Abstract
Background:
Spinal arachnoid webs (SAW) occur when abnormally thickened bands of arachnoid membranes commonly located dorsal to the thoracic spine cause blockage of normal cerebrospinal fluid (CSF) flow, resulting in focal cord compression and myelopathy. The pathognomonic MR finding for SAW is the “positive scalpel sign” comprised of an enlarged dorsal CSF space with a normal ventral subarachnoid space. The main differential diagnostic consideration for SAW is idiopathic spinal cord herniation (ISCH); however, for ISCH, MR studies classically demonstrate ventral displacement of the spinal cord through an anterior dural defect. Here, we describe a 60-year-old female with an atypical SAW at the T3-T4 level (i.e., the preoperative MR failed to demonstrate the “positive scalpel sign”). Nevertheless, at surgery, intraoperative ultrasonography confirmed that SAW was present and was decompressed/marsupialized/removed.
Case Description:
A 60-year-old female presented with sensory impairment to both lower extremities. The thoracic MR images showed an enlarged dorsal CSF space at the T3-T4 level but without the “scalpel sign” suggesting “interruption” of CSF flow by thickened bands of focal dorsal arachnoidal tissues. Although the initial preoperative diagnosis was ISCH, intraoperative ultrasound (IOUS) confirmed the presence of a thickened arachnoid band, confirming the diagnosis of a SAW that was appropriately decompressed/resected.
Conclusion:
Correctly, establishing the preoperative diagnosis of a SAW based on MR imaging may sometimes be difficult as the typical “scalpel sign” may not be present in all patients. Notably, in cases like this one, IOUS may critically confirm the diagnosis of SAW thus leading to appropriate SAW decompression/removal.
Keywords: Arachnoid web, Idiopathic spinal herniation, Intraoperative ultrasound, Scalpel sign, Spinal arachnoid web

INTRODUCTION
Spinal arachnoid webs (SAWs), sometimes considered a subset of spinal arachnoid cysts, must be differentiated from idiopathic spinal cord herniation (ISCH) as causes of myelopathy and dorsal cord compression/ventral cord migration. SAW is comprised thickened bands of focal dorsal arachnoidal tissues resulting in focal cord indentation. On both MR/Myelo-CT studies, they yield the typical “positive scalpel sign” defined by a widened dorsally compressive cerebrospinal fluid (CSF) collection.[10] Alternatively, on MR imaging, ISCH results in ventral displacement of the spinal cord through an anterior dural defect.[12] Here, we present a 60-year-old female with a T3-T4 SAW and show how at surgery utilizing intraoperative ultrasound (IOUS) confirmed the diagnosis of a SAW that could then be surgically decompressed/removed.
CASE PRESENTATION
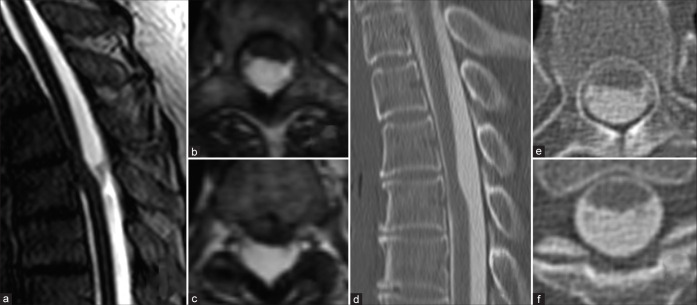
A 60-year-old female presented with sensory impairment to both lower extremities of 1-year duration. As the thoracic MR/Myelo-CT studies, both revealed at the T3-T4 level, a dorsally widened subarachnoid space resulting in ventral cord compression/shift the, and an inadequately defined anterior subarachnoid space. Based on these findings, the preoperative diagnosis was ISCH, and not a SAW [Figure 1].
Figure 1:
MRI T2 CISS revealed anterior subarachnoid space of the spinal cord was not clear and relatively focal ventral shifting of the spinal cord. (a-c) Sagittal image of CT myelography showed ventral CSF space but coronal image of CT myelography showed interruption of ventral CSF space. (d-f). Abbreviation; CISS: Constructive interference in steady state, CT: Computed tomography, CSF: Cerebrospinal fluid.
Surgery with intraoperative ultrasound (IOUS) confirmed a T3-T4 SAW
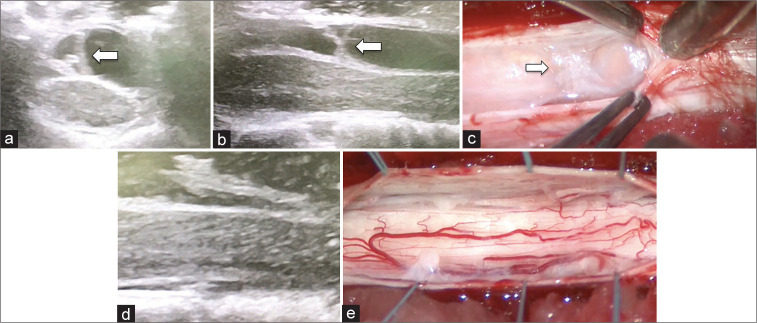
A laminectomy was performed at the T3-4 level. However, before opening the dura, intraoperative ultrasound (IOUS) confirmed the presence of thickened dorsal arachnoid bands consistent with SAW, and no evidence of idiopathic ventral spinal cord herniation [Figures 2a and b]. On opening the dura, a SAW characterized by an arachnoid membrane on the dorsal side of the spinal cord was identified, marsupialized, resected, and dissected, thus restoring normal CSF flow [Figures 2c-e]. Postoperatively, the patient’s sensory disturbance improved.
Figure 2:
IOUS revealed partition structures over the spinal cord (a and b; arrow) and ventral shifting of the spinal cord. (a and b) After opening dura, thickened arachnoid membrane was observed in dorsal space of the spinal cord. (c; arrow) The arachnoid band was dissected and restoration of the space for CSF flow was confirmed. (d and e) Abbreviation; IOUS: Intraoperative ultrasound, CSF: Cerebrospinal fluid.
DISCUSSION
Differentiating ISCH from SAW
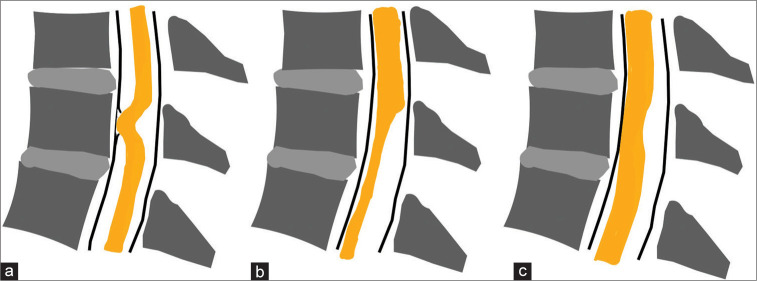
ISCH may sometimes mimic SAW, but is attributed to a deficit of the inner layer of the dura mater (i.e., with herniation of the cord through that defect). Key radiological findings for ISCH include focal distortion/rotation of the spinal cord with no ventral subarachnoid CSF space (i.e., the MR/Myelo-CT studies show that the cord is pulled ventrally over a short segment as seen on MR/Myelo-CT studies) [Figure 3a].
Figure 3:
Illustration depicting typical findings of idiopathic spinal cord herniation (a), arachnoid web (b) and our case (c). (a) Spinal cord is deviated anteriorly with spinal cord protrusion through dural defect. The ventral subarachnoid space is not preserved and the dorsal surface of the spinal cord is deformed in C-shape. (b) Scalpel-sign deformity of the dorsal surface of the spinal cord. The ventral subarachnoid space is preserved. (c) The dorsal surface of the spinal cord is slightly deformed in C-shape. The ventral subarachnoid space of the spinal cord is not visible on MRI but is preserved on CT myelogra.
Diagnosing and treating SAW
SAWs are rare and are considered variants of spinal arachnoid cyst.[1,9] SAWs are caused by dorsally thickened bands of arachnoid that result in widening of the dorsal subarachnoid space in the shape of a scalpel (i.e., “Positive scalpel sign”) and dorsal cord compression, while the ventral subarachnoid space is preserved [Figure 3b]. Interestingly, Schultz et al. found that all six patients with SAW all had the “positive scalpel sign” and preserved ventral subarachnoid spaces, while the five patients with ISCH had focal dorsal indentations of the cord with ventral interruption of CSF flow.[11] They commonly block dorsal CSF flow and may result in syrinx formation.[2,5] Over 90% of the patients with SAW improve following decompressive laminectomies with resection/dissection/marsupialization of the arachnoidal bands.[8] Notably, IOUS should be applied to confirm the restoration of normal CSF flow before closing, and some cases may warrant placement of a shunt or stent.[2,4] In the present case, the MR and Myelo-CT studies led to the wrong preoperative diagnosis of ISCH [Figure 3c].[3] However, intraoperative ultrasound IOUS confirmed the presence of a T3-T4 SAW that was then appropriately managed with resection/dissection/removal resulting in the restoration of normal CSF flow.[6,7]
CONCLUSION
Here, we have reported a 60-year-old female who presented with bilateral sensory impairment to both lower extremities over a year’s duration. Despite the preoperative diagnosis of ISCH based on MR/Myelo CT studies, intraoperative ultrasound (IOUS) confirmed a SAW at the T3-T4 level.
Footnotes
How to cite this article: Nagashima Y, Nishimura Y, Ito H, Oyama T, Nishii T, Gonda T, et al. Atypical radiographic case of arachnoid web without scalpel sign. Surg Neurol Int 2022;13:108.
Contributor Information
Yoshitaka Nagashima, Email: nagashima4251@gmail.com.
Yusuke Nishimura, Email: yusukenishimura0411@gmail.com.
Hiroshi Ito, Email: diamonddust.h@gmail.com.
Takahiro Oyama, Email: oyama.takahiro.0504@gmail.com.
Tomoya Nishii, Email: tomoya.nishii@gmail.com.
Tomomi Gonda, Email: kgmvegelife@gmail.com.
Hiroyuki Kato, Email: herohiro1027@gmail.com.
Ryuta Saito, Email: ryuta@med.nagoya-u.ac.jp.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ben Ali H, Hamilton P, Zygmunt S, Yakoub KM. Spinal arachnoid web-a review article. J Spine Surg. 2018;4:446–50. doi: 10.21037/jss.2018.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodbelt AR, Stoodley MA. Syringomyelia and the arachnoid web. Acta Neurochirurg (Wien) 2003;145:707–11. doi: 10.1007/s00701-003-0071-9. discussion 11. [DOI] [PubMed] [Google Scholar]
- 3.Chang HS, Nagai A, Oya S, Matsui T. Dorsal spinal arachnoid web diagnosed with the quantitative measurement of cerebrospinal fluid flow on magnetic resonance imaging. J Neurosurg Spine. 2014;20:227–33. doi: 10.3171/2013.10.SPINE13395. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara Y, Manabe H, Izumi B, Shima T, Adachi N. Microscope and fiberscope-assisted subarachnoid-subarachnoid (S-S) bypass: A novel surgical technique to reestablish cerebrospinal fluid flow in treating dorsal spinal arachnoid webs, diagnosed by cine-MRI. Clin Spine Surg. 2018;31:58–64. doi: 10.1097/BSD.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 5.Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006;29:251–63. doi: 10.1007/s10143-006-0029-5. discussion 64. [DOI] [PubMed] [Google Scholar]
- 6.Kelly PD, Zuckerman SL, Yamada Y, Lis E, Bilsky MH, Laufer I, Barzilai O. Image guidance in spine tumor surgery. Neurosurg Rev. 2020;43:1007–17. doi: 10.1007/s10143-019-01123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura Y, Thani NB, Tochigi S, Ahn H, Ginsberg HJ. Thoracic discectomy by posterior pedicle-sparing, transfacet approach with real-time intraoperative ultrasonography: Clinical article. J Neurosurg Spine. 2014;21:568–76. doi: 10.3171/2014.6.SPINE13682. [DOI] [PubMed] [Google Scholar]
- 8.Nisson PL, Hussain I, Härtl R, Kim S, Baaj AA. Arachnoid web of the spine: A systematic literature review. J Neurosurg Spine. 2019;31:175–84. doi: 10.3171/2019.1.SPINE181371. [DOI] [PubMed] [Google Scholar]
- 9.Paramore CG. Dorsal arachnoid web with spinal cord compression: Variant of an arachnoid cyst? Report of two cases. J Neurosurg. 2000;93(Suppl 2):287–90. doi: 10.3171/spi.2000.93.2.0287. [DOI] [PubMed] [Google Scholar]
- 10.Reardon MA, Raghavan P, Carpenter-Bailey K, Mukherjee S, Smith JS, Matsumoto JA, et al. Dorsal thoracic arachnoid web and the “scalpel sign”: A distinct clinical-radiologic entity. AJNR Am J Neuroradiol. 2013;34:1104–10. doi: 10.3174/ajnr.A3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz R, Jr, Steven A, Wessell A, Fischbein N, Sansur CA, Gandhi D, et al. Differentiation of idiopathic spinal cord herniation from dorsal arachnoid webs on MRI and CT myelography. J Neurosurg Spine. 2017;26:754–9. doi: 10.3171/2016.11.SPINE16696. [DOI] [PubMed] [Google Scholar]
- 12.Summers JC, Balasubramani YV, Chan PC, Rosenfeld JV. Idiopathic spinal cord herniation: Clinical review and report of three cases. Asian J Neurosurg. 2013;8:97–105. doi: 10.4103/1793-5482.116386. [DOI] [PMC free article] [PubMed] [Google Scholar]