Abstract
Endometrial cancer (EC) is the second most common gynecological malignancy worldwide, the first in developed countries [Sung et al. in CA Cancer J Clin 71:209–249, 2021]. Although a majority is diagnosed at an early stage with a low risk of relapse, an important proportion of patients will relapse. Better knowledge of molecular abnormalities is crucial to identify high-risk groups in early stages as well as for recurrent or metastatic disease for whom adjuvant treatment must be personalized. The objective of this guide is to summarize the current evidence for the diagnosis, treatment, and follow-up of EC, and to provide evidence-based recommendations for clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12094-022-02799-7.
Keywords: Endometrial cancer, Guideline, Diagnosis, Treatment
Introduction
Endometrial cancer (EC) is the most common gynecological cancer, following cervical cancer, in developed countries. Most patients are diagnosed at an early stage with a low risk of relapse. In the last 30 years, incidence has increased in a proportion of 1% per year, associated with higher mortality. Age at diagnosis and comorbidities (e.g. diabetes, hypertension and obesity) make treatment of EC challenging and might increase mortality. The high rate of cure in initial stages with an OS at 5 years around 80–85% have created the false belief that EC is a low-risk disease. Yet, advanced stages and some histologies are associated with poor prognosis.
Methodology
This guideline is based on relevant published studies and with the consensus of ten EC treatment expert oncologists from GEICO (Spanish Group for Investigation in Ovarian Cancer) and SEOM (Spanish Society of Medical Oncology). The Infectious Diseases Society of America-US Public Health Service Grading System for Ranking Recommendations in Clinical Guidelines [2] has been used to assign levels of evidence and grades of recommendation.
Diagnosis
The most frequent symptom of EC is abnormal uterine bleeding. In postmenopausal women or those with risk factors, metrorrhagia should always be investigated. Transvaginal ultrasound (TVUS) is usually performed given its availability and low cost. The cutoff level of 3 mm for exclusion of EC in women with postmenopausal bleeding is widely recommended [II, B], whereas no established consensus for premenopausal women [3] has been established. Histologic confirmation is always required. Blind endometrial biopsy is preferred although false negative results are frequent. In such cases, hysteroscopy with targeted biopsy could be recommended. In patients who cannot tolerate an office biopsy or for those with an unsuccessful office procedure, dilation and curettage, with or without hysteroscopy, is an option.
Preoperative imaging and histologic features help in tailoring the surgical approach to avoid unnecessary lymphadenectomy (LND) in low-risk patients [4]. TVUS can accurately evaluate myometrial invasion in most cases (80%) but it is less sensitive for cervical stromal invasion. Contrast-enhanced magnetic resonance imaging is the best method for detecting myometrial invasion or cervical involvement and it is highly recommended especially when conservative fertility preservation treatment is planned and in inoperable patients referred to radical radiation. At least an abdomino-pelvic computerized tomography scan (CTscan) must be performed to rule out lymph node (LN) or distant metastasis. Positron emission tomography/CTscan can also be employed. Thorax CTscan should also be performed as part of the initial assessment to exclude lung metastases in high-risk cases. The role of serum tumor markers is unclear [IV, B].
Hereditary endometrial cancer
Around 5% of EC cases have an inherited mutation in Mismatch Repair (MMR) genes, namely MLH1, MSH2, MSH6, PMS2 or EPCAM [5]. The diagnosis of Lynch Syndrome is based on the detection of the germline mutation. Screening of Lynch Syndrome is currently recommended for all EC cases with no limitations regarding the age or the histology type [6] [IIA]. Screening of Lynch syndrome is based in the detection of MMR protein loss (MMR deficiency). When MMR-D is present, to rule out a non-hereditary cause, the MLH1 methylation or, more rarely, a BRAF mutation can be performed. If none of these are identified, a germline analysis of MMR genes must be undertaken. When carriers are identified, they must be advised of the specific lifetime risk of colorectal cancer ranging from 20% in PMS2 to 70% in MLH1 and other Lynch syndrome tumors. Moreover, this should prompt the direct mutation analysis of relatives to help identify carriers and to offer women prophylactic salpingo-ophorectomy and hysterectomy once childbearing is completed [IV, B].
Other less frequent EC hereditary syndromes can be caused by PTEN germline mutations in Cowden syndrome, (< 1%) [7], germline mutation in BRCA1/2 (1%) with controversy regarding the association to serous histology [8] and germline TP53 mutations in Li-Fraumeni syndrome (< 1%).
Screening
There are no high-quality data to support the efficacy of screening with imaging, tissue sampling, or cervical cytology for reducing EC mortality. Thus, in women with average or high-risk for endometrial cancer without abnormal bleeding, routine screening is not recommended [II, A]. This includes patients on tamoxifen, although there are no reliable data in women with extended therapy beyond five years.
Women with Lynch syndrome have a lifetime risk of endometrial cancer of 13–71%. Annual endometrial sampling, TVUS and CA125 beginning at age 30–35 or 5–10 years prior to the earliest age of first diagnosis of Lynch-associated cancer of any kind in the family is recommended [IV, B].
Pathology and molecular biology of EC
Epithelial EC is divided into different histologic subtypes:
Endometrioid carcinoma.
Serous carcinoma.
Clear cell carcinoma.
Uterine carcinosarcoma.
Other: mucinous, neuroendocrine, undifferentiated, dedifferentiated carcinomas.
Endometrioid adenocarcinomas (EEC) are the most frequent subtype (≅ 80%). EEC is a heterogeneous subgroup that varies from indolent to very aggressive carcinomas. After tumor stage, the next most informative prognostic division in EEC is between high grade (grade 3) and low grade (grade 1–2). Despite the three-grade classification is widely used, a binary FIGO (International Federation of Gynecology and Obstetrics) grade is recommended: low grade (1–2) vs high grade (3) [9].
Serous carcinomas (SC) are the second most frequent endometrial carcinomas (< 10%). This subtype is aggressive and is frequently associated with deep myometrial involvement and lymphovascular invasion. More than 90% of SC are associated with TP53 mutations.
Clear cell carcinomas (CCC) are a rare subgroup (1–6%) characterized by the clearing of tumor cell cytoplasm. Patients with CCC are more likely to present with a higher FIGO stage than EEC and have a poorer prognosis [10].
Uterine carcinosarcoma (UCS) is a highly aggressive tumor with a mixture of malignant epithelial and mesenchymal/sarcomatous components. UCS is very rare (≅ 1.5%). Next generation sequencing (NGS) analyses have revealed that UCS are serous-like tumors. The sarcoma dominance (presence of > 50% of sarcomatous element) is associated with worse prognosis [11].
Eventually EEC can coexist in the presence of SC or CCC, when one of these components are present in at least 5% it is classified as a mixed carcinoma.
Every subtype has been associated with different molecular alterations [12]. Table 1.
Table 1.
Frequency of most common targeted alterations in endometrial cancer according to histological subtype
| EEC | SC | CCC | UCS | |
|---|---|---|---|---|
| PTEN mutation |
64–80% G1–3 52–82% G1/G2 62–90% G3 |
2–3% | 0–21% | 11–33% |
| PI3KCA mutation |
22–59% G1–G3 38–54% G1/G2 45–59% G3 |
15–35% | 24–36% | 22–40% |
| PIK3R1 mutation |
9–43% G1–G3 19–38% G1/G2 31–41% G3 |
5–8% | 7–18% | 6–20% |
| KRAS mutation |
19–43% G1–G3 17–23% G1/G2 7–33% G3 |
2–6% | 2–14% | 10–17% |
| FGFR2 mutation |
10–18% G1–G3 11–13% G1/G2 14–16% G3 |
8% | 0% | 0–2% |
| CTTNB1 mutation |
19–37% G1–G3 24–28% G1/G2 19–40% G3 |
0–3% | 0% | 0–5% |
| MMR-d |
34–35% G1–G3 34% G1/G2 44% G3 |
0–3% | 11–14% | 3–6% |
| ARID1A mutation |
39–55% G1–G3 39–47% G1/G2 39–60% G3 |
7–11% | 14–21% | 10–24% |
| P53 mutation |
5–14% G1–G3 6–10% G1/G2 21–35% G3 |
59–93% | 28–46% | 44–91% |
| ERBB2 amplification |
1% G1–G3 3% G1/G2 4% G3 |
26–44% | 11% | 9% |
| POLE mutation |
13–15% G1–G3 11% G1/G2 20% G3 |
0–2% | 2–7% | 3–4% |
Modified from Urick and Bell [12]
Molecular classification
In 2013, The Cancer Genome Atlas (TCGA) Research Network published an integrated genomic analysis of 373 EC. The analyses identified four prognostic categories of EC: POLE (ultra-mutated) (7%), microsatellite instability (MSI)/hypermutated (28%), copy number low/microsatellite stable (39%), and serous-like/copy number high (26%) [13].
POLE ultra-mutated is a subgroup characterized by somatic mutations in polymerase epsilon DNA polymerase (POLE) exonuclease domain which results in a high mutation rate. POLE is more frequently presented in low-grade and high-grade EEC. This subtype has an excellent prognosis and rarely recurs.
Microsatellite instability high (MSI-h) subtype is characterized by a deficiency in the MMR system that leads to an hypermutated status. Most MSI-h tumors are EEC.
The copy number (CN) low subgroup also called microsatellite stable, includes most of low-grade endometrioid tumors.
The CN high subgroup is frequently harbors TP53 suppressor gene mutations and includes almost all SC and most of mixed type carcinomas and carcinosarcomas.
The complexity of the TCGA classification led to establishing more practical and feasible molecular classifications. The Leiden/TransPORTEC and the Vancouver/PROMISE studies generated two new classifications that are similar in molecular alterations. Although they are not identical to TCGA, these new classifications are preferred for clinical practice. They established four subgroups according to MSI status, POLE and TP53 mutations. These four subgroups defined in thePORTEC study are:
POLE-mutant.
Microsatellite instable.
p53 abnormal.
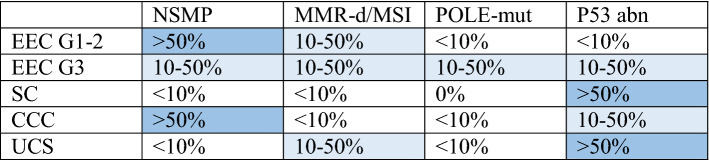
Table 2.
Relationship between histotype and molecular classification
Modified from Huvila J et al. [15]
Staging and risk assessment
FIGO 2009 is the staging system currently recommended (Table 3). EC is surgically staged [16]. Risk groups have been designed based on clinicopathological factors associated at risk of recurrence to identify patients who may benefit from adjuvant therapy. Currently, well-defined clinicopathologic prognostic factors include: histological subtype, tumor differentiation grade, FIGO stage, age, depth of myometrial invasion and lymphovascular space invasion (LVSI) [17]. All these factors must be reported in the pathology report. LVSI should be categorized as (1) absent, (2) focal or (3) substantial. Focal LVSI is defined as the presence of a single focus and substantial LVSI as the presence of diffuse or multiple foci invasion or the identification of tumor cells in at least 5 lymphovascular spaces. Only substantial LVSI has been identified as prognostic for recurrence. Given its prognostic relevance, it is highly recommended to categorize tumors according to molecular classification [III,A], especially in the heterogenous high-grade EC subgroup that ranges from indolent POLEmut to highly aggressive p53 abnormal tumors. Molecular classification may be not required in low-risk EEC. According to the risk of relapse, EC can be subdivided into five risk categories integrating molecular markers (Table 4) [18].
Table 3.
2009 FIGO staging system for endometrial cancer
| Stage I | Tumor confined to corpus uteri, including endocervical glandular involvement |
| IA | Tumor limited to the endometrium or invading less than half the myometrium |
| IB | Tumor invading one half or more of the myometrium |
| Stage II | Tumor invading the stromal connective tissue of the cervix but not extending beyond the uterus |
| Stage III | Tumor involving serosa, adnexa, vagina, or parametrium |
| IIIA | Tumor involving the serosa and/or adnexa (direct extension or metastasis) |
| IIIB | Vaginal involvement (direct extension or metastasis) or parametrial involvement |
| IIIC1 | Regional lymph node metastasis to pelvic lymph nodes |
| IIIC2 | Regional lymph node metastasis to para-aortic lymph nodes, with or without positive pelvic lymph nodes |
| Stage IV | Tumor invading bladder and/or bowel mucosa, and or distant metastasis |
| IVA | Tumor invading the bladder mucosa and/or bowel mucosa |
| IVB | Distant metastasis (includes metastasis to inguinal lymph nodes, intra-peritoneal disease, lung, liver, or bone) |
Table 4.
Prognostic risk groups—treatment recommendation
| Risk group | Previous description | Molecular adapted | |
|---|---|---|---|
| Low | Stage I endometrioid G1–2, < 50% myometrial invasion, LVSI negative |
Stage I–II POLEmut endometrial carcinoma,no residual disease Stage IA MMRd/NSMP endometrioid carcinoma G1-2, with no or focal LVSI |
No adjuvant treatment |
| Intermediate |
Stage I endometrioid, G1–2, ≥ 50% myometrial invasion, LVSI negative Stage I endometrioid G3, < 50% myometrial invasion and LVSI negative |
Stage IB MMRd/NSMP endometrioid carcinoma G1-2, with no or focal LVSI Stage IA MMRd/NSMP endometrioid carcinoma G3, with no or focal LVSI Stage IA p53abn without myometrial invasion Stage IA non-endometrioid (serous, clear cell, undifferentiated carcinoma, carcinosarcoma, mixed) without myometrial invasion |
BVT |
| High-intermediate |
Stage I EEC with substancial LVSI regardless of grade and myometrial invasion Stage I EEC, G3, ≥ 50% myometrial invasion, regardless of LVSI Stage II |
Stage I MMRd/NSMP endometrioid carcinoma, with LVSI Stage IB MMRd/NSMP endometrioid carcinoma G3 Stage II MMRd/NSMP endometrioid carcinoma |
Surgical staging negative: VBT No surgical staging: PRT + VBT Consider CT for high grade or substantial LVSI |
| High |
Stage III-IVA EEC optimally debulked Non-endometrioid EC (serous or clear cell or undifferentiated carcinoma, or carcinosarcoma) |
Stage III–IVA MMRd/NSMP endometrioid carcinoma with no residual disease Stage I–IVA p53abn endometrial carcinoma with myometrial invasion, with no residual disease Stage I–IVA NSMP/MMRd serous, undifferentiated carcinoma, carcinosarcoma, with myometrial invasion, with no residual disease |
EBRT concurrent or sequential with CT CT alone as alternative |
| Advanced metastatic | Stage III–IVA with residual disease or Stage IVB |
Stage III–IVA with residual disease of any molecular type Stage IVB regardless molecular type |
CT (RT can be considered depending on residual disease) |
p53abn p53 abnormal, POLEmut polymerase-mutated, LVSI lymphovascular space invasion, MMRd mismatch repair deficient, NSMP non-specific molecular profile
For stage III–IVA POLEmut endometrial carcinoma and stage I–IVA MMRd or NSMP clear cell carcinoma with myometrial invasion, insufficient data are available to allocate these patients to a prognostic risk group in the molecular classification. Prospective registries are recommended
Surgical treatment
Early stages
All patients with newly diagnosed EC should be considered for surgery. Surgical staging is necessary for an accurate prognostic stratification and for adjuvant treatment decisions. Standard surgery in early stages is total hysterectomy with bilateral salpingo-oophorectomy without vaginal cuff resection. Peritoneal cytology, although considered a poor prognosis factor, is not mandatory for FIGO staging [19].
Minimally invasive surgery (laparoscopic or robotic) is the preferred surgical approach. Laparoscopic-assisted vaginal hysterectomy has been associated with lower peri and post-operative morbidity compared to laparotomy with similar oncologic outcomes [20]. Robotic surgery could be an alternative to laparoscopic approach, especially in those patients who have a high risk of conversion to laparotomy (e.g. obese patients). Additional routes (laparotomy or vaginal) can be individualized based on patient and tumor specific factors (e.g. uterine size, known adhesive disease or anesthesia limitations). Tumor rupture or morcellation should be avoided due to intra-peritoneal cells spillage risk.
LN evaluation provides prognostic information and could determine the adjuvant therapy. As the risk of LN involvement increases with tumor grade, high-risk histology, and depth of myometrial invasion, systematic pelvic and para-aortic LND in all patients is not recommended. Two randomized clinical trials demonstrated that systematic LND was not associated with overall survival (OS) or recurrence-free survival benefit in early-stage EC [21, 22]. In those patients considered for LN staging, uterine factors are used to assess the risk of retroperitoneal LN metastasis. If pelvic LN involvement is detected, para-aortic LN staging should be considered.
Sentinel node biopsy (SLND) has been introduced as an alternative to LND for LN staging, although there are no randomized clinical trials comparing outcomes between these two approaches. Multiple prospective cohort studies have demonstrated the feasibility of this technique with high sensitivity for detection of positive LN, in association with lower rates of post-operative morbidity (eg, lymphedema and cellulitis) [23].
In SC, UCS or undifferentiated EC omentectomy should be included as a part of the staging procedure because of the high risk of omental metastases.
Recommendations:
Standard surgical treatment in early stages EC is total hysterectomy and bilateral salpingo-oophorectomy without vaginal cuff resection with a minimally invasive surgery approach [I,A].
In low-risk EC, systematic LND is not recommended [II, A]. In intermediate and high-risk group, LND is recommended to guide surgical staging and adjuvant therapy [II,C]. SNLB can be considered for staging purposes [III,A].
Omentectomy should be performed in serous, carcinosarcoma, and undifferentiated endometrial carcinoma [IV,B].
Advanced stages
In stage III-IV EC determination of whether cytoreduction is feasible is highly recommended. Surgical tumor debulking with complete macroscopic disease resection should be considered only in patients with good performance status and acceptable morbidity [III,B] [24]. Visible or palpable LN should be removed.
Palliative surgery could be considered in patients with good performance status and metastatic disease [IV,A].
Fertility sparing therapy
Conservative management to preserve reproductive function and delay surgery until childbearing completion should only be performed in specialized centers. It should only be offered to patients with low-grade EEC without myometrial invasion [V,A] [25].
Initial work-up with pelvic and abdominal imaging and endometrial sampling is necessary to assess cancer grade and depth of myometrial invasion. Close follow-up and confirmation of lesion regression are also mandatory.
The most common approach is progestin therapy (medroxyprogesterone acetate; 400–600 mg/day or megestrol acetate; 160–320 mg/day or an intrauterine device containing levonorgestrel) [IV,B].
Adyuvant treatment
Radiotherapy
Pelvic radiation (PRT) after surgery in stage I EC provides locoregional control without improvement in OS or disease-free survival (DFS). A randomized trial comparing vaginal brachytherapy (VBT) and observation in women with stage IA, grade 1 and 2 EEC showed no OS beneft in VBT group. VBT was associated with an increase in genitourinary symptoms [26]. The results of PORTEC-2 trial, comparing VBT vs PRT in the high–intermediate-risk group, showed that there were no differences in pelvic or vaginal recurrences, DFS and distant metastasis, VBT being less toxic [27]. VBT in combination with PRT was compared to VBT alone in patients with intermediate risk [28]. Addition of PRT improved locoregional control without any impact on OS. Acute gastrointestinal and urinary toxicity was superior in the combination group. Postoperative RT has been considered standard in high-risk EC group, although a comparative study of adjuvant radiation versus no treatment in this group of patients has not been conducted.
Chemotherapy
Results of two old prospective randomized trials comparing external beam RT (EBRT) to chemotherapy (CT) did not show differences in DFS an OS [29, 30]. CT reduced the risk of distant recurrences, but not that of local relapses. These observations provided the rationale for a combined CT/RT approach.
The pooled analysis of NSGO-EC-9501/EORTC-55991 and MaNGO ILIADE-III trials demonstrated that combined treatment (four cycles of platinum-based CT, given either before or after RT) improves DFS and showed a trend towards improved OS [31]. The limitation of these studies is that 25–40% of the patient population was stage III or incompletely surgically staged. The type of CT used and the number of cycles are other concerns that preclude generalization of these results.
In PORTEC-3 trial, EBRT was compared with chemoradiation (two cycles of cisplatin with EBRT, followed by four cycles of carboplatin and paclitaxel). The combined approach improved DFS and OS. The magnitude of benefit was greater in stage III and SC, but adverse events were more frequent with CT/RT [32]. Molecular analysis of PORTEC-3 trial participants suggested no benefit of CT for MMRd carcinomas [33]. GOG 258 trial compared CT (carboplatin and paclitaxel) vs CT-RT (two cycles of cisplatin with EBRT, followed by four cycles of carboplatin and paclitaxel) in stages III to IVA and stage I–II SC or CCC. There were no differences in DFS and OS [34], however the rates of locoregional relapses were higher with CT alone. In GOG 249, which included stage I EEC high–intermediate-risk group, stage I SC and CCC or stage II patients of any histology, CT (carboplatin-paclitaxel) plus VBT demonstrated similar DFS as EBRT, but more acute toxicity in chemotherapy arm [35].
Adjuvant treatment. Recommendations (Table 4)
Incorporation of the molecular classification for adjuvant treatment decisions is encouraged, especially in high-grade tumors or high-risk disease where adjuvant chemotherapy is being considered. If molecular classification is not available, EC risk classification should be based on pathologic features.
Low-risk patients do not require adjuvant treatment [I,A].
VBT is recommended for intermediate-risk patients [I,A].
In the intermediate–high risk group, VBT is recommended in patients with surgical staging and node negative [III,B]. In patients with no surgical nodal staging, PRT and VBT is recommended [III,B]. Although adjuvant CT in intermediate high-risk group is not recommended, it can be considered in selected cases, especially for high grade and/or substantial LVSI [III, B].
In high-risk disease:
Adjuvant CT with EBRT (concurrent or sequential) is recommended [I,A]. Alternative option could be CT alone. [I,B].
p53abn identifies a high-risk group regardless of stage (except stage IA), histology and grade [IV,B].
POLEmut is associated with excellent prognosis and adjuvant therapy might be avoided in stage I-II disease [IV,B].
Treatment of metastatic or recurrent disease
For pelvic isolated relapses or single metastatic sites, surgical resection, radiotherapy or ablative therapy should be considered [IV,A], as well as systemic therapy although its benefit is uncertain [IV,B].
In patients with recurrent unresectable or metastatic disease, CT and HT are therapeutic options. Enrollment in clinical trials is strongly recommended [V,B].
Systemic treatment
Hormonal agents evaluated include progestogens alone or alternated with tamoxifen, tamoxifen alone, aromatase inhibitors and fulvestrant. Confirmation of hormone-receptor status by biopsy should be considered at the time of recurrence [IV,B]. The response rate (RR) in CT-naive patients is about 10–25% [36]. Hormonal therapy could be an appropriate therapeutic alternative for patients who are low-grade, hormone-receptor positive, without rapid progressive metastatic disease [37] [II,A]. The treatment of choice are progestogens (megestrol acetate 160 mg QD or medroxyprogesterone acetate 200 mg QD) or progestogens alternating with tamoxifen [III,A].
For more aggressive diseases, chemotherapy is the treatment of choice. Several combinations have been tested. GOG 209 showed equivalence of carboplatin-doxorrubicin-paclitaxel and carboplatin-paclitaxel with a PFS of 12–14 months and OS of 32 months, with a better toxicity profile for the latter [38]. Based on these results, the standard chemotherapy treatment for advanced or recurrent EC is the combination of carboplatin-paclitaxel [I, A]. For patients with late relapses (i.e. more than 6 months after last platinum), rechallenge with CT may be of beneficial [V,C].
Immunotherapy
Several anti PD-1 and anti PD-L1 checkpoint inhibitors have shown activity in EC. Pembrolizumab showed activity in a phase II trial including patients with MMR-D tumors with 20% complete responses and 33% partial responses among EC patients [39, 40]. The phase II KEYNOTE-158 multicohort study evaluated the antitumor activity and safety of pembrolizumab in patients previously treated for advanced MSI-h/MMR-D non-colorectal cancer with 27 different histologies [41]. Among 49 patients with MSI-h/MMR-D EC, RR was 57.1%, and 16.3% of patients had a complete response. Median PFS was 25.7 months, and median OS was not reached.
The phase I GARNET trial evaluated the safety and activity of dostarlimab. The MSI-h cohort included 104 patients [42]. Approximately half of patients had received 2 or more prior lines of therapy. RR was 42.3%, 12.7% of patients had a complete response, and 29.6% had a partial response. With a median follow-up of 11.6 months, the estimated likelihood of maintaining a response was 96.4% at 6 months and 76.8% at 12 months in the MSI-h cohort.
The combination of Lenvatinib plus pembrolizumab showed promising activity in a phase II study in unselected patients with EC who had progressed to at least one previous line of treatment [43]. The phase III KEYNOTE-775 study evaluated the combination of lenvatinib plus pembrolizumab versus treatment of physician´s choice following platinum-based therapy in advanced EC [44]. 827 patients with advanced EC (unselected for MMR) were included, and about 85% of patients had MMR-proficient tumors. The combination of lenvantinib and pembrolizumab showed, regardless of MMR status, statistically significant improvements in OS (17.4 vs 12.0 months in MMR-proficient patients, and 18.3 vs 11.4 months in all-comers), PFS (6.5 vs 3.8 months in MMR-proficient patients, and 7.2 vs 3.8 months in all-comers). However, the combination of lenvatinib and pembrolizumab was associated with considerable toxicity; grade 3 treatment-related adverse events occurred in 88.9% of patients, and 33% of patients discontinued treatment because of treatment-related adverse events.
The combination of pembrolizumab and lenvatinib should be considered for second-line treatment of EC [I,A], particularly for MMR-proficient tumors, whereas Dostarlimab or pembrolizumab can be also considered for second-line therapy of MMR-D EC [II,B].
Targeted therapies
Better knowledge of driver mutations across different EC subtypes has led to the development of multiple clinical trials with antiangiogenic agents, anti-HER2-targeted agents, PI3Kinase/mTOR, CDK4/6 and MEK inhibitors showing activity but without strong evidence to recommend its use. Inhibitors of other targets like PARP, WEE1, and the PI3K/AKT/mTOR pathway are subjects of thorough study.
Follow up
Surveillance in EC is aimed at the early detection of recurrent disease. Most recurrences are diagnosed within 3 years of primary treatment. The most common site of recurrence is the pelvis, especially in the vaginal vault while distant relapses represent one-third of cases.
There is no evidence that any specific posttreatment surveillance strategy is associated with improved survival.
The TOTEM study [45] assessed the role of intensive (INT) vs minimalist follow-up (MIN). EC patients were included in two different cohorts: 1) low-risk group (FIGO IA G1-2) or 2) high-risk group (IA G3 or ≥ IB). The rate of relapse was 12.3%. No differences in OS were seen. According to TOTEM trial MIN strategy (clinical examination every 6/12 months for low-risk group and clinical examination and CTscan every 6/12 months for high-risk group) could be recommended for the follow-up of FIGO I-II EEC [I,B].
Surveillance consists mainly of monitoring symptoms and physical examination including: a speculum and pelvic examination every 3–6 months for 2 years, and every 6–12 months thereafter. Patients with low-risk endometrial cancer can be followed less frequently: 6–12 months for first 2 years, then yearly thereafter. Vaginal cytology is not routinely recommended as most vaginal recurrences are detected with clinical examination alone [I,A]. CA-125 may be used in surveillance for those patients who have an elevated CA-125 prior to treatment, advanced disease or serous endometrial cancer [46].
In high-risk non-endometrioid or FIGO III-IV tumors, imaging may be helpful, chest/abdominal/pelvic CT every 6 months during the first 3 years, and every 6 to 12 months for 2 additional years is recommended [IV,A]. Additional imaging considerations include whole body PET/CT in selected patients who may be candidates for surgery or locoregional therapy and/or pelvic MRI for patients who retain their uterus [47].
Following treatment, endometrial cancer patients should be counseled on the impact of obesity, lifestyle and nutrition [48] [IV,A].
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
MQV, MCEE, CGR and MJRP declare that they have no conflict of interest. MPBG reports advisory/speaker honoraria from GSK, MSD, Astra Zeneca, Clovis Oncology, Pharmamar and Roche outside the submitted work. JDAC reports advisory/speaker honoraria from GSK, Clovis Oncology, Astra Zeneca and Roche and a grant from GSK outside the submitted work. LG reports advisory/speaker honoraria from GSK, Astra Zeneca, MSD, Clovis Oncology and advisory honoraria from Pharmamar outside the submitted work. JAPF reports advisory honoraria from GSK, Ability Pharma, Astra Zeneca, Roche, Pharmamar; grants from GSK and Pharmamar; speaker honoraria from GSK, Astra Zeneca, MSD, Clovis Oncology and Pharmamar outside the submitted work. IRN reports advisory honoraria from GSK, Clovis Oncology, Pharmarmar and Astra Zeneca; speaker honoraria from GSK and Pharmarmar, and non-financial support from Astra Zeneca outside the submitted work. ASB reports advisory/speaker and grants from MSD and GSK outside the submiteed work.
Ethical approval
The current manuscript has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. P. Barretina-Ginesta, M. Quindós, J. D. Alarcón, C. Esteban, L. Gaba, C. Gómez, J. A. Pérez Fidalgo, I. Romero, A. Santaballa, and M. J. Rubio-Pérez have contributed equally to the writing of the manuscript.
Contributor Information
María Pilar Barretina-Ginesta, Email: mpbarretina@iconcologia.net.
María Quindós, Email: maria.quindos.varela@sergas.es.
Jesús Damián Alarcón, Email: jesus.alarcon@ssib.es.
Carmen Esteban, Email: carmen.estebanesteban@gmail.com.
Lydia Gaba, Email: lgaba@clinic.cat.
César Gómez, Email: cgomezraposo@gmail.com.
José Alejandro Pérez Fidalgo, Email: japfidalgo@msn.com.
Ignacio Romero, Email: iromero@fivo.org.
Ana Santaballa, Email: anasantaballa@gmail.com.
María Jesús Rubio-Pérez, Email: mjesusrubio63@gmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33(2):139–144. doi: 10.1086/321805. [DOI] [PubMed] [Google Scholar]
- 3.Timmermans A, Opmeer BC, Khan KS, Bachmann LM, Epstein E, Clark TJ, et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(1):160–167. doi: 10.1097/AOG.0b013e3181e3e7e8. [DOI] [PubMed] [Google Scholar]
- 4.Lin MY, Dobrotwir A, McNally O, Abu-Rustum NR, Narayan K. Role of imaging in the routine management of endometrial cancer. Int J Gynaecol Obstet. 2018;143:109–117. doi: 10.1002/ijgo.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bercow AS, Eisenhauer EL. Screening and surgical prophylaxis for hereditary cancer syndromes with high risk of endometrial and ovarian cancer. J Surg Oncol. 2019;120(5):864–872. doi: 10.1002/jso.25645. [DOI] [PubMed] [Google Scholar]
- 6.https://cap.objects.frb.io/protocols/cp-femalereproductive-endometrium-18protocol-4100.pdf.. Accessed August 2018
- 7.Dörk T, Hillemanns P, Tempfer C, Breu J, Fleisch MC. Genetic susceptibility to endometrial cancer: risk factors and clinical management. Cancers (Basel) 2020;12(9):2407. doi: 10.3390/cancers12092407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith ES, Da Cruz PA, Cadoo KA, Abu-Rustum NR, Pei X, et al. Endometrial cancers in BRCA1 or BRCA2 germline mutation carriers: assessment of homologous recombination DNA repair defects. JCO Precis Oncol. 2019;29:3. doi: 10.1200/PO.19.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soslow RA, Tornos C, Park KJ, Malpica A, Matias-Guiu X, Oliva E, et al. Int J Gynecol Pathol. 2019;38:S64–S74. doi: 10.1097/PGP.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varughese J, Hui P, Lu L, Yu H, Schwarz PE. Clear cell cancer of the uterine corpus: the association of clinicopathologic parameters and treatment on disease progression. J Oncol. 2011;2011:628084. doi: 10.1155/2011/628084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzaki S, Klar M, Matsuzaki S, Roman L, Sook AK, Matsuo K. Uterine carcinosarcoma: contemporary clinical summary, molecular updates, and future research opportunity. Gynecol Oncol. 2021;160(2):586–601. doi: 10.1016/j.ygyno.2020.10.043. [DOI] [PubMed] [Google Scholar]
- 12.Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19(9):510–521. doi: 10.1038/s41568-019-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Cancer Genome Atlas Research Network et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen T-T, Wang T-L, Fader A, Shih I-M, Gaillard S. Molecular classification and emerging targeted therapy in endometrial cancer. Int J Gynecol Pathol. 2020;39:26–35. doi: 10.1097/PGP.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huvila J, Pors J, Thompson EF, Gilks CB. Endometrial carcinoma: molecular subtypes, precursors and the role of pathology in early diagnosis. J Pathol. 2021;253:355–365. doi: 10.1002/path.5608. [DOI] [PubMed] [Google Scholar]
- 16.Lewin SN. Revised FIGO staging for endometrial cancer. Clin Obstet Gynecol. 2011;54:215–218. doi: 10.1097/GRF.0b013e3182185baa. [DOI] [PubMed] [Google Scholar]
- 17.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- 18.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 19.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Galaal K, Bryant A, Fisher AD, et al. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2012;12(9):CD006655. doi: 10.1002/14651858.CD006655.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 22.Kitchener H, Swart AM, Qian Q, ASTEC Study Group et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384–392. doi: 10.1016/S1470-2045(17)30068-2. [DOI] [PubMed] [Google Scholar]
- 24.Barlin JN, Puri I, Bristow RE. Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecol Oncol. 2010;118(1):14–18. doi: 10.1016/j.ygyno.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Fan Z, Li H, Hu R, et al. Fertility-preserving treatment in young women with grade 1 presumed stage IA endometrial adenocarcinoma: a meta-analysis. Int J Gynecol Cancer. 2018;28(2):385–393. doi: 10.1097/IGC.0000000000001164. [DOI] [PubMed] [Google Scholar]
- 26.Sorbe B, Nordström B, MäenpääJ Kuhelj J, Kuhelj D, Okkan S, et al. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: a controlled randomized study. Int J Gynecol Cancer. 2009;19(5):873–878. doi: 10.1111/IGC.0b013e3181a6c9df. [DOI] [PubMed] [Google Scholar]
- 27.Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of highintermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 28.Sorbe B, Horvath G, Andersson H, Boman K, Lundgren C, Pettersson B. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma—a prospective randomized study. Int J Radiat Oncol Biol Phys. 2012;82:1249–1255. doi: 10.1016/j.ijrobp.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese gynecologic oncology group study. Gynecol Oncol. 2008;108:226–233. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer–results from two randomised studies. Eur J Cancer. 2010;46(13):2422–2431. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(3):295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.León-Castillo A, de Boer SM, Powell ME, Mileshkin LR, Mackay HJ, Leary A, et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol. 2020;38(29):3388–3397. doi: 10.1200/JCO.20.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380(24):2317–2326. doi: 10.1056/NEJMoa1813181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37(21):1810–1818. doi: 10.1200/JCO.18.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ethier JL, Desautels DN, Amir E, MacKay H. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol Oncol. 2017;147(1):158–166. doi: 10.1016/j.ygyno.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007;17(5):964–978. doi: 10.1111/j.1525-1438.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG oncology/GOG0209) J Clin Oncol. 2020;38(33):3841–3850. doi: 10.1200/JCO.20.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microstallite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oaknin A, Tinker AV, Gilbert L, Samouëlian V, Mathews C, Brown J, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 2020;6(11):1–7. doi: 10.1001/jamaoncol.2020.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981–2992. doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colombo N, Casado A, Santin AD, Coomba E, Miller DS, et al. A multicenter, open-label, randomized, phase 3 study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician´s choice in patientes with advanced endometrial cancer: study 309/KEYNOTE-775. Presented at the Society of Gynecologic Oncology 2021 Virtual Annual Meeting on Women’s Cancer. Abstract 37/ID 11512.
- 45.Zola P, Ciccone G, Piovano E, Fuso K, Peirano E, Di Cuonzo D, et al. Intensive vs minimalist follow-ip in patients treated for endometrial cancer: A multicentric randomized controlled trial (The TOTEM study- NCT00916708) J Clin Oncol. 2021;39:15. [Google Scholar]
- 46.Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: society of gynecologic oncology recommendations. Gynecol Oncol. 2017;146(1):3–10. doi: 10.1016/j.ygyno.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Reinhold C, Ueno Y, Akin EA, Bhosale PR, Dudiak KM, Expert Panel on GYN and OB Imaging et al. ACR appropriateness criteria® pretreatment evaluation and follow-up of endometrial cancer. J Am Coll Radiol. 2020;17(11S):S472–S486. doi: 10.1016/j.jacr.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton CA, Pothuri B, Arend RC, Backes FJ, Gehrig PA, Soliman PT, et al. Endometrial cancer: a society of gynecologic oncology evidence-based review and recommendations, part II. Gynecol Oncol. 2021;160(3):827–834. doi: 10.1016/j.ygyno.2020.12.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.