Abstract
Background:
Lower urinary tract symptoms (LUTS) are associated with frailty phenotype, a risk factor for functional decline. Our objective was to determine the association between baseline LUTS and 2-year risk of new functional limitation among older men.
Methods:
We analyzed data from the Osteoporotic Fractures in Men (MrOS) study with baseline at Year 7 and follow-up through Year 9. Participants included 2716 community-dwelling men age ≥71 years without any baseline self-reported functional limitation. LUTS severity (American Urologic Association Symptom Index) was classified as none/mild (score 0–7), moderate (8–19), and severe (20–35). At baseline and follow-up, men reported their ability to complete several mobility, activities of daily living (ADLs), and cognition-dependent tasks. Risk was estimated for 3 incident functional limitation outcomes: 1) mobility (any difficulty walking 2–3 blocks or climbing 10 steps), 2) ADL (any difficulty bathing, showering, or transferring), and 3) cognition-dependent (any difficulty managing money or medications). We used Poisson regression with a robust variance estimator to model adjusted risk ratios (ARR) and 95% confidence intervals (CI) controlling for age, site, and comorbidities; other demographic/lifestyle factors did not meet criteria for inclusion.
Results:
Overall, the 2-year risk was 15% for mobility, 10% for ADLs, and 4% for cognition-dependent task limitations. Compared to none/mild LUTS, risk of incident mobility limitations was increased for moderate (ARR=1.35, 95% CI: 1.12,1.63) and severe LUTS (ARR=1.98, 95% CI: 1.48,2.64). Men were also at higher risk for incident ADL limitations if they reported moderate (ARR=1.32, 95% CI: 1.05,1.67) and severe LUTS (ARR=1.62, 95% CI: 1.07,2.43). Results were somewhat attenuated after adjusting for the frailty phenotype but remained statistically significant. LUTS were not associated with incident cognition-dependent task limitations.
Conclusions:
LUTS severity is associated with incident mobility and ADL limitations among older men. Increased clinical attention to risk of functional limitations among older men with LUTS is likely warranted.
Keywords: Functional health status, Aging, Epidemiology, Disability, Benign Prostatic Hyperplasia
INTRODUCTION
Lower urinary tract symptoms (LUTS) affect almost half of men after age 70.1,2 Older men with greater LUTS severity are more likely to be depressed2, phenotypically frail3, and to report difficulties with mobility, activities of daily living (ADLs), and instrumental activities of daily living (iADLs).2,4,5 LUTS is also associated with greater risk of falls and some studies show a positive association between LUTS and fractures in older men.6,7 However, current first line medications for male LUTS8 are not designed to prevent these important geriatric outcomes. Rather, they narrowly focus on reducing symptom severity by targeting prostatic enlargement, smooth muscle dysfunction, and detrusor overactivity, which may contribute to even greater systemic risks for older men.9–13 To our knowledge, no prior studies have examined the longitudinal association between LUTS and incident functional limitation. Understanding whether LUTS precedes functional limitation is an important first step towards understanding the prognostic value of LUTS in older men, for identifying potentially modifiable risk factors for co-occurring geriatric syndromes, and for weighing potential urinary symptom benefits and functional adverse events of current and future interventions for LUTS in older men.
In order to address this gap in knowledge, we evaluated the association of baseline LUTS severity, overall and by storage and voiding subscores, with incident self-reported functional limitations (mobility, ADLs, and cognition-dependent tasks) in a large cohort of older, community-dwelling men. We hypothesized that men with more severe LUTS at baseline would have a higher risk of developing new functional limitations within 2 years.
METHODS
Participants
The Osteoporotic Fractures in Men (MrOS) study is a multicenter cohort study of 5,994 community-dwelling men age 65 years or older at enrollment as previously described.14 Briefly, this cohort was designed to collect comprehensive data to study older men’s health, including urologic symptoms, with a particular focus on falls and fractures. Men were recruited from March 2000 to April 2002 from six geographic regions in the US. Surviving participants were invited to return to the clinic during Year 7 (March 2007 – March 2009) and to complete a questionnaire during Year 9 (March 2009 – February 2011). Year 7 was the first visit at which men were asked about ADLs and functional cognition-dependent task limitations. The analytic cohort included 2,716 men who attended the Year 7 clinic visit and completed the Year 9 questionnaire, completed self-reported functional assessments at both Year 7 and 9, and completed LUTS assessments and reported no functional limitation in any domain at Year 7 (Supplemental Figure 1). All participants gave written informed consent and Institutional Review Boards at each participating institution approved the study.
LUTS Assessment
LUTS were assessed during Year 7 with the validated, widely used 7-item American Urological Association Symptom Index (AUASI)15, including individual items on urinary frequency, urgency, intermittency, straining, weak urinary stream, incomplete bladder emptying, and nocturia. Responses to each item are on an ordinal scale with values ranging from 0 to 5 (higher = more frequent symptoms) and total scores range from 0 to 35. For example, to evaluate the storage symptom of urgency men were asked “Over the past month, how often have you found it difficult to postpone urination?” and to evaluate the voiding symptom of incomplete emptying men were asked “Over the past month, how often have you had a sensation of not emptying your bladder completely after you finish urinating?” Response options included “Not at all”, “Less than 1 time in 5”, “Less than half the time”, “About half the time”, “More than half the time”, or “Almost always”. The AUASI has clinically relevant categories of 0 to 7 (none/mild), 8 to 19 (moderate), and 20 to 35 (severe).8 In addition to the total score, we calculated validated AUASI subscores separately for storage symptoms (urgency, frequency, nocturia) and for voiding symptoms (incomplete emptying, intermittency, weak stream, straining).16 These subscores were categorized using percentiles rather than other categories because we did not want to make assumptions about their distribution in our study population.
Functional Limitation Assessment
Functional status was assessed at Year 7 and again at Year 9. Specifically, men reported their perceived difficulty and ability to complete the following tasks without help from others and without using any special aids: walking 2 to 3 blocks on level ground, climbing ten steps without resting, managing money or medications, bathing/showering, and transfers (“difficulty getting in and out of beds or chairs”). Response options included “Some difficulty”, “Much difficulty”, and “Unable to do it” and if they reported that they did not do a task or were unable, they were asked whether or not this was due to a health or physical problem.
We focused on three distinct domains of functional limitation: 1) mobility limitation, 2) ADL limitation, and 3) cognition-dependent task limitation. Mobility limitation was defined as having any difficulty or inability to walk 2 to 3 blocks or climb ten steps.17,18 ADL limitation was defined as any difficulty or inability to bathe, shower, or transfer in and out of beds or chairs. Cognition-dependent task limitation was defined as having any difficulty or inability to manage money or medications.
Other Measurements
All covariate measures were collected at the Year 7 visit except demographics which were collected at enrollment. These included age, clinic site and selected variables from five groups: demographics (education, race, and marital status), anthropometrics (BMI and waist circumference), health-related behaviors (smoking, alcohol intake, and physical activity), cardiovascular comorbidities (self-reported history of myocardial infarction or angina, heart failure, and hypertension), and other medical comorbidities (self-reported history of diabetes mellitus, prostate cancer, chronic obstructive pulmonary disease, and stroke or Parkinson’s disease). Additional covariate details are in the Supplemental Methods S1.
Statistical Analysis
In this analytic cohort, defined in part by the absence of any functional limitation at Year 7, the primary independent variable was LUTS severity at Year 7 and the 3 primary dependent variables were incident mobility limitation, incident ADL limitation, and incident cognition-dependent task limitation at Year 9, respectively. We first compared distributions of established LUTS and functional limitation risk factors across clinical categories of LUTS severity. We then modeled associations of overall LUTS severity (clinical categories and approximately equal fourths or rough quartiles of total AUASI score) and storage and voiding subscores (approximately equal thirds or rough tertiles) with incident functional limitations using risk ratios (RR) estimated with a modified Poisson regression model with robust error variance.19
To identify and control for confounding factors, we applied a change in estimate criteria.20 First, we forced age and study site into the model. Next, we fit a full multivariable model including age, site, and variables forming 5 categories of potential confounders: demographics, anthropometrics, health-related behaviors, cardiovascular comorbidities, and other medical comorbidities. We then successively removed a category of variables from the full model and each time calculated the % change in the beta coefficients compared to the full model, with a change of ≥5% used to indicate important confounding.21 Only cardiovascular and other medical comorbidities met this criteria for confounding and were therefore included in models with mobility limitation or ADL limitation as the dependent variable, and no groups met this criteria for inclusion in models with cognition-dependent task limitation as the dependent variable. We further adjusted for sleep apnea as a possible confounder, but adjustment did not materially change the beta coefficients so it was not retained. The final multivariable model for all dependent variables included age (continuous in years), site, and self-reported myocardial infarction or angina, heart failure, hypertension, diabetes mellitus, prostate cancer, stroke or Parkinson’s disease, and chronic obstructive pulmonary disease.
To visualize potentially complex interactions, we plotted the estimated probability of new functional limitations by potential effect modifiers, stratified by LUTS severity. We then tested for effect modification of the main associations by including a cross product term with age, any LUTS treatment (medication or surgery), neurologic disease (stoke or Parkinson’s disease), or diabetes and LUTS severity as a three level variable (none/mild, moderate, severe).
We conducted sensitivity analyses further adjusting for variables that could be confounders or mediators as outlined in our conceptual model (Figure 1), including: Geriatric Depression Scale (GDS), SF-12 mental component score (MCS), multimorbidity, self-reported general health status, cognitive function (3MS and Trails B; both continuous), frailty phenotype, and LUTS medication use (α-antagonist, 5α-reductase inhibitor, or anti-cholinergic use). To maximize adjustment for frailty, we simultaneously included each of the following frailty phenotype criterion as separate variables: gait speed (continuous), grip strength (continuous), PASE (continuous), exhaustion (SF-12 question that asks “Did you have a lot of energy?”; categorical), and unintentional weight loss of ≥5% since prior visit (binary). We conducted additional sensitivity analyses excluding men with urinary incontinence (at least weekly). In additional sensitivity analyses, we used stabilized inverse probability of censoring weights (IPCW) to account for potential bias due to loss of follow-up by modeling each participant’s probability of having a non-missing outcome at follow-up as a function of all demographic and baseline clinical characteristics included in Table 1.22 Weights were constructed using the LOGISTIC procedure in SAS version 9.4 and were then applied to subsequent models evaluating the associations of LUTS with incident functional limitations.
Figure 1.
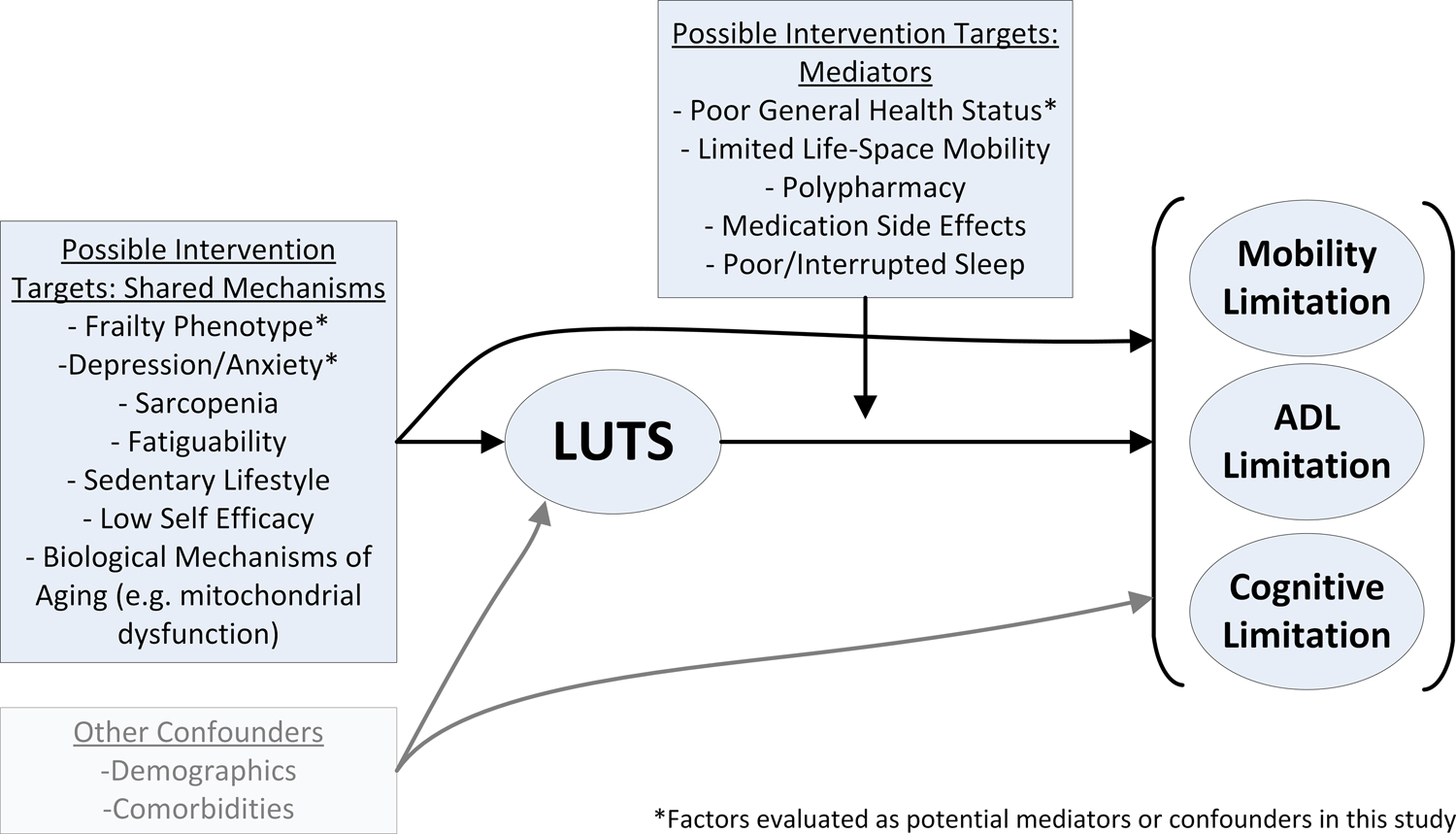
Conceptual model of potential shared mechanisms and mediators of the association between lower urinary tract symptoms (LUTS) and incident functional limitations.
Table 1.
Characteristics of 2716 MrOS participants, by clinical LUTS categories at baseline (Year 7).
| Variable | None/Mild (AUASI <8) |
Moderate (AUASI 8–19) |
Severe (AUASI ≥20) |
|---|---|---|---|
| Sample Size, n (%) | 1512 (56) | 1045 (38) | 159 (6) |
| Demographics | |||
| Age, years, mean (SD) | 78 (5) | 79 (5) | 79 (5) |
| College education, n (%) | 900 (60) | 650 (62) | 90 (57) |
| Married status, n (%) | 1232 (82) | 850 (81) | 120 (76) |
| Self-reported race, n (%) | |||
| White, not Hispanic or Latino | 1356 (90) | 960 (92) | 147 (93) |
| Black or African-American | 43 (3) | 23 (2) | 2 (1) |
| Asian | 65 (4) | 37 (4) | 2 (1) |
| Native Hawaiian or Pacific Islander | 1 (0) | 1 (0) | 0 (0) |
| American Indian or Alaskan Native | 2 (0) | 0 (0) | 1 (1) |
| Multiracial | 19 (1) | 8 (1) | 5 (3) |
| Other | 26 (2) | 16 (2) | 2 (1) |
| Hispanic ethnicity, n (%) | 33 (2) | 19 (2) | 3 (2) |
| Biometrics, mean (SD) | |||
| BMI, kg/m2 | 26.8 (4) | 26.7 (4) | 27.1 (3) |
| Waist circumference, cm | 100 (10) | 100 (10) | 102 (9) |
| Walking speed, m/s | 1.2 (0.2) | 1.2 (0.2) | 1.1 (0.2) |
| Maximum Grip Strength, kg | 40 (8) | 40 (8) | 40 (8) |
| Questionnaires | |||
| Self-reported General Health Status, n (%) | |||
| Excellent | 641 (42) | 341 (33) | 36 (23) |
| Good | 795 (53) | 602 (58) | 90 (57) |
| Fair, Poor, or Very Poor | 75 (5) | 102 (10) | 33 (21) |
| Geriatric Depression Scale, mean (SD) | 1.2 (2) | 1.6 (2) | 2.6 (3) |
| SF-12 MCS, mean (SD) | 57 (5) | 56 (6) | 54 (8) |
| Health-Related Behaviors | |||
| Smoking, n (%) | |||
| Never | 635 (42) | 423 (41) | 66 (42) |
| Past | 847 (56) | 610 (58) | 89 (56) |
| Current | 30 (2) | 12 (1) | 4 (3) |
| Drinking, n (%) | |||
| <1 drink/week | 698 (46) | 440 (42) | 74 (47) |
| 1–14 drinks/week | 737 (49) | 558 (54) | 75 (47) |
| >14 drinks/week | 75 (5) | 43 (4) | 10 (6) |
| PASE score, mean (SD) | 147 (67) | 139 (66) | 136 (60) |
| Comorbidities and Medication Use, n (%) | |||
| Myocardial Infarction or Angina | 256 (17) | 190 (18) | 38 (24) |
| Heart Failure | 56 (4) | 51 (5) | 18 (11) |
| Prostate Cancer | 268 (18) | 152 (15) | 23 (15) |
| Stroke or Parkinson’s Disease | 85 (6) | 58 (6) | 11 (7) |
| Hypertension | 756 (50) | 564 (54) | 84 (53) |
| Diabetes Mellitus | 181 (12) | 145 (14) | 27 (17) |
| Chronic Obstructive Pulmonary Disease | 103 (7) | 108 (10) | 20 (13) |
| Diuretic Medication Use | 389 (26) | 273 (26) | 43 (27) |
| Multimorbidity * | |||
| 0 chronic conditions | 830 (55) | 511 (49) | 64 (40) |
| 1 chronic condition | 462 (31) | 341 (33) | 53 (33) |
| 2 chronic conditions | 163 (11) | 131 (13) | 22 (14) |
| ≥3 chronic conditions | 57 (4) | 62 (6) | 20 (13) |
| Frailty Phenotype ** , n (%) | |||
| Robust (0 criteria met) | 553 (37) | 341 (33) | 42 (27) |
| Intermediate (1–2 criteria met) | 786 (52) | 567 (54) | 87 (55) |
| Frail (≥3 criteria met) | 173 (11) | 137 (13) | 29 (18) |
| Cognitive function, mean (SD) | |||
| Teng Mini-Mental Status score | 93 (6) | 93 (5) | 93 (6) |
| Trails-Making Test part B, s | 116 (58) | 114 (54) | 121 (58) |
| LUTS Treatments, n (%) | |||
| α-Antagonist | 199 (13) | 317 (30) | 66 (42) |
| 5α-Reductase Inhibitor | 83 (6) | 123 (12) | 24 (15) |
| Anti-Cholinergic | 25 (2) | 39 (4) | 8 (5) |
| Self-reported BPH Surgery | 168 (11) | 123 (12) | 24 (15) |
AUASI American Urological Association Symptom Index; n sample size; SD standard deviation; s seconds; BMI body mass index; SF-12 MCS Short Form 12 Mental Component Summary; PASE Physical Activity Scale for the Elderly
Cumulative number of the following chronic medical conditions: stroke, Parkinson’s disease, myocardial infarction, angina, chronic obstructive pulmonary disease, heart failure, diabetes mellitus, osteoporosis, osteoarthritis, hyperthyroidism or hypothyroidism.
Frailty phenotype status based on cumulative number of criteria met: shrinking/sarcopenia, weakness, exhaustion, slowness, and low physical activity.
P-value <0.05 was considered statistically significant. All analyses were performed using STATA version 15.1 (StataCorp LLC, College Station, TX). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
RESULTS
General characteristics of the 2716 community-dwelling men in this prospective analytic cohort are reported by LUTS severity category in Table 1. Within this analytic cohort, 56% of men had none/mild LUTS, 38% had moderate LUTS, and 6% had severe LUTS. Mean time between the baseline and follow-up visit was 2.0 (standard deviation 0.12) years. During follow-up, the cumulative incidence of new limitations was 15% for mobility, 10% for ADLs, and 4% for cognition-dependent tasks.
Associations between baseline LUTS severity and incident mobility limitations are reported in Table 2. Overall, 11% of men with none/mild LUTS, 17% of men with moderate LUTS, and 26% of men with severe LUTS developed new mobility limitations during follow-up. Compared to men with none/mild LUTS, men with moderate and severe LUTS were 35% and 98% more likely to develop incident mobility limitations, respectively. A similar graded association was observed between quartiles of total AUASI score and incident mobility limitation, although there was some evidence of a non-linear association and of increased risk among men with total AUASI scores from 4 to 7, compared to 0 to 3. When storage and voiding subscores were examined separately, both were positively associated with risk of incident mobility limitation and RRs were of similar magnitude. When considering variables with an unclear role (potential mediators or confounder), further adjustment one at a time for LUTS medication use, self-reported general health status, and GDS as well as all 5 frailty phenotype components produced smaller RRs that remained statistically significant, whereas further adjustment for multimorbidity did not materially change the results (Supplemental Table 1). For example, after adding baseline gait speed, grip strength, PASE, exhaustion, and unintentional weight loss to the final multivariable model, the RR for severe LUTS compared to none/mild LUTS attenuated from 1.98 to 1.60.
Table 2.
Association of lower urinary tract symptoms (LUTS) severity at baseline with incident mobility limitation*.
| Age and site-Adjusted | Multivariable§ Adjusted | ||||
|---|---|---|---|---|---|
|
|
|||||
| AUASI Score Range† | # Incident Limitation /Total (%) | Risk Ratio (95% CI)‡ | Risk Ratio (95% CI)‡ | P value | |
| Clinical LUTS Categories | |||||
| None/Mild | 0–7 | 173/1512 (11.4) | Ref. | Ref. | <0.001 |
| Moderate | 8–19 | 179/1045 (17.2) | 1.47 (1.21, 1.77) | 1.35 (1.12, 1.63) | |
| Severe | 20–35 | 42/159 (26.4) | 2.20 (1.64, 2.95) | 1.98 (1.48, 2.64) | |
|
| |||||
| Total AUASI | |||||
| Quartile 1 | 0–3 | 70/712 (9.8) | Ref. | Ref. | <0.001 |
| Quartile 2 | 4–7 | 103/800 (12.9) | 1.32 (0.99, 1.74) | 1.31 (0.99, 1.73) | |
| Quartile 3 | 8–12 | 88/608 (14.5) | 1.50 (1.12, 2.01) | 1.37 (1.03, 1.83) | |
| Quartile 4 | 13–35 | 133/596 (22.4) | 2.13 (1.64, 2.78) | 1.96 (1.51, 2.55) | |
|
| |||||
| Storage Subscore | |||||
| Tertile 1 | 0–3 | 135/1228 (11.0) | Ref. | Ref. | <0.001 |
| Tertile 2 | 4–6 | 127/848 (15.0) | 1.40 (1.12, 1.75) | 1.34 (1.08, 1.68) | |
| Tertile 3 | 7–15 | 132/640 (20.6) | 1.81 (1.46, 2.24) | 1.64 (1.32, 2.04) | |
|
| |||||
| Voiding Subscore | |||||
| Tertile 1 | 0–1 | 118/1108 (10.6) | Ref. | Ref. | <0.001 |
| Tertile 2 | 2–4 | 110/732 (15.0) | 1.37 (1.08, 1.74) | 1.33 (1.05, 1.68) | |
| Tertile 3 | 5–20 | 166/876 (19.0) | 1.69 (1.36, 2.09) | 1.56 (1.25, 1.93) | |
Defined as having any difficulty or inability for either walking 2–3 blocks or climbing 10 steps.
American Urological Association Symptom Index (AUASI) score range is 0 to 35 and equals the sum of 2 validated subscores based on symptom type: storage (urgency, frequency, nocturia) and voiding (intermittency, weak stream, straining, incomplete emptying). Higher score indicates more frequent symptoms. Total AUASI score was categorized using clinically validated thresholds for none/mild (0–7), moderate (8–19), and severe (20–35) LUTS and approximately into equal fourths (quartiles). Storage and voiding subscores were categorized approximately into equal thirds (tertiles) of the subscore distribution.
Risk ratios and 95% confidence intervals calculated using Poisson regression. P values calculated using the Wald test.
Adjusted for age, site, and history of myocardial infarction or angina, heart failure, hypertension, diabetes, chronic obstructive pulmonary disease, prostate cancer, and stroke or Parkinson’s disease.
Baseline LUTS severity was also positively associated with incident ADL limitations (Table 3). During follow-up, 8% of men with none/mild LUTS, 12% of men with moderate LUTS, and 15% of men with severe LUTS developed new ADL limitations. Compared to men with none/mild LUTS, men with moderate and severe LUTS were 32% and 62% more likely to develop incident ADL limitations, respectively. When associations were examined by quartiles of total AUASI score, only men in the highest quartile had an increased risk of new ADL limitations compared to men in the lowest quartile. When subscores were examined separately, men in the middle or highest category of storage subscores were more likely to develop incident ADL limitations compared to the lowest category. Men in the highest category of voiding subscores, but not middle category, had a higher risk of incident ADL limitation. Adjustment for other potential confounders somewhat attenuated the RR estimates and led to wide confidence intervals that included 1 (Supplemental Table 1).
Table 3.
Association of lower urinary tract symptoms (LUTS) severity at baseline with incident ADL limitation*.
| Age and site-Adjusted | Multivariable§ Adjusted | ||||
|---|---|---|---|---|---|
|
|
|||||
| AUASI Score Range† | # Incident Limitation /Total (%) | Risk Ratio (95% CI)‡ | Risk Ratio (95% CI)‡ | P value | |
| Clinical LUTS Categories | |||||
| None/Mild | 0–7 | 125/1512 (8.2) | Ref. | Ref. | 0.02 |
| Moderate | 8–19 | 122/1045 (11.7) | 1.37 (1.09, 1.73) | 1.32 (1.05, 1.67) | |
| Severe | 20–35 | 24/159 (15.1) | 1.74 (1.16, 2.63) | 1.62 (1.07, 2.43) | |
|
| |||||
| Total AUASI | |||||
| Quartile 1 | 0–3 | 54/712 (7.6) | Ref. | Ref. | 0.003 |
| Quartile 2 | 4–7 | 71/800 (8.9) | 1.15 (0.82, 1.60) | 1.13 (0.81, 1.57) | |
| Quartile 3 | 8–12 | 58/608 (9.5) | 1.25 (0.89, 1.78) | 1.18 (0.84, 1.67) | |
| Quartile 4 | 13–35 | 88/596 (14.8) | 1.80 (1.31, 2.47) | 1.72 (1.25, 2.36) | |
|
| |||||
| Storage Subscore | |||||
| Tertile 1 | 0–3 | 97/1228 (7.9) | Ref. | Ref. | 0.01 |
| Tertile 2 | 4–6 | 92/848 (10.8) | 1.40 (1.07, 1.82) | 1.37 (1.05, 1.79) | |
| Tertile 3 | 7–15 | 82/640 (12.8) | 1.54 (1.17, 2.02) | 1.46 (1.11, 1.93) | |
|
| |||||
| Voiding Subscore | |||||
| Tertile 1 | 0–1 | 89/1108 (8.1) | Ref. | Ref. | 0.03 |
| Tertile 2 | 2–4 | 71/732 (9.7) | 1.16 (0.86, 1.55) | 1.14 (0.85, 1.52) | |
| Tertile 3 | 5–20 | 111/876 (12.6) | 1.49 (1.15, 1.93) | 1.43 (1.10, 1.86) | |
Defined as having any difficulty or inability for bathing or showering and transfers in and out of beds or chairs.
American Urological Association Symptom Index (AUASI) score range is 0 to 35 and equals the sum of 2 validated subscores based on symptom type: storage (urgency, frequency, nocturia) and voiding (intermittency, weak stream, straining, incomplete emptying). Higher score indicates more frequent symptoms. Total AUASI score was categorized using clinically validated thresholds for none/mild (0–7), moderate (8–19), and severe (20–35) LUTS and approximately into equal fourths (quartiles). Storage and voiding subscores were categorized approximately into equal thirds (tertiles) of the subscore distribution.
Risk ratios and 95% confidence intervals calculated using Poisson regression. P values calculated using the Wald test.
Adjusted for age, site, and history of myocardial infarction or angina, heart failure, hypertension, diabetes, chronic obstructive pulmonary disease, prostate cancer, and stroke or Parkinson’s disease.
Baseline LUTS severity was not associated with incident cognition-dependent task limitations (Supplemental Table 2), although confidence intervals were wide and included clinically meaningful effect estimates above and below 1. When subscores were examined separately, associations were not significant, and RR were in the opposite direction for storage versus voiding LUTS.
Additional sensitivity analyses are reported in Supplemental Table 1 and Supplemental Table 3. We also observed evidence of an interaction between age and overall LUTS severity for associations with all three functional limitation outcomes (mobility: P for interaction = 0.04; ADL: P for interaction = 0.02; cognition-dependent tasks: P for interaction = 0.04; Figure 2 and Supplemental Table 4). Although men in the highest quartile of total AUASI score had an increased risk of incident mobility limitation in all age groups, the RR estimates were greatest among men in the lowest age tertile (71–76 years) compared to the middle (77–80 years) and highest (81–98 years) age tertiles. Conversely, the association between overall LUTS severity and incident ADL limitation was strongest among men in the middle age tertile. Among men in the lowest age tertile, higher total AUASI was associated was an increased risk of incident cognition-dependent task limitation (Supplemental Figure 2), although confidence intervals were wide. We did not observe evidence of a consistent interaction of LUTS severity with LUTS treatment, neurologic disease, or diabetes.
Figure 2.
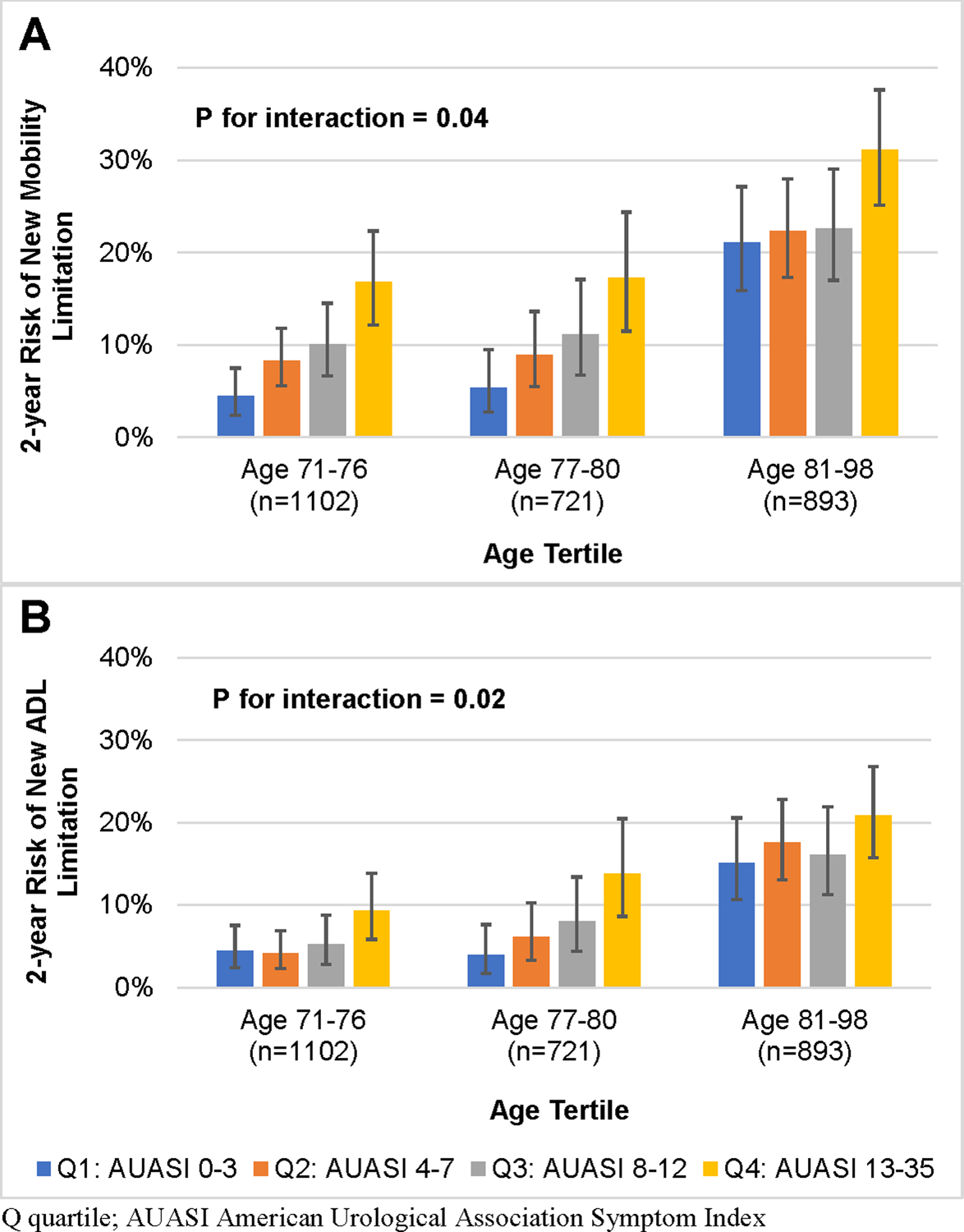
2-year risk of A) new mobility and B) activities of daily living (ADL) limitation by quartile of AUASI score, stratified by age.
DISCUSSION
In this prospective cohort study, we found that older community-dwelling men with greater LUTS severity had a higher risk of developing new mobility and ADL limitations within two years. These associations were independent of age and comorbidities, appeared similar when comparing highest versus lowest tertiles for both storage and voiding LUTS subtypes, and persisted among men without urinary incontinence or cognitive impairment. Observed associations appeared to vary by age group and were partially, but not completely, explained by greater phenotypic frailty, depressive symptoms, and poorer self-related health status among men with more severe LUTS. Conversely, few men developed new cognition-dependent task limitations during the 2-year follow-up and LUTS severity was not consistently associated with this outcome.
Several cross-sectional studies report that men with LUTS report greater functional limitation compared to men without LUTS. Consistent with the earliest publications 3 decades ago,23,24 subsequent studies have confirmed that men with LUTS have lower scores on the SF-36 physical functioning subscale.25–30 The SF-36 physical functioning subscale summarizes the amount of self-reported limitation due to health in 10 tasks that include a range of concepts, including mobility, physical activity, and a single ADL (“bathing or dressing yourself”).31 Two of the tasks assessed in this subscale, ability to “walk several blocks” and “climb one flight of stairs”, are similar to our mobility limitation outcome and may explain previously observed cross-sectional associations. Despite the consistency of these cross-sectional associations, there remains a key lack of longitudinal studies examining the association between LUTS severity and geriatric outcomes, such as change in functional status. Our study fills this knowledge gap.
If LUTS caused new mobility and ADL limitation, one would expect a lower risk of these outcomes, as well as their surrogates or proxies, among men receiving effective LUTS treatments in clinical trials. Although we are unaware of any LUTS trials that formally assessed change in mobility or ADL limitation, a secondary data analysis of the Medical Therapy of Prostatic Symptoms trial, which compared placebo, doxazosin, finasteride, and doxazosin plus finasteride (combination therapy), suggested a modestly slower decline in the SF-36 physical functioning subscale among men randomized to finasteride compared to placebo (difference of change from baseline to year 4 = 2.20, 95% CI 0.17, 4.57, P = 0.05).32 However, a similar association was not observed among men randomized to doxazosin or combination therapy compared to placebo despite greater decreases in LUTS severity in these treatment arms32,33, suggesting that the effect of finasteride on SF-36 was not mediated by LUTS severity. Data from other randomized clinical trials have also reported mixed results for the effect of medication or surgery on improving activity limitation due to LUTS.34–38 In our analysis, associations were minimally attenuated after adjustment for LUTS medication use, which is consistent with the hypothesis that mobility and ADL limitations are part of the natural history of LUTS, independent of available pharmacologic therapies. Taken together, interventions that improve LUTS severity alone do not consistently prevent co-existing physical function decline among older men with LUTS and do not explain associations observed in this analysis.
Our results have important implications for future research. First, the underlying mechanism of this association is uncertain and, based on our sensitivity analyses, does not appear to be affected by current pharmacologic LUTS therapies. Second, the greater prevalence of phenotypic frailty among older men with LUTS does not fully explain the observed associations with incident functional limitations. Our team previously reported that older men with LUTS are more likely to exhibit phenotypic frailty and slower Timed-Up-and-Go tests3,39, both risk factors for new functional limitation. The direction of these relationships remain under investigation, although we have hypothesized in our prior work that LUTS and frailty frequently co-occur due to a shared underlying biological mechanism. Understanding the role of frailty is important because findings from the growing field of geroscience suggest that human healthspan and chronic diseases or syndromes of aging, including LUTS, frailty, and functional limitation, may be amenable to treatment by targeting the fundamental biological processes of aging that drive aging-related pathophysiology.40 Third, the greater prevalence of depression and poor self-reported health among older men with LUTS2 also failed to fully explain the observed associations. Furthermore, the association between LUTS and depression appears bidirectional based on prior studies41–43 which adds to the complexity of modeling this potential confounder. Lastly, we were surprised that adjustment for self-reported physical activity did not change the results in this study. Based on qualitative studies, older men with LUTS report their symptoms as a barrier to several health-related behaviors known to improve mood and prevent functional limitation, including physical activity.44,45 Physical activity is also associated with both LUTS and functional limitations in several prospective studies.46,47 Although physical activity measured via the PASE does not appear to explain observed associations between LUTS severity and functional limitations, this finding does not eliminate the possibility that a supervised exercise intervention may decrease risk of incident functional limitations among older men with LUTS. Confirmatory studies with comprehensive and repeated measures of key co-occurring age-related conditions, particularly frailty and depression, are needed to determine their contribution to the observed relationship between LUTS and risk of functional limitations.
There are also important clinical implications of our findings, regardless of whether LUTS causes functional limitations or they share an underlying biological mechanism. Based on these results, we propose that LUTS is a previously under-appreciated prognostic indicator of accelerated phenotypic aging that occurs sooner than, or independent of, other urologic (e.g., urinary incontinence) and non-urologic (e.g., multimorbidity, frailty phenotype, falls, etc.) indicators. Accordingly, the presence of LUTS in older men should motivate clinicians to actively screen for functional limitations. Given the heterogeneity of LUTS etiologies in older men, clinicians should also consider known age-related systemic causes of LUTS that contribute to risk of functional limitation, such as cardiovascular disease, diabetes, volume overload states, primary sleep disorders, and nocturnal polyuria48, before empirically treating urologic causes with medications that may further increase risk of functional limitations.
We recognize several limitations to our study. First, MrOS is a cohort of predominantly healthy, White, community-dwelling older men. Thus, the results may not be generalizable to younger men or to institutionalized, less healthy, or more racially diverse men. Second, this is an observational study and residual confounding may explain the observed associations. For example, malnutrition may be associated with both worse LUTS and higher risk of new functional limitations. However, our results were not substantially altered by considering demographic or clinical variables, including history of comorbidities. Third, a relatively small number of men in this community-dwelling cohort reported severe LUTS (AUASI 20–35) which limited our ability to evaluate non-linear associations that may manifest with greater LUTS severity. We also did not calculate LUTS trajectories and therefore did not evaluate LUTS progression as a risk factor for new functional limitations, a different yet clinically relevant research question. Fourth, fewer men with moderate and severe LUTS in our study sample reported less anti-cholinergic medication use compared to a contemporaneous Medicare sample49 and we did not have access to data regarding behavioral LUTS interventions, such as pelvic physical therapy. Lastly, very few men developed cognition-dependent task limitations, which limited our power to detect potentially clinically meaningful associations.
In conclusion, older men with greater LUTS severity have an increased risk of incident mobility and ADL limitations within 2 years. Observed associations were not entirely explained by consideration of frailty phenotype components and were independent of LUTS medication use, multimorbidity, cognitive impairment, and urinary incontinence. While additional studies are investigating the mechanistic basis of this association, clinicians treating older men should be aware that greater LUTS severity is a prognostic marker of phenotypic aging and identifies a group of men at increased risk of mobility and ADL limitations.
Supplementary Material
Supplemental Methods S1. Other Measurements.
Supplemental Figure 1. Study flowchart.
Supplemental Figure 2. 2-year risk of new cognition-dependent task limitation by tertile of AUASI score, stratified by age.
Supplemental Table 1. Association of LUTS severity at baseline with incident mobility limitation, ADL limitation, and cognition-dependent task limitation after adjusting for potential mediators.
Supplemental Table 2. Association of lower urinary tract symptoms (LUTS) severity at baseline with incident cognition-dependent task limitation.
Supplemental Table 3. Sensitivity analyses of association of overall LUTS severity at baseline with incident mobility limitation, ADL limitation, and cognition-dependent task limitation.
Supplemental Table 4. Association of AUASI score at baseline with incident mobility limitation, ADL limitation, and cognition-dependent task limitation, stratified by age tertile.
Supplementary References S1.
KEY POINTS.
Older men with lower urinary tract symptoms have increased risk of developing mobility and activities of daily living (ADL) limitations, perhaps due to greater frailty phenotype.
Why does this matter?
Clinicians should be aware that LUTS severity is a prognostic marker for future mobility and ADL limitations in older men.
ACKNOWLEDGMENTS
Funding:
This work was supported by grants to SRB from the National Institute of Diabetes, Digestive, and Kidney Disorders (grant number 1K12DK111028) and the National Institute on Aging (grant numbers 1R03AG067937 and K76AG074903) and the UCSF Claude D. Pepper Older Americans Independence Center funded by National Institute on Aging (grant number P30 AG044281 to KC). The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and the NIH Roadmap for Medical Research (grant numbers U01 AR45580, U01 AR45614, U01 AG042145, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, R01 AG066671, and UL1 TR000128).
Sponsor’s Role:
The study funders had no role in the design, methods, subject recruitment, data collections, analysis or preparation of this paper.
Footnotes
Conflict of Interest: The authors have no conflicts.
The data presented in this manuscript has been accepted for a virtual oral presentation at the International Conference on Frailty and Sarcopenia Research on September 29th, 2021.
REFERENCES
- 1.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in america project: benign prostatic hyperplasia. The Journal of urology. 2008;179(5 Suppl):S75–80. doi: 10.1016/j.juro.2008.03.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor BC, Wilt TJ, Fink HA, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68(4):804–809. doi: 10.1016/j.urology.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Bauer SR, Scherzer R, Suskind AM, et al. Co-Occurrence of Lower Urinary Tract Symptoms and Frailty among Community-Dwelling Older Men. Journal of the American Geriatrics Society. 2020. doi: 10.1111/jgs.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuotio M, Tammela TL, Luukkaala T, Jylhä M. Association of urgency symptoms with self-rated health, mood and functioning in an older population. Aging clinical and experimental research. 2007;19(6):465–471. doi: 10.1007/bf03324732. [DOI] [PubMed] [Google Scholar]
- 5.Trueman P, Hood SC, Nayak US, Mrazek MF. Prevalence of lower urinary tract symptoms and self-reported diagnosed ‘benign prostatic hyperplasia’, and their effect on quality of life in a community-based survey of men in the UK. BJU international. 1999;83(4):410–415. doi: 10.1046/j.1464-410x.1999.00966.x. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi N, Chan L, Cumming RG, Blyth FM, Naganathan V. A systematic review of the association between lower urinary tract symptoms and falls, injuries, and fractures in community-dwelling older men. The aging male : the official journal of the International Society for the Study of the Aging Male. 2016;19(3):168–174. doi: 10.3109/13685538.2016.1169399. [DOI] [PubMed] [Google Scholar]
- 7.Pesonen JS, Vernooij RWM, Cartwright R, et al. The Impact of Nocturia on Falls and Fractures: A Systematic Review and Meta-Analysis. The Journal of urology. 2019:101097ju0000000000000459. doi: 10.1097/ju.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 8.Lerner LB, McVary KT, Barry MJ, et al. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE PART I-Initial Work-up and Medical Management. The Journal of urology. 2021;206(4):806–817. doi: 10.1097/ju.0000000000002183. [DOI] [PubMed] [Google Scholar]
- 9.Oelke M, Becher K, Castro-Diaz D, et al. Appropriateness of oral drugs for long-term treatment of lower urinary tract symptoms in older persons: results of a systematic literature review and international consensus validation process (LUTS-FORTA 2014). Age and ageing. 2015;44(5):745–755. doi: 10.1093/ageing/afv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welk B, McArthur E, Fraser LA, et al. The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist: a population based cohort study. BMJ (Clinical research ed). 2015;351:h5398. doi: 10.1136/bmj.h5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of Suicidality and Depression With 5alpha-Reductase Inhibitors. JAMA internal medicine. 2017;177(5):683–691. doi: 10.1001/jamainternmed.2017.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic Drug Exposure and the Risk of Dementia: A Nested Case-Control Study. JAMA internal medicine. 2019. doi: 10.1001/jamainternmed.2019.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welk B, McArthur E. Increased risk of dementia among patients with overactive bladder treated with an anticholinergic medication compared to a beta-3 agonist: a population-based cohort study. BJU international. 2020;126(1):183–190. doi: 10.1111/bju.15040. [DOI] [PubMed] [Google Scholar]
- 14.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Barry MJ, Fowler FJ Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of urology. 1992;148(5):1549–1557; discussion 1564. [DOI] [PubMed] [Google Scholar]
- 16.Barry MJ, Williford WO, Fowler FJ Jr., Jones KM, Lepor H Filling and voiding symptoms in the American Urological Association symptom index: the value of their distinction in a Veterans Affairs randomized trial of medical therapy in men with a clinical diagnosis of benign prostatic hyperplasia. The Journal of urology. 2000;164(5):1559–1564. http://ac.els-cdn.com/S0022534705670280/1-s2.0-S0022534705670280-main.pdf. [PubMed] [Google Scholar]
- 17.Cawthon PM, Orwoll ES, Peters KE, et al. Strong Relation Between Muscle Mass Determined by D3-creatine Dilution, Physical Performance, and Incidence of Falls and Mobility Limitations in a Prospective Cohort of Older Men. The journals of gerontology Series A, Biological sciences and medical sciences. 2019;74(6):844–852. doi: 10.1093/gerona/gly129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. Journal of the American Geriatrics Society. 2009;57(3):492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Daniel R, Pearce N. Outcome modelling strategies in epidemiology: traditional methods and basic alternatives. Int J Epidemiol. 2016;45(2):565–575. doi: 10.1093/ije/dyw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S, Pearce N. Statistical foundations for model-based adjustments. Annu Rev Public Health. 2015;36:89–108. doi: 10.1146/annurev-publhealth-031914-122559. [DOI] [PubMed] [Google Scholar]
- 22.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 23.Fowler FJ Jr., Wennberg JE, Timothy RP, Barry MJ, Mulley AG Jr., Hanley D Symptom status and quality of life following prostatectomy. JAMA : the journal of the American Medical Association. 1988;259(20):3018–3022. https://jamanetwork.com/journals/jama/articlepdf/372123/jama_259_20_030.pdf. [PubMed] [Google Scholar]
- 24.Garraway WM, Russell EB, Lee RJ, et al. Impact of previously unrecognized benign prostatic hyperplasia on the daily activities of middle-aged and elderly men. The British journal of general practice : the journal of the Royal College of General Practitioners. 1993;43(373):318–321. https://bjgp.org/content/bjgp/43/373/318.full.pdf. [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts RO, Jacobsen SJ, Rhodes T, Girman CJ, Guess HA, Lieber MM. Natural history of prostatism: impaired health states in men with lower urinary tract symptoms. The Journal of urology. 1997;157(5):1711–1717. https://www.sciencedirect.com/science/article/pii/S0022534701648420. [DOI] [PubMed] [Google Scholar]
- 26.Engstrom G, Henningsohn L, Walker-Engstrom ML, Leppert J. Impact on quality of life of different lower urinary tract symptoms in men measured by means of the SF 36 questionnaire. Scandinavian journal of urology and nephrology. 2006;40(6):485–494. doi: 10.1080/00365590600830862. [DOI] [PubMed] [Google Scholar]
- 27.Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the Health Professionals Follow-up Study. Urology. 2002;59(2):245–250. https://www.goldjournal.net/article/S0090-4295(01)01506-0/fulltext. [DOI] [PubMed] [Google Scholar]
- 28.Girman CJ, Jacobsen SJ, Tsukamoto T, et al. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology. 1998;51(3):428–436. doi: 10.1016/s0090-4295(97)00717-6. [DOI] [PubMed] [Google Scholar]
- 29.Coyne KS, Wein AJ, Tubaro A, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU international. 2009;103 Suppl 3:4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]
- 30.Robertson C, Link CL, Onel E, et al. The impact of lower urinary tract symptoms and comorbidities on quality of life: the BACH and UREPIK studies. BJU international. 2007;99(2):347–354. doi: 10.1111/j.1464-410X.2007.06609.x. [DOI] [PubMed] [Google Scholar]
- 31.Bohannon RW, DePasquale L. Physical Functioning Scale of the Short-Form (SF) 36: internal consistency and validity with older adults. J Geriatr Phys Ther. 2010;33(1):16–18. [PubMed] [Google Scholar]
- 32.Fwu CW, Eggers PW, Kaplan SA, Kirkali Z, Lee JY, Kusek JW. Long-term effects of doxazosin, finasteride and combination therapy on quality of life in men with benign prostatic hyperplasia. The Journal of urology. 2013;190(1):187–193. doi: 10.1016/j.juro.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 33.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. The New England journal of medicine. 2003;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 34.Girman CJ, Kolman C, Liss CL, Bolognese JA, Binkowitz BS, Stoner E. Effects of finasteride on health-related quality of life in men with symptomatic benign prostatic hyperplasia. Finasteride Study Group. The Prostate. 1996;29(2):83–90. doi:. [DOI] [PubMed] [Google Scholar]
- 35.O’Leary MP, Roehrborn CG, Black L. Dutasteride significantly improves quality of life measures in patients with enlarged prostate. Prostate cancer and prostatic diseases. 2008;11(2):129–133. doi: 10.1038/sj.pcan.4500990. [DOI] [PubMed] [Google Scholar]
- 36.Haltbakk J, Hanestad BR, Hunskaar S. Use and misuse of the concept of quality of life in evaluating surgical treatments for lower urinary tract symptoms. BJU international. 2003;91(4):380–388. doi: 10.1046/j.1464-410x.2003.04077.x. [DOI] [PubMed] [Google Scholar]
- 37.MacDonagh RP, Cliff AM, Speakman MJ, O’Boyle PJ, Ewings P, Gudex C. The use of generic measures of health-related quality of life in the assessment of outcome from transurethral resection of the prostate. British journal of urology. 1997;79(3):401–408. doi: 10.1046/j.1464-410x.1997.34017.x. [DOI] [PubMed] [Google Scholar]
- 38.Bruskewitz R, Girman CJ, Fowler J, et al. Effect of finasteride on bother and other health-related quality of life aspects associated with benign prostatic hyperplasia. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1999;54(4):670–678. doi: 10.1016/s0090-4295(99)00209-5. [DOI] [PubMed] [Google Scholar]
- 39.Bauer SR, Jin C, Kamal P, Suskind AM. Association Between Lower Urinary Tract Symptoms and Frailty in Older Men Presenting for Urologic Care. Urology. 2020. doi: 10.1016/j.urology.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang CL, Wu MP, Ho CH, Wang JJ. The bidirectional relationship between anxiety, depression, and lower urinary track symptoms: A nationwide population-based cohort study. Journal of psychosomatic research. 2017;100:77–82. doi: 10.1016/j.urology.2017.07.039 [DOI] [PubMed] [Google Scholar]
- 42.Hakkinen JT, Shiri R, Koskimaki J, Tammela TL, Auvinen A, Hakama M. Depressive symptoms increase the incidence of nocturia: Tampere Aging Male Urologic Study (TAMUS). The Journal of urology. 2008;179(5):1897–1901. doi: 10.1016/j.juro.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 43.Obayashi K, Saeki K, Negoro H, Kurumatani N. Nocturia increases the incidence of depressive symptoms: a longitudinal study of the HEIJO-KYO cohort. BJU international. 2017;120(2):280–285. doi: 10.1111/bju.13791. [DOI] [PubMed] [Google Scholar]
- 44.Suen LKP, Cheng HL, Yeung SKW, et al. Qualitative insights into the experiences of living with moderate-to-severe lower urinary tract symptoms among community-dwelling ageing males. PloS one. 2017;12(10):e0187085. doi: 10.1371/journal.pone.0187085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wareing M Lower urinary tract symptoms: a hermeneutic phenomenological study into men’s lived experience. Journal of clinical nursing. 2005;14(2):239–246. doi: 10.1111/j.1365-2702.2004.01003.x. [DOI] [PubMed] [Google Scholar]
- 46.Parsons JK, Kashefi C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. European urology. 2008;53(6):1228–1235. doi: 10.1016/j.eururo.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA : the journal of the American Medical Association. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J. Evaluation and treatment of lower urinary tract symptoms in older men. The Journal of urology. 2013;189(1 Suppl):S93–s101. doi: 10.1016/j.juro.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Welliver C, Feinstein L, Ward JB, et al. Trends in Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia, 2004 to 2013: the Urologic Diseases in America Project. The Journal of urology. 2020;203(1):171–178. doi: 10.1097/ju.0000000000000499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods S1. Other Measurements.
Supplemental Figure 1. Study flowchart.
Supplemental Figure 2. 2-year risk of new cognition-dependent task limitation by tertile of AUASI score, stratified by age.
Supplemental Table 1. Association of LUTS severity at baseline with incident mobility limitation, ADL limitation, and cognition-dependent task limitation after adjusting for potential mediators.
Supplemental Table 2. Association of lower urinary tract symptoms (LUTS) severity at baseline with incident cognition-dependent task limitation.
Supplemental Table 3. Sensitivity analyses of association of overall LUTS severity at baseline with incident mobility limitation, ADL limitation, and cognition-dependent task limitation.
Supplemental Table 4. Association of AUASI score at baseline with incident mobility limitation, ADL limitation, and cognition-dependent task limitation, stratified by age tertile.
Supplementary References S1.


