magidson JF, O’Cleirigh C, et al. A pilot study of a nurse-delivered cognitive behavioral therapy intervention (Ziphamandla) for adherence and depression in HIV in South Africa. J Health Psychol. 2018;23(6):776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolton P, Bass J, Betancourt T, et al. Interventions for depression symptoms among adolescent survivors of war and displacement in northern Uganda: a randomized controlled trial. JAMA. 2007;298(5):519–527. [DOI] [PubMed] [Google Scholar]
- 34.Bolton P, Bass J, Neugebauer R, et al. Group interpersonal psychotherapy for depression in rural Uganda: a randomized controlled trial. JAMA. 2003;283(23):3117–3124. [DOI] [PubMed] [Google Scholar]
- 35.Jalal B, Kruger Q, Hinton D. Culturally adapted CBT (CA-CBT) for traumatised indigenous South Africans (Sepedi): a randomised pilot trial comparing CA-CBT to applied muscle relaxation. Intervention. 2020;18:61–65. [Google Scholar]
- 36.Murray LK, Familiar I, Skavenski S, et al. An evaluation of trauma focused cognitive behavioral therapy for children in Zambia. Child Abuse Negl. 2013;37:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray LK, Dorsey S, Skavenski S, et al. Identification, modification, and implementation of an evidence-based psychotherapy for children in a low-income country: the use of TF-CBT in Zambia. International Journal of Mental Health Systems. 2013;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dow DE, Mmbaga BT, Gallis JA, et al. A group-based mental health intervention for young people living with HIV in Tanzania: results of a pilot individually randomized group treatment trial. BMC Public Health. 2020;20(1):1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell K, Dorsey S, Gong W, et al. Treating maladaptive grief and posttraumatic stress symptoms in orphaned children in Tanzania: Group-based trauma-focused cognitive–behavioral therapy. J Trauma Stress. 2014;27(6):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray LK, Skavenski S, Kane JC, et al. Effectiveness of Trauma-Focused Cognitive Behavioral Therapy Among Trauma-Affected Children in Lusaka, Zambia: A Randomized Clinical Trial. JAMA pediatrics. 2015;169(8):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burleson JA, Kaminer Y, Dennis ML. Absence of iatrogenic or contagion effects in adolescent group therapy: findings from the Cannabis Youth Treatment (CYT) study. Am J Addict. 2006;15 Suppl 1:4–15. [DOI] [PubMed] [Google Scholar]
- 43.Kieling C, Baker-Henningham H, Belfer M, et al. Child and adolescent mental health worldwide: evidence for action. Lancet. 2011;378:1515–1525. [DOI] [PubMed] [Google Scholar]
- 44.McKay M, Bahar O, Ssewamala FM. Implementation science in global health settings: Collaborating with governmental & community partners in Uganda. Psychiatry Res. 2020;283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celletti F, Wright A, Palen J, et al. Can the deployment of community health workers for the delivery of HIV services represent an effective and sustainable response to health workforce shortages? Results of a multicountry study. AIDS. 2010;24 Suppl 1:S45–57. [DOI] [PubMed] [Google Scholar]
- 46.Rasschaert F, Philips M, Van Leemput L, et al. Tackling Health Workforce Shortages During Antiretroviral Treatment Scale-up-Experiences From Ethiopia and Malawi. JAIDS. 2011;57:S109–S112. [DOI] [PubMed] [Google Scholar]
- 47.Wainberg ML, Scorza P, Shultz JM, et al. Challenges and opportunities in global mental health: a research-to-practice perspective. Current Psychiatry Reports. 2017;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naidoo N, Railton JP, Khosa SN, et al. Fidelity of HIV programme implementation by community health workers in rural Mopani district, South Africa: a community survey. BMC Public Health. 2018;18(1):1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durlak JA, DuPre EP. Implementation Matters: A Review of Research on the Influence of Implementation on Program Outcomes and the Factors Affecting Implementation. Am J Community Psychol. 2008;41(3–4):327–350. [DOI] [PubMed] [Google Scholar]
- 50.Maticka-Tyndale E, Barnett JP. Peer-led interventions to reduce HIV risk of youth: A review. Eval Program Plann. 2010;33(2):98–112. [DOI] [PubMed] [Google Scholar]
- 51.Weiner B, Belden C, Bergmire D, Johnston M. The meaning and measurement of implementation climate. Implementation Science. 2011;6(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kershner S, Flynn S, Prince M, et al. Using data to improve fidelity when implementing evidence-based programs. J Adolesc Health. 2014;54(3):S29–S36. [DOI] [PubMed] [Google Scholar]
- 53.Donenberg GR, Cohen MH, Ingabire C, et al. Applying the Exploration Preparation Implementation Sustainment (EPIS) Framework to the Kigali Imbereheza Project for Rwandan Adolescents Living With HIV. J Acquir Immune Defic Syndr. 2019;82 Suppl 3(Suppl 3):S289–s298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jewkes R, Nduna M, Levin J, et al. Impact of Stepping Stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. Br Med J. 2008;337:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller WR, Yahne CE, Moyers, et al. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72(6):1050–1062. [DOI] [PubMed] [Google Scholar]
- 56.Achenbach T. Manual for the Youth Self-Report and 1991 Profile. Department of Psychiatry University of Vermont; 1991. [Google Scholar]
- 57.Betancourt TS, Meyers-Ohki SE, Stulac SN, et al. Global Mental Health Programs for Children and Families Facing Adversity: Development of the Family Strengthening Intervention in Rwanda. In: Corbin J, Ed. Children and Families Affected by Armed Conflicts in Africa: Implications and Strategies for Helping Professionals in the United States. Washington, D.C.: National Association of Social Workers Press; 2012:113–142. [Google Scholar]
- 58.Steinberg A, Brymer MJ, Decker KB, et al. The University of California at Los Angeles post-traumatic stress disorder reaction index. Current Psychiatry Reports. 2004;6(2):96–100. [DOI] [PubMed] [Google Scholar]
- 59.Wilson IB, Lee Y, Michaud J,et al. Validation of a New Three-Item Self-Report Measure for Medication Adherence. AIDS Behav. 2016;20(11):2700–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parienti J-J, Das-Douglas M, Massari V. Not all missed doses are the same: Sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3(7):e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson IB, Tie Y, Padilla M, et al. Performance of a short, self-report adherence scale in a probability sample of persons using HIV antiretroviral therapy in the United States. AIDS. 2020;34(15):2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–181. [DOI] [PubMed] [Google Scholar]
- 63.Bryk A, Raudenbush S. Hierarchial Linear Model: Applications and Data Analysis Methods. Newbury Park: SAGE; 1992. [Google Scholar]
- 64.Cheng S-T, Chan ACM. The multidimensional scale of perceived social support: dimensionality and age and gender differences in adolescents. Pers Individ Dif. 2004;37(7):1359–1369. [Google Scholar]
- 65.Eschenbeck H, Kohlmann C-W, Lohaus A. Gender Differences in Coping Strategies in Children and Adolescents. Journal of Individual Differences. 2007;28(1):18–26. [Google Scholar]
- 66.Cheng Y, Li X, Lou C, et al. The Association Between Social Support and Mental Health Among Vulnerable Adolescents in Five Cities: Findings From the Study of the Well-Being of Adolescents in Vulnerable Environments. J Adolesc Health. 2014;55(6, Supplement):S31–S38. [DOI] [PubMed] [Google Scholar]
- 67.Babinski DE, Sibley MH, Ross JM, et al. The effects of single versus mixed gender treatment for adolescent girls with ADHD. J Clin Child Adolesc Psychol. 2013;42(2):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karver MS, Handelsman JB, Fields S, et al. Meta-analysis of therapeutic relationship variables in youth and family therapy: The evidence for different relationship variables in the child and adolescent treatment outcome literature. Clin Psychol Rev. 2006;26(1):50–65. [DOI] [PubMed] [Google Scholar]
- 69.Donenberg GR, Fabri M, Ingabire C, et al. Empowering Rwandan Young Adults: A Case Example Using Youth Leaders. International Society of Traumatic Stress Studies. November 2015; New Orleans, LA./References> [Google Scholar]