Abstract
Laponite is a clay-based material composed of synthetic disc-shaped crystalline nanoparticles with a large and highly ionic surface area. These characteristics enable the intercalation and dissolution of biomolecules in Laponite-based drug delivery systems. Furthermore, Laponite’s innate physicochemical properties and architecture enable the development of tunable pH-responsive drug delivery systems. Laponite’s coagulation capacity and cation exchangeability, determine its exchange capabilities, drug encapsulation efficiency, and release profile. These parameters have been exploited to design highly controlled and efficacious drug delivery platforms for sustained drug release. In this review, we give an overview of how the properties and specific interactions of various Laponite–polymer composite and drug moieties, have been leveraged to design efficient delivery of a number of therapeutics.
Keywords: Laponite, clay nanoparticles, drug delivery, biomaterials, drug carriers, nanotherapeutics
1. Introduction
Natural clays have been utilized throughout history for a myriad of applications including in filtration, as cosmetics, and within the fields of architecture, and healthcare.[1] Within healthcare, clay’s use dates back to 2500 BC, when it was employed as an absorbent for both gastrointestinal diseases as well as other topical ailments and wound treatments [2]. Natural clay minerals are classified according to their fundamental structures, consisting of sheets of tetrahedral silicate and octahedral hydroxide sheets (e.g. kaolin, smectite, illite, and chlorite group).[3] Even though natural clay minerals have been used throughout history, scientific exploration and synthesis of these materials began only in the 1930s.[4] The enhanced purity, control of the structure, and superior material properties of synthetic clays over natural clays have led to the use of a variety of clay-based materials in industrial and pharmaceutical fields.[5] In the 1960s, new types of synthetic hectorite, part of the trioctahedral subgroup of the smectites, started to be produced as nanoparticles at an industrial scale with applications in household products, surface coatings, agriculture, ceramics, enamels, and the oil industry.[6, 7] Specifically, in this period, the paint industry introduced the term Laponite which is a registered trademark owned and actively protected by the manufacturers of Laponite to describe a group of synthetic hectorite-like clay minerals with great colloidal properties.[8] Of specific relevance to the biomedical world, synthetic nanoparticles are inherently free of microorganisms. Consequently, in the 1970s, Laponite particles became of particular interest to the pharmaceutical industry, and as raw materials used in cosmetics and personal care products. [9][10][11]
Laponite is a synthetic trioctahedral clay mineral and belongs to the smectite clay group.[6] Clays within the smectite group have a 2:1 layered structure, with an octahedral sheet positioned between two tetrahedral sheets (TOT).[12] This structure generates sheets with 1 nm thickness and varying widths.[13] Synthetic production techniques have been able to achieve a well-controlled diameter of 25–30 nm for Laponite materials.[14] Laponite is negatively charged (~0.2–0.6 charge/formula unit) due to isomorphic substitution of magnesium ions (Mg2+) with lithium ions (Li+) in the lattice structure forming a net negative charge on the surface that is balanced by cations such as sodium ions.[15][16] The size and charge of the molecules impact their passage through clay systems. Water can become tightly associated with the clay surface and results in slow dynamics.[17] The diffusion of larger molecules depends on the ratio between their size and the spacing of the clay particles.[18] Moreover, for biomedical applications, synthetic nanoparticles have the advantage of being free of microorganisms. In the 1970s, Laponite particles were introduced and garnered significant attention within the pharmaceutical industry, such as cosmetics and personal care products.[11] These favorable physicochemical properties of Laponite particles make them strong drug carrier candidates for controlled drug release purposes.[9, 10, 11, 12, 13] Nevertheless, a recent search for “Laponite” and “drug delivery” in the PubMed database (September 2021) revealed only 77 relevant papers published from 2005 to this date. It is important to note that more than 70% of these 77 papers were published as of 2016, demonstrating that Laponite has become an increasingly attractive nanoparticle-based drug delivery system in recent years due to its unique characteristics, such as pH responsiveness. This review is a comprehensive analysis of Laponite as a drug delivery system and provides a strong resource for the readers.
Although other clay subgroups have been widely studied for drug delivery applications,[24][25][26][27] the main emphasis of this review article is physicochemical features of Laponite with a focus on synthetic Laponite clays as a potential therapeutic drug carrier.[28] We first introduce the background on clay materials, including synthetic Laponite, and describe the essential properties that render Laponite an attractive material for pharmaceutical applications. We discuss the characteristics affecting drug loading capacity and drug release profiles. Additionally, we elaborate on the more recent applications of Laponite-based materials for targeted drug delivery. Finally, we present the studies centered around the development of advanced drug delivery systems addressing specific translational applications for targeted drug delivery. We invite readers interested in non-pharmaceutical applications of clay materials, to the Handbook of Clay Science for an in-depth review.[28]
2. Laponite characteristics
2.1. Physicochemical properties of Laponite
Laponite (LAP) is a white synthetic smectite clay, a layered hydrous magnesium silicate that forms transparent colloidal dispersions in water.[29] Laponite has octahedral layers of magnesium oxide sandwiched between two parallel trioctahedral structure and the following formula: (Si4)IV(Mg3-yLiy)VIO10(OH)2 · yM+ · nH2O
The octahedral sheet is composed of closely packed oxygens and hydroxyls in which magnesium atoms are arranged in octahedral coordination. This tightly formed structure is the key factor to keep Laponite stable in such a small size.[30] The negative surface of Laponite in solid form is balanced by the interlayer sodium ions.[31]When Laponite powder disperses in aqueous media, water gets to the inter-layer of Laponite, causing it to swell. This induces dissociation of Na+ ions and leads to their diffusion into the media due to the osmotic gradient. [30] The release of Na+ ions leads to a permanent negative charge on the faces of Laponite nanoparticles.[6]This property is exploited to load cationic drugs, usually molecules with protonated amino groups, that can be released in aqueous saline solutions (Table 1).[29][32][33] As described more extensively in section 3.3.3, the capacity of Laponite to hold exchangeable cations is called the cation exchange capacity (CEC) which is an inherent characteristic of Laponite.[34] Another important physicochemical property of Laponite is its swellability which depends on the chemical potential of its aqueous solution (the potential to absorb or release energy based on the concentration of Laponite in the solution), as well as the cations present in the interlayer space.[35] The dispersion, aggregation, or coagulation of Laponite can be changed by adjusting its concentration in an aqueous solution or the ionic strength of the solution itself.[33] The dispersion of clay in solution is described as the critical coagulation concentration (ck), which is determined by the type of counter ion used. For example, in an aqueous sodium chloride solution, the Laponite-based clay has a ck of less than 10mmol/L. On the other hand, phosphate, which is common in many biological buffers, strongly buffers the pH of the solution and can affect the ck by affecting the thickness of the ionic layer surrounding the clay (Table 1). Therefore, due to these characteristics of clay materials being extremely hydratable, diffusion of therapeutics through clay-based colloidal suspensions or gels can be widely explored for drug release applications. Especially for hydrophilic molecules, or even for hydrophobic molecules encapsulated within micro- or nanoparticles presenting hydrophilic surfaces.[17] However, water diffusion within clay-based colloidal suspensions or gels is dependent on the age of the solution. The viscosity of the solution increases over time due to the dynamics of clay particle diffusion, limiting water diffusion.
Table 1.
Critical properties of Laponite-based clays.
| Property | Common Values | Comment |
|---|---|---|
|
| ||
| Charge | ~0.2–0.6 charge/formula unit | NA |
| pH | Coagulation by salts pH>6 | pH can serve as a signal to release bound drug molecule |
| Spontaneous coagulation pH<4 | ||
| Cation Exchange Capacity | Mg2+, Li+, Na+ 115 mEq/100g for hectorite | Total specific amount of charge that is balanced by exchangeable cations; determines amount of drug able to be loaded |
| Critical Coagulation Concentration | Ck~10 mmol/L in NaCl | NA |
| Thixotropy | Monotonic increase in viscosity | Stiffening of gels containing Laponite over time |
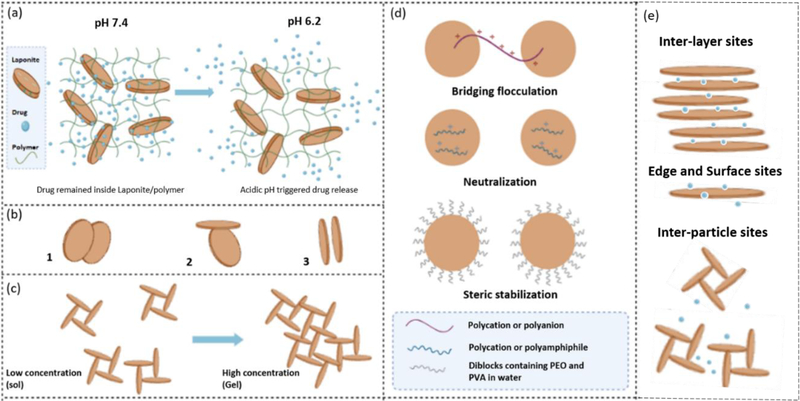
Laponite can interact with organic molecules through different mechanisms, depending on the pH of the medium, electrostatic characteristics and size of the adjoining molecule.[36]These drug molecule interactions occur in inter-particle locations, inter-layer sites or at the surface or the edges of the Laponite particles. Multiple mechanisms such as the formation of Van der Waals bonds cause inter-particle localization of drugs with uncharged, nonpolar, aromatic or alkyl groups. Drugs with cation exchange capabilities (e.g. those containing amin, ring NH and heterocyclic nitrogen groups), cation/water bridging potential (e.g. those containing carboxylate, amins, carbonyl and alcoholic groups) and those which can form Van der Waals bonds can be loaded in inter-layer sites. Mechanisms such as cation exchange capabilities, cation/water bridging potential and hydrogen bonding (e.g. for drugs with amins, carbonyl, carboxyl, heterocyclic nitrogen and phenyl hydroxyl groups) can lead to the surface adsorption of the drugs. Finally, drugs with groups that allow protonation (e.g. those with amins, heterocycle N, carbonyl and carboxylate groups) and ligand exchange (e.g. those with carboxylate or phenolate) can be adsorbed to the edges of Laponite nanoparticles. This shows the broad range of molecules that can interact with Laponite and confirms the extensive potential of Laponite as a drug carrier system.[36]
Microenvironmental conditions also have a significant impact on gelation time. Laponite gelation, for instance, occurs when weak bonds form amid the rim with positive charges and the face with negative charges of the Laponite crystal, leading to the aggregation of Laponite particles.[33] The gelation time is found to increase significantly with decreasing salt and Laponite concentration.[33] The increase in ionic strength leads to a decrease in electrostatic repulsion between the Laponite particles, which consequently helps the formation of aggregates. When salt concentrations go above 11 mM in this case, gelation happens abruptly.[33] However, in Laponite concentrations above 10 g/L, salt concentration does not have any significant role in gelation time.[33] To make homogenous ionic solutions with Laponite, it must first be dispersed in salt-free water, followed by an increase in the ionic strength of the solution.[33]
The orientation of clay particles is commonly referred to as the “house-of-cards” structure, or “T configuration”. This orientation is generated by preferential interactions between the negative charge on the basal plane and the positive-charge of the edge of the particles (Figure 1b, 1c).[14] However, this only applies to lower salt concentrations (<ck) or pH values lower than 11 (the point of zero charges [p.z.c.] for the edges of Laponite particles), a condition in which the positive charge at the edges can have a significant effect on the system. On the other hand, at higher salt concentrations, face to face Van der Waals interactions can dominate and generate band-type aggregates or stacked configurations, which can still transition into the house-of-cards structure (Figure 1c). However, when the salt concentration is too high, it will cause excessive aggregation and precipitation of the clay solution. While the “house of cards” structure is widely accepted for gelification of Laponites and is governed by attractive forces, it is shown that Wigner glasses form when Laponite nanodiscs are kept apart due to electrostatic repulsion at low ionic strength.[31] Depending on the orientation of the clay particles within the gel or nanocomposite, the permeability of water and diffusion of small molecules through the system can change. Well-oriented structures of clay particles can be used as a barrier to gases and liquids,[37] while randomly oriented particles can present a sufficiently tortuous path to decrease permeability.[38]
Figure 1.
Physicochemical characteristics of Laponite. a) pH-responsive characteristics of Laponite-polymer regulate drug release in pathophysiological conditions (acidic).[56] b) Different interactions of Laponite nanodiscs: 1) Overlapping coins configuration, 2) House of card configurations, 3) Configuration of stacked Laponite nanodiscs. c) The structure of Laponite networks at lower and higher particle concentrations.[14] d) Laponite-polymer conjugates/system. Schematics of flocculation and stabilization mechanisms in clay-polymer systems highlighting the role of the clay (circles) and polymers: Polyethylene oxide (PEO) and Polyvinyl alcohol (PVA).[12] e) Potential interactions of drug/Laponite systems: drug molecules can be loaded in inter-particle locations, inter-layer sites or can be adsorbed on the surface or edges depending on the size and charge of drug molecules. Reproduced with permission,[6][35]Adapted with permission.[33]
Given the affinity of Laponite and ions, various structures can be formed by merely changing the pH, ionic strength, or concentration of clay particles, in turn affecting the properties of gels containing the clay particles.[39] This dependence on salt concentration is not straightforward, as systems can have a narrow window of ion concentrations at which increased mechanical properties (e.g., viscosity, strength) are observed. Weak sols (colloid gels) are present on both extreme salt concentration values of some smectite clays, while others see a re-emergence of a gel region at higher salt concentrations.[40]
2.2. Laponite – polymer conjugates
When polymers are combined with Laponite in a solution, flocculation (a process by which unstabilized particles combine to form clusters) or stabilization can occur depending on the concentration of polymer and their interactions with the clay.[41,42] Polymers can flocculate colloidal dispersions at low concentrations via two methods: forming bridges between the Laponite nanoparticle and neutralizing the charge. In a bridging process of flocculation, polymers bind to the surface of two neighboring Laponite nanoparticles. Flocculation through charge neutralization occurs when charges of opposite surfaces of Laponite nanoparticles and polymers neutralize each other. Polyanions produce more effective flocculation of clay dispersions compared with polycations. Polyanions attach to few sites on particles, leaving larger parts of the macromolecules floating free in solution, which can form bridges between adjacent nanoparticles (Figure 1d). However, in the case of polycations, a longer part of the polymer molecule interacts with the negatively charged surface of Laponite; rendering bridging between nanoparticles not as effective as with polyanions (Figure 1d). Alternatively, high concentrations of polymers can re-stabilize dispersion by steric stabilization (Figure 1d). If the solvent is suitable for the polymer and promotes polymer coil expansion, high chain density can occur in the space between Laponite nanoparticles, with solvent penetrating the area, preventing precipitation.[12] Destabilization can occur with the addition of a lower quality solvent for the polymer as the polymer coil contracts (Figure 1d).[43] Hence, polymer structure, as well as charges, are important parameters to be considered when selecting polymers to develop Laponite-based drug delivery systems. Besides, pH-sensitive or stabilizing polymers can also impact the release and stability of drug delivery systems.[44] Natural (e.g., alginate) and synthetic (e.g., Pluronic (F68) and poly(lactide-co-glycolide) (PLGA)) polymers are commonly employed to improve the loading capacity and sustained release of drug-loaded Laponite.[42] As detailed in this section, Laponite characteristics, permeability, diffusion behaviors, and its ability to form polymer conjugates all lend themselves to drug delivery applications.
3. Laponite as a drug carrier
The drugs’ therapeutic efficacy often depends on the high administration’s dosage and frequency, resulting in severe side effects and toxicity.[45] To tackle this issue, drug carriers that deliver a sufficient amount of the drug to the site of action while maintaining a therapeutic level during the treatment period have been frequently practiced.[46] Selecting a suitable drug carrier is essential in various pathologies, including infections, cancer, and inflammatory diseases.[47] Besides, the drug encapsulation strategies can also potentially improve the delivery of compounds with poor solubility, low stability, and high cytotoxicity under physiological conditions.[48]
Laponite is an exciting drug carrier to encapsulate cationic compounds due to its interlayer spaces with a large surface area (330 m2/g) and high negative charge (−39.5 mV for 1 wt% Laponite suspension), allowing the adsorption of positively charged drug molecules.[49] In these Laponite incorporated drug carriers, the release of the molecule occurs through a controlled ion-exchange mechanism.[50] In the following section, we discuss the characteristics which affect drug encapsulation and release from Laponite.
3.1. Characteristics Affecting Drug Encapsulation Efficiency (EE)
The EE is defined as the ratio of the intercalated drug molecule to the total amount of the added drug. The main mechanism of Laponite drug uptake is through intercalation. However, some fractions can be adsorbed on the surface of the particle.[51] As an exfoliated clay mineral, a large surface area and the repulsive electrostatic forces between negatively charged interlayers create sufficient space to encapsulate drug molecules.[52] Drug intercalation leads to an increase in layer spacing (d-spacing). The highly ionic Laponite layer can hold drug molecules via two potential mechanisms depending on the physicochemical properties of the compounds: (I) ion-dipole interaction with hydrophilic molecules and (II) ion-exchange reaction with ionic compounds.[53] The competition between the drug compound and cations (such as Ca2+, Na+, K+) dictates the EE.[54] In the following sections, we explain various factors that affect the EE of the drug in Laponite.
3.1.1. Drug compound and Laponite net charge
The key mechanism of drug intercalation is cation exchange (also see Section 3.3), which depends on both drug and Laponite nanoparticles’ net charge. The main variable that influences Laponite and drug compound charges is the pH.[53] Laponite’s pH-responsive behavior is attributed to the negative charge on its surfaces and the pH-dependent charge of the edges.[55] This pH-dependent property enables Laponite to be protonated or deprotonated based on the microenvironment acidity or alkalinity.[56] Further, owing to the differently charged edges and surfaces, Laponite can therefore adsorb charged species and water independent of each other.[57] On the other hand, the drug molecule’s net charge determines the strength of electrostatic interactions between a drug and Laponite.[52] The higher electrostatic forces between the drug and Laponite increase the encapsulation efficiency. For example, tetracycline is positively charged at acid conditions with pH values lower than 3.3, zwitterionic at the pH range between 3.3 to 7.7, whereas at pH 7.7 to 9.5, it acquires a net charge of −1, which drops to −2 at pH > 9.5. As a result, tetracycline’s intercalation efficiency decreases by increasing the pH level due to the electrostatic repulsive interaction between the negatively charged layer of Laponite and tetracycline (Figure 2).[53] Apart from pH, ionic strength can also affect drug molecules’ net charge via modulating the pKa. Ghadiri et al. showed higher pKa of tetracycline due to the release of Na+ from Laponite, expanding the pH range favorable to encapsulation. A decrease in tetracycline loading was observed in high pH conditions due to a higher net negative value of the drug.[53]
Figure 2.
Impact of pH level on drug intercalation and release. a, b) release curve of tetracycline from Laponite system was assessed in saliva solution. Laponite–tetracycline (L–T) composites were prepared at various pH. c) Schematic representation of the intercalation process of tetracycline between exfoliated Laponite layers and cation exchange process which lead to drug release, Reproduced with permission. Copyright 2013, The Royal Society of Chemistry Advances.[53]
3.1.2. Drug concentration
The process of drug intercalation into Laponite depends upon the concentration of both the Laponite and the drug.[36] Laponite XLG has about 60 meq/100 g CEC, meaning that 100 g Laponite can hold up to 1.38 g of Na+ (23 meq/mg).[58] Assuming complete exchange between all intercalated Na+ ions and drug molecules, the amount of drug encapsulated is related to the molecular weight and osmolarity of the drug. In the case of tetracycline, 26.7 g tetracycline (molecular weight = 444.4 g/mol) could be intercalated in 100 g Laponite (Na+ molecular weight 23g/mol).[53] When the drug concentration exceeds the maximum loading capacity of Laponite, EE will be reduced. For instance, increasing the tetracycline concentration from 0.1 wt% to 0.2 and 0.3 wt% in Laponite dispersion (1 wt%), reduced EE of tetracycline from 99.2% to 97.7%, and 95.5%, respectively.[53]
3.1.3. Laponite concentration
EE is also affected by the Laponite concentration in a drug delivery system [41]; however, the impact of Laponite concentration on EE is still unresolved and described by multiple competing theories. The first theory suggests that increasing the amount of Laponite in the carrier increases the EE. As an example, the EE of pure chitosan is generally around 31%. However, when increasing the amount of Laponite from 10 mg/mL to 40 mg/mL in a Laponite/chitosan sample loaded with ofloxacin, the EE increased from 50% to 91%.[41] Similarly, by increasing the amount of Laponite from 1 to 4 mg/mL in Laponite/chitosan carrier loaded with doxorubicin (DOX), the EE increased from 72% to 98%.[59] Based on these findings, it can be concluded that increasing the amount of Laponite can result in better drug dispersion within its layers, leading to an increase in the EE of the drug.
The second theory contradicts the previous one by claiming that increasing the amount of Laponite in carriers can decrease the EE. For instance, researchers found that by increasing Laponite concentration from 3 to 10 mg/mL in Laponite/chitosan loaded with amoxicillin (AMX), the EE decreased from 10% to 3%.[42] Based on these outcomes, the researchers proposed that a higher concentration of Laponite may lead to aggregation of the Laponite discs into clumps that lower the drug molecules’ ability to access the interlayer space of Laponite, leading to a lower loading efficacy. These contradictory results suggest that an optimum Laponite concentration is needed, depending on the type of loaded drugs.[60]
3.2. Drug loading characterization
A robust strategy for the characterization of drug incorporation in a Laponite layer is critical to ensure quality, safety, and rational development of drug delivery systems for medical applications. The most commonly used methods to demonstrate drug incorporation are Fourier-transform infrared spectroscopy (FTIR), x-ray diffraction (XRD), scanning electron microscopy (SEM), and zeta potential. The aforementioned techniques can be leveraged to measure the size, shape, composition, purity, surface property stabilities, and dispersion state of nanometric systems. The characterization of drug intercalation and its impact on the d-spacing (the distance between planes of clay) was studied by Ordikhani et al. In this study, the XRD pattern of Laponite demonstrated a peak at 2θ=6.57° related to (001) plane with d-spacing of 13.43 A°. This specific peak dropped to 3.79° for the chitosan/Laponite nanocomposite film, suggesting an increase in Laponite particles’ plane spacing. No peaks related to Laponite in the XRD pattern were detected after adding a drug to the Laponite-chitosan nanocomposite, demonstrating significant exfoliation of Laponite nanoparticles in the chitosan matrix.[61] Kuen et al. also employed XRD and tested formulations of donepezil, an acetylcholinesterase inhibitor used in treating Alzheimer’s disease, with three smectites: Laponite, saponite, and montmorillonite.[62] Their results indicated that complete intercalation of donepezil into the smectite interlayer space leads to the loss of the peaks in the XRD pattern related to donepezil crystalline.[62] In the same study, the authors used FTIR to demonstrate that the donepezil molecules were stabilized inside the clay’s layer in an amorphous form, not crystalline, and confirmed an unordered, uniform distribution of the drug within Laponite layers. It was shown that the drug’s chemical structure did not deteriorate throughout the loading process, and the functional groups of the drug remained active.[62] Laponite has two main characteristic peaks at 1000 cm−1 and 700 cm−1, related to stretching from Si–O and bending from O–Si–O. By adding a drug to Laponite, a slight increase in wavenumber can be seen which is due to the molecular interactions between Laponite and drug.[63]
As mentioned earlier, net charge plays an important role in the drug’s intercalation (EE %); thus, the zeta potential measurement is critical to quantify the drug’s charge. Ghadiri et al. showed that tetracycline-Laponite had a greater negative charge as the pH level increased from 3 to 7.[53] Consequently, the increase in negative charge reduced the intercalation of tetracycline. Additionally, the release curves (Figure 1 a and b) revealed that the microenvironment pH also affects the drug release profile by enhancing Laponite solubility. For example, changing the pH from 7.2 to 5.2 increased the release of tetracycline from 15.4% to 52.3% in 72 hours.[53]
SEM is another technique used to demonstrate drug incorporation into Laponite.[64] In a study published by Yang et al. SEM images revealed that the addition of the drug ofloxacin to chitosan/Laponite carrier causes a high degree of surface roughness compared to chitosan alone.[41] That phenomenon may be due to the leakage of drug crystals from the chitosan network to the surface of the carrier. Interestingly, Laponite addition also has an impact on the microstructure of the carriers as an increased amount of Laponite significantly improved the regularity of the surface structure, which can be attributed to a more favorable drug loading after Laponite incorporation. Release studies of the aforementioned system showed that higher concentrations of Laponite resulted in a decrease in the initial burst of ofloxacin and a slower release rate in both pH levels.[41]
3.3. Characteristics affecting drug release
When drug molecules are uniformly incorporated in a drug carrier, release happens via diffusion or the carrier’s degradation. When the rate of drug diffusion is higher than degradation, the release is mostly governed by diffusion. A burst release could also happen as the portion of the drug on the surface of the drug carrier dissolves quickly if not covalently bonded. Thus, the general mechanism of release for intercalated drug molecules from drug carriers requires water diffusion into the carrier and drug diffusion out of the carrier. Any factor that can influence water absorption and drug diffusion will have a direct impact on the release rate. As Wang et al. showed the incorporation of AMX within PLGA containing Laponite nanofibers yielded a slower release of AMX compared to carriers made of only Laponite or PLGA.[42] In this example, the drug release route starts from the Laponite reservoir, followed by the PLGA network, which acts as a physical obstacle and delays the drug release. On the other hand, it has been demonstrated that in chitosan/Laponite composites at acidic pH, the drug was released faster compared to composites at physiological pH, a result that is attributed to the cationic charge of chitosan in acidic pH and an increase in water absorption.[41] Below, we describe the factors which affect drug release efficacy.
3.3.1. Physicochemical properties of drug compounds
The chemical structure of drugs is an important factor in defining their release profile within a Laponite complex. Protonated and hydrophilic drug molecules are more strongly retained in the interlayer spaces of Laponite structures due to strong interfacial interactions with Laponite’s negative surface charges.[65] Laponite possesses high hydrophilicity, swelling properties, and cation exchange capacity.[6] Therefore, drug molecules with effective hydrogen bonds, significant electrostatic interactions, and high positive charge have a higher retention capacity and a better profile for sustained drug release.[66]
3.3.2. Microenvironment’s pH
Physiological variability in pH levels, such as those that occur pathologically in cancer or inflammation, have been used as a strategy to target specific organs and stimulate the release of drugs.[67] Low pH values are typically observed in inflamed, ischemic, and neoplastic tissues, a trait that can be exploited by the use of pH-sensitive compounds for the active release of the drug into target tissues.[44] Laponite has negative surface charge and positive pH sensitive edge.[68] The presence of amphoteric groups such as MgOH, LiOH and SiOH in Laponite nanodiscs [30] which can be protonated and deprotonated depending on the environmental pH result in pH dependency of the edges.[10] Altering the pH level is a reversible mechanism to mediate the release of loaded molecules and should be considered as a critical factor when designing drug carrier systems.[55] As electrostatic interaction between drug molecule and Laponite in different pH is governed by protonation and deprotonation of Laponite, the pH responsive release of drug is reversible by adjusting the pH. Consequently, Laponite can be utilized as a pH-responsive drug carrier to improve control over drug release. The release of cationic drugs such as DOX from Laponite occurs via an ion-exchange mechanism, which is more rapid in the presence of high H+ concentrations. For example, it has been demonstrated that with DOX/Laponite nanohybrids, at physiological pH (7.4), 10.5 ± 2.9% of DOX was released, while dropping the pH to 5.4, increased the release rate.[59]
3.3.3. Cation exchange capacity (CEC)
The de-intercalation of a drug from the Laponite layer strongly depends on the drug release amount.[54] The protonated drug is substituted with cations in solution, and the rate of this replacement is controlled by the physicochemical properties of both the drug and clay.[55] A higher clay CEC indicates a stronger interaction between the drug and clay, which can cause a slower drug release.[53] Donepezil’s release profile from Laponite XLG (a grade of Laponite that forms highly thixotropic gels), saponite, and montmorillonite demonstrated this relationship. The release of these clays after 180 minutes was as follows: Laponite 37%, saponite 15%, and montmorillonite 7%.[62] To improve the drug release, Eudragite® E-100, a large cationic molecule, was included in the release media to facilitate the substitution of the drug due to its stronger interaction with the negative face of the clay. The addition of Eudragite® E-100 increased the rate of release to 61%, 23%, and 12% for Laponite XLG, saponite, and montmorillonite, respectively. Coating the clay-drug hybrid with Eudragite® E-100 could also increase the drug release to 83%, 83%, and 43% for Laponite XLG, saponite, and montmorillonite, respectively.[62] Thus, controlled drug delivery can be achieved to various degrees of success, depending on the type of clay used.[69]
3.3.4. Swelling behavior
As with all biomaterials, rehydration is an important step leading to the release of encapsulated drugs from Laponite.[70] Research has shown that incorporation of Laponite can modulate the swelling behavior of hydrogels containing the clay.[57] For example, the swelling behavior of chitosan was measured at pH 1.2 (gastric pH) and pH 7.4 (intestinal pH).[71] The swelling ratio of chitosan beads in pH of 1.2 was higher than that in pH of 7.4; thus chitosan bead completely dissolved within 3 hours in acidic pH. The addition of Laponite to the chitosan system helps to overcome chitosan’s dissolution and quick release of the drug.[61] These characteristics make Laponite a favorable candidate for targeting release at the lower intestine’s slightly alkaline environment.[50]
The greater charge density of Laponite produces a more hydrophilic system and infiltration of water molecules.[57] However, in another study, it has been reported that the increase in the Laponite content can lead to more crosslinked hydrogels and more tortuous paths for diffusion, which would inhibit water penetration into the hydrogel.[57] By adding a highly hydrophilic cation in the release media, the swelling ratio increased.[36] Park et al. showed this by the addition of Eudragite®-E100 (a molecule with a high number of hydrophilic groups such as NH4+) to release media.[62]
Similar findings have been reported by Chen et al. for the pH-dependent swelling behavior of nanocomposite containing poly(dimethylaminoethyl methacrylate) (PDMAEMA) network crosslinked by Laponite XLS and carboxymethyl chitosan.[72] The presence of a high concentration of NH4+ in the hydrogel in acidic pH leads to disruption of the hydrogen bonds and consequently increases the swelling ratio due to the repulsion forces exerted by the NH4+ molecules. As the pH rises to 7, the swelling ratio decreases due to the reduction of the NH4+ molecules. However, for even higher pH values (> 8), the swelling ratio increases due to the repulsion that is exerted from the -COO− ions deriving from the -COOH groups. In contrast, due to the abundance of hydrogen bonds in the hydrogels at acidic pH, the swelling ratio reduces.[72]
3.3.5. Concentration of Laponite
The concentration of Laponite not only affects the loading efficiency but also directly influences the release profile of an intercalated drug. For example, Yang et al. showed that the release rate of ofloxacin was significantly reduced with increased Laponite content,[41] possibly due to a slower degradation rate or limited diffusion.[73] In another study, both the swelling of gellan gum methacrylate, a photocrosslinkable hydrogel, and the diffusion of the loaded compound was affected by the incorporation of the Laponite and its interaction with the compound. It was shown that the diffusion of the drug was reduced by increasing the concentration of Laponite from 0.1 to 1 %.[74]
3.3.6. Drug solubility
Drug solubility in water is a factor that affects the release rates and the bioavailability of the drug [75]. Water-insoluble drugs can be challenging to deliver due to their slow-release rate in aqueous media and low bioavailability.[76] Jung et al. showed that the solubility of itraconazole (ITA) is enhanced through incorporation into Laponite.[54] Drug release was initiated when cations were exchanged with ITA stored in the Laponite interlayers. The release rate can be controlled by using different cation species. Simple cations such as Na+ and Ca2+ showed slower release rates in comparison to hydrophobic organic cations due to the difficulty of ion exchange reactions with smaller-sized cations.[48] However, the release of ITA improved in a Eudragite® E-100 solution due to the higher charge of Eudragite® E-100 relative to simple cations, resulting in greater exposure of the layered edges of Laponite, and subsequently greater release of ITA.[48] Another study indicated that the water solubility of indigo dye, which is a nonpolar compound, was improved when introduced into Laponite.[77] Lipid-based formulations have also been combined with Laponite which plays the role of the solid-state carrier to improve the loading capacity of poorly water-soluble compounds. Two different publications from Dening and coworkers examine how the addition of Laponite can improve the performance of a lipid-based formulation. They showed the intercalation of the antipsychotic drug Blonanserin into laponite reached up to 20% w/w, which is 7-fold more than the traditional lipid-based formulation and surprisingly higher compared to 11% w/w drug intercalation achieved with montmorillonite although laponite possesses lower CEC.[78][79] In the second study Dening et al. used spray drying to encapsulate Miglyol® 812, a mixture of medium chain caprylic/ capric triglycerides in Laponite. The Laponite-lipid hybrid not only reduced the partitioning of triglycerides but also affected the lipolytic product release by retaining a large fraction of the lipolytic products.[80]
3.4. Degradation of Laponite
Degradation and cytotoxic profile of a material dictates its suitability for biomedical application. In low pH, Laponite degrades and releases sodium, aqueous silica (Si(OH)4), magnesium, and lithium ions into the solution. Using Laponite as a drug carrier may raise biocompatibility concerns due to the nanostructure of the clay, which may trigger a series of biological effects.[1] These concerns can be mitigated by an enhanced comprehension concerning the mechanism of Laponite degradation and clearance of the resulting by-products. Complete clearance from the body can significantly lower the risks associated with using the nanomaterial.[18]
As discussed earlier, the amount of released Mg2+ is correlated with the amount of Laponite released in the medium, which defines its dissolution rate.[6] It is known that, in acidic conditions, Laponite starts to degrade and release Mg2+ according to the following equation:
Researchers studied the degradation rate of different ratios of Laponite/alginate in the cell culture medium. The result showed that a loss of mass of 19% for alginate alone was observed after 48 h; however, 28% and 48% mass loss was observed with 50:50 and 20:80 (Laponite/Alginate) composites, respectively. The nanocomposite with high clay mineral concentration (80:20) resulted in a very low rate of degradation of 5.5% after 48 h.[81] Similarly, incubation of Laponite/poly (ethylene oxide) (PEO) nanocomposites with 40% to 70% of Laponite clay minerals in phosphate-buffered saline (PBS) over 21 days lead to the decline in mass loss from 47% to 23%, respectively.[82]
3.5. Biocompatibility of Laponite
So far, a standard method for the evaluation of Laponite biocompatibility as a drug carrier does not exist.[83] The researchers used different methodology to evaluate Laponite biocompatibility often upon degradation. For example, the biocompatibility of a Laponite crosslinked PEO films for its application in controlled cell adhesion was investigated in mouse preosteoblast cells using Multitox-Fluor Multiplex Cytotoxicity Assay Kit with selected ratios of polymer:Laponite.[82] The authors demonstrated that by increasing Laponite concentration from 40 to 70%, cell viability stayed unchanged, indicating that Laponite is not cytotoxic.[82]
In another study, the same authors added different concentration of chitosan (0, 4 and 11%) to the Laponite crosslinked PEO, to further control cell adhesion, antibacterial properties and mineralization for bone repair.[84] They found that the addition of chitosan did not have any negative effects on the cytotoxicity of the material, and cell viability remained high within the 14-day duration of the study in mouse preosteoblast cells.[84] However, addition of chitosan improved the structural integrity and delayed the release profile of albumin used as a sample drug. In an additional study by Gaharwar et al., the biocompatibility of an injectable hemostatic gel comprising different ratios of gelatin/Laponite (ranging from 0:1 to 1:0) was investigated for out-of-hospital wound treating. The formulations containing a total solid concentration of 9 wt% and Laponite ratio of 75 wt% (i.e., 9NC75) are non-cytotoxic to macrophages. Similarly, ~100% cell viability was observed for all the formulations tested including pure gelatin (9NC0) and pure Laponite (9NC100) as determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The formulations have also been demonstrated in vivo biocompatibility without signs of inflammation after subcutaneous implantation in rats for 28 days.[85]
Electrospun Laponite-doped PLGA nanofibers have also been used as scaffold materials.[42] The biocompatibility of the nanofibers was evaluated by culturing fibroblasts and epithelial cells on the nanofiber scaffolds and assessing cellular activity.[86] Cell attachment and proliferation of cells cultured on the nanofibers were improved compared with those on Laponite free PLGA nanofibers; which was credited as a result of improved hydrophilicity and protein adsorption provided by the PLGA.[86]
In a different study, electrospun Laponite-doped PLGA nanofibers with encapsulated AMX were investigated as a drug release system.[42] This research demonstrated that porcine vascular endothelial cells (PIEC) cultured on either PLGA or PLGA/Laponite/AMX nanofibers had no significant difference in viability after 8 hours. After 72 hours, proliferation on PLGA and PLGA/Laponite/AMX were significantly higher compared with those cultured on coverslips (p < 0.05). Both PLGA and PLGA/Laponite/AMX showed similar cytocompatibility, and the utilization of Laponite/AMX did not affect cell viability. Cell morphology studies also demonstrated that after 72 hours of culture, cells had a similar phenotype in both PLGA and PLGA/Laponite/AMX.[42] Also, cells were able to penetrate and migrate within both scaffolds, corroborating the results of the MTT cell viability assay.[42]
The biocompatibility of Laponite (1 mg/ml) has been measured using human epithelial carcinoma cells using an MTT assay.[73] The cells showed similar behavior in the presence of Laponite compared to the PBS control. This study aimed to examine cell apoptosis in the presence of DOX. It was found that carcinoma cells underwent apoptosis after exposure to free DOX or intercalated DOX in the Laponite solution.[73]
Systemic toxicity of the Laponite has also been investigated by Wang et al. in combination with intramuscular stimulation.[87] Laponite was incubated with saline, and after 3 days was injected intraperitoneally to the rodent model. All general toxic effects such as appetite, body weight, and survival were monitored, and compared with the control (injected with pure saline) to ensure that the difference in experimental groups were not statistically significant.[88] The authors also looked into intramuscular stimulation tests where they locally injected the Laponite, saline, and alcohol to the back of the rats and monitored the animals for any signs of erythema, edema, and necrosis around the injection site. The injection sites showed no signs of rejection when contrasted with the control group, whereas the alcohol injection exhibited significant skin damage. The treated animals were then sacrificed, and their organs were tested histologically. All the examined organs confirmed the biocompatibility of the injected material, and no signs of severe abnormality were observed.[88]
Application of Laponite:alginate composites to bone defects in mouse models demonstrated the bone formation ability of the system.[81] Although the study did not report on the biocompatibility of the tested systems, in particular, no inflammation or severe foreign body response was reported either, suggesting biosafety of Laponite composites as bone grafts.[81] Avery et al. used a gel-based non-invasive embolic agent made of gelatin and Laponite to address endovascular embolization. Their results demonstrated the hemocompatibility of the gelatin/Laponite composite, and the study did not report any foreign body reaction or in vivo toxicity.[89] Table 3 summarizes various studies that assessed the biocompatibility of Laponite or composites with Laponite components.
Table 3.
Summary of various Laponite-based drug delivery studies
| Therapeutic Agent | Intercalation Mechanism | Charge / pH | Laponite concentration | pH release | Co-polymer | Method of preparation | ||
|---|---|---|---|---|---|---|---|---|
| Small molecules | Antibacterial agents | Tetracycline | Predominately cation exchange under acidic pH, adsorption | (+) / pH < 3.3 |
10 mg/ml | 5.2 & 7.2 | NA | Dispersion method |
| Zwitterionic / 3.3<pH <7.7 | ||||||||
| (−1) / 7.7 <pH<9.5 | ||||||||
| (−2) / pH>9.5 | ||||||||
| Ofloxacin | Cationic dye adsorption under acidic pH | (+) / pH<5.9 |
0,10, 20, 30 & 40 mg/ml | 1.2 & 7.4 | Chitosan | Ionic gelation method | ||
| Zwitterionic / 5.9<pH<9.2 | ||||||||
| (−) / pH>9.2 | ||||||||
|
| ||||||||
| Amoxicillin | H-bond & electrostatic interaction | pH 7.4 | 3, 5, & 10 mg/ml | 7.4 | PLGA | Electrospinning | ||
|
| ||||||||
| Ciprofloxacin | Cation exchange under acidic pH | (+) / pH < 6.1 |
1 mg/ml | 7.4 | PCL | Electrospinning | ||
| Zwitterionic / 6.1 <pH <8.7 | ||||||||
| (−) / pH>8.7 | ||||||||
|
| ||||||||
| Vancomycin | Cationic exchange under acidic pH | (+) / acidic pH | 0.05, 0.1, 0.25, 0.5 & 1 mg/ml | 7.4 | Chitosan | Electrophoretic deposition | ||
|
| ||||||||
| Itraconazole | Cation exchange under acidic pH | (+)/pH<3.7 | 10 mg/ml | 1.2 | NA | Mixed to result in the ion exchange reaction | ||
|
| ||||||||
| Anti-Inflammatory | Theophylline | Electrostatic interaction, H-bond, Van der Waals | 1.2 | 40 mg/ml | 6.8 | Alginate | Agitation, mixing | |
|
| ||||||||
| Dexamethasone | Physisorption, H-bond | NA | 15 mg/ml | 7.4 | Hyaluronate/no polymer | Stirring and solvent evaporation | ||
|
| ||||||||
| Brimonidine | H-bond, Van der Waals | NA | 10mg/ml | 7.4 | NA | Stirring and solvent evaporation | ||
|
| ||||||||
| Anti-Cancer | Doxorubicin | H-bond & electrostatic interaction | (+) / acidic pH | 1, 4, 6.12 mg/ml | 7.4, 6.5 5.5 & 5 | Alginate/ no polymer | Mixing followed by ion crosslinking / DOX loaded on LAP nanodiscs (no polymer) / reaction LAP with PEG-FA. DOX encapsulated in LAP-PEG-FA nanodiscs / Conjugation of LAP with P-gp inhibitor TPGS, then encapsulation of DOX | |
|
| ||||||||
| Methotrexate | Surface adsorption H-bond | (−)/ pH>4.7 | 2,4,6 & 8 mg/ml | 7.4, 6.5 & 5 | oHA-His | Conjugation | ||
|
| ||||||||
| O2 (generated by benzoyl peroxide) | Adsorption | NA | 30% w/v | hypoxia condition (1% O2) | Alginate | Mixing benzoyl peroxide with alginate then with LAP, then solution was 3D printed into grid like structures | ||
|
| ||||||||
| Large molecules | Inflammatory cytokine | GM-CSF, FlT3L, IL-15, IL-2, CCL20 | Pre-adsorption of proteins (non-covalent) | NA | 0.1–0.5 mg/ml | 7.4 | Click-Alginates (Tetrazine - Norborene) | Mixing LAP with protein + alginates, followed by cryopolymerization |
|
| ||||||||
| Growth factors | FGF4 | Pre-adsorption of proteins | NA | LAP/Hep: 3.8/19, 7.6/19 & 15.2/19 mg/ml | NA | Heparin | Separate dissolution of LAP & heparin. Mixing with FGF4 | |
|
| ||||||||
| VEGF | Pre-adsorption of proteins | NA | 0.5 & 1 wt% 3% w/v | 7.4 | Alginate | Mixing VEGF with LAP mix with alginate solution | ||
|
| ||||||||
| BMP-2 | adsorption | 1 & 5 mg/ml 2% w/v | 7.4 | Hyaluronate/ Hyaloronic acid | Mixing BMP-2 with LAP, then mixing it with HA-ADH (Hyaluronate-adipic dihydrazide) / Functionalization of Hyaloronic acid with Bisphosphonate and then addition of LAP | |||
The low cytotoxicity and biocompatibility of Laponite, along with the advantage of prolonged-release, can be used in the field of ophthalmology.[29] In 2016 Fraile et al. first demonstrated in vitro application of Laponite as a drug carrier for dexamethasone in ocular pathologies.[29] Subsequently, Prieto et al. demonstrated good tolerability in rabbit models, where no inflammation and foreign body encapsulation was observed upon Laponite nanoparticles administration. Most importantly, their study suggested the transparency of Laponite nanoparticles in the gel state, which is desirable for ocular applications.[90] Encouraged by the aforementioned studies, the same group recently developed a Laponite-based drug delivery formulation of brimonidine for the treatment of glaucoma. Their study for the first time demonstrated that treatment with a brimonidine/Laponite formulation was neuroprotective in a rat model of glaucoma for up to 6 months.[91]
4. Laponite-based drug delivery systems
Targeted and sustained drug delivery systems are engineered worldwide for delivering therapeutic agents for clinically relevant periods (i.e, hours to months depending on the mechanism of action) to a targeted diseased area within the body.[92] Targeted delivery specifically helps maintain therapeutic drug levels at the relevant tissue environment, thereby reducing damage to the healthy tissues due to cytotoxic side effects.[93] To develop an efficient drug delivery system that also enables sustained release, it is crucial to increase bioavailability by enhancing the delivery system solubility in hydrophilic environments, enabling a targeted drug release for the required duration of treatment.[94] Sustained drug delivery provides a continuous release of therapeutics at a predetermined ratio for a fixed period,[66] enabling stable plasma levels of drugs, less frequent dosing, reduced side effects,[95] increased efficacy, and prolonged delivery.[96]
Hybrid clay-based materials have gained increasing attention for sustained delivery of therapeutic owing to inherent properties showed by these materials, including large surface area, decreased toxicity, and high absorption capacity.[97] For instance, the addition of Laponite, a synthetic clay material, to chitosan was reported to increase the sustainability of the release system.[41] This was attributed to Laponite’s role as a crosslinker with chitosan as a result of its exfoliated particles, which strengthen the chitosan network and lead to a sustained release rate.[41] In another study, the incorporation of Laponite with chitosan films and their integration into silicate pallets facilitated a more prolonged controlled release of a glycopeptide antibiotic, vancomycin, when compared to its release from chitosan alone, showing great promise in orthopedic applications.[61] Various other drug delivery platforms, particularly for wound healing applications and anti-tumor therapies, have taken advantage of Laponite-based drug delivery systems as well, and the increasing number of studies exploiting Laponite further demonstrate its potential as an effective material for sustained and targeted drug delivery applications.[63] In the aforementioned drug delivery system, Laponite is used as an excipient however, Laponite can also directly affect the action of another molecules. Dening et al. showed that Laponite can be used for the treatment and prevention of obesity due to its concentration-dependent property to reduce lipid absorption. The application of Laponite in Sprague-Dawley rats led to the increased weight loss compared to a control group [i.e., phosphate buffered saline (PBS)]. The result of this study was statistically equivalent to the commonly prescribed anti-obesity drug, Orlistat, over a 2- week dosing period. [98] In another study, it was demonstrated that Laponite selectively bind to Free Fatty Acid (FFA) due to electrostatic attraction between the positively charged Laponite edge and the negatively charged FFA. This electrostatic binding results in the inhibition activity of gastrointestinal lipase to digest dietary fat.[99]
In the field of pharmacology and drug development, drug molecules are classified as small and large molecules. While the majority of the current on-the-market drugs are small molecules, large molecules, also known as biologics, are quickly rising in popularity and importance.[100] Laponite has been used in different studies for the delivery of both small molecules and large molecules. In this section (4.1) and the following section (4.2) we intend to showcase some of the drugs that have been used in Laponite drug delivery systems and describe the behavior of these systems. These examples alongside the information on structural, drug loading and drug release capabilities of Laponite that have been described throughout the review can guide the readers in selecting Laponite as their potential delivery model.
4.1. Small molecules
Laponite has been used to improve the delivery of chemotherapeutic drugs such as DOX, a pH-responsive compound, in preclinical studies with or without the use of a polymer. Wang et al. encapsulated DOX in the interlayers of Laponite with a high EE of 98.3 ± 0.77%.[101] The Laponite/DOX complex displayed a potent anti-tumor effect as a result of the increased cellular uptake of DOX by MCF-7 cells. In addition, it was shown that the hydrodynamic size of the Laponite/DOX complex was different in PBS (pH 7.4) compared with its size in acetic acid-sodium acetate buffer (pH 5.4).[59] In PBS buffer, Laponite/DOX complexes were observed to be bigger, prolonging the release of the drug. Accordingly, the drug diffusion in acidic pH was faster than at physiological pH, where smaller Laponite/DOX complexes were formed. Considering the fact that the pH of tumor sites is lower than that of normal tissues (pH 5.0 – 6.0), Laponite/DOX complexes show great promise as carriers in tumor therapy applications.[73] In addition, Chen et al. showed that the addition of polymers such as oligomeric hyaluronic acid-aminophenylboronic acid (oHA-APBA) could improve the tumor-targeting efficiency of DOX/Laponite. oHA is a degradation product of hyaluronic acid (HA) and can effectively suppress tumors by inhibiting CD44 clustering, which enhances the cellular uptake of the drug. As a result, Laponite/OHA-APBA/DOX complex prolonged drug release for up to 7 days while free DOX was released in 6 hours.[102]
In another study, a self-assembled layer-by-layer method was used to coat Laponite with DOX, forming DOX/Laponite nanohybrids[59]. Strong polyanions - poly sodium styrene sulfonate (not pH-dependent) - and weak polycations - polyallylamine hydrochloride (pH-dependent) - were used as the layers. In a physiological pH environment, a faster drug release was observed. Under these conditions, the polycation was lower-charged and the electrostatic repulsion between polymer chains was reduced. This resulted in a coiled conformation, thus, preserving the thickness of the layers, as opposed to a more extended conformation due to greater charge density.[59] Therefore, at pH 7.4, the drug was released at a slower rate than at acidic pH.[59] In another study, Gonçalves et al. fabricated hybrid hydrogels by incorporating Laponite nanodiscs into alginate (AG) hydrogels and loaded them with DOX (Figure 5). [103] The AG hydrogel showed a burst release of DOX upon gel formation (EE=80 ± 11%), inducing severe toxicity in cells surrounding the tumor. In addition, the incorporation of 0.2 wt% Laponite into the AG hydrogel significantly improved encapsulation of DOX up to 99 ± 1 wt%.[103] Researchers have also explored the effect of Laponite on DOX release from these hydrogels using dynamic light scattering (DLS). Their analysis indicated that Laponite was successfully incorporated into the gels and a smaller average peak size was observed at 38 nm. Moreover, the Laponite/alginate hydrogel exhibited improved stability, EE, and release profile.[104] Another interesting study by Motealleh and Kehr utilized the alginate-Laponite hydrogels to chemically modify the tumor microenvironment. More specifically, benzoylperoxide was incorporated into alginate - Laponite hydrogels, which yielded a sustained release of oxygen for 14 days and reversed the effects of hypoxia. Consequently, the proliferation of malignant Colo-818 cells decreased while the viability of healthy fibroblasts showed an increase.[105] In other recent studies, Laponite nanodiscs were functionalized and loaded with DOX. In one of these studies, Wu et al. created a polyethylene glycol-linked by FA (PEG-FA) dendrimer conjugated with Laponite to target SK-OV-3 tumors in mice.[106] In another study, Jiang et al. modified Laponite nanodiscs with a P-gp inhibitor (D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS)), which efficiently encapsulated and released DOX in a DOX-resistant MCF-7/ADR cell environment.[107] In both studies, modified nanodiscs increased the survival rate of the mice while decreasing the toxicity of DOX in organs.[106,107]
Figure 5.
(a) Schematic of a transdermal electronic wound dressing containing pH-responsive hydrogel. The electrostatic interaction between negative charges on the surfaces of Laponite and positively charged chitosan nanoparticles is weakened in a basic pH environment, leading to the release of the drug. (b) FITC (as a drug model) was released from ChPs incorporated in PEGDA/Laponite hydrogel at different pH levels. (c) The release of FITC was controlled via pH variations using an electrical field. Reproduced with permission. Copyright 2018, Advanced Healthcare Materials.[55]
While all aforementioned anti-tumor Laponite-based delivery systems focus on the use of a single anti-cancer agent, a combination of compounds with synergistic effects could also be considered. For example, Li et al. developed a process in which they synthesized an injectable hydrogel consisting of low molecular weight heparin (5% w/v) and poloxamer 407 (17.5% w/v) incorporated with Laponite (2.5% w/v) and DOX.[108] The hypothesis that the synergistic chemotherapeutic action of heparin and DOX is superior to each of the molecules alone was confirmed both in vitro and in vivo. Specifically, the hybrid heparin-poloxamer 407-Laponite hydrogels exhibited the best sustained release profile of DOX and anti-tumor effect in vitro as well as the best pharmacodynamic effect in vivo.[108]
The synergistic effect of multiple anti-cancer drugs has also been studied in vitro by Becher et al. A mixture of cisplatin (10wt%), 4-fluorouracil (45wt%), and cyclophosphamide (45wt%) was encapsulated simultaneously in nanohydrogels comprised of sodium polyacrylate–Laponite nanodiscs. This system showed approximately 10 times lower IC50 in MCF-7 cells after 24 and 48 h compared with the nanohydrogels containing each drug.[51]
An important parameter pertaining to synergistic drug combinations is their release profiles, particularly their sequential release. An interesting study on the co-delivery and the sequential release of two anti-cancer agents was conducted by Zhang et al. DOX and MTX were loaded onto Laponite nanodiscs with high loading efficiency of ~85% and ~80%, respectively.[109] This dual-drug/Laponite delivery system displayed remarkable pH and temperature responsiveness along with the sequential release of MTX (approx. 70% after 120h) and DOX (approx. 20% after 120h). This sequential release pattern also demonstrated the tuning capabilities Laponite offers both as an individual drug carrier and in hybrid Laponite-based hydrogels, which is desirable in anti-cancer treatments.[109]
Targeted drug delivery can also be achieved by modifying Laponite nanodiscs with different ligands. The folate receptor appears to be a promising target for cancer treatment as it is highly overexpressed on the surfaces of many tumor types.[110] Folic acid (FA) can be grafted to the surface of aminated Laponite nanodiscs by covalent bonds. In a previous study, DOX was successfully loaded into FA-modified Laponite, achieving a high EE of 92.1%. This system also enabled pH-responsive drug release.[110] In another study, DOX drug carriers targeted for liver cancer cells were prepared by conjugation of polyethylene glycol (PEG)-lactobionic acid to Laponite nanodiscs. It was shown that hepatocytes, as well as numerous human carcinoma cell lines overexpressed asialoglycoprotein receptors on their surface, resulting in a high affinity of these cells to galactose. Thus, galactose-bearing lactobionic acid has been used as a targeting agent in the mentioned study by Chen et al. This system achieved a high EE of 91.5% and sustained drug release with pH-responsiveness.[111] In another study, the Laponite surface was functionalized with an amphiphile PEG-poly(lactic acid) (PEG-PLA) copolymer using a self-assembly process and loaded with DOX (the EE of 85%), which demonstrated sustained drug release property in a pH-responsive manner.[101]
CD44, a transmembrane glycoprotein, is another promising target for cancer treatment that is overexpressed in several solid tumors, such as breast cancer, gastric carcinoma, hepatocarcinoma, and melanoma. Thus, targeting CD44 receptors can increase the amount of the drug that cancer cells endocytose. Jiang et al. conjugated HA, which has the binding site for CD44 molecule, onto Laponite and showed high EE of DOX (85.1 ± 1.5%), and significantly enhanced anti-tumor activity of DOX in CD44-overexpressed HeLa cells.[102]
The development of drug delivery systems for the controlled release of antibiotics is critical to prevent antibiotic resistance.[88,89] In a study conducted by Kiaee et al., a hybrid UV-cross-linkable hydrogel consisting of poly (ethylene glycol) diacrylate (PEGDA) and Laponite embedded with drug-encapsulated chitosan nanoparticles (ChPs) was developed (Figure 5).[55] The pH level was altered by applying an electric field, where the movement of positive ions away from the anode led to an alkaline environment at the proximity of the anode. The hydrogel containing the drug carriers was placed on the anode, and the cathode electrode remained uncovered. In general, the strength of the interaction between Laponite and ChPs is pH-dependent and determines the drug release rate. Due to this fact, the presence of Laponite in the hydrogel facilitated the formation of an electrostatic interaction between the positively charged ChPs and the negatively charged Laponite surfaces, which led to extended-release of the drugs encapsulated in ChPs.[55]
Various electrospun fibers combined with Laponite have also been investigated for translational antibacterial applications. Wang et al. fabricated electrospun sheets of Amoxicillin/Laponite/Poly(Lactic-co-Glycolic Acid) (AMX/LAP/PLGA) nanofibers via incorporation of AMX/Laponite nanodiscs into PLGA nanofibers, which led to an EE of 9.76 ± 0.57%.[42] Sustained drug release was achieved for the duration of 2 weeks demonstrating effective antibacterial activity and non-compromised cytocompatibility (Figure 6). Electrospun fibers of polycaprolactone (PCL) have also been loaded with ciprofloxacin (Cip)/Laponite complex [114]. The PCL/Laponite/Cip nanofibers demonstrated sustained release for 14 days, rendering the Cip/Laponite complex a good candidate for treating wound infections.[114]
Figure 6.
Schematic showing the preparation of (a) AMX/PLGA and (b) AMX/Laponite/PLGA nanofibers. (c) Drug release of PLGA/LAP/AMX in PBS (pH 7.4 at 37°C) and changes in EE of AMX in response to different Laponite concentrations, Reproduced with permission, Copyright 2014, ACS Applied Materials & Interfaces.[42]
In another study,[115] addition of Laponite into porous poly(L-lactic acid) (PLLA) membrane yielded a controlled drug delivery profile. Tetracycline hydrochloride was effectively encapsulated into Laponite nanodiscs with an EE of 85.3%. The addition of tetracycline/Laponite into porous PLLA membrane reduced the initial burst release, which is generally observed with conventional polymer membrane-based delivery systems, and extended the release of the drug.[115]
The antibacterial effect of tetracycline has also been leveraged by a previous study and implemented by the incorporation of tetracycline into 1% Laponite as a method for in situ drug delivery. The researchers created a Laponite-tetracycline composite by intercalating tetracycline into Laponite nanoparticles. They performed drug release studies in a simulated saliva solution and confirmed a 72-hour extended-release, and observed an enhanced antibacterial activity. Interestingly, the tetracycline release rate increased from 15.4% to 52.3% as the pH was reduced from 7.2 to 5.2. Additionally, the antibacterial effect of tetracycline remained unchanged after its incorporation into Laponite.[53]
The antibacterial effect of Laponite/mafenide composites in the form of gel or film for wound healing applications has also been reported by Ghadiri et al.[116] As in the case of tetracycline, mafenide was also intercalated into the layers of Laponite nanoparticles [116]. Remarkably, the dissolution of Laponite caused the release of Mg+2, which reduced the cytotoxicity of mafenide and improved the wound healing process.[116]
In a recently reported application, alginate-Laponite beads were utilized for the delivery of anti-inflammatory molecule, theophylline. This system bypassed the rapid release of the drug in the stomach and provided a prolonged release in the small intestine, increasing the efficacy of the drug and reducing the side effects.[34]
4.2. Large molecules
Similar approaches used for small molecules have been implemented on large molecules, such as proteins, to exploit the adsorption properties of Laponite. In a recent study, Koshy et al. created a nanocomposite drug delivery carrier in which the protein was initially incorporated into the Laponite.[117] Here, Laponite was integrated into an injectable click alginate hydrogel to hinder the burst release of the protein.[117] Using this technique, the release of various therapeutically relevant proteins with different molecular weights (12–35 kDa) was studied.[117] Hydrogels were successfully loaded with protein/Laponite, and slow release of the bioactive proteins was achieved.[117] More specifically, as demonstrated in Figure 7, the incorporation of Laponite into the alginate hydrogels altered the burst release behavior in all cases, resulting in sustained release of the proteins. Further, Koshy et al., using the same alginate hydrogels, tested the consequence of varying quantities of Laponite on the GM-CSF and IL-2 release. Their studies showed that as the Laponite concentration increased, slower release of the encapsulated protein was observed, demonstrating that alginate-Laponite systems are tunable hydrogels for controlled protein release.[117]
Figure 7.
Laponite-containing click alginate cryogels a) System formation and protein release schematics. b) Release profiles of IL-2 and GM-CSF from click alginate cryogels. c) Release profiles of FGF4 from Heparin/Laponite in different ratios.[118] Reproduced with permission. Copyright 2019, International Journal of Biological Macromolecules.[108] Copyright 2018, ACTA Biomaterial.[117]
Similarly, the impact of different amounts of Laponite on protein release was studied by Wang et al., who formulated injectable Heparin/Laponite hydrogels for the delivery of fibroblast growth factor 4 (FGF4) to facilitate protection and regeneration after spinal cord injury (SCI). They tested hydrogels with different Heparin-Laponite ratios (Figure 7) and found that a ratio of 2.5x Laponite: Heparin (7.6 + 19 mg/mL) exhibited the most desired sustained release profile locally, which may also serve as a neuroprotective for SCI.[108] Overall, the studies of Koshy et al. and Wang et al. demonstrate that by altering the amount of Laponite, customized release profile of proteins can be attained independent of the matrix material. [118]
The high tunability and sustained protein release properties of the alginate-Laponite combination have also been exploited for the delivery of VEGF. In one example, Page et al. reported that the incorporation of Laponites in alginate hydrogels prolonged VEGF release, resulting in an increased blood vessel growth. This finding can be particularly useful in wound healing applications.[119] A follow-up study by Zhang et al. investigated the alginate-Laponite system which enabled sustained release of VEGF over 28 days and showed an enhanced generation of microvessels in the dental pulp in mice. Additionally, Zhang et al. encapsulated VEGF in the alginate-Laponite system and observed viability rates to be over 85% of human dental pulp stem cells. This finding highlights the cytocompatibility of Laponite and illustrates its potential in dental applications.[120] Li et al. showed an extended release of large quantities of an insulin-like growth factor-1 (IGF1) mimetic protein over a four-week period from a Laponite/Alginate composite.[121] The release rate is primarily controlled by ionic exchange and can be further increased by low environmental pH, which is typical for injured tissues (Figure 8a, 8b). This group used a rodent Achilles tendon injury model to showcase the applicability of Laponite carrier for a highly extended and localized release of biological drugs in vivo. The release profile along with well-defined biodegradation and biocompatibility characteristics make the composite of Laponite a promising system for drug delivery and regenerative medicine and exemplifies the potential of Laponite systems for drug delivery applications (Figure 8c, 8d). [121]
Figure 8.
a) Laponite (gray discs) adsorb drug molecules (red) and reside inside a biodegradable alginate network (blue lines) with micro-sized cavities (gray bubbles). b) The alginate network degrades slowly over time, leading to the release of drugs. Subject to localized stimuli such as the change in pH (green region), the clay particles are expected to release most of the loaded drugs locally. c) Extended and localized drug release in a rat model of Achilles tenotomy. d) Images of H&E stained histological sections 1 week and 2 weeks after implantation on the site of Achilles tendon injury. Copyright 2018, Advanced Healthcare Materials. [121]
Besides alginate, various other polymers, such as HA, have also been combined with Laponite for delivering proteins. Zhang et al.[122] created nanocomposite hydrogels loaded with BMP-2. The hydrogels were injected into rats and promoted proliferation and differentiation of bone marrow MSCs, resulting in enhanced osteoinduction. Increased induction of bone regeneration in mice was also reported by Kim et al., where smaller amounts of BMP-2 was incorporated into nanocomposite hydrogels via a bisphosphonate crosslinker boosted osteoinduction.[123] Both studies highlight the importance of the mechanical properties of Laponite in adapting hydrogels for bone repair applications.
5. Clinical Translation of Laponite
Laponite or Laponite derivatives can be used as drug additives or excipients to drug formulations along with active pharmaceutical components. [124] Formulation excipients such as Laponite are used to achieve a specific dosage of the active ingredient and to improve the drug product stability. [124] Overall, these excipients can be utilized for various functions for instance as a protective agent, rheology modifier, and bulking agents. [125] Laponite has been mainly used in drug products as a rheology modifier to improve the stability of the drug molecules and also help to achieve desired viscosity, shelf stability and ease of application. Laponite and other rheology modifiers are commonly used to adjust the overall viscosity of the drug formulation and also to control non-Newtonian behaviors such as shear thinning or shear thickening. [124] Since drug additives such as Laponites are an essential component of therapeutic products, they must be evaluated for their safety and stability. This mainly because any negative such as drug- excipient, excipient-excipient and even package- excipient interactions can cause serious side effects in patients. This necessitates to study the biocompatibility of Laponite as an excipient to determine the safety and stability of it before designing the drug formulation. There are several stability testing procedures available where the excipients, such as Laponites, are subject to extreme conditions of temperature, humidity and other environmental settings. These studies are designed to provide evidence on how Laponite structure changes during these extreme conditions. Stability testing enables the recommended storage conditions, retest periods and shelf life to be established for drug product containing Laponite. This information is provided in Material Safety Data Sheet (MSDS) available by the manufacturer. For example, In MSDS of Laponite RD made by BYK Additive Inc, it was claimed that Laponite is chemically stable at normal condition, however, prolonged exposure of Laponite containing products to air and moisture can affect the chemical stability of Laponite. Moreover, it is demonstrated that Laponite is incompatible with strong acids and oxidizing agent. Based on stability studies, hazardous polymerization doesn’t occur during extreme conditions.[126] After stability test, the excipients are further tested for assuring safety, which is the most important feature of any formulation intended to be used in humans or animals. The safety assurance of excipients helps to design an effective and safe dosage form. Thus, for Laponite to be used in a formulation it must be highly stable, safe and efficacious, and above all it must comply with the expected performance in the formulation.[127] In section 3.5 we discussed in-depth into Laponite biocompatibility and its biodegradation byproducts.
6. Conclusions and future perspectives
To date, the field of controlled drug release is focused on the exploration of various materials to serve as drug carriers. Although various grades of Laponite have been used extensively in the cosmetic and chemical industries, Laponite has been less explored in biological research. Laponite possesses properties that enable the formation of effective drug carriers, indicating potential pharmaceutical uses. Herein, we highlighted the most important parameters for engineering Laponite nanoparticle-based drug delivery systems. As we described above, ion exchange is of critical importance, since it determines the uptake of drug molecules. Ion exchange should be determined experimentally for each system and serves as the critical parameter for the modification and optimization of the materials (including hybrid materials). As is evident from the studies presented in this review, the translational research performed with Laponite is centered on wound healing, anti-tumor drug delivery (small molecules), and, more recently, protein delivery. In this review, we provided a comprehensive picture of the advantages and the unique properties of Laponite nanocarriers as drug delivery systems. The limited number of existing research papers highlights the exciting opportunities and room for novel ideas that could lead to the development of innovative Laponite-based biomaterials.
One of the main limitations of Laponite as a drug carrier is the low delivery yield for highly charged molecules. Due to chemisorption and the ionic interactions between the highly charged molecule and Laponite, the amount of the molecule released is much lower compared to the amount that is loaded. As such, higher loading of the therapeutic molecule is required in order to reach the desired release profile and amount of the drug. Due to high cost of some active compounds, this limitation may increase the fabrication cost significantly. In some cases, the interaction between the active molecule and Laponite can be disrupted or masked by using polymers such as Polyethyleneimine and Polylysine which their high positive charge can compete with the interaction between Laponite and the drug molecule.[128] Alternatively, the strength of the interaction between the drug and Laponite can be adjusted by changing the pH which will result to the change of the charge of the active molecule. However, this may be not favorable where physiological pH is required for the release of the molecule. Moreover, Laponite-drug hybrid systems showed great result in vitro, however, in vivo results are not as promising due to the complications involved in real physiological conditions. Although Laponite-drug hybrid systems showed great result in vitro, in vivo results are not as promising due to the complications involved in real physiological conditions which may derive due to poor or inconsistent characterization of the interactions between the biological system and the materials and consequently result to misleading outcomes and incorrect interpretation of the day. Key role in avoiding this pitfall plays the selection of the appropriate characterization of the material in relation to the intended application. The importance of the correct characterization is also highlighted by nanotechnology regulatory science research plan guidance documents of the US Food and Drug Administration (FDA) state. Last but not least, reproducibility issues in translational industry reports related to the standardization of the aforementioned characterization techniques may also lead to inaccurate and unreliable outcomes which may be reflected to failures in clinical trials affecting the health care system and the patients.[129] Additionally, toxicological studies are needed in order to demonstrate the safety and effectiveness of Laponite-drug systems in clinical outcomes.[124] Nevertheless, further characterization of these materials and their degradation process, both in vitro and in vivo, is necessary in order to assess their biological impact on the human body.
Figure 3.
Degradation mechanism of Laponite; (a) water penetrates into interlayers of Laponite via diffusion through empty space and upon hydrolysis and condensation reaction with metal-oxygen bonds which results into the presence of OH- [130] (b) subsequently OH− attacks the silica glass network of Laponite on the surface, leading to Si–O–Si dissociation and c) releasing soluble silica as Si(OH)4.[83]
Figure 4.
Enhanced tumor drug delivery using a Laponite/alginate (LP/AG) system. (a) DOX-loaded LP/AG crosslinked with Ca+2 released drug at the tumor site and enabled improved anti-tumor efficacy, detected via fluorescent imaging. DAPI-stained cells are in blue and DOX exhibits red fluorescence. (b) DOX-loaded LP/AG showed a sustained release profile in physiological environments. (c) Cell viability study for cells exposed to AG, LP/AG, AG-DOX, and LP/AG-DOX hydrogels for 24, 48, and 72 hours. Reproduced with permission. Copyright 2014, ACTA Biomaterialia,[103] Copyright 2014, Macromolecular Bioscience.[104]
Table 2.
Summary of Laponite biocompatibility studies
| Cell Type | Duration of Experiment | Type of Cell Study | Control | Concentration | Observation | Reference |
|---|---|---|---|---|---|---|
| Primary human dermal fibroblasts | 3 days | Cell proliferation (MTS assay) | No treatment | Extracts of Laponite according to ISO10993.5 | Laponite extract did not induce cytotoxicity and increased the cell growth (110%) | [63] |
| Primary human dermal fibroblasts | 2 days | Scratch test (proliferation) | No treatment | Extracts of Laponite according to ISO10993.5 | Laponite extract did not make any significant difference in gap closure | [63] |
| CAL-72 cells (osteosarcoma) and NIH-3T3 fibroblast cells | 2 days | resazurin reduction assay (metabolic activity) | No treatment | 0.2 – 2.5 μM | Laponite and amphiphile PEG−PLA modified Laponite did not display any cytotoxicity | [101] |
| L929 mouse fibroblasts and porcine iliac artery endothelial cells | 8 h and 3 days | MTT assay | Coverslip | Laponite:PLGA: 1%, 3%, 5% of total concentration of 25% solid | Increase in adhesion and proliferation of cells on Laponite-doped PLGA nanofibers was observed. | [86] |
| L929 cells and porcine iliac artery endothelial cells | 8 h and 3 days | Cell morphology observation (SEM) | Coverslip | Laponite:PLGA. 1%, 3%, 5% of total concentration of 25% solid | Increase in adhesion and proliferation of cells on Laponite-doped PLGA nanofibers was observed. | [86] |
| HeLa cells (human cervical cancer) and L929 cells | 2 days | Resazurin reduction assay | PBS | Modified Laponite-NH2/DOX, and Modified Laponite-FA/DOX at a DOX concentration of 10 mg/ml | Modified Laponite-FA/DOX specifically targeted cancer cells with overexpressed FA receptors. | [110] |
| Human epidermoid carcinoma (KB) cells | 14 days | Cell viability test | PBS | Laponite/DOX | Therapeutic efficacy of Laponite/DOX nanodiscs was improved in comparison with free DOX. | [73] |
| MC3T3-E1 subclone 4 mouse preosteoblast cells | 24h | Multitox-Fluor Multiplex Cytotoxicity Assay Kit | Tissue culture polystyrene (TCPS) plates | Cells were seeded on 70–100 μm thick PEO/Laponite nanocomposites with up to 35 mg/ml of silicate | No acute toxicity to Laponite nanodiscs was reported. | [82] |
| Human Mesenchymal Stem Cells (P5) | Up to 8 h for cell attachment and up to 7 days for cell proliferation assays | MTT assay | TCP and cover slips | PLGA and Laponite-doped w/1%, 3%, and 5% Laponite relative to PLGA | Osteoblast differentiation was not disturbed using Laponite composites. | [86] |
| CAL-72 cells (osteosarcoma) | 48h | Resazurin reduction assay | Control experiment was done in the absence of LP and LP/DOX/AG | Laponite ranged in size from 20 to 50 nm, while the Laponite/DOX/AG particles were around 200 nm | Cytotoxicity was only observed in samples with DOX release. | [103] |
| Human mesenchymal stem cells (MSCs) | 12 days | Alamar blue assay | TCP | Nanocomposite hydrogels were 2–3.5% silicate (Laponite), 3–1.5% PEO, 95% water. Nanocomposite films were 40–70% silicate, 60–30% PEO. | Increase in Laponite concentration enhanced the attachment and proliferation of MSCs significantly. | [87] |
| Rat MSCs | 14 days | Resazurin reduction assay | TCP | Various Laponite bioceramics | Hemocompatibility and biocompatibility was observed. | [88] |
Acknowledgements
G. Kiaee and N. Dimitrakakis contributed equally to this work. The authors acknowledge funding from the National Institutes of Health (HL140951, HL137193). We would also like to thank Dr. W. Glindmeyer, Lindsay Brownell and Dr. Sasan Jalili for helpful discussions and critical feedback.
Contributor Information
Gita Kiaee, Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston, MA 02115, USA..
Nikolaos Dimitrakakis, Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston, MA 02115, USA..
Shabnam Sharifzadeh, Dana-Farber Cancer Institute, Boston, MA 02115, USA..
Han-Jun Kim, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90024, USA..
Reginald K. Avery, Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston, MA 02115, USA.
Kamyar Mollozadeh Moghaddam, Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston, MA 02115, USA..
Reihaneh Haghiniaz, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90024, USA..
Ezgi Pinar Yalcintas, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90024, USA..
Natan Barros, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90024, USA..
Solmaz Karamikamkar, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90024, USA..
Alberto Libanori, Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA 90095, USA.
Ali Khademhosseini, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90024, USA..
Parastoo Khoshakhlagh, Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston, MA 02115, USA..
References
- [1].Carretero MI, Gomes CSF, Tateo F, in Dev. Clay Sci, Elsevier, 2006, pp. 717–741. [Google Scholar]
- [2].Gaskell EE, Hamilton AR, Future Med. Chem. 2014, 6, 641. [DOI] [PubMed] [Google Scholar]
- [3].Bergaya F, Lagaly G, in Handb. Clay Sci. (Eds: Bergaya F, G.B. T-D in Lagaly CS), Elsevier, 2013, pp. 1–19. [Google Scholar]
- [4].Carniato F, Gatti G, Bisio C, New J. Chem. 2020, 44, 9969. [Google Scholar]
- [5].Alberto L, Rodrigues DS, Figueiras A, Veiga F, Mendes R, Freitas D, César L, Nunes C, Cavalcanti E, Maria C, Colloids Surfaces B Biointerfaces 2013, 103, 642. [DOI] [PubMed] [Google Scholar]
- [6].Thompson DW, Butterworth JT, 1992, 15, 236. [Google Scholar]
- [7].Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK, Beilstein J. Nanotechnol. 2018, 9, 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Christidis GE, Aldana C, Chryssikos GD, Gionis V, Kalo H, Stöter M, Breu J, Robert J-L, Miner. 2018, 8, DOI 10.3390/min8080314. [DOI] [Google Scholar]
- [9].BARRY BW, J. Texture Stud. 1970, 1, 405. [DOI] [PubMed] [Google Scholar]
- [10].Tomás H, Alves CS, Rodrigues J, Nanomedicine Nanotechnology, Biol. Med. 2018, 14, 2407. [DOI] [PubMed] [Google Scholar]
- [11].Neumann BS, Rheol. Acta 1965, 4, 250. [Google Scholar]
- [12].Lagaly G, Dé Ká Ny I, Colloid Clay Science, 2014. [Google Scholar]
- [13].Aguiar AS, Michels L, da Silva FG, Kern C, Gomide G, Ferreira CM, Depeyrot J, Aquino R, da Silva GJ, Appl. Clay Sci. 2020, 193, 105663. [Google Scholar]
- [14].Ruzicka B, Zaccarelli E, Soft Matter 2011, 7, 1268. [Google Scholar]
- [15].Negrete-Herrera N, Putaux JL, Bourgeat-Lami E, Prog. Solid State Chem. 2006, 34, 121. [Google Scholar]
- [16].Negrete-Herrera N, Putaux JL, David L, Bourgeat-Lami E, Macromolecules 2006, 39, 9177. [Google Scholar]
- [17].Duval FP, Porion P, Faugère A-M, Van Damme H, J. Colloid Interface Sci. 2001, 242, 319. [Google Scholar]
- [18].Petit L, Barentin C, Colombani J, Ybert C, Bocquet L, Langmuir 2009, 25, 12048. [DOI] [PubMed] [Google Scholar]
- [19].Kiaee G, Javar HA, Kiaee B, Kiaei S, Silico Vitr. Pharmacol. 2016, 2. [Google Scholar]
- [20].Patel VF, Liu F, Brown MB, J. Control. Release 2011, 153, 106. [DOI] [PubMed] [Google Scholar]
- [21].Sadeqi A, Nejad HR, Kiaee G, Sonkusale S, in 2018 40th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE, 2018, pp. 5737–5740. [DOI] [PubMed] [Google Scholar]
- [22].Khoshakhlagh P, Rabiee SM, Kiaee G, Heidari P, Miri AK, Moradi R, Moztarzadeh F, Ravarian R, Carbohydr. Polym. 2016, 157, 1261. [DOI] [PubMed] [Google Scholar]
- [23].Viseras C, Cerezo P, Sanchez R, Salcedo I, Aguzzi C, Appl. Clay Sci. 2010, 48, 291. [Google Scholar]
- [24].Dong J, Cheng Z, Tan S, Zhu Q, Expert Opin. Drug Deliv. 2021, 18, 695. [DOI] [PubMed] [Google Scholar]
- [25].Rapacz-Kmita A, Szaraniec B, Mikołajczyk M, Stodolak-Zych E, Dzierzkowska E, Gajek M, Dudek P, Acta Bioeng. Biomech. 2020, 22, DOI 10.37190/abb-01523-2019-03. [DOI] [PubMed] [Google Scholar]
- [26].Murugesan S, Scheibel T, Adv. Funct. Mater. 2020, 30, DOI 10.1002/adfm.201908101. [DOI] [Google Scholar]
- [27].Jayrajsinh S, Shankar G, Agrawal YK, Bakre L, J. Drug Deliv. Sci. Technol. 2017, 39, 200. [Google Scholar]
- [28].Bergaya F, Theng BKG, Lagaly G, Handbook of Clay Science, Elsevier, 2006. [Google Scholar]
- [29].Fraile JM, Garcia-Martin E, Gil C, Mayoral JA, Pablo LE, Polo V, Prieto E, Vispe E, Eur. J. Pharm. Biopharm. 2016, 108, 83. [DOI] [PubMed] [Google Scholar]
- [30].Lapasin R, Grassi M, Abrami M, Šebenik U, Colloids Surfaces A Physicochem. Eng. Asp. 2020, 602, DOI 10.1016/j.colsurfa.2020.125126. [DOI] [Google Scholar]
- [31].Becher TB, Braga CB, Bertuzzi DL, Ramos MD, Hassan A, Crespilho FN, Ornelas C, Soft Matter 2019, 15, 1278. [DOI] [PubMed] [Google Scholar]
- [32].Phuoc TX, Chen RH, Opt. Lasers Eng. 2011, 49, 396. [Google Scholar]
- [33].Mongondry P, Tassin JF, Nicolai T, Colloids Interfaces Sci. 2005, 283, 397. [DOI] [PubMed] [Google Scholar]
- [34].Nandi U, Trivedi V, Douroumis D, Mendham AP, Coleman NJ, Pharmaceuticals 2020, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Valencia GA, Djabourov M, Carn F, Sobral PJA, Colloid Interface Sci. Commun. 2018, 23, 1. [Google Scholar]
- [36].Singh S, Hussain A, Tebyetekerwa M, Sachi Das S, Hussain K, Faruk A, Mike T, Curr. Pharm. Des. 2018, DOI 10.2174/1381612825666190402165845. [DOI] [PubMed] [Google Scholar]
- [37].Ruiz-Hitzky E, Van Meerbeek A, in Dev. Clay Sci, Elsevier, 2006, pp. 583–621. [Google Scholar]
- [38].Karthikeyan CS, Nunes SP, Schulte K, Eur. Polym. J. 2005, 41, 1350. [Google Scholar]
- [39].Sheikhi A, Afewerki S, Oklu R, Gaharwar AK, Khademhosseini A, Biomater. Sci. 2018, 6, 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mourchid A, Delville A, Levitz P, Faraday Discuss. 1995, 101, 275. [Google Scholar]
- [41].Yang H, Hua S, Wang W, Wang A, Composite Hydrogel Beads Based on Chitosan and Laponite: Preparation, Swelling, and Drug Release Behaviour, 2011. [Google Scholar]
- [42].Wang S, Zheng F, Huang Y, Fang Y, Shen M, Zhu M, Shi X, ACS Appl. Mater. Interfaces 2012, 4, 6393. [DOI] [PubMed] [Google Scholar]
- [43].Elzbieciak M, Wodka D, Zapotoczny S, Nowak P, Warszynski P, Langmuir 2010, 26, 277. [DOI] [PubMed] [Google Scholar]
- [44].Yoshida T, Lai TC, Kwon GS, Sako K, 2013, DOI 10.1517/17425247.2013.821978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kiaee B, Kiaei S, Silico Vitr. Pharmacol. 2016, 1. [Google Scholar]
- [46].Mostafalu P, Tamayol A, Rahimi R, Ochoa M, Khalilpour A, Kiaee G, Yazdi IK, Bagherifard S, Dokmeci MR, Ziaie B, Sonkusale SR, Khademhosseini A, Small 2018, 14, 1703509. [DOI] [PubMed] [Google Scholar]
- [47].Suresh R, Borkar SN, Sawant VA, Shende VS, Dimble SK, Nanoclay Drug Delivery System, n.d. [Google Scholar]
- [48].Jung J, Dong S, Lee S, Kim K, Yoon D, Lee K, 1999, 187, 209. [DOI] [PubMed] [Google Scholar]
- [49].Gadige P, Bandyopadhyay R, Soft Matter 2018, 14, 6974. [DOI] [PubMed] [Google Scholar]
- [50].Roozbahani M, Kharaziha M, Emadi R, Int. J. Pharm. 2017, 518, 312. [DOI] [PubMed] [Google Scholar]
- [51].Becher TB, Mendonça MCP, de Farias MA, Portugal RV, de Jesus MB, Ornelas C, ACS Appl. Mater. Interfaces 2018, 10, 21891. [DOI] [PubMed] [Google Scholar]
- [52].Aray Y, Marquez M, Rodríguez J, Coll S, Simón-Manso Y, Gonzalez C, Weitz DA, J. Phys. Chem. 2003, DOI 10.1021/jp0302257. [DOI] [Google Scholar]
- [53].Ghadiri M, Hau H, Chrzanowski W, Agus H, Rohanizadeh R, RSC Adv. 2013, 3, 20193. [Google Scholar]
- [54].Jung H, Kim H, Bin Y, Hwang S, Choy J, Appl. Clay Sci. 2008, 40, 99. [Google Scholar]
- [55].Kiaee G, Mostafalu P, Samandari M, Sonkusale S, Adv. Healthc. Mater. 2018, 1800396. [DOI] [PubMed] [Google Scholar]
- [56].Wang J, Wang G, Sun Y, Wang Y, Yang Y, Yuan Y, Li Y, Liu C, RSC Adv. 2016, 6, 31816. [Google Scholar]
- [57].Li P, Hoon N, Hui D, Yop K, Hee J, Appl. Clay Sci. 2009, 46, 414. [Google Scholar]
- [58].“Laponite XLG,” 2013. [Google Scholar]
- [59].Xiao S, Castro R, Maciel D, Gonçalves M, Shi X, Rodrigues J, Tomás H, Mater. Sci. Eng. C 2016, 60, 348. [DOI] [PubMed] [Google Scholar]
- [60].Ruzicka B, Zaccarelli E, Zulian L, Angelini R, Sztucki M, Moussaïd A, Narayanan T, Sciortino F, Nat. Mater. 2010, 10, DOI 10.1038/NMAT2921. [DOI] [PubMed] [Google Scholar]
- [61].Ordikhani F, Dehghani M, Simchi A, J. Mater. Sci. Mater. Med. 2015, 26, 269. [DOI] [PubMed] [Google Scholar]
- [62].Park JK, Bin Choy Y, Oh J-M, Kim JY, Hwang S-J, Choy J-H, Int. J. Pharm. 2008, 359, 198. [DOI] [PubMed] [Google Scholar]
- [63].Ghadiri M, Chrzanowski W, Lee WH, Rohanizadeh R, RSC Adv. 2014, 4, 35332. [Google Scholar]
- [64].Amini F, Bahador A, Kiaee B, Kiaee G, Amini K, Med. Commun. Biosci. Biotech. Res. Comm 2017, 10, 28. [Google Scholar]
- [65].Martínez Martínez V, López Arbeloa *F, Bañuelos Prieto J, Arbeloa López AT, Arbeloa IL, Langmuir 2004, DOI 10.1021/LA049675W. [DOI] [PubMed] [Google Scholar]
- [66].Yang J-H, Lee J-H, Ryu H-J, Elzatahry AA, Alothman ZA, Choy J-H, Appl. Clay Sci. 2016, 130, 20. [Google Scholar]
- [67].Schmaljohann D, Adv. Drug Deliv. Rev. 2006, 58, 1655. [DOI] [PubMed] [Google Scholar]
- [68].Choi D, Heo J, Aviles Milan J, Oreffo ROC, Dawson JI, Hong J, Kim YH, Mater. Sci. Eng. C 2021, 118, 111440. [DOI] [PubMed] [Google Scholar]
- [69].Ghadiri M, Chrzanowski W, Rohanizadeh R, RSC Adv. 2015, 5, 29467. [Google Scholar]
- [70].Brazel CS, Peppas NA, Eur. J. Pharm. Biopharm. 2000, 49, 47. [DOI] [PubMed] [Google Scholar]
- [71].Hejazi R, Amiji M, J. Control. Release 2003, 89, 151. [DOI] [PubMed] [Google Scholar]
- [72].Chen Y, Xu W, Xiong Y, Peng C, Liu W, Zeng G, Peng Y, J. Mater. Res. 2013, 28, 1394. [Google Scholar]
- [73].and Shige Wang X, Wu Yilun, Guo Rui, Huang Yunpeng, Wen Shihui, Shen Mingwu, Wang Jianhua, Shi, Langmuir 2013. [DOI] [PubMed] [Google Scholar]
- [74].Pacelli S, Paolicelli P, Moretti G, Petralito S, Di S, Vitalone A, Antonietta M, Eur. Polym. J. 2016, 77, 114. [Google Scholar]
- [75].Ghasemian E, Rezaeian B, Alaei S, Vatanara A, Ramezani V, Pharm. Sci. 2015, 21, 136. [Google Scholar]
- [76].Alaei S, Ghasemian E, Vatanara A, Powder Technol. 2016, 288, 241. [Google Scholar]
- [77].Lezhnina MM, Grewe T, Stoehr H, Kynast U, Angew. Chemie - Int. Ed. 2012, 51, 10652. [DOI] [PubMed] [Google Scholar]
- [78].Dening TJ, Rao S, Thomas N, Prestidge CA, Int. J. Pharm. 2017, 526, 95. [DOI] [PubMed] [Google Scholar]
- [79].Dening TJ, Thomas N, Rao S, Van Looveren C, Cuyckens F, Holm R, Prestidge CA, Mol. Pharm. 2018, 15, 4148. [DOI] [PubMed] [Google Scholar]
- [80].Dening TJ, Joyce P, Prestidge CA, J. Pharm. Sci. 2019, 108, 295. [DOI] [PubMed] [Google Scholar]
- [81].Ghadiri M, Chrzanowski W, Lee WH, Fathi A, Dehghani F, Rohanizadeh R, Appl. Clay Sci. 2013, 85, 64. [Google Scholar]
- [82].Gaharwar AK, Schexnailder PJ, Kline BP, Schmidt G, Acta Biomater. 2011, 7, 568. [DOI] [PubMed] [Google Scholar]
- [83].Mousa M, Evans ND, Oreffo ROC, Dawson JI, Biomaterials 2018, 159, 204. [DOI] [PubMed] [Google Scholar]
- [84].Gaharwar AK, Schexnailder PJ, Jin Q, Wu CJ, Schmidt G, ACS Appl. Mater. Interfaces 2010, 2, 3119. [DOI] [PubMed] [Google Scholar]
- [85].Gaharwar AK, Avery RK, Assmann A, Paul A, McKinley GH, Khademhosseini A, Olsen BD, ACS Nano 2014, 8, 9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang S, Castro R, An X, Song C, Luo Y, Shen M, Tomás H, Zhu M, Shi X, J. Mater. Chem. 2012, 22, 23357. [Google Scholar]
- [87].Gaharwar AK, Kishore V, Rivera C, Bullock W, Wu CJ, Akkus O, Schmidt G, Macromol. Biosci. 2012, 12, 779. [DOI] [PubMed] [Google Scholar]
- [88].Wang C, Wang S, Li K, Ju Y, Li J, Zhang Y, Li J, Liu X, Shi X, Zhao Q, PLoS One 2014, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Avery RK, Albadawi H, Akbari M, Zhang YS, Duggan MJ, Sahani DV, Olsen BD, Khademhosseini A, Oklu R, Sci. Transl. Med. 2016, 8, DOI 10.1126/scitranslmed.aah5533. [DOI] [PubMed] [Google Scholar]
- [90].Prieto E, Vispe E, De Martino A, Idoipe M, Rodrigo MJ, Garcia-Martin E, Fraile JM, Polo-Llorens V, Mayoral JA, Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 535. [DOI] [PubMed] [Google Scholar]
- [91].Rodrigo MJ, Cardiel MJ, Fraile JM, Mendez-Martinez S, Martinez-Rincon T, Subias M, Polo V, Ruberte J, Ramirez T, Vispe E, Luna C, Mayoral JA, Garcia-Martin E, Biomater. Sci. 2020, 8, 6246. [DOI] [PubMed] [Google Scholar]
- [92].Petrak K, Drug Discov. Today 2005, 10, 1667. [DOI] [PubMed] [Google Scholar]
- [93].Li K, Wang S, Wen S, Tang Y, Li J, Shi X, Zhao Q, ACS Appl. Mater. Interfaces 2014, DOI 10.1021/am502094a. [DOI] [PubMed] [Google Scholar]
- [94].Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK, Int. J. Pharm. 2013, 447, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mostafalu P, Kiaee G, Giatsidis G, Khalilpour A, Nabavinia M, Dokmeci MR, Sonkusale S, Orgill DP, Tamayol A, Khademhosseini A, Adv. Funct. Mater. 2017, 27, 1. [Google Scholar]
- [96].Adrover A, Paolicelli P, Petralito S, Di Muzio L, Trilli J, Cesa S, Tho I, Casadei MA, Pharmaceutics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Li H, Li M, Wang Y, Zhang W, Chem. - A Eur. J. 2014, 20, 10392. [DOI] [PubMed] [Google Scholar]
- [98].Joyce P, Dening TJ, Meola TR, Wignall A, Ulmefors H, Kovalainen M, Prestidge CA, ACS Appl. Bio Mater. 2020, 3, 7779. [DOI] [PubMed] [Google Scholar]
- [99].Joyce P, Dening TJ, Meola TR, Gustafsson H, Kovalainen M, Prestidge CA, Eur. J. Pharm. Sci. 2019, 135, 1. [DOI] [PubMed] [Google Scholar]
- [100].Dimasi JA, Feldman L, Seckler A, Wilson A, Clin. Pharmacol. Ther. 2010, 87, 272. [DOI] [PubMed] [Google Scholar]
- [101].Wang G, Maciel D, Wu Y, Rodrigues J, Shi X, Yuan Y, Liu C, Tomás H, Li Y, ACS Appl. Mater. Interfaces 2014, 6, 16687. [DOI] [PubMed] [Google Scholar]
- [102].LTingting Jiang X. S., Chen Guangxiang, Polymers (Basel). 2019, DOI 10.3390/polym11010137. [DOI] [Google Scholar]
- [103].Gonçalves M, Figueira P, Maciel D, Rodrigues J, Qu X, Liu C, Tomás H, Li Y, Acta Biomater. 2014, 10, 300. [DOI] [PubMed] [Google Scholar]
- [104].Gonc M, Figueira P, Maciel D, Shi X, Tom H, Macromol. Biosci. 2014, 110. [DOI] [PubMed] [Google Scholar]
- [105].Motealleh A, Kehr NS, J. Mater. Chem. B 2020, 8, 4195. [DOI] [PubMed] [Google Scholar]
- [106].Wu Y, Li K, Kong L, Tang Y, Li G, Jiang W, Shen M, Guo R, Zhao Q, Shi X, Bioconjug. Chem. 2020, 31, 2404. [DOI] [PubMed] [Google Scholar]
- [107].Jiang T, Zhang C, Sun W, Cao X, Choi G, Choy JH, Shi X, Guo R, Chem. - A Eur. J. 2020, 26, 2470. [DOI] [PubMed] [Google Scholar]
- [108].Li J, Pan H, Qiao S, Li Y, Wang J, Liu W, Pan W, Int. J. Biol. Macromol. 2019, 134, 63. [DOI] [PubMed] [Google Scholar]
- [109].Zheng L, Zhou B, Qiu X, Xu X, Li G, Lee WYW, Jiang J, Mater. Sci. Eng. C 2019, 99, 1407. [DOI] [PubMed] [Google Scholar]
- [110].Wu Y, Guo R, Wen S, Shen M, Zhu M, Wang J, Shi X, Mater. Chem. B 2014, 2, 7410. [DOI] [PubMed] [Google Scholar]
- [111].Chen G, Li D, Li J, Cao X, Wang J, Shi X, Guo R, New J. Chem. 2015, 39, 2847. [Google Scholar]
- [112].Lakshminarayanan R, Ye E, Young DJ, Li Z, Loh XJ, Adv. Healthc. Mater. 2018, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sharifzadeh S, Boersma MJ, Kocaoglu O, Shokri A, Brown CL, Shirley JD, Winkler ME, Carlson EE, 2017, 2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kalwar K, Zhang X, Bhutto MA, Dali L, Shan D, Mater. Res. Express 2017, 4, 125401. [Google Scholar]
- [115].Peng Q, Xu P, Xiao S, Fibers Polym. 2018, 19, 477. [Google Scholar]
- [116].Ghadiri M, Chrzanowski W, Rohanizadeh R, J. Mater. Sci. Mater. Med. 2014, 25, 2513. [DOI] [PubMed] [Google Scholar]
- [117].Koshy ST, Zhang DKY, Grolman JM, Stafford AG, Mooney DJ, Acta Biomater. 2018, 65, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang C, Gong Z, Huang X, Wang J, Xia K, Ying L, Shu J, Yu C, Zhou X, Li F, Liang C, Chen Q, Theranostics 2019, 9, 7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Page DJ, Clarkin CE, Mani R, Khan NA, Dawson JI, Evans ND, bioRxiv 2019, DOI 10.1101/566943. [DOI] [PubMed] [Google Scholar]
- [120].Zhang R, Xie L, Wu H, Yang T, Zhang Q, Tian Y, Liu Y, Han X, Guo W, He M, Liu S, Tian W, Acta Biomater. 2020, 113, 305. [DOI] [PubMed] [Google Scholar]
- [121].Li J, Weber E, Guth-Gundel S, Schuleit M, Kuttler A, Halleux C, Accart N, Doelemeyer A, Basler A, Tigani B, Wuersch K, Fornaro M, Kneissel M, Stafford A, Freedman BR, Mooney DJ, Adv. Healthc. Mater. 2018, 7, DOI 10.1002/adhm.201701393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang Y, Chen M, Dai Z, Cao H, Li J, Zhang W, Biomater. Sci. 2020, 8, 682. [DOI] [PubMed] [Google Scholar]
- [123].Kim YH, Yang X, Shi L, Lanham SA, Hilborn J, Oreffo ROC, Ossipov D, Dawson JI, Nat. Commun. 2020, 11, DOI 10.1038/s41467-020-15152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Liu B, Li J, Lei X, Miao S, Zhang S, Cheng P, Song Y, Wu H, Gao Y, Bi L, Pei G, RSC Adv. 2020, 10, 25652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chaudhari SP, Patil PS, Int. J. Adv. Pharmacy, Biol. Chem. 2012, 1, 21. [Google Scholar]
- [126].BYK Additives & Instruments, 2013, 1. [Google Scholar]
- [127].Pifferi G, Restani P, Farmaco 2003, 58, 541. [DOI] [PubMed] [Google Scholar]
- [128].Zheng M, Pan M, Zhang W, Lin H, Wu S, Lu C, Tang S, Liu D, J. Cai, Bioact. Mater. 2021, 6, 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Mahmoudi M, Nat. Commun. 2021, 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bunker BC, J. Non. Cryst. Solids 1994, 179, 300. [Google Scholar]