Abstract
Background:
Understanding the correlates of disengagement from HIV care and treatment failure during second-line antiretroviral therapy (ART) could inform interventions to improve clinical outcomes among people living with HIV (PLHIV).
Methods:
We conducted a retrospective cohort study of PLHIV aged >15 years who started second-line ART at a tertiary centre in Nigeria between 2005 and 2017. Participants were considered to have disengaged from care if they had not returned within a year after each clinic visit. Cox proportional hazards models were used to investigate factors associated with: i) viral failure (HIV-1 RNA >1,000 copies/mL), ii) immunologic failure (CD4 count decrease or <100 cells/mm3), and iii) severe weight loss (>10% of bodyweight), after >6 months of second-line ART.
Results:
Among 1031 participants, 33% (341) disengaged from care during a median follow-up of 6.9 years (IQR 3.7–8.5). Of these, 26% (89/341) subsequently re-entered care. Disengagement was associated with male gender, age <30, lower education level and low CD4 count at second-line ART initiation. Among participants with endpoint assessments available, 20% (112/565) experienced viral failure, 32% (257/809) experienced immunologic failure, and 23% (190/831) experienced weight loss. A lower risk of viral failure was associated with professional occupations compared to elementary: adjusted hazard ratio 0.17 (95% confidence interval 0.04–0.70).
Conclusion:
Adverse outcomes were common during second-line ART. However, re-engagement is possible and resources should be allocated to focus on retaining PLHIV in care and providing services to trace and re-engage those who have disengaged from care.
Introduction
Protease inhibitor-based regimens remain the combination of choice for second-line antiretroviral therapy (ART) for people living with HIV (PLHIV).1 Most individuals starting such regimens have already experienced first-line treatment failure and, therefore, are at increased risk of subsequent treatment failure during second-line ART, compared to people who maintained viral suppression on first-line ART.2 Whilst many studies have investigated first-line ART failure, fewer have described outcomes on second-line ART, particularly in relation to engagement in care. There remains a need to understand the correlates of disengagement from care and treatment failure during second-line protease inhibitor-based therapy in order to inform optimized management and prevent adverse clinical outcomes.
Continuous engagement in care is a key requirement for the successful management of HIV, which requires lifelong adherence to ART. A meta-analysis of over 70,000 patients in the early years of the ART programmes in sub-Saharan Africa (2000–2007), estimated retention in care to be 75% at one year and 62% at two years after ART initiation.3 Death was thought to account for about 40% of attrition. A subsequent update that included the period 2008 to 2013, suggested an improvement in retention with 81% in care at one year and 71% at two years after initiating ART among the sub-Saharan African cohorts.4
In Nigeria, varying definitions of retention have been used, which complicates interpretation of the existing literature. One analysis conducted early in the implementation of the national ART programme considered participants to be lost to follow-up if they had missed a clinic appointment and had not returned within 60 days of that missed visit.5 By that definition, 26% of 5,760 patients across five centres, including the site for the present study, were deemed lost to follow-up. The median follow-up period was around seven months and most of the attrition occurred immediately after the first clinic visit. The largest study of its kind in Nigeria evaluated loss to follow-up in over 50,000 people who had initiated first-line ART between 2004 and 2011 and had returned to clinic at least once to collect ART.6 Overall, 28% were considered lost to follow-up by 2012 as they had not collected ART from pharmacy within two months following the last scheduled appointment on at least one occasion.
Less is known about engagement in care during second-line therapy despite the particular vulnerability of PLHIV on second-line regimens to viral failure and other adverse events.2,7 The diagnosis of clinical, immunologic or viral failure requires engagement in care for clinical assessment, CD4 count measurement and HIV viral load testing, respectively. The World Health Organization (WHO) defines clinical failure in adults as, after at least six months of ART, a new or recurrent stage IV condition (such as wasting syndrome with severe weight loss of >10% of bodyweight) or certain stage III conditions, including pulmonary TB.8 Immunologic failure is defined by a poor CD4 response during ART of <100 cells/mm3 and/or a decrease compared to baseline levels. Viral failure is usually defined as a viral load >1,000 copies/mL, ideally confirmed by a second measurement.8
We conducted a retrospective cohort study to describe engagement in care and treatment failure among second-line ART recipients attending a tertiary hospital in central Nigeria. A pragmatic approach to follow-up was taken in this study, which recognised that a period of disengagement may not represent permanent loss-to-follow-up, and so participants could contribute data to the analysis if they returned to care.
Methods
Participants were selected from the second-line treatment cohort of PLHIV at the University of Abuja Teaching Hospital. As previously described, the ART programme is delivered by the AIDS Care and Treatment in Nigeria (ACTION) project, which is a collaboration between the Institute of Human Virology Nigeria (IHVN), the Federal Ministry of Health of Nigeria, the Institute of Human Virology of the University of Maryland and other local organisations.9,10 Second-line protease inhibitor therapy has been available in this setting since 2005 for people who have failed NNRTI-based first-line regimens. There have been major changes in ART programming over this time, including the CD4 threshold at which ART is initiated, the recommended antiretroviral agents, and the availability of laboratory monitoring. The preferred protease inhibitor is ritonavir-boosted lopinavir, though in the early years of the second-line programme (2005–2008) some people were started on older protease inhibitor agents (such as saquinavir, indinavir and nelfinavir), and in the most recent period (2013–2016) atazanavir has also been used.
The Nigerian national guidelines for HIV treatment and care from 2007 and 2010 recommended routine follow-up every 3–6 months during ART, with a clinical evaluation including weight and adherence assessment at every visit.11,12 Routine blood tests (e.g. full blood count, renal and liver function) and a CD4 count were recommended every 6 months. While a six-monthly HIV-1 RNA “viral load” quantitation was listed as “desirable”, it was recognised that the availability of laboratory tests may differ between healthcare facilities. In the 2016 national guidelines, the viral load monitoring recommendation changed to “essential” every 12 months (and remained “desirable” every six months).13 This was the year that routine viral load monitoring was introduced at the study site. Prior to that, viral load testing was not performed routinely, but could be requested on a case-by-case basis if treatment failure was suspected despite apparently good adherence.
The IHVN database of prospectively collected clinical and laboratory information was used to identify potential participants. The medical notes at the University of Abuja Teaching Hospital were also reviewed to determine eligibility and to collect additional data. Participants were included if they were aged >15 years and had initiated second-line protease inhibitor-based ART. The potential time-in-cohort for each participant started at the switch to second-line ART and ceased at death, transfer of care to another treatment centre or study end (25 May 2017). People who had switched to second-line ART less than one year before the study end were excluded to allow at least one year of potential follow-up for all participants. Participants were considered to be in-care for one year following each clinic visit. If they did not re-attend clinic within the year they were then considered to have disengaged from care. However, no one was considered completely “lost to follow-up”, and if a participant returned to clinic after a period of disengagement then they were considered to have re-entered care. In this way, each person’s total potential time-in-cohort was subdivided into a series of consecutive periods of time either in-care or out-of-care. Engagement in care was summarised via the proportion of potential time spent in-care and out-of-care, the number of distinct periods in-care, the number of clinic attendances per year, and the frequency of clinical and laboratory monitoring. The characteristics of participants who were continuously in-care were compared to those who had disengaged at any point.
Three types of treatment failure were considered, each defined after a minimum of six months following second-line ART switch. The primary treatment failure outcome of interest was viral failure, defined as HIV-1 RNA >1,000 copies/mL. Secondary outcomes were immunologic failure, defined as CD4 count <100 cells/mm3 or any decrease in CD4 count from the “baseline” value (at second-line ART switch); and severe weight loss, defined as a decrease of >10% of bodyweight compared to the baseline value.
Risk factors for treatment failure were investigated during the first in-care period using Cox proportional hazards models to estimate hazard ratios (HR) and associated 95% confidence intervals (CI). Age, sex and year at the time of second-line ART switch were included a priori. Multivariable models were fitted parsimoniously. Variables associated with the outcome of interest in univariable analyses (p<0.1) were included in the multivariable analyses and retained if they remained significant (p<0.05). If two factors that were associated with the outcome of interest in univariable analysis were also strongly associated with each other, then the one with the strongest association with the outcome of interest was carried forward for the final model. The other was included instead in a sensitivity analysis. For analyses of each endpoint, individuals without the necessary clinical assessments during routine care at the time of and after second-line ART switch were excluded.
Sensitivity analyses were performed to examine the effect of different definitions of time-in-care, treatment failure and the population included. The study definition of time-in-care as up to 12 months after the last clinic attendance was varied to allow up to 6, 24 or 36 months in-care after the last clinic attendance. The viral load threshold for viral failure was varied from the study definition of >1,000 copies/mL to >400 or >5,000 copies/mL. Immunologic failure was varied from the study definition of any decrease in CD4 count or a CD4 count of <100 cells/mm3, to a >20% decrease in CD4 count, or to just an absolute CD4 count of <100 cells/mm3. Weight loss of >10% was varied to require both >10% and >5kg loss, and to >15% loss. The population included was varied to either include the whole cohort as the denominator, or just those in whom it was possible to ascertain treatment failure, e.g. those with viral load data for the viral failure endpoint, those with CD4 data for immunologic failure, and those with weight data for the weight loss endpoint. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, US).
Ethical approval was granted by the Institute of Human Virology Nigeria Institutional Review Board, the National Health Research Ethics Committee of Nigeria (NHREC/01/01/2007), the University of Maryland, Baltimore Institutional Review Board (HP-00066914), and the University College London Research Ethics Committee (14865/002).
Results
Study participants
Over 9,000 people have been enrolled in the HIV treatment and care programme at the University of Abuja Teaching Hospital. The IHVN database query identified 1,111 individuals as potential second-line ART recipients. One person was excluded as the medical notes could not be found, 28 had not received a protease inhibitor, and five were aged under 15 at the start of second-line ART. A further 46 people were excluded from the analysis as they had less than one year of potential follow-up, having started second-line ART after 26 May 2016. This left 1,031 adults and adolescents eligible for inclusion in the analysis.
Two-thirds of the cohort (671/1031, 65.1%) were women and the median age was 36 (interquartile range [IQR] 31–42). The majority (720, 71.8%) were educated to at least secondary school level and 466 (47.1%) were in professional or skilled employment (Table 1). Around one quarter were not in paid employment (264, 26.7%, in the “other” occupation group: 156 housewives, 61 students, 38 unemployed people and nine retirees). Most participants were married (688, 69.4%) and the median number of dependants was three (IQR 1–4). The median travel time to the clinic was 90 minutes (IQR 45–120), although the maximum journey time was over ten hours. First-line ART containing nevirapine or efavirenz had been given for a median duration of 2.5 years (IQR 1.5–4.1) prior to second-line switch. Three quarters of participants (768, 74.5%) had a HIV-1 RNA measured during first-line therapy (median 56,872 copies/mL, IQR 12,831–178,658). The median CD4 count at switch to second-line ART was 173 cells/mm3 (IQR 80–294) in women and 116 cells/mm3 (IQR 52–205) in men (p<0.0001).
Table 1.
Participant characteristics overall and stratified by engagement in care status
| All n=1031 | Disengaged n=341 | Stayed in-care n=690 | p value* | |
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Male, n (%) | 360 (34.9) | 142 (41.6) | 218 (31.6) | 0.002 |
| Female, n (%) | 671 (65.1) | 199 (58.4) | 472 (68.4) | |
| Age | ||||
| years, median (IQR) | 36 (31–42) | 35 (30–42) | 36 (31–42) | 0.06 |
| <30, n (%) | 202 (19.6) | 85 (24.9) | 117 (17.0) | 0.02 |
| 30–39, n (%) | 488 (47.3) | 151 (44.3) | 337 (48.8) | |
| 40–49, n (%) | 253 (24.5) | 76 (22.3) | 177 (25.7) | |
| ≥50, n (%) | 88 (8.5) | 29 (8.5) | 59 (8.6) | |
| Year ** | ||||
| 2005–2008, n (%) | 290 (28.1) | 127 (37.2) | 163 (23.6) | <0.0001 |
| 2009–2012, n (%) | 451 (43.7) | 154 (45.2) | 297 (43.0) | |
| 2013–2016, n (%) | 290 (28.1) | 60 (17.6) | 230 (33.3) | |
| Viral load ** | ||||
| log10 copies/mL, median (IQR) | 4.75 (4.10–5.25) | 4.82 (4.13–5.31) | 4.71 (4.10–5.23) | 0.25 |
| CD4 count ** | ||||
| cells/mm3, median (IQR) | 151 (64–261) | 125 (48–246) | 161 (75–267) | 0.003 |
| Weight ** | ||||
| kg, median (IQR) | 61.0 (53.0–71.0) | 59.0 (51.3–68.0) | 62.0 (54.8–72.1) | 0.0002 |
| Education | ||||
| None/Primary, n (%) | 283 (27.4) | 98 (29.5) | 185 (27.6) | 0.03 |
| Secondary, n (%) | 384 (38.3) | 141 (42.5) | 243 (36.2) | |
| Post-secondary, n (%) | 336 (33.5) | 93 (28.0) | 243 (36.2) | |
| Occupation | ||||
| Professional, n (%) | 95 (9.6) | 25 (7.6) | 70 (10.6) | 0.11 |
| Skilled, n (%) | 371 (37.5) | 118 (35.8) | 253 (38.3) | |
| Elementary, n (%) | 260 (26.3) | 101 (30.6) | 159 (24.1) | |
| Other, n (%) | 264 (26.7) | 86 (26.1) | 178 (27.0) | |
| Marital status | ||||
| Single, n (%) | 173 (17.4) | 65 (19.5) | 108 (16.4) | 0.12 |
| Married, n (%) | 688 (69.4) | 217 (65.2) | 471 (71.5) | |
| Other, n (%) | 131 (13.2) | 51 (15.3) | 80 (12.1) | |
| Dependants | ||||
| median (IQR) | 3 (1–4) | 3 (1–4) | 3 (1–4) | 0.80 |
| Travel time to clinic | ||||
| minutes, median (IQR) | 90 (45–120) | 90 (45–150) | 85 (45–120) | 0.03 |
| First-line ART duration | ||||
| years, median (IQR) | 2.5 (1.5–4.1) | 2.1 (1.3–3.1) | 2.6 (1.6–4.5) | <0.0001 |
p value for Chi-square or Kruskal-Wallis test, as appropriate.
Year, viral load (HIV-1 RNA), CD4 count and weight at start of second-line therapy.
Missing (all, in-care, disengaged): education - 28, 19, 9; occupation - 41, 30, 11; marital status- 39, 31, 8; dependants - 89, 60, 29; travel time - 6, 4, 2; first-line antiretroviral therapy (ART) duration - 114, 65, 49; viral load - 319, 203, 116; CD4 count - 75, 45, 30; weight - 67, 51, 16.
Second-line ART regimens
Most participants started ritonavir-boosted lopinavir as part of second-line ART (846, 82.1%). Other protease inhibitors started included ritonavir-boosted atazanavir in 167 (16.2%), mainly from 2013 onwards. Older agents had been started between 2005 and 2008, including saquinavir in 12 participants (1.2%), indinavir in five (0.5%), and nelfinavir in one (0.1%). The most common nucleos(t)ide reverse transcriptase inhibitor (NRTI) agents prescribed with the protease inhibitor were tenofovir disoproxil fumarate, zidovudine, lamivudine and emtricitabine, which were prescribed in some combination in 805 (78.1%) participants. Other combinations, including abacavir, stavudine and/or didanosine were also used.
Engagement in-care
About 95% of participants (983/1031) returned to the clinic at least once after starting second-line ART. Seventeen cohort members (1.6%) died at a median of 5.2 months (IQR 1.1–24.7) after switching to second-line ART. Seventy people (6.8%) formally transferred care to another treatment facility.
Overall, the median potential time in-cohort (from second-line initiation to death, transfer or study end) was 6.9 years (IQR 3.7–8.5), but only two-thirds of participants (690/1031, 66.9%) were continuously in-care for all of their potential follow-up time. Participants who spent time out-of-care were more likely to be male, aged under 30, to have started ART earlier, to have lower baseline CD4 counts at the start of second-line ART, and were less likely to be educated to post-secondary level, compared to those who remained continuously in-care (Table 1). For participants who remained in-care continuously, the first (and by definition, only) in-care period lasted a median of 5.8 years (IQR 3.4–7.9), compared to a median of 2.3 years (IQR 1.3–4.0) for the first in-care period of those who subsequently disengaged.
Of the 341 (33.1%) participants who became out-of-care, one quarter (89/341, 26.1%) later returned to the clinic for further periods in-care (up to five separate in-care periods in one individual). The characteristics of those who did and did not re-enter care after a period of disengagement were similar, with the exception of the CD4 count at the start of second-line ART, which was lower in those who did not return to care: median 116 cells/mm3 (IQR 41–232) compared to those who later returned to care: 146 cells/mm3 (IQR 87–314), p=0.01. Participants who started second-line ART with a CD4 count of <50 cells/mm3 had the highest proportion of disengagement (43%, 80/186) and the lowest proportion of subsequent re-entry to care. Only 15% (12/80) of this group who had disengaged later returned to clinic during the follow-up period (Table 2).
Table 2.
Disengagement and return to care, by CD4 count at second-line ART initiation
| CD4 count (cells/mm3) | Disengaged (% of all participants) | p value* | Re-engaged (% of disengaged) | p value* |
|---|---|---|---|---|
|
| ||||
| <50 | 80/186 (43.0%) | 12/80 (15.0%) | ||
| 50–200 | 131/420 (31.2%) | 0.002 | 40/131 (30.5%) | 0.04 |
| >200 | 100/350 (28.6%) | 28/100 (28.0%) | ||
p value for Chi-square test
The total time spent in-care for all periods for all participants was 4,773 person-years, representing 75.4% of the total 6,333 person-years that could potentially have been contributed by the cohort. The median number of clinic attendances per year was 4.4 (IQR 3.6–4.8).
Ascertainment of treatment failure
Not everyone had an HIV viral load, CD4 count and weight recorded during second-line therapy. The ascertainment of immunologic or weight failure also required baseline measurements prior to second-line initiation for comparison. Sufficient measurements to assess viral, immunologic and weight failure were available for 582 (56.5%), 822 (79.7%) and 840 (81.5%) people, respectively. Overall, treatment failure could not be assessed through any of the three approaches for 122 (11.8%) participants, including nine people who died and 17 who transferred care to another centre (median time in-care: 6.6 months, IQR 6.0 – 9.0). Among the whole cohort, assessments of weight, CD4 count and viral load were recorded a median (IQR) of 2.5 (1.9–3.1), 1.2 (1.0–1.5), and 0.26 (0.16–0.38) times per year of second-line therapy, respectively.
Viral failure
Viral failure was detected in 112 participants (19.8% of those with viral load data, 10.9% of the cohort), at a median of 2.6 years (IQR 1.2–4.9) after switch to second-line ART. The median viral load at viral failure was 28,103 copies/mL (IQR 5,520–124,340). Most people (85/112, 75.9%) did not have a repeat viral load measurement to confirm viral failure. In univariable analyses (Table 3) participants with higher levels of education and more professional occupations were less likely to experience viral failure. Participants with lower baseline weight and those who started second-line ART in later years were more likely to experience viral failure. Baseline viral load was strongly associated with viral failure, with over 50% increase in the hazard of viral failure for every log10 increment in baseline HIV-1 RNA measurement. Results from the multivariable model confirmed the greatest risk of viral failure was among people with elementary occupations, high viral loads at first-line ART failure, and those starting second-line ART more recently.
Table 3.
Risk factors for treatment failure in univariable and multivariable Cox regression models
| Viral failure | Immunologic failure | Weight loss | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted HR (95% CI) n=565 |
Adjusted HR* (95% CI) n=394 |
Unadjusted HR (95% CI) n=809 |
Adjusted HR* (95% CI) n=809 |
Unadjusted HR (95% CI) n=831 |
Adjusted HR* (95% CI) n=831 |
|
|
| ||||||
| Sex | ||||||
| Male | reference | reference | reference | reference | reference | reference |
| Female | 1.12 (0.76–1.66) | 1.10 (0.65–1.87) | 0.90 (0.70–1.16) | 0.77 (0.58–1.01) | 1.37 (1.01–1.88) | 1.31 (0.94–1.82) |
| Age | ||||||
| per 5 years | 0.96 (0.85–1.07) | 0.91 (0.78–1.07) | 1.02 (0.95–1.10) | 1.01 (0.94–1.09) | 0.92 (0.85–1.01) | 0.93 (0.85–1.03) |
| Year ** | ||||||
| 2005–2008 | reference | reference | reference | reference | reference | reference |
| 2009–2012 | 1.18 (0.74–1.89) | 1.14 (0.62–2.10) | 0.93 (0.70–1.23) | 0.87 (0.66–1.15) | 1.19 (0.87–1.64) | 1.16 (0.84–1.59) |
| 2013–2016 | 2.54 (1.48–4.35) | 3.14 (1.53–6.43) | 0.83 (0.58–1.19) | 0.78 (0.54–1.12) | 0.52 (0.29–0.93) | 0.51 (0.28–0.93) |
| Viral load ** | ||||||
| per log10 copies/mL | 1.56 (1.19–2.04) | 1.48 (1.13–1.93) | 0.97 (0.87–1.08) | - | 0.97 (0.86–1.09) | - |
| CD4 count ** | ||||||
| per 50 cells/mm3 | 0.99 (0.94–1.04) | - | 1.11 (1.09–1.13) | 1.12 (1.10–1.14) | 1.02 (0.98–1.05) | - |
| Weight ** | ||||||
| per 5kg | 0.92 (0.85–0.99) | - | 0.98 (0.93–1.02) | - | 1.05 (0.99–1.10) | 1.06 (1.00–1.11) |
| Occupation | ||||||
| Professional | 0.19 (0.06–0.61) | 0.17 (0.04–0.70) | 0.56 (0.33–0.94) | - | 0.84 (0.47–1.50) | - |
| Skilled | 0.65 (0.42–1.02) | 0.56 (0.33–0.95) | 0.77 (0.57–1.05) | - | 1.10 (0.76–1.60) | - |
| Other | 0.60 (0.36–1.01) | 0.33 (0.16–0.68) | 0.95 (0.68–1.31) | - | 1.10 (0.73–1.64) | - |
| Elementary | reference | reference | reference | - | reference | - |
| Education | ||||||
| None/primary | reference | - | reference | - | reference | - |
| Secondary | 0.80 (0.52–1.24) | - | 1.05 (0.77–1.43) | - | 1.09 (0.77–1.57) | - |
| Post-secondary | 0.45 (0.28–0.75) | - | 0.96 (0.70–1.32) | - | 0.78 (0.54–1.15) | - |
| Marital status | ||||||
| Single | reference | - | reference | - | reference | - |
| Married | 0.85 (0.51–1.43) | - | 0.85 (0.62–1.17) | - | 0.66 (0.46–0.94) | - |
| Other | 0.83 (0.40–1.72) | - | 0.58 (0.35–0.96) | - | 0.97 (0.60–1.56) | - |
| Dependants | ||||||
| per dependant | 1.05 (0.99–1.11) | - | 0.99 (0.95–1.04) | - | 0.95 (0.90–1.01) | - |
| Travel time to clinic | ||||||
| per hour | 1.02 (0.90–1.15) | - | 0.94 (0.85–1.03) | - | 1.03 (0.93–1.13) | - |
HR - hazard ratio, CI - confidence interval
adjusted for sex, age, year and other variables as shown
Year, viral load (HIV-1 RNA), CD4 count and weight at start of second-line therapy.
Immunologic failure
Immunologic failure occurred in 257 participants (31.8% of those with CD4 count data, 24.9% of the cohort). It was more common among participants with higher CD4 counts at second-line ART switch with an adjusted HR of 1.11, 95% CI 1.09–1.13, for every 50 cells/mm3 higher baseline value (Table 3). There was a trend towards women having a lower hazard of immunologic failure than men once this was adjusted for baseline CD4 count (adjusted HR 0.77, 95% CI 0.58–1.01, p=0.06).
Weight loss
Weight loss was observed in 190 participants (22.9% of those with weight data, 18.4% of the cohort). Weight loss was 37% more common in women in univariable analysis, though the effect was attenuated after controlling for other factors (Table 3). People starting second-line ART between 2013 and 2016, had around half the risk of weight loss compared to those starting between 2005 and 2008. In the univariable analysis, married people had two thirds of the risk of weight loss of single people but the marital status did not remain significant in multivariable analyses.
Correlation between different types of treatment failure
At least one type of treatment failure occurred in 422 (40.9%) participants, but there was little overlap between the different types of treatment failure (Figure 1). Of those with viral failure, 51/112 (46%) did not have any other type of treatment failure, 56/112 (50%) also had immunologic failure, and 25/112 (22%) also had weight loss.
Figure 1.
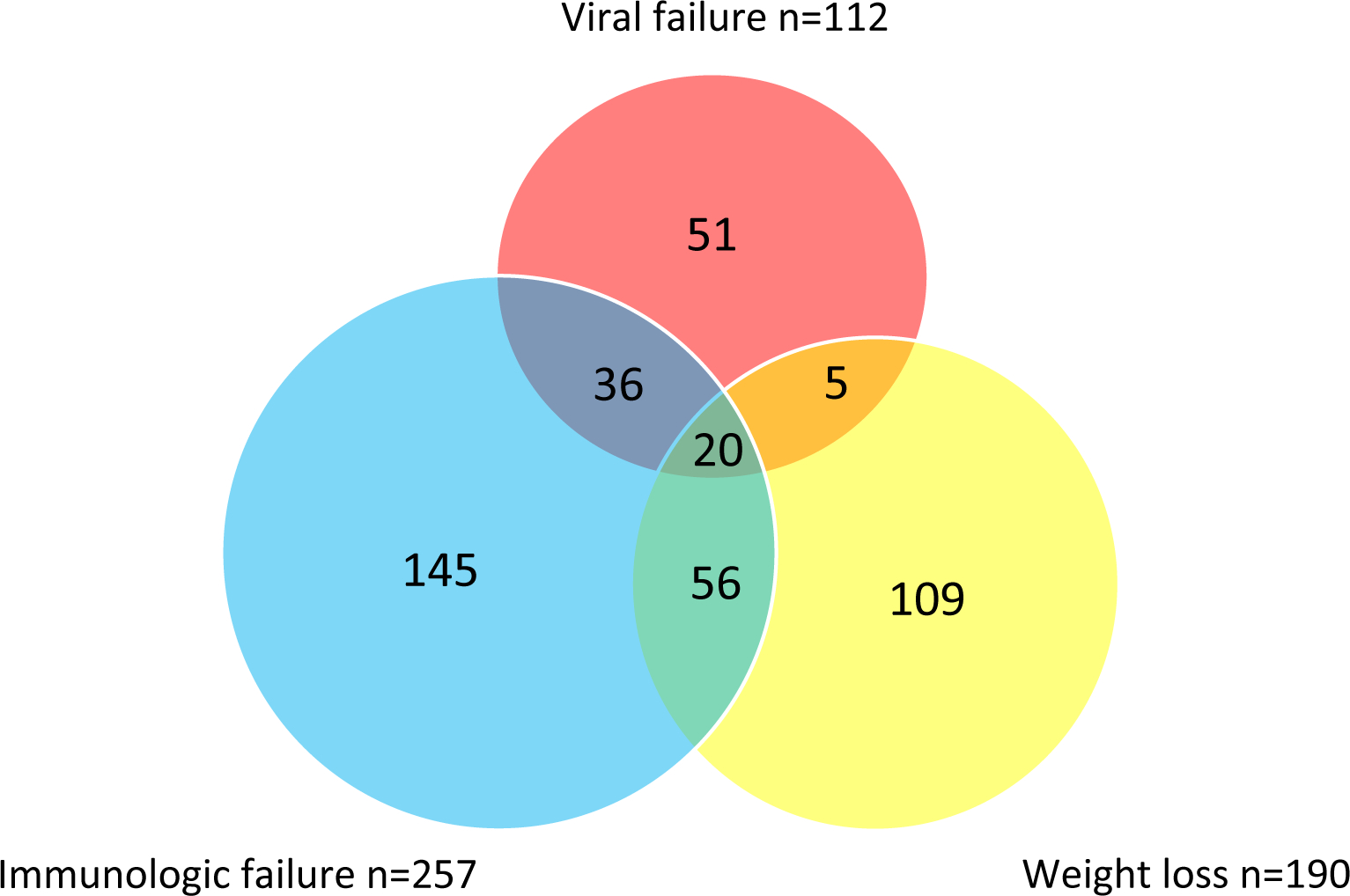
Venn diagram of treatment failure events
Sensitivity analyses
A series of sensitivity analyses were performed to check the robustness of the findings to differences in study definitions; conclusions from all analyses were similar. The number of treatment failure events using different definitions of treatment failure and time in-care are shown in Supplementary table 1. The multivariable Cox regression models for viral failure using different definitions of time in-care, viral load threshold and population included are shown in Supplementary tables 2–3. The corresponding analyses are shown for immunologic failure in Supplementary tables 4–5 and for weight loss in Supplementary tables 6–7. Changing the definition of disengagement from a 12-month absence from clinic to six months since the last attendance would result in 55.5% (572/1031) of participants being considered to have disengaged during follow-up.
The decision to exclude the 46 people who had less than one year of potential follow-up before the study end was tested by examining their outcomes. One had experienced viral failure, two had immunologic failure and one had weight loss. If the definition of disengagement was revised to allow six months in-care since last clinic attendance, then seven (15.2%) of the 46 would be considered to have disengaged.
Discussions
In this study of PLHIV in Nigeria, one third of the cohort members at least transiently disengaged from care after switching to second-line ART and 40% experienced treatment failure. Disengagement was more common in men, young people, those with a lower education level, and those with a lower CD4 count at switch to second-line ART. One of the strengths of the study is that follow-up was not censored at a pre-defined threshold for loss-to-follow-up, but rather participants could become out-of-care then later return to care and contribute more data. Importantly, we found that one quarter of participants who had disengaged from care later returned to clinic. Participants with a very low CD4 count of <50 cells/mm3 were more likely to disengage from care and were less likely to re-enter care following disengagement. This is concerning as these people who were profoundly immunosuppressed were most in need of lifesaving care. However, these data show that it is possible to re-engage people and strategies are needed to increase the proportion of PLHIV that return to care, perhaps via dedicated services to trace and welcome back those who have disengaged.
In the absence of a standard definition in the literature, engagement in care may be determined by the number and proportion of missed appointments, the visit constancy (i.e. whether a person attended clinic within a specified period) or by identifying gaps in clinic attendance that exceed a particular length of time.14 These varying definitions hamper direct comparisons with other studies. Furthermore, the bulk of the literature describes retention during first-line ART,3,6,14–16 which is known to be significantly impacted by the deaths of patients soon after ART initiation, so may not be applicable to engagement in care during second-line treatment. On the other hand, studies published to date usually have a much shorter observation period over which participants can become lost to follow-up. For instance, a multicentre study in Nigeria that included the present study site, reported retention in care of 74% of first-line ART recipients,5 but this was over a median follow-up of around seven months, so may have missed the attrition that can occur later in the course of ART as a result of treatment fatigue and a return to health.14 Indeed we found that people who had started second-line ART in earlier years were more likely to become out of care, which is likely to reflect a longer potential follow-up period, prior to the end of the study.
Among participants with sufficient data to assess treatment failure, one fifth was diagnosed with viral failure, one third had immunologic failure and one quarter had severe weight loss, although these measures of treatment failure often identified different individuals. This mirrors the results from previous first-line ART studies from sub-Saharan Africa that have shown clinical and immunologic failure to be poor predictors of viral failure during first-line ART.10,17–19
Viral failure was identified less often than one might expect from other second-line studies,20–25 particularly when considering the long follow-up period of this analysis. However, the low frequency of viral load measurements, which was only done in 55% of participants, meant that this is likely to be an underestimate of the true proportion of the cohort that had viral failure. This is also indicated by the fact that participants who started second-line ART in the 2013–2016 period, when viral load testing was performed more frequently, were more than twice as likely to have viral failure compared to those starting in the earliest study period.
People with professional occupations were about five times less likely to experience viral failure than those with elementary jobs. Likewise, a higher level of education was associated with a lower risk of viral failure. This may reflect an improved knowledge of HIV and the importance of adhering to ART, or it may be that those participants with higher socioeconomic status had lifestyles that were more conducive to adhering to a daily regimen. The baseline viral load, measured prior to switch to second-line ART, was strongly associated with the risk of second-line viral failure. This is likely to reflect poor adherence to ART during first-line therapy that continued during second-line, as compared to participants with an undetectable viral load who had presumably switched to second-line therapy for other reasons, such as clinical or immunologic failure, or toxicity. This finding is similar to that observed in the SECOND-LINE and EARNEST trials, as well as observational studies.22,26,27
High CD4 count at the start of second-line ART was the strongest predictor of immunologic failure. This has been reported previously with pre-treatment CD4 count and first-line immunologic failure,28–30 however unlike these studies we did not find an association between immunologic failure and low CD4 count. This may reflect differences between first-line and second-line ART recipients. For example, people with advanced disease and low CD4 counts on first-line therapy may be less likely to survive to make the switch to second-line. In general, women started second-line ART with less advanced infection, as evidenced by higher baseline CD4 counts compared to men. When adjusted for CD4 count, there was a trend towards women having a lower hazard of immunologic failure, although this was not statistically significant.
Weight is an important predictor of survival in PLHIV.31–34 Participants who switched to second-line ART most recently, between 2013 and 2016, were less likely to have weight loss and this may reflect better overall health. The test and treat strategy introduced in that period meant that ART was started earlier in the natural history of the infection, without waiting for clinical and immunologic deterioration. There is a paucity of data on weight loss during second-line ART in low and middle income countries and it is not clear whether it is as important as weight loss following first-line ART initiation in terms of associated mortality.35,36 However, it is plausible that weight loss may be a particular problem in this setting with the widespread use of the protease inhibitor lopinavir, which can cause diarrhoea and lipid abnormalities.37 There was a trend towards weight loss being more common in women. The Nigeria Demographic and Health Survey reported that women are generally more at risk of chronic energy deficiency than men, particularly during their reproductive years, for reasons that include poor intake and uneven household food distribution.
This retrospective cohort analysis has some limitations. It may not have captured all deaths, which in previous studies have accounted for a significant proportion of attrition.3,15 However, one might expect a lower mortality among people switching to second-line regimens who have already survived first-line therapy, compared to people who have just initiated ART for the first time. The deaths that were recorded in the present study did tend to occur early after switch to second-line ART. It is possible that many of the people who disengaged and did not return to care had in fact died out of care. While contact tracing was utilized to ascertain reasons for non-attendance at clinic visits, it was not always possible to make contact in order to differentiate loss to follow-up due to death or any other reason. This strengthens the case for increased efforts to promote retention in care and re-engagement, particularly in people with low CD4 counts.
As the Nigerian national guidelines recommend follow-up every 3–6 months, it was felt that a disengagement definition of six months would be too strict and would likely capture participants whose appointments had been scheduled a few days after this period, or those in whom there may be missing data for some attendances. Therefore, one year was selected as an appropriate cut-off to define disengagement. This resulted in 33% of the cohort considered to have disengaged, compared to 55% had a stricter six-month definition been used. By definition, those considered disengaged using a six-month cut-off but not the one-year cut-off had returned to care within the year. However, only one quarter of the people considered disengaged using a one-year cut-off ever attended the clinic again. Exclusion of participants with less than one year of exposure to second-line ART may have introduced some bias to our results. However, we found that these participants were less likely to disengaged from care than other cohort participants when applying the stricter definition for disengagement within six months that could be applied to participants with limited observation time. Our primary findings may therefore slightly overestimate the proportion of participants who disengaged from care.
With regard to the treatment failure outcomes, selection bias may have been introduced by differences in monitoring of viral load, CD4 count and weight. The study definition of viral failure only required a single measurement, rather than two consecutive measurements as recommended by the WHO,8 because in most cases repeat viral load measurements had not been performed. The lack of routine genotypic resistance testing means it is not known whether virological failure was associated with drug resistance. We have previously reported extensive NRTI and NNRTI resistance at the time of first-line failure at this centre, so it is likely that the protease inhibitor was the only fully active agent in many second-line regimens.9 Further work is required to determine the prevalence of major protease resistance at second-line failure in this cohort.
The study definition of immunologic failure included a decrease in CD4 from baseline or <100, similar to the WHO definition.8 While it is recognised that a decrease in CD4 from a high level may not represent a significant deterioration, this cohort initiated second-line therapy with a median CD4 count of 151, and so would be expected to have an increase of 50–100 cells/mm3 during successful therapy, according to national guidelines.11–13 With regard to the weight loss definition, there were no other anthropometric measurements to contextualise the weight loss observed, and weight loss may have been a positive outcome if some participants were overweight at baseline.
During the study period there were few management options in the case of second-line treatment failure. Efforts concentrated on optimising adherence and it was recognised that a second-line regimen may have only partial activity. There were no third-line regimens available through the ART programme at that time. Salvage therapy, which may have included darunavir and/or raltegravir, would have to be sourced privately and was prohibitively expensive for most people. In 2018, the second-generation integrase inhibitor dolutegravir was rolled out for first-line therapy and for second-line therapy following failure of a NNRTI-based regimen. Some of the existing second-line PI recipients may have been switched to a third-line dolutegravir regimen, and further research is underway to examine the success of this strategy. We previously reported that the HIV-1 subtypes circulating in this cohort had a high prevalence of the integrase L74I variant, prior to integrase inhibitor exposure in this population.38 This variant has been associated with the emergence of major integrase resistance in trial participants with subtype A infection and may confer reduced susceptibility to this class.39 It is hoped that a regimen containing two NRTIs and dolutegravir will be efficacious even in the context of NRTI resistance, as was the case when dolutegravir was used as a second-line agent in the NADIA trial.40 However, studies into the outcomes of protease inhibitor therapy remain important, as protease inhibitors are recommended for second-line therapy following first-line dolutegravir failure.1
This in-depth analysis of a large second-line HIV treatment cohort in Nigeria shows that disengagement from care and treatment failure were common over several years of follow-up. Improved understanding of the long-term patterns of engagement in care may aid planning of ART programmes. Knowledge of the risk factors associated with adverse outcomes on second-line protease inhibitor-based regimens allows healthcare practitioners to identify people at risk and target resources effectively. This may include interventions aimed at improving engagement, return to care and adherence to second-line therapy, as well as increasing the availability of third-line ART regimens.
Supplementary Material
Acknowledgments
We are grateful to the patients and staff at the University of Abuja Teaching Hospital. This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of U2G GH002099-01, PA GH17-1753. K.E.B. is supported by Wellcome Trust Clinical Ph.D. fellowship in Clinical Science (WT170461); T.A.C. was supported by a cooperative agreement between the U.S. Army and the Henry M. Jackson Foundation for the Advancement of Military Medicine (W81XWH-18-2-0040: the views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, or Henry M. Jackson Foundation for the Advancement of Military Medicine); R.K.G. is supported by Wellcome Trust Senior Fellowship in Clinical Science (WT108082AIA); N.N. is supported by the National Institutes of Health (R01 AI147331-01); C.A.S. has received funding for membership of Advisory Boards, Speaker panels and for preparation of educational materials from Gilead Sciences, Viiv Healthcare and Janssen-Cilag.
Source of Funding:
This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of U2G GH002099-01, PA GH17-1753.
Footnotes
Conflicts of Interest: K.E.B. is supported by Wellcome Trust Clinical Ph.D. fellowship in Clinical Science (WT170461); T.A.C. is supported by a cooperative agreement between the U.S. Army and the Henry M. Jackson Foundation for the Advancement of Military Medicine (W81XWH-18-2-0040: the views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, or Henry M. Jackson Foundation for the Advancement of Military Medicine); R.K.G. is supported by Wellcome Trust Senior Fellowship in Clinical Science (WT108082AIA); N.N. is supported by the National Institutes of Health (R01 AI147331-01); C.A.S. has received funding for membership of Advisory Boards, Speaker panels and for preparation of educational materials from Gilead Sciences, Viiv Healthcare and Janssen-Cilag. For the remaining authors none were declared.
References
- 1.WHO. Update of recommendations on first- and second-line antiretroviral regimens. Geneva, Switzerland: World Health Organization. 2019. (WHO/CDS/HIV/19.15); [Google Scholar]
- 2.Kiweewa F, Esber A, Musingye E, et al. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLoS One. 2019;14(2):e0211344. doi: 10.1371/journal.pone.0211344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. Oct 16 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr. May 1 2015;69(1):98–108. doi: 10.1097/QAI.0000000000000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charurat M, Oyegunle M, Benjamin R, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS One. May 11 2010;5(5):e10584. doi: 10.1371/journal.pone.0010584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meloni ST, Chang C, Chaplin B, et al. Time-Dependent Predictors of Loss to Follow-Up in a Large HIV Treatment Cohort in Nigeria. Open Forum Infect Dis. Sep 2014;1(2):ofu055. doi: 10.1093/ofid/ofu055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier D, Iwuji C, Derache A, et al. Virological Outcomes of Second-line Protease Inhibitor-Based Treatment for Human Immunodeficiency Virus Type 1 in a High-Prevalence Rural South African Setting: A Competing-Risks Prospective Cohort Analysis. Clin Infect Dis. Apr 15 2017;64(8):1006–1016. doi: 10.1093/cid/cix015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2013. WHO Guidelines Approved by the Guidelines Review Committee. [PubMed] [Google Scholar]
- 9.El Bouzidi K, Datir RP, Kwaghe V, et al. Deep sequencing of HIV-1 reveals extensive subtype variation and drug resistance after failure of first-line antiretroviral regimens in Nigeria. J Antimicrob Chemother. Nov 6 2021;doi: 10.1093/jac/dkab385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndembi N, Murtala-Ibrahim F, Tola M, et al. Predictors of first-line antiretroviral therapy failure among adults and adolescents living with HIV/AIDS in a large prevention and treatment program in Nigeria. AIDS Res Ther Nov 3 2020;17(1):64. doi: 10.1186/s12981-020-00317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National guidelines from HIV and AIDS treatment and care in adolescents and adults (Federal Ministry of Health, Abuja, Nigeria: ) (2007). [Google Scholar]
- 12.National guidelines from HIV and AIDS treatment and care in adolescents and adults (Federal Ministry of Health, Abuja, Nigeria: ) (2010). [Google Scholar]
- 13.National guidelines for HIV prevention treatment and care (Federal Ministry of Health, Abuja, Nigeria: ) (2016). [Google Scholar]
- 14.Ugoji C, Okere N, Dakum P, et al. Correlates of patient retention in HIV care and treatment programs in Nigeria. Current HIV Research. 2015;13:300–307. [DOI] [PubMed] [Google Scholar]
- 15.Geng EH, Odeny TA, Lyamuya R, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. Apr 01 2016;62(7):935–944. doi: 10.1093/cid/civ1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan AS, Mwaringa SM, Ndirangu KK, Sanders EJ, de Wit TF, Berkley JA. Incidence and predictors of attrition from antiretroviral care among adults in a rural HIV clinic in Coastal Kenya: a retrospective cohort study. BMC Public Health. May 10 2015;15:478. doi: 10.1186/s12889-015-1814-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joram SL, Paul G, Moses K, et al. Misdiagnosis of HIV treatment failure based on clinical and immunological criteria in Eastern and Central Kenya. BMC Infect Dis. Jun 2 2017;17(1):383. doi: 10.1186/s12879-017-2487-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunda DW, Kidenya BR, Mshana SE, Kilonzo SB, Mpondo BC. Accuracy of WHO immunological criteria in identifying virological failure among HIV-infected adults on First line antiretroviral therapy in Mwanza, North-western Tanzania. BMC Res Notes. Jan 17 2017;10(1):45. doi: 10.1186/s13104-016-2334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanapathipillai R, McGuire M, Mogha R, Szumilin E, Heinzelmann A, Pujades-Rodriguez M. Benefit of viral load testing for confirmation of immunological failure in HIV patients treated in rural Malawi. Trop Med Int Health. Dec 2011;16(12):1495–500. doi: 10.1111/j.1365-3156.2011.02874.x [DOI] [PubMed] [Google Scholar]
- 20.Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. May 15 2012;26(8):929–38. doi: 10.1097/QAD.0b013e328351f5b2 [DOI] [PubMed] [Google Scholar]
- 21.Stockdale AJ, Saunders MJ, Boyd MA, et al. Effectiveness of protease inhibitor/nucleos(t)ide reverse transcriptase inhibitor-based second-line antiretroviral therapy for the treatment of HIV-1 infection in sub-Saharan Africa: systematic review and meta-analysis. Clin Infect Dis. Dec 20 2018;doi: 10.1093/cid/cix1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edessa D, Sisay M, Asefa F. Second-line HIV treatment failure in sub-Saharan Africa: A systematic review and meta-analysis. PLoS One. 2019;14(7):e0220159. doi: 10.1371/journal.pone.0220159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onyedum CC, Iroezindu MO, Chukwuka CJ, Anyaene CE, Obi FI, Young EE. Profile of HIV-infected patients receiving second-line antiretroviral therapy in a resource-limited setting in Nigeria. Trans R Soc Trop Med Hyg. Oct 2013;107(10):608–14. doi: 10.1093/trstmh/trt071 [DOI] [PubMed] [Google Scholar]
- 24.Osinusi-Adekanmbi O, Stafford K, Ukpaka A, et al. Long-term outcome of second-line antiretroviral therapy in resource-limited settings. J Int Assoc Provid AIDS Care. Jul-Aug 2014;13(4):366–71. doi: 10.1177/2325957414527167 [DOI] [PubMed] [Google Scholar]
- 25.Dapiap SB, Adelekan BA, Ndembi N, Murtala-Ibrahim F, Dakum PS, Aliyu AT. Immunological and clinical assessment of adult HIV patients following switch to second-line antiretroviral regimen in a large HIV Program in North-central Nigeria. Journal of AIDS and HIV Research. 2017;9(5):106–116. doi: 10.5897/jahr2017.0416 [DOI] [Google Scholar]
- 26.Boyd MA, Moore CL, Molina J-M, et al. Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: an exploratory analysis. The Lancet HIV. 2015;2(2):e42–e51. doi: 10.1016/s2352-3018(14)00061-7 [DOI] [PubMed] [Google Scholar]
- 27.Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. Jul 17 2014;371(3):234–47. doi: 10.1056/NEJMoa1311274 [DOI] [PubMed] [Google Scholar]
- 28.Vanobberghen FM, Kilama B, Wringe A, et al. Immunological failure of first-line and switch to second-line antiretroviral therapy among HIV-infected persons in Tanzania: analysis of routinely collected national data. Trop Med Int Health. Jul 2015;20(7):880–92. doi: 10.1111/tmi.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yirdaw KD, Hattingh S. Prevalence and Predictors of Immunological Failure among HIV Patients on HAART in Southern Ethiopia. PLoS One. 2015;10(5):e0125826. doi: 10.1371/journal.pone.0125826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gesesew HA, Ward P, Woldemichael K, Mwanri L. Immunological failure in HIV-infected adults from 2003 to 2015 in Southwest Ethiopia: a retrospective cohort study. BMJ Open. Aug 17 2018;8(8):e017413. doi: 10.1136/bmjopen-2017-017413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Sande MA, Schim van der Loeff MF, Aveika AA, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. J Acquir Immune Defic Syndr. Oct 1 2004;37(2):1288–94. doi: 10.1097/01.qai.0000122708.59121.03 [DOI] [PubMed] [Google Scholar]
- 32.Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. Mar 15 2006;42(6):836–42. doi: 10.1086/500398 [DOI] [PubMed] [Google Scholar]
- 33.Tang AM, Forrester J, Spiegelman D, Knox TA, Tchetgen E, Gorbach SL. Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. Oct 1 2002;31(2):230–6. doi: 10.1097/00126334-200210010-00014 [DOI] [PubMed] [Google Scholar]
- 34.Kibuuka H, Musingye E, Mwesigwa B, et al. Predictors of All-Cause Mortality among People with HIV in a Prospective Cohort Study in East Africa and Nigeria. Clin Infect Dis. Dec 3 2021;doi: 10.1093/cid/ciab995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biadgilign S, Reda AA, Digaffe T. Predictors of mortality among HIV infected patients taking antiretroviral treatment in Ethiopia: a retrospective cohort study. AIDS Res Ther May 18 2012;9(1):15. doi: 10.1186/1742-6405-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bussmann H, Wester CW, Ndwapi N, et al. Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. AIDS. Nov 12 2008;22(17):2303–11. doi: 10.1097/QAD.0b013e3283129db0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croxtall JD, Perry CM. Lopinavir/Ritonavir: a review of its use in the management of HIV-1 infection. Drugs. Oct 1 2010;70(14):1885–915. doi: 10.2165/11204950-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 38.El Bouzidi K, Kemp SA, Datir RP, et al. High prevalence of integrase mutation L74I in West African HIV-1 subtypes prior to integrase inhibitor treatment. J Antimicrob Chemother. Jun 1 2020;75(6):1575–1579. doi: 10.1093/jac/dkaa033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez C, van Halsema CL. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential. HIV AIDS (Auckl). 2019;11:179–192. doi: 10.2147/HIV.S184642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paton NI, Musaazi J, Kityo C, et al. Dolutegravir or Darunavir in Combination with Zidovudine or Tenofovir to Treat HIV. N Engl J Med. Jul 22 2021;385(4):330–341. doi: 10.1056/NEJMoa2101609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


