Abstract
Developmental changes that occur throughout childhood have long been known to impact drug disposition. However, pharmacokinetic studies in the pediatric population have historically been limited due to ethical concerns arising from incorporating children into clinical trials. As such, much of the early work in the field of developmental pharmacology was reliant on difficult-to-interpret in vitro and in vivo animal studies. Over the last two decades, our understanding of the mechanistic processes underlying age-related changes in drug disposition has advanced considerably. Progress has largely been driven by technological advances in mass spectrometry-based methods for quantifying proteins implicated in drug disposition, and in silico tools that leverage that data to predict age-related changes in pharmacokinetics. This review summarizes our current understanding of the impact of childhood development on drug disposition, particularly focusing on research of the past 20 years, but also highlighting select examples of earlier foundational research. Equally important to the studies reviewed herein are the areas that we cannot currently describe due to the lack of research evidence; these gaps provide a map of drug disposition pathways for which developmental trends still need to be characterized.
Keywords: developmental trajectory, developmental expression, ontogeny, drug biotransformation, drug transporters, absorption, renal excretion
Introduction
Over the past decades, many efforts have been made to predict age-related alterations in pharmacokinetics of drugs in the pediatric population. In addition to dedicated pharmacokinetic studies conducted in pediatric populations, extrapolations from investigations in adults have also been used to estimate dose-exposure relationships in children, including approaches based on changes in body size, body composition, body maturation, organ volume maturation. In addition, research studies utilizing juvenile animal models and postmortem human pediatric tissue have also provided insights into the disposition pathways underlying differences in pharmacokinetics in children relative to adults.
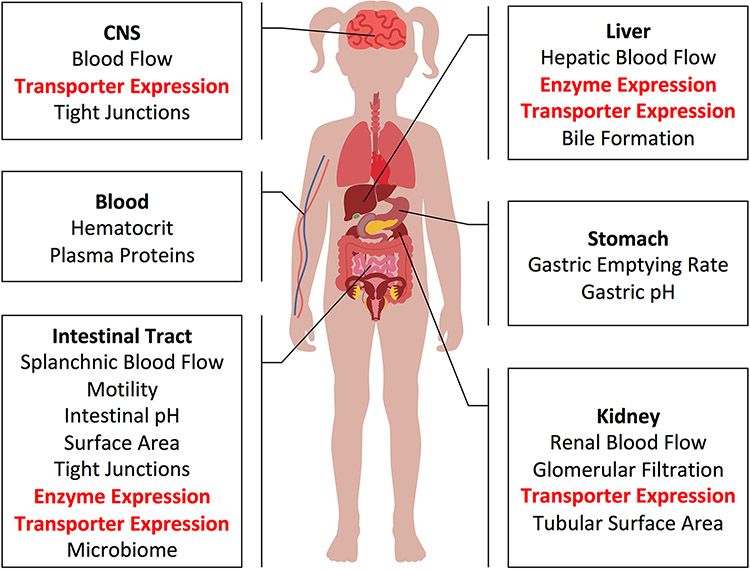
Accumulated evidence to date indicates that there is no single uniform pattern describing the developmental trajectory of all processes contributing to drug disposition. Rather, each physiologic process, including drug-metabolizing enzyme (DME) and transporter expression and function, has its own unique developmental trajectory. This reality makes predictions of drug exposure without any prior pediatric clinical pharmacokinetic experience complex. This challenge can be overcome by mechanistic modelling approaches, such as physiologically based pharmacokinetic (PBPK) models, which present an elegant way to combine a multitude of known age-dependent systems (i.e., physiological) related parameters with drug-related parameters in an attempt to accurately extrapolate and/or simulate drug exposure in children. This approach has successfully been used to simulate clinical studies for estimating drug dosing in the pediatric population and has contributed to the approval of pediatric dosing guidelines for valganciclovir and nilotinib by the United States Food and Drug Administration [12, 13]; more recent publications have illustrated to potential to infer drug-drug interaction potential in children based on adult data [1]. The extension of PBPK modeling and simulation to a wider array of drugs demands accurate systems-based parameters. To that end, a quantitative characterization of changes in the proteins and processes governing drug disposition (highlighted in Figure 1) is underway. Much progress has been made but still much more work lies ahead.
Figure 1.
Processes governing drug disposition that may change through childhood developments. Areas in which LC-MS/MS protein quantification have provided substantial new information have in the last 10 years are highlighted in red.
This review supplements past knowledge with select illustrative examples of some of the more important advances that have been made in describing age-related changes in physiological processes and DME/transporter expression, with a particular emphasis on developments from the past 20 years. It highlights the information gaps and limitations in our understanding of developmental trajectories of drug disposition pathways (mostly from post-conception to childhood period), and offers a roadmap towards future, more accurate mechanistic predictions of pediatric drug exposure in drug development, as well as in the hospital and outpatient setting.
Enteral Absorption
As with adults, most medications are administered orally to children, and the first organ of pharmacokinetic significance that drugs encounter is typically the stomach. The low pH environment of the stomach is favorable for the dissolution of weak bases, which constitute the majority of marketed drugs [2]. Amniotic fluid swallowed in utero results in a neutral gastric pH in children at birth, but the fasted pH of the stomach begins to decline thereafter [3]. While there is some debate over the exact timing, fasted gastric pH drops to values comparable to those seen in adults within the first few weeks of life [4-8]. However, because pH can increase substantially in the postprandial period, timing of meals may be an even greater determinant of gastric pH in neonates than age-related alterations in basal hydrochloric acid secretion [9, 10], an important fact considering the frequency of feeding by neonates. Elevated gastric pH, regardless of the underlying mechanism, has been proposed to explain the increased oral bioavailability of benzylpenicillin, an acid-labile drug, in neonates. Higher gastric pH may also reduce the solubility of some poorly soluble weak bases and delay or reduce their subsequent absorption. This mechanism has been suggested to explain reduced or non-existent exposure to orally administered ketoconazole in neonates [11, 12]. Dramatically smaller stomach volumes, particularly in the first weeks of life, may further exacerbate impaired drug dissolution [13].
The pattern and rate at which the contents of the stomach are emptied into the small intestine (i.e. gastric emptying) can also influence drug absorption. While some evidence suggests that gastric emptying may be slower in pre-term infants and neonates, a more recent meta-analysis did not observe a significant effect of age (postnatal or gestational) on gastric emptying time [14]. However, as with pH, gastric emptying is determined by several factors, including the timing and composition of meals. Indeed, gastric emptying half-time of formula was found to be about 60% greater than that of breast milk in newborns [15]. For acid-labile drugs, increased residence time in the stomach could result in increased degradation. Additionally, because absorptive (e.g., uptake transporters) and restrictive processes (e.g., DMEs and efflux transporters) in the small intestine may be saturable, prolongation of the release of drug from the stomach into the small intestine may avert these processes from becoming overwhelmed and less efficient.
Following gastric emptying, drugs next enter the small intestine, the major site for the absorption of most orally administered medications. The general primacy of the small intestine in oral drug absorption principally derives from its expansive surface area resulting from numerous tissue folds, villi (i.e. multicellular finger-like projections) and an epithelial apical brush border [16]. While villi mostly mature by 20 weeks of gestation [17, 18], some evidence suggests villous surface area and density moderately increase in the first few months of life, a function of widening villi and crypt elongation [19].
Enterocyte height has also been shown to modestly increase (by 14%) from infancy into adulthood [20], suggesting the cells undergo a postnatal maturation process. It is difficult to predict the impact of changes in enterocyte height on drug absorption, but if microvillous length or brush border density are also subject to developmental changes in cellular morphology, then changes in absorptive surface area would be anticipated. Notably, breast milk versus formula feeding has not been shown to alter villous length or enterocyte height in infants [20]. Increases in intestinal absorptive surface area are also attributable to increasing diameter and length of the organ; the length of the small intestine increases from approximately 300 cm at term to around 600 cm in adulthood [21]. Collectively, the effect of age-related increases in small intestinal surface area on drug bioavailability have not been conclusively established, but they are expected to increase the fraction absorbed for some drugs whose absorption is dependent on passive partitioning across the intestinal epithelium.
Another important contributor to efficient absorption of drugs and nutrients is the extensive perfusion of the intestines. Once molecules cross the intestinal epithelium and enter the microvasculature, the high rate of villous blood flow rapidly draws the solutes away from the intestine and into the portal vein. This reduces partitioning of absorbed drug back into the enterocytes and ensures the maintenance of a favorable concentration gradient for absorption, with concentrations in the intestinal lumen exceeding microvascular drug concentrations. Mesenteric blood flow velocity is decreased during the first week of life [22, 23], which may affect the rate and extent of absorption. This may be particularly relevant for acidic drug molecules that are frequently ionized under the pH conditions of the small intestine; normal pH ranges for the small intestine do not differ appreciably between children and adults [24, 25].
Small intestinal motility also has the capacity to impact oral drug absorption. Relatively delayed gastrointestinal transit time in infants has been posited as an explanation for reduced time to reach peak plasma concentrations (Tmax) of cisapride in infants under 42 weeks compared to adults [26]. However, this interpretation is complicated by a simultaneous reduction in cisapride half-life, another potential contributor to the observed reduction in Tmax. Furthermore, some reports challenge the idea that there are substantial age-related changes in small intestinal transit time [27-29], and a relatively recent meta-regression analysis found no significant increase in basal intestinal motility from the neonatal period to adulthood [30]. Interestingly, it has been reported that intestinal transit in preterm infants may be reduced but evidence is limited [27].
In addition to the contents from the stomach, the small intestine also receives bile from the liver and digestive enzyme-rich secretions from the pancreas. Bile, a solution consisting of various lipids and surfactants (e.g. bile acids), aids in the dissolution and absorption of nutrients and lipophilic drugs. Much of the research related to the maturation of bile acid synthesis and secretion was conducted decades ago with the foundational research reviewed and contextualized by Murphy and Signer [31]. Rapid maturation of the processes governing bile acid synthesis and secretion occur in the first months of life with age-related effects being most evident in neonates and pre-term infants [32, 31]. Bile acids require a threshold concentration, ~1 to 2 mM under simulated in vivo conditions, to form the micelles necessary to aid in absorption of fats[31]. Studies in low birth weight infants and healthy neonates suggest bile acid concentrations in the duodenum are frequently below the critical micellar threshold concentrations [33, 34] and result in a disproportionate reduction in fat absorption for highly lipophilic compounds. Insufficient bile acid concentrations have been suggested to result in poor absorption of fat-soluble vitamins ( summarized in the review by Murphy and Signer [31]), and pleconaril [35]. It is worth noting that bile salts are present in breast milk and may offset lower concentrations of endogenously produced bile acids in the neonatal duodenum [36, 37]. Further studies are required to determine whether breastfed pre-term and term neonates have a greater capacity to absorb poorly soluble lipophilic drugs than those who are formula fed. Following the neonatal period, total bile acid concentrations in the duodena reach those seen in adults [33, 31]. However, the proportions of specific bile acids are altered throughout infancy [32, 38, 39]. This is likely the result of both normal liver development and the maturation of the intestinal microbiome, which is responsible for bile acid deconjugation and the formation of secondary bile acids. In addition to affecting bile acid homeostasis, intestinal flora can also impact oral bioavailability through direct drug metabolism. Historical examples include the therapeutic bioactivation of sulfasalazine and the metabolic inactivation of digoxin [40-42], but more recent investigations reveal that a broad range of drugs are subject to presystemic biotransformation by gut microbiota [43]. Furthermore, it is not surprising that an individual’s unique gut microbiome may contribute to inter-individual variability in the nature and extent of drug biotransformation and clearance beyond that related to the host genome [44, 43]. Although rapidly occurring changes in the gut microbiome after birth and following perturbations associated with antibiotic use represent additional challenges to be considered, initial efforts are beginning to recognize the potential of pharmacokinetic modeling to incorporate the contribution of the intestinal microbiota to overall drug disposition [45, 46].
In addition to the intestinal microbiota, several host proteins support intestinal barrier function, including efflux transporters and DMEs. Cytochrome P450 3A4 (CYP3A4) is the most notable among these DMEs due to its high expression in the small intestine and broad substrate specificity for lipophilic drugs [47, 48]. Several studies have characterized the developmental trajectories of CYPs 3A4, 3A5 and 3A7 mRNA [49, 50], but data more closely related to activity are more relevant clinically. Two studies using immunoassay methodologies to quantify CYP3A4 protein in small intestinal biopsies demonstrated that low amounts of the enzyme are present at birth, but expression increases throughout early childhood [49, 50]. Conversely, CYP3A4 mRNA expression in the duodenum has been shown to decline with age [51, 50]. Delayed maturation of CYP3A4 in intestinal mucosa and liver accounts for the increased bioavailability of midazolam (by approximately 3-fold) in premature newborns relative to adults [52].
Relatively less is known about the developmental trajectories of other DMEs isoforms in the small intestine. The catalytic activity of CYP1A1 in biopsies from the small intestine of children appears to increase by approximately 3-fold on average from infancy to adolescence [53]. Intestinal expression of CES2 protein, as determined via immunoblotting, and mRNA expression have been shown to increase throughout infancy to near adult levels [50]. Conversely, some evidence suggests that the expression pattern for another DME, GSTA1, may decrease over childhood. In pediatric small intestinal biopsies, the glutathionylation rate of the GSTA1 substrate, busulfan, was 77% greater in 1 to 3 year old children than in those 9 to 17 years of age [54].
Working in concert with DMEs, efflux transporters in the apical membrane of intestinal enterocytes extrude drug molecules from the cytosol and cellular phospholipid bilayer. To-date, our knowledge of intestinal transporter protein ontogeny is mainly based on assessments of transporter gene transcription and transporter localization via immunohistochemistry. It is important to note that, while expression of transporter genes may be activated at birth, the functioning of the transporter and its contribution to drug absorption will depend on its protein expression and any additional post-translational modifications that may be required for proper membrane localization. Functional activity and specific membrane localization of transporters in cellular membranes is governed by complex cellular trafficking processes that are directed in part by glycosylation of the transporter proteins in the endoplasmic reticulum and Golgi apparatus [55, 56]. These processes are not necessarily captured in proteomic analysis of tissue homogenates or even plasma membrane fractions.
An array of apical efflux transporters exists in the small intestine. Notable transporters include permeability glycoprotein (P-gp), multidrug resistance protein (MRP) 2 and breast cancer resistance protein (BCRP). Owing to its wide substrate profile, P-gp is one of the most broadly relevant efflux transporters in the human intestine. Expression of P-gp in enterocytes, as determined via immunohistochemical staining, is detectable after 12 weeks of gestation [57]. Data suggest that mRNA expression reaches adult levels at, or shortly after, birth and is marked by a considerable inter-individual variation [51, 58, 59]. The findings from mRNA studies are supported by the observation that the oral bioavailability of cyclosporine, a prototypical P-gp substrate, does not change from infancy to 18 years of age [60]. However, because cyclosporine is also a CYP3A4 substrate, interpretation of these findings is complicated and may conflict with observations of increasing CYP3A4 protein expression throughout early childhood [49]. Nevertheless, functional studies of another P-gp substrate, aliskiren, have similarly revealed its pharmacokinetic and safety profile to be comparable between children aged 6-17 years old and adults [61]. In the small intestine, MRP2 appears to have a similar developmental trajectory as P-gp; its mRNA levels are detected in neonatal tissue, appear constant through adulthood and are characterized by substantial inter-individual variation [59, 62, 63]. Lastly, protein expression and cellular localization of BCRP has been detected as early as 6 weeks of gestation and remain constant out to 28 weeks [57, 63].
Contrasting with efflux transporters, apical membrane bound transporters including peptide transporter 1 (PEPT1), organic cation transporter (OCT) 3, organic anion transporting polypeptides (OATP) 1A2 and OATP2B1 play a role in facilitating the uptake of drug molecules into the intestinal epithelium. In the case of OATP2B1, the anticipated impact of its ontogeny on the distribution of substrates is complicated by reports of basolateral localization which would presumably result in uptake into the intestinal epithelium from the blood [64]. Significantly higher mRNA levels (over 2-fold, as judged by the median values) of SLCO2B1, the gene encoding for OATP2B1, have been detected in neonates compared to adults [59], suggesting that levels decrease as children mature. On the other hand, investigations into small intestinal expression of SLC15A1, the gene encoding for PEPT1, revealed mRNA levels are comparable between neonates and older children, with tissues from the former exhibiting only marginally lower (0.8-fold) relative mRNA expression [65]. Specific apical localization of PEPT1 was also demonstrated in both neonatal and adult tissue [65, 66]. Some basolateral efflux transporters, such as MRP1, can theoretically work in conjunction with apical uptake transporters in the vectorial transit of drugs across the intestinal epithelium by extruding transporter-imported molecules from the cytosol into systemic circulation [67]. The limited data that describe MRP1 ontogeny come from immunoblotting tissue studies; at 7 weeks of gestation, expression is comparable to that observed in adult specimens [57].
The benefits of consolidation and integration of new data describing the developmental trajectories of multiple processes involved in drug absorption are now being realized in the form of comprehensive PBPK absorption models for drugs like acetaminophen, theophylline and ketoconazole, but also illustrate that some knowledge deficits persist [68].
Distribution
Distribution is the reversible exchange of drug from one site in the body to another. These sites may reflect anatomical compartments, such as organs or tissues, or they may represent mathematical “apparent” spaces such as those used in compartmental pharmacokinetic modeling (i.e. central and peripheral compartments). In either case, developmental changes in physiological process such as body fat/water composition, plasma protein homeostasis and drug transporter expression will impact the rate and extent of drug distribution. It is important to stress that changes in volume of distribution do not affect the overall exposure of drug in blood/plasma, however they can impact the shape of the concentration-time curve, effectively resulting in changed trough and peak drug blood/plasma concentrations.
Infants and neonates have larger total body-water space relative to their weight than older children or adults. As such, dosing strategies based upon proportional reductions in dose by weight for hydrophilic drugs may result in relatively reduced plasma concentrations in the very young. Notable examples include antibiotics such as linezolid and gentamicin [69, 70]. In the case of gentamicin, one study observed mean peak concentrations of 1.58, 2.03 and 2.81 μg/mL in the 1.5-5, 5-10, and >10 year age groups, respectively, following a 1 mg/kg dose [70]. This may be clinically significant considering the established relationship between peak concentrations and efficacy/toxicity [71, 72]. It is also important to note that age-related changes in the renal clearance of gentamicin and linezolid, in addition to distributional phenomena, will also affect drug concentrations in infants.
Reductions in amounts of circulating plasma proteins (e.g. albumin and α1-acid glycoprotein) and increased plasma concentrations of bilirubin (a competing ligand for albumin) in neonates and young infants may also reduce total concentrations of highly protein-bound drugs in circulation as a greater portion of drug molecules will partition out of the plasma [73, 74]. As a result, the fraction of unbound drug in plasma will increase and therapeutic drug monitoring approaches that rely on total concentrations may be misleading. Increased fraction unbound in the range of 2- to as much as 4-fold in neonates and infants has been observed for cyclosporine, diazepam, thiopental and sufentanil [75-77]. Complicating issues further is the fact that neonates possess relatively (up to 20-30%) higher hematocrit levels than adults [78, 79]. This has the potential to translate into increased blood concentrations for drugs that accumulate in erythrocytes. However, this may not necessarily be the case if uptake transporters or specific cellular binding proteins that may themselves be subject to maturation trends contribute to drug sequestration in the erythrocytes. Therapeutic drug monitoring approaches that rely upon whole blood rather than plasma measurements (e.g. cyclosporine) may be more challenging to interpret in this context, as the ratio of blood to free plasma concentrations may be impacted. Fortunately, hematocrit levels decline to adult levels within the first few weeks of life and drugs with whole blood monitoring are infrequently administered during this brief period [78, 79].
Changes in the expression and activity of transporters in the liver and kidneys may also influence the apparent volume of distribution (Vd) of substrate drugs as reviewed by Grover and Benet [80]. Furthermore, hepatic uptake transporters located in the sinusoidal membranes of hepatocytes may be of additional clinical significance as the liver is often a site of drug action, biotransformation and toxicity. However, due to issues of ethics and feasibility, experiments evaluating the ontogeny of transporter-mediated effects on drug disposition in children have been limited. Currently, our understanding of drug tissue distribution is mainly based on findings from animal studies, in vitro systems or human tissues collected post-mortem.
Studies applying quantitative proteomic approaches or immunoblot analysis to characterize expression of OATP1B3 and OCT1 respectively have revealed that neonates and infants have significantly lower hepatic abundance of these transporters compared to older children (by 60 and 80% on average, respectively), who possess amounts comparable to adults [81, 82]. However, it is important to note that a complete understanding of the developmental trajectory for a given protein is dependent on a sufficient sample size for each age group or developmental stage. For example, a separate study that analyzed a greater number of pediatric liver samples, but using immunoblotting techniques, reported relatively high OATP1B3 expression at birth, which then declined for a period of time before increasing again over the preadolescent age range [83]. Caution also should be taken in interpreting these studies given that age-related changes in transporter activity are superimposed upon other sources of inter-individual variability (e.g. genetics, epigenetics, concomitant medications). For example, age-dependent changes in OATP1B1 expression are only evident in the carriers of the reference allele, but not when the ontogeny of hepatic OATP1B1 expression is analyzed in a combined (non-genotype stratified) manner [82]. Indeed, genetic variation has also been established to be a significant contributor to variability in morphine [84], simvastatin [85], pravastatin [86], and rosuvastatin exposure in children [87]. As for the transporters that shuttle drugs and metabolites out of the hepatocytes back into the blood compartment (i.e., MRP3, MRP4, OAT7), very little is known about their ontogeny. The expression of MRP3 was shown to increase by approximately 2-fold on average from neonates to adults [82], and is marked by significant inter-individual variability in all age groups. While dedicated pediatric pharmacokinetic studies describing the ontogeny of MRP3 substrates are missing, transporter proteomic data may inform the impact of MRP3 ontogeny on the disposition of 25-hydroxy-vitamin D3 glucuronide, a metabolite of the prohormone and therapeutic agent 25-hydroxy-vitamin D3 [88].
The blood-brain barrier is a gateway for passage of drugs and metabolites between vascular and neural compartments in the brain. Its permeability in the pediatric population could be assessed via measurements of brain extracellular fluid (ECF) drug concentrations, however such measurements are not usually performed due to relative brain ECF inaccessibility. Instead, drug concentrations in the cerebrospinal fluid (CSF) can be measured as a surrogate. One study evaluating CSF uptake of imipenem, a drug that predominantly transits passively across the blood-brain barrier [89], observed no difference in blood brain barrier permeability across the age range studied (4 months to 11 years of age)[90]. Because the central nervous system (CNS) is an important site of action for many drugs, age-related patterns in the expression of efflux transporters in the blood-brain and blood-CSF barriers may be of clinical importance. Given the limited availability of sufficient amount of human tissue to conduct quantitative proteomic investigations, the monitoring of radiolabeled compounds using positron emission tomography (PET) and the measurement of central pharmacodynamic responses have been proposed as potential means to characterize the ontogeny of P-gp in the blood brain barrier [91]. However, there are considerable practical challenges to conducting PET imaging studies in the pediatric population including requiring exposure to radiation and sedative drugs, which may be required to reduce the movement of pediatric subjects while obtaining measurements [92]. As such, much of our current knowledge comes from antibody-based in vitro analyses.
A recent immunohistochemical study reported higher intensity staining of P-gp and BCRP, and decreased staining intensity of MRP1 and MRP2, in adults relative to children under 3 years of age [93]. While the results for P-gp and BCRP mirror findings from an earlier investigation, the observed trend for MRP1 ontogeny was reversed; the cellular localization pattern of all transporters evaluated in the earlier study was found to be similar to that of adults [94]. A third investigation into the developmental trajectory of P-gp in the brain demonstrated that infants between 3 to 6 months exhibit comparable levels of P-gp protein to adults, complicating our understanding of its ontogeny [95]. Nevertheless, for the neonate, it is possible that reduced expression of P-gp contributes to increased CNS exposure to morphine, a P-gp substrate, and may account for observations of greater morphine sensitivity in neonates compared to older infants and adults [96-98]. These findings may also be explained in part by developmental changes impacting systemic morphine disposition, such as reduced OCT1 expression [99, 63], contributing to reduced hepatic morphine uptake [100] and reduced UGT2B7-mediated glucuronidation [101]. However, greater apparent analgesia was observed in neonates under 10 days of age who were exposed to similar circulating concentrations of morphine as older infants [102]. This supports the hypothesis that age-related changes in systemic pharmacokinetics alone cannot fully account for differences in morphine sensitivity. Indeed, differences in distribution into the CNS, morphine pharmacodynamics or the perception of pain in the very young have all been posited as additional contributors to increased morphine sensitivity in the very young [102].
In addition to factors driving changes in gene transcription, it appears that developmental differences in post-translational factors may also contribute to developmental trajectories of transporter function. For example, age-dependent changes in the ratio of glycosylated OATP1B3 to total OATP1B3 protein concentrations have been reported in pediatric liver and could have functional consequences in terms of transporter activity [83]. Furthermore, decreased N-linked glycosylation of NTCP, MRP2 and OATPs 1B1, 1B3 and 2B1 have been reported in the presence of non-alcoholic fatty liver disease, with potential implications for altered disposition of drugs in obese (> 95th percentile for body mass index) children and adolescents as has been reported for pravastatin [86].
Hepatic Biotransformation
Enzymatic biotransformation is the major route of systemic elimination for approximately 75% of drugs [103], and hepatic biotransformation represents the primary site of metabolism for most drugs due to the high degree of blood perfusion and DME abundance in liver. Furthermore, due to direct blood flow from the intestines, the liver also contributes to first-pass metabolism wherein drug is cleared before ever reaching the systemic circulation. Because transdermal and subcutaneous administration bypasses hepatic first-pass metabolism, bioavailability by these routes is not affected by developmental changes in hepatic DME activity.
DMEs are classified as either Phase I enzymes that metabolize drug molecules via oxidation/reduction/hydrolysis or Phase II enzymes that conjugate polar substituents to substrates. From a developmental perspective, Phase I and Phase II DMEs have historically been classified according to three general developmental trajectories: Class I DMEs are most abundant in the fetus and decline after birth, Class II DMEs have relatively constant expression from gestation to adulthood, and Class III DMEs are expressed to a relatively low extent in fetal liver and increase after birth [104]. An overview of the ontogeny of Phase I DMEs (including the impact of additional factors such as pharmacogenetics, concurrent critical illness and other confounding factors) has been summarized in a recent review [105], and ontogeny functions have now been published for several CYPs and UGTs [106-109]. Multiple data types have been utilized for the characterization of DME and (and transporter) developmental trajectories, and not all are equally informative for accurate characterization of changes in drug clearance in vivo after birth. For example, changes in mRNA expression in vitro provide an assessment of developmental activation of DME gene expression, but do not necessarily translate in functional catalytic activity. Similarly, measurement of protein content by immunochemical or quantitative proteomic approaches provides a measure of protein content, but again does not necessarily equate with functional in vitro activity; for example, CYP apoprotein may be detected by either technique, but catalytic activity require the presence of a prosthetic heme group as well as an accessory oxidoreductase protein for electron transfer [110]. Extrapolation of enzyme activity in vitro to clearance in vivo is also not without challenges as data regarding the developmental trajectories of critical scaling factors, such as microsomal protein per gram of liver (MPPGL) or cytosolic protein per gram liver (CPPGL) derived from pediatric tissues, are extremely limited [105]. Recognizing these limitations, Upreti and Wahlstrom [109], conducted a comprehensive analysis of published in vitro and in vivo data relevant to CYP ontogeny and determined that similar developmental trajectories are derived from in vitro or in vivo data for CYP2A6, CYP2D6 and CYP2E1 (or differ only at very young ages), whereas the two data types generate quite different patterns for CYP1A2 and CYP3A4. CYP2C9 and CYP2C19 represent intermediate situations wherein the developmental trajectories are similar for in vitro and in vivo data at the extremes of age but are quite different between the ages of 0.5 and 10 years. They further observed that, in general, when differences between in vitro and in vivo trajectories are present, in vivo activity is greater than that observed in vitro and pediatric activity exceeds adult activity before returning to adult values during adolescence.
The analysis conducted by Upreti and Wahlstrom provides a comprehensive resource of literature describing CYP ontogeny, and reviewers are referred to that paper for references to the primary literature [109]. A summary of parameter estimates for a number of CYP and other DME isoforms in the liver is also provided in Table 1. Rather than recapitulate the findings from each study in detail , we will instead discuss some additional issues relevant to interpretation of ontogeny data. First, several exogenous factors may influence typical developmental trajectories. For example, formula feeding may accelerate developmental increases in CYP1A2 activity such that adult expression levels are achieved sooner in formula-fed infants compared to infants solely fed breast milk, as revealed by the latter group demonstrating >3-fold longer elimination half-life [111]. The effects of infant diet were confirmed in a longitudinal phenotyping study conducted using caffeine as a probe [112]. Second, in in vitro studies where individual level data are provided, it is not unusual to observe considerable inter-individual variability (approximately 300-fold) in protein expression as has been reported for CYP2B6 [113], and this extensive inter-individual variability in protein content or activity may obscure modest increases in average protein expression with increasing age throughout childhood (CYP2B6 is considered invariant with age in the Upreti and Wahlstrom analysis). Indeed, the percentage of samples with detectable CYP2B6 protein has been reported to increase with postnatal age (from 64% in fetal samples to 95% in samples from children >10 years of age) [114], and after birth, protein expression of CYP2B6 steadily increases, reaching adult levels by age 1 [113]. These in vitro data are consistent with the developmental trajectory of efavirenz pharmacokinetics, a CYP2B6 substrate, in newborns [115].
Table 1.
Published Sigmoid Emax Models Describing Drug Metabolizing Enzyme and Transporter Ontogenies in the Liver
| Enzyme or Transporter |
Source | Data Type | Birth | Adult | Age50 (y) | Exp or h | Age Cap (y) | Reference Number |
||
|---|---|---|---|---|---|---|---|---|---|---|
| E0 (pmol/mg) | Fbirth | Max (pmol/mg) |
Fadult or Fmax | |||||||
| ADH1A | in vitro | Protein-QP | ND | 426 | 0.842 | 0.84 | [137] | |||
| ADH1B | in vitro | Protein-QP | 1822 | 5220 | 0.775 | 40.6 | [137] | |||
| ADH1C | in vitro | Protein-QP | 21 | 2598 | 1.03 | 2.1 | [137] | |||
| ALDH1A1 | in vitro | Protein-QP | 209 | 394 | 0.900 | 244 | [137] | |||
| CES1 | in vitro | Protein-QPe | 0.20 | 1 | 1.10 | 0.56 | [128] | |||
| CYP1A2 | in vitro | Protein-IR | 0.01 | 1.0 | 5.2 | 0.5 | [109] | |||
| in vivo | Activity | 0.16 | 1.5 | 0.20 | 2.0 | 10.0d | [109] | |||
| in vitro | Hybridh | 0.08 | 1.05 | 1.69 | 1.1 | [108] | ||||
| in vivo | Activity | 0 | 1.6 | 1.05g | 5.7 | 3.76d,g | [107] | |||
| CYP2A6 | in vitro | Protein-IR | 0.01 | 1.0 | 0.02 | 1.8 | [109] | |||
| in vivo | Activity | 0.15 | 1.1 | 0.04 | 0.8 | [109] | ||||
| CYP2B6 | in vitro | Hybridh | 0.1 | 1 | 1 | 1 | [108] | |||
| in vitro | Protein-IR | 1.0 | 1.0 | ND | ND | [109] | ||||
| in vivo f | Activity | 1.0 | 1.0 | ND | ND | [109] | ||||
| CYP2C8 | in vitro | Hybridh | 0.3 | 1 | 0.02 | 1 | [108] | |||
| in vitro | Protein-IR/mRNA | 0.03 | 1.0 | 0.02 | 1.3 | [109] | ||||
| in vivo | Activity | 0.15 | 2.7 | 0.15 | 4.0 | 0.5d | [109] | |||
| CYP2C9 | in vitro | Hybridh | 0.17 | 1 | 0.016 | 0.53 | [108] | |||
| in vitro | Protein-IR | 0.01 | 1.0 | 0.02 | 1.2 | [109] | ||||
| in vivo | Activity | 0.01 | 2.2 | 0.01 | 1.2 | 0.3d | [109] | |||
| CYP2C19 | in vitro | Hybridh | 0.3 | 1 | 0.28 | 2.44 | [108] | |||
| in vitro | Protein-IR | 0.11 | 1.1 | 0.21 | 1.5 | [109] | ||||
| in vivo | Activity | 0.16 | 2.3 | 0.30 | 2.0 | 0.8d | [109] | |||
| CYP2D6 | in vitro | Hybridh | 0.036 | 1.0 | 0.1 | 1 | [108] | |||
| in vitro | Activity | 0.07 | 1.0 | 0.02 | 2.6 | [109] | ||||
| in vivo f | Activity | 1.00 | 1.0 | ND | ND | [109] | ||||
| CYP2E1 | in vitro | Hybridh | 0.086 | 1.074 | 0.226 | 0.496 | [108] | |||
| in vitro | Protein-IR | 0.03 | 1.0 | 0.14 | 0.6 | [109] | ||||
| CYP3A | in vivo | Activity | 0.05 | 1.7 | 0.10 | 1.3 | 2.5d | [109] | ||
| CYP3A4/5 | in vitro | Hybridh | 0 | 1.061 | 0.66 | 0.78 | [108] | |||
| CYP3A4 | in vitro | Protein-IR | 0.10 | 1 | 6.30 | 0.7 | [109] | |||
| in vivo | Activity | 0 | 1 | 2.07g | 3.9 | [107] | ||||
| CYP3A5 | in vitro | Protein-IR/QP | 1.0 | 1.0 | 1.0 | 1.0 | [109] | |||
| FMO3 | in vitro | Protein-QP | 1.75 | 31.22 | 0.80 | 0.49 | [134] | |||
| SULT1A3 | in vitro | Protein-QP | 85.26 | 96.83 | 0.9094 | 166.5 | [144] | |||
| SULT1B1 | in vitro | Protein-QP | 37.75 | 116.8 | 0.9092 | 166 | [144] | |||
| UGT1A1 | in vitro | Protein-QP | 0.27 | 1 | 7.5 | 0.5 | [99] | |||
| UGT1A4 | in vitro | Activity | 0.235 | 1.25 | 0.502 | 2.77 | [106]a | |||
| in vitro | Protein-QP | 0.01 | 1 | 3.6 | 0.9 | [99] | ||||
| UGT1A6 | in vitro | Protein-QP | 0.03 | 1 | 10.3 | 0.6 | [99] | |||
| UGT1A9 | in vitro | Protein-QP | 0 | 1 | 8.2 | 0.5 | [99] | |||
| UGT1A9/2B7 | in vitro | Activity | 0 | 1.1 | 2.18 | 0.063 | [106]a | |||
| UGT2B7 | in vitro | Protein-QP | 0.02 | 1 | 2.6 | 0.4 | [99] | |||
| UGT2B17 | in vitro | Activity | 0.612 | 2.08 | 17.4 | 40.6 | [106]a | |||
| in vitro | Protein-QP | 0.11 | 1.31 | 13.5 | 7.5 | [139] | ||||
| Mb 0.10 | Mb 1.75 | Mb 13.6 | Mb 14.9 | |||||||
| Fb 0.05 | Fb 0.65 | Fb 10.7 | Fb 1.8 | |||||||
| OCT1 | in vitro | Protein-QP | 0.58 | 3.98 | 0.47 | 0.92 | [82]c | |||
| OATP1B3 | in vitro | Protein-QP | 0.50 | 1.14 | 0.58 | 4.87 | [82]c | |||
| P-gp | in vitro | Protein-QP | 0.15 | 0.41 | 2.94 | 0.78 | [82]c | |||
Significant figures provided as they are presented in respective source material. Protein-QP and -IR refer to protein expression as determined by quantitative proteomics and immunoreactive methods respectively.
Developmental trajectories for UGT 1A1, 1A3, 1A6, 1A9, 2B4, 2B7, 2B10 and 2B15 activities described in reference by one-phase decay equations
Data also presented separately for males (M) and females (F)
Expression of BCRP, MRP2, BSEP, MATE1 not influenced by age
Ontogeny equation applicable up to an age cap due to average adult clearance not representing maximum clearance.
Represents collective expression of both microsomal and cytosolic protein
Upreti et al state “in vivo data used to verify, but not develop, in vivo ontogeny”
Refers to postmenstrual age
Ontogeny models from this were collectively developed from both in vitro expression and activity data but specific use of expression and/or activity data unclear for specific isoforms
Third, rapid increases in CYP2D6 expression and activity have been observed after birth. Studies relying on dextromethorphan and tramadol urinary metabolite ratios as probes of CYP2D6 activity have demonstrated that CYP2D6 phenotype is concordant with genotype after approximately two weeks of age [116, 117], but it does not necessarily follow that clearance of CYP2D6 substrates reaches adult values within that time frame. On the other hand, plasma data confirm that clearance of the CYP2D6 substrate, tramadol, increases rapidly after birth and that the clinical consequences of developmental changes in acquisition of CYP2D6 activity may be limited to premature newborns [118]. It has now become apparent that consideration of genetic variation is much more important than ontogeny as a factor contributing to variation in clearance of CYP2D6 substrates. Nevertheless, consideration of CYP2D6 ontogeny and genetic variation alone is insufficient to characterize the disposition of substrates like tramadol [119], and other as yet unknown factors must be identified to address the current limitations (e.g. under-prediction) of PBPK models to describe tramadol pharmacokinetics in children [120].
A final issue involves new knowledge regarding the developmental trajectories of CYP3A4 and CYP3A7. CYP3A4 expression and activity is characterized by a large degree of inter-individual variability, and genetic variation generally is not regarded to be clinically significant source of the observed variability [121]. As apparent from the ontogeny functions described above, expression of hepatic CYP3A4 protein follows the Class III DME pattern [122, 123], and ontogeny may represent a significant source of inter-individual variability in the clearance of compounds administered in the early postnatal period, such as midazolam, sildenafil and fentanyl. In contrast, CYP3A7, exhibits a Class I DME developmental trajectory with predominantly fetal expression that declines rapidly to very low or absent levels shortly after birth in most individuals, exceptions being individuals who possess the CYP3A7*1C allele, which has been associated with persistence of CYP3A7 protein expression in adulthood [124]. Although CYP3A7 shares a similar substrate profile with CYP3A4 and CYP3A5, it is generally less active towards prototypic CYP3A substrates such as midazolam [125]. Due to relatively high abundance, however, its role in the clearance of CYP3A substrates, especially in the most premature newborns, may be underappreciated. Transcription factors regulating CYP3A4 developmental expression, such as the pregnane X-receptor (PXR) and constitutive androstane receptor (CAR) also appear to be developmentally regulated [126], more recent data implicate age-dependent changes in cytosine methylation of the CYP3A7 and CYP3A4 promoter regions as a mechanism for the “developmental switch” that occurs at birth. Increasing methylation of critical cytosine residues in CYP3A7 promoter region is associated with decreased transcription and silencing of CYP3A7 and the opposite scenario is true for CYP3A4 [127].
While CYPs are recognized as the most broadly important DMEs, around 25% of metabolically eliminated drugs are subject primarily to non-CYP mediated biotransformation [103]. Notable non-CYP Phase I DMEs include carboxylesterases (CESs), flavin-containing monooxygenases (FMOs), dehydrogenases and aldehyde oxidase, and data describing the developmental trajectories of these pathways have been published in recent years.
Both CES1 and CES2 are expressed in the liver, with CES1 being the more abundantly expressed isoform. In two separate studies, each relying on a different method of protein quantitation (immunoblotting and mass spectrometry), expression of both CES1 and CES2 were observed to be considerably lower in liver tissue isolated from neonates relative to older subjects [128, 129]. While the absolute expression of CES1 and CES2 varied between the two studies, the developmental trajectories were similar. Supporting these findings was an in vitro study that observed oseltamivir, a CES1 substrate, was hydrolyzed by S9 fractions of neonatal liver tissue with less efficiency than S9 fractions of tissue from older infants [130]. Further refinement of our understanding in CES ontogeny throughout childhood may provide particular benefit in the dosage selection for the CES1 substrate methylphenidate in attention deficit disorders [131].
Biotransformation of another common attention deficit disorder medication, amphetamine, is partially mediated by another non-CYP DME, FMO3 [132]. The FMO3 isoform is largely absent in the fetal liver; expression increases after birth and is detectable in most children by age 2, with adult levels achieved around age 11 [133]. The postnatal ontogeny of FMO3 has been described using a sigmoid Emax model [134]; parameter estimates are provided in Table 1. Somewhat analogous to the switching that occurs between CYP3A4 and CYP3A7 following birth, another FMO isoform, FMO1, is expressed in the fetus, declines soon after birth, and is generally undetectable in the adult liver [133].
Other Phase I enzyme families are of generally less importance in drug metabolism to date, but developmental trajectories for some pathways have been characterized to an extent. Relative aldehyde oxidase (AO) activity in children has been estimated from the urinary ratio of pyridine to 1-methylnicotinamide, and is reported to be only 10 to 15% of adult activity in neonates. Adult levels of activity have been observed by one year of age [135]. A small investigation into protein expression of AO, applying immunoblotting methods to liver tissue, yielded findings that are generally consistent with the in vivo activity data [136]. Using targeted proteomic approaches, protein abundance data for alcohol and aldehyde dehydrogenases has also been characterized. Protein abundances in the liver increase from the neonatal period into early childhood for ADH1A, ADH1B, ADH1C, and ALDH1A1 by 3-, 8-, 146-, and 3-fold, respectively. Interestingly, expression of ADH1A expression then declines approximately 40% from early childhood levels by adulthood [137]. Ontogeny functions are presented in Table 1.
Encouraged by recent advances in targeted proteomics and a renewed appreciation for their role in the disposition of drugs, more efforts have been directed towards characterizing the ontogeny of Phase II DMEs. Phase II biotransformation activities are mediated by uridine 5’-diphospho-glucuronosyltransferases (UGTs), sulfotransferases (SULTs), glutathione S-transferases (GSTs) and N-acetyltransferases (NATs). Each of these enzyme families is known to have multiple isoforms with unique developmental trajectories. Ontogeny for key Phase II enzymes in the liver can also be found in Table 1.
Perhaps most well described of these enzymes in the pediatric population is UGT1A1, which is involved in the metabolism of both exogenous (e.g., acetaminophen, ibuprofen, warfarin) and endogenous compounds (e.g., bilirubin). According to a recent targeted proteomic analysis, protein abundance of UGT1A1 is low at birth but reaches adult levels in early childhood, effectively resulting in approximate 8-fold increase in protein expression [99]. This finding is in general agreement with observations that UGT1A1 activity is lower in the newborn liver but increases quickly with adult levels achieved in early childhood [106, 138]. However, it is notable that one study did not observe age-related changes in the expression of UGT1A1, nor for the closely related UGT1A6 isoform, when immunoblotting methods were used to determine protein abundance [138]. Other UGT1A isoforms were measured by Bhatt et al. using targeted proteomic methods; UGT1A4, 1A6 and 1A9 protein expression was respectively 55-, 35- and 33-fold greater in adult liver tissue compared to that of neonates and enzymatic activity generally followed the protein expression pattern [99].
Involved in the metabolism of morphine, naloxone and mycophenolic acid, UGT2B7 also demonstrates differential expression and activity with age. Protein expression gradually, but substantially (~8-fold), increases from the neonatal period into adulthood [99]. A similar developmental trajectory was observed for UGT2B15 with protein expression increasing by 3-fold from the neonatal period to adulthood [99]. A separate targeted proteomic study by Bhatt et al. observed limited UGT2B17 protein expression in children younger than 9 years old, with approximately 10-fold increase in expression from 9 years of age to adulthood [139]. It is notable that while Bhatt et al. observed generally concordant trends between protein expression of the investigated UGT isoforms and enzymatic activity, the age-related activity trends observed by Badée et al. using different probe substrates frequently do not align with the targeted proteomic data from the other studies. Badée et al. also investigated the ontogeny of enzyme activity for two UGT isoforms, UGT2B4 and UGT2B10, that were not evaluated in the Bhatt et al. targeted proteomic studies; these two enzymes appeared to have a moderately (up to 2-fold) greater enzyme activities in early childhood relative to adulthood [106]. Sulfotransferases (SULTs) represent another important enzyme family involved in Phase II metabolism. In addition to their role in xenobiotic metabolism, a number of endogenous molecules are substrates for SULTs and their importance in fetal development has long been recognized [140-142]. Notably, the sulfonation rates for dopamine and estradiol, markers of SULT1A3/4 and SULT1E1 respectively, are greater in fetal relative to adult liver tissue [143]. While SULT1A3 protein expression remains constant from birth to 70 years, expression of SULT1E1 was shown to decline from birth into adulthood. Protein expression of SULT1A1, SULT1B1 and SULT2A1 in liver cytosol samples from neonates was 24, 19, and 38% of the adult levels. Notably, SULT1A1 and SULT2A1 showed highest protein expression in early childhood (between 1 to 6 years of age), followed by a decrease thereafter [144]. The importance of the decrease in SULT1A1 activity and concurrent increase in UGT1A6 expression with increasing age are illustrated by the developmental shift in acetaminophen conjugation wherein the sulfonated metabolite is primarily formed in newborns but the glucuronide metabolite predominates in adults [145]. It is important to note that because SULT1A1 is relatively less efficient at catalyzing its respective reaction, newborns and young infants have a longer acetaminophen half-lives than do older children and adults [145], an observation that now can be captured using contemporary PBPK models that take SULT and UGT ontogeny into consideration [146, 144].
GSTs are Phase II enzymes involved in metabolism of cisplatin, busulfan and endogenous compounds such as leukotrienes and prostaglandins [147]. Targeted proteomic data describing GST ontogeny is limited. Results obtained using immunohistochemistry and radioimmunoassays have revealed hepatic GSTA1 and GSTA2 are detectable as early as 10 weeks gestation and expression levels increase by 1.5 and 4-fold respectively to adult levels by early childhood [148, 149]. While GSTM expression is low during gestation, it rapidly increases (by approximately 5-fold) after birth, and hepatic levels of GSTM in neonates and infants approximate that of adults. On the other hand, GSTP1 expression is highest in fetal liver samples (10-22 weeks gestational age), followed by a reduction in expression during the second and third trimesters [148, 149]. While neonates continue to express up to 4-5 fold less GSTP1 protein relative to fetus, this protein becomes absent in the adult liver [148, 149].
Renal and Biliary Excretion
The kidneys are the primary organs through which xeno- and endobiotic excretion occurs. Three major processes dictate clearance of drugs via the kidney: passive filtration through the glomerulus, secretion (passive and active) in the proximal tubule, and/or reabsorption (passive and active) in the proximal or distal tubule. Although nephron development is complete by 36 weeks of gestation [150], the maturation of the kidneys continues further into childhood. At birth, the kidneys contain the same number of nephrons as in adults (approximately 1,000,000 per kidney); however age-related differences in anatomy, morphology and functionality can readily be observed [151]. Although premature infants have some capacity to form new nephrons after birth, there is concern that individuals born preterm may not ultimately achieve similar amounts of nephrons to individuals born at term [152, 153]. Between birth and 12 years of age, the kidneys almost double in length and correspondingly increase in weight. On a structural level, it can be observed that the average diameter of a glomerulus in a newborn is approximately one-third of the adult. In the first 3 months after birth, the radius of small pores in the glomerulus increases by approximately 25%, with accompanying increase in proportion of large pores relative to small pores [154, 155]. A noticeable development of proximal tubules also occurs; the average length of proximal tubules at birth is approximately 10% of that of an average adult. The variability in the length of proximal tubules is also greater at birth, with over an 11-fold difference between the shortest and longest measured proximal tubule in tissue samples, as compared to the approximately 2-fold variability observed in adults [156].
The age-dependent changes in kidney structure described above are, not surprisingly, accompanied with changes in kidney functioning. Glomerular filtration rate (GFR) increases abruptly after birth and continues to increase until the child’s growth is complete. When standardized to a body weight of 70 kg using an allometric power model with a coefficient of 0.75, the GFR at 1 year of age is predicted to be at 90% of the adult GFR value, which is estimated at 121.2 mL/min [157]. It is important to note that, relative to healthy newborns, premature newborns display significantly reduced GFR that follows its own developmental trajectory [158]. With respect to tubular reabsorption, newborns have much lower urine concentrating ability (600 mOsm/kg water), a value that increases slowly to 900 mOsm/kg water during the first month of life, and reaching 1200 mOsm/kg in adolescence [158]. Renal clearance of drugs is generally reduced in the young, and especially in pre-term infants. For example, fluconazole half-life is over 3-fold longer in premature than in term infants [159]. Similarly, multiple studies over the past 15 years have demonstrated that vancomycin and amikacin clearances are reduced in premature infants and increase with postnatal age; evidence suggests that clearance of these compounds can serve as a proxy for the ontogeny of GFR [160-162].
Age-dependent changes in clearance of p-amino hippurate (PAH), a substrate for renal transport and a prototypical marker of renal plasma flow, have also been well documented. Immediately after birth, plasma clearance of PAH is low and then steadily increases to values comparable to adults around 2 years of age [163, 164]. To date, it is unclear whether this is a result of increasing plasma flow to the kidney, age-dependent changes in the expression of relevant transporters (e.g. basolateral uptake OAT1/3 and apical efflux MRP2/4), or a combination of both factors.
Recently, age-dependent changes in expression of transporters localized on the basolateral and apical membranes of proximal tubule epithelial cells have been characterized in pediatric tissues using targeted proteomics. The expression of basolateral uptake (OAT1, OAT3 and OCT2) and apical efflux (P-gp) transporters were significantly lower in term newborns and infants compared to children, adolescents and adults. A sigmoid Emax model was fit to the relative expression data and provided Age50 values of 20, 31, 4.4, and 4.0 weeks post-natal age for OAT1, OAT3, OCT2 and P-gp respectively [165]. Expression of other apical efflux transporters (MATE1, MATE2-K, BCRP, MRP2 and MRP4) was consistent throughout the investigated age continuum [165]. The importance of understanding and integrating transporter ontogeny into PBPK models for renally eliminated drugs was illustrated in a recent in silico study. Including or excluding maturation trends for transporters involved in active tubular secretion predicted substantially different renal clearance values for several hypothetical drugs assessed, and these differences were most pronounced in children less than 2 years of age [166]. Based on these findings, it should be possible to hypothesize which medications (i.e. substrates of these transporters) may be subject to age-dependent changes in renal clearance and identify suitable candidate drugs for prospective pharmacokinetic studies to evaluate the clinical significance of these maturation trends.
In addition to the kidney, the liver is also capable of mediating the secretion of drug molecules. In general, hepatic excretion of drugs and metabolites into the bile is enabled by some of the same properties that permit drug excretion in the renal proximal tubules. Tight junctions in the biliary canaliculi, countercurrent blood flow, expression of drug transporters and osmotic gradients favor the biliary excretion of drugs and metabolites [167]. Recent investigations using targeted proteomic techniques have offered some clearer insight into the maturation of drug transporters in the hepatic canalicular membrane. According to recent targeted proteomic data, expression of P-gp in neonates, infants, children (1-12 years) and adolescents (>12 to 16 years) were approximately 30, 40, 70 and 75% that of adult values. A sigmoid Emax model was also fit to the protein abundance data (parameter estimates provided in Table 1) and seemed to reasonably describe the developmental trajectory of hepatic P-gp expression. Mean abundances of other canalicular efflux transporters including MRP2, BCRP and MATE1 were not significantly different across the same 4 age groups [82]. A separate study employing targeted proteomics, but extending the analysis to fetal samples, also observed no significant changes in BCRP and P-gp expression from birth to adulthood. However, expression of P-gp was ~2-fold less in fetal livers compared to those of adults, and MRP2 expression was ~3-fold greater in adults than in the fetus or term infants under 18 weeks of age [168].
Bile acids are synthesized in hepatocytes from cholesterol by CYP isoforms and are exported across the canalicular membrane by transporters, most notably the bile salt salt exporter pump (BSEP). Other transporters also play a role in bile acid homeostasis including the apical sodium bile acid transporter (ASBT) and the heteromeric organic solute transporters (OSTs) that are involved with the vectorial reabsorption of bile acids from the ileum and directly from the bile ducts [167, 169]. While these are not typically considered drug transporters per se, their effects on canalicular bile acid concentrations have the potential to impact hepatobiliary secretion of drugs. Two relatively recent proteomics studies demonstrated modest but non-significant trends of progressively increasing BSEP expression after birth [82, 168]. However, one of these studies also evaluated fetal tissue and demonstrated a statistically significant greater abundance of BSEP in the livers from term infants and adults compared to fetal livers [168]. Unfortunately, interpreting the ontogeny of bile acid transporters in terms of quantitative estimations of their impact on pharmacokinetics is challenging given their indirect relationship with drug disposition.
Discussion and Vision for the Future
Knowledge of age-dependent changes in various physiological processes relevant to drug disposition from birth to adulthood, especially developmental trajectories of enzymes and transporters, has greatly expanded over the past 2 decades as summarized in a recent comprehensive review [170]. This new knowledge has led to an increasing number of new applications impacting regulatory and clinical decision-making processes, such as initial dose selection for pediatric clinical trials [171], extrapolation of a model developed for one compound to other compounds metabolized by the same elimination pathway ([172], potential for drug-drug interactions [173, 1], and pediatric regulatory approvals [174]. Particularly interesting are the use of population PK approaches to improve the quality of PBPK models [175] and the use of PBPK modeling and simulation to identify covariates that can be used to improve the quality of population PK models ([176], with further potential application for model-informed precision dosing ([177]. Despite these advances, critical gaps in our understanding remain; several challenges and opportunities are summarized below:
Characterization of developmental trajectories for key scaling factors used in PBPK models. A model has been published characterizing the change in liver volume between birth and 18 years of age, and the pediatric model also predicts liver volume in adults with precision and accuracy that exceeds almost all published adult models [178]. In contrast, as indicated earlier data regarding the developmental trajectory of MPPGL for scaling in vitro intrinsic clearance of unbound drug to hepatic clearance (CLH) are extremely limited, consisting of only include only five samples with ages between 2 and 13 years of age ([179]. Similarly, the CPPGL value used for pediatric extrapolations is derived from adult tissues as no corresponding value for the pediatric age range is currently available. Unpublished data from our group indicate that MPPGL values vary little with increasing postnatal age, but absolute values are dependent on tissue source (flash frozen or perfused versus autopsy tissue; manuscript in preparation).
Developmental trajectories for unknown unknowns. A challenge for the future is to identify additional factors, currently unknown or poorly understood, with the potential to modulate known developmental trajectories of processes governing drug disposition. Examples include accessory proteins and co-factors for DMEs, such as cytochrome P450 oxidoreductase, uridine diphosphate glucuronic acid [180] and enzymes catalyzing the biosynthesis of 3′-phosphoadenosine 5′-phosphosulfate (PAPS), and glycosyltransferases involved in glycosylation and membrane localization of transporters.
Leveraging intensive opportunistic sampling to characterize developmental trajectories of biotransformation pathways at the individual level in vivo. Existing developmental trajectories, especially those derived from in vitro data, are derived from a single time point (age) from and individual sample. Much more valuable information could be gained from multiple samples collected longitudinally in each patient. A brief report describing patterns of indomethacin acylglucuronide formation and O-demethylation in individual patients in a neonatal intensive care setting illustrate the potential for comprehensive urine collections, albeit labor intensive (collection of all diapers up to at least 7 days after the last dose of drug and binned into 12-hour intervals), to provide novel insights into the acquisition of drug biotransformation activity after birth. In this study we found that study participants could be assigned to one of three patterns of cumulative acylglucuronidation/O-demethylation excretion in urine, with a higher percentage of the administered dose being recovered in newborns in whom O-demethylation was the predominant biotransformation pathway [181]. More importantly, these insights would not have been gained if we had limited our collection interval to a more common protocol of 24-hour collections. One would expect that similar intensive sampling using discarded plasma samples and dried blood spots collected with routine clinical blood draws will provide an equally rich dataset for characterization of developmental trajectories in vivo, especially the degree of inter-individual variability in the rate and extent of the trajectories and the impact of systemic exposure and clinical response.
Use of endogenous biomarkers of DME and transporter activity in longitudinal studies and opportunistic sampling protocols further characterize developmental trajectories of important clearance pathways. In a recently published paper, Smits et al summarized available data regarding CYP3A and OATP1B1/3 biomarkers in healthy term and preterm infants [182], and a potential biomarker of CYP2D6 activity has been described in a pediatric study [183]. Although available sample volumes may limit application of newer technologies like exosome-based liquid biopsies ([184] in opportunistic sampling protocols involving extremely young patients, incorporation of biomarker data holds considerable potential for creation of avatars of actual patients and clinical application of a pediatric version of the “virtual twin” approach [185].
Use of disease-/patient population-specific data. Scaling factors, such as liver mass, utilize estimates of height and weight derived from growth curves derived from “normal” developing males and females. Patients with conditions like cerebral palsy, anorexia nervosa, and obesity, among others, may not necessarily demonstrate typical growth trajectories. Preliminary data indicate that growth patterns for children with type 2 diabetes mellitus differ from those presented in Center for Disease Control and Prevention growth charts [186]. The implications for PBPK modeling and simulation remain to be determined.
Developmental trajectories for targets of drug action. The focus of this paper has been the ontogeny of processes governing drug disposition, but knowledge of the developmental trajectories of receptors, neurotransmitter re-uptake pumps, ion channels and other drug targets is critically important as the next step to ensure safe and effective use of medications throughout the age continuum. In the simplest sense, the risk benefit ratio for a medication will be unacceptable if the presumed target mediating the therapeutic response is not expressed at the age/developmental stage that a medication is administered, and further investigation in this area is of critical importance for the future.
Establishing the relationship between systemic drug exposure and therapeutic response as a function of drug target expression. This issue directly follows from the issue above, and in our opinion represents a major opportunity for the next generation of pediatric clinical pharmacology investigators. Analogous to the strategy of administering the same dose of medication or phenotyping probe to a study population and investigating the pharmacogenetic or developmental factors contributing to the observed variability in systemic exposure/clearance or other measure of drug disposition phenotype, a study design to identify genetic and developmental factors contributing a drug/therapeutic response requires administration of the same systemic exposure to the study cohort. In this context, there will be a need for tools to individualize dose and minimize inter-individual variability in systemic exposure so that the confounding effects of exposure on drug response can be reduced. More specifically, relevant signals will be easier to detect if noise related to poor response due simply to low/inadequate systemic exposure can be eliminated. The concept of concentration-controlled clinical trials is not new, having been proposed by Carl Peck and colleagues in the early 1990s [187] and discussed further over the years [188], largely in the context of clinical trials conducted for regulatory purposes; the application we propose is somewhat different – as a means to identify important genetic and developmental factors influencing the exposure-response relationship in pediatrics, and advances in PK modeling made over the past 30 years now lead to considerable optimism for meaningful progress in this area in the foreseeable future.
Future advances should involve collaboration between the academic, regulatory, clinical and industry communities, with the data generated by these endeavors freely accessible in a centralized global repository. Similar databases have been proposed by the FDA and other researchers [189]. Such a repository would facilitate the continual development of ever more refined population PK, PBPK and hybrid models optimally developed and prospectively validated for the specific intended purpose providing a better understanding of the sources of variability in drug exposure throughout childhood with the objective of optimizing treatment for children of all ages.
Funding Information
BDC is supported by the NIH grant [T32 HD069038] “Research Fellowship Program in Pediatric Clinical/Developmental Pharmacology”.
Footnotes
Conflicts of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Lang J, Vincent L, Chenel M, Ogungbenro K, Galetin A Impact of hepatic cyp3a4 ontogeny functions on drug-drug interaction risk in pediatric physiologically-based pharmacokinetic/pharmacodynamic modeling: Critical literature review and ivabradine case study. Clin Pharmacol Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Charifson PS, Walters WP. Acidic and basic drugs in medicinal chemistry: A perspective. J Med Chem. 2014; 57(23):9701–9717. [DOI] [PubMed] [Google Scholar]
- 3.Avery GB, Randolph JG, Weaver T Gastric acidity in the first day of life. Pediatrics. 1966; 37(6):1005–1007. [PubMed] [Google Scholar]
- 4.Sondheimer JM, Clark DA, Gervaise EP. Continuous gastric ph measurement in young and older healthy preterm infants receiving formula and clear liquid feedings. J Pediatr Gastroenterol Nutr. 1985; 4(3):352–355. [DOI] [PubMed] [Google Scholar]
- 5.Kelly EJ, Newell SJ, Brownlee KG, Primrose JN, Dear PR. Gastric acid secretion in preterm infants. Early Hum Dev. 1993; 35(3):215–220. [DOI] [PubMed] [Google Scholar]
- 6.Kelly EJ, Chatfield SL, Brownlee KG, Ng PC, Newell SJ, Dear PR, Primrose JN. The effect of intravenous ranitidine on the intragastric ph of preterm infants receiving dexamethasone. Arch Dis Child. 1993; 69(1 Spec No):37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith LJ, Kaminsky S, D'souza SW. Neonatal fat digestion and lingual lipase. Acta Paediatr Scand. 1986; 75(6):913–918. [DOI] [PubMed] [Google Scholar]
- 8.Omari TI, Davidson GP. Multipoint measurement of intragastric ph in healthy preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003; 88(6):F517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu G, Zheng QS, Li GF. Similarities and differences in gastrointestinal physiology between neonates and adults: A physiologically based pharmacokinetic modeling perspective. Aaps j. 2014; 16(6):1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agunod M, Yamaguchi N, Lopez R, Luhby AL, Glass GB. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am J Dig Dis. 1969; 14(6):400–414. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas JM, Bouzom F, Hugues C, Ungell AL. Oral drug absorption in pediatrics: The intestinal wall, its developmental changes and current tools for predictions. Biopharm Drug Dispos. 2017; 38(3):209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Den Anker JN, Van Lingen RA, Koster M, Heykants J, Sauer PJ. Insufficient ketoconazole concentrations in preterm infants with fungal infections. Eur J Pediatr. 1993; 152(6):538. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi SV, Rodriguez W, Khan M, Polli JE. Considerations for a pediatric biopharmaceutics classification system (bcs): Application to five drugs. AAPS PharmSciTech. 2014; 15(3):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonner JJ, Vajjah P, Abduljalil K, Jamei M, Rostami-Hodjegan A, Tucker GT, Johnson TN. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm Drug Dispos. 2015; 36(4):245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavell B Gastric emptying in infants fed human milk or infant formula. Acta Paediatr Scand. 1981; 70(5):639–641. [PubMed] [Google Scholar]
- 16.Helander HF, Fändriks L Surface area of the digestive tract - revisited. Scand J Gastroenterol. 2014; 49(6):681–689. [DOI] [PubMed] [Google Scholar]
- 17.Lebenthal A, Lebenthal E The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. 1999; 23(5 Suppl):S3–6. [DOI] [PubMed] [Google Scholar]
- 18.Moxey PC, Trier JS. Development of villus absorptive cells in the human fetal small intestine: A morphological and morphometric study. Anat Rec. 1979; 195(3):463–482. [DOI] [PubMed] [Google Scholar]
- 19.Cummins AG, Catto-Smith AG, Cameron DJ, Couper RT, Davidson GP, Day AS, Hammond PD, Moore DJ, Thompson FM. Crypt fission peaks early during infancy and crypt hyperplasia broadly peaks during infancy and childhood in the small intestine of humans. J Pediatr Gastroenterol Nutr. 2008; 47(2):153–157. [DOI] [PubMed] [Google Scholar]
- 20.Thompson FM, Catto-Smith AG, Moore D, Davidson G, Cummins AG. Epithelial growth of the small intestine in human infants. J Pediatr Gastroenterol Nutr. 1998; 26(5):506–512. [DOI] [PubMed] [Google Scholar]
- 21.Weaver LT, Austin S, Cole TJ. Small intestinal length: A factor essential for gut adaptation. Gut. 1991; 32(11):1321–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinussen M, Brubakk AM, Linker DT, Vik T, Yao AC. Mesenteric blood flow velocity and its relation to circulatory adaptation during the first week of life in healthy term infants. Pediatr Res. 1994; 36(3):334–339. [DOI] [PubMed] [Google Scholar]
- 23.Martinussen M, Brubakk AM, Vik T, Yao AC. Mesenteric blood flow velocity and its relation to transitional circulatory adaptation in appropriate for gestational age preterm infants. Pediatr Res. 1996; 39(2):275–280. [DOI] [PubMed] [Google Scholar]
- 24.Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH, Rasmussen SN. Measurement of gastrointestinal ph and regional transit times in normal children. J Pediatr Gastroenterol Nutr. 1990; 11(2):211–214. [DOI] [PubMed] [Google Scholar]
- 25.Barbero GJ, Runge G, Fischer D, Crawford MN, Torres FE, Gyorgy P Investigations on the bacterial flora, ph, and sugar content in the intestinal tract of infants. J Pediatr. 1952; 40(2):152–163. [DOI] [PubMed] [Google Scholar]
- 26.Kearns GL, Robinson PK, Wilson JT, Wilson-Costello D, Knight GR, Ward RM, Van Den Anker JN. Cisapride disposition in neonates and infants: In vivo reflection of cytochrome p450 3a4 ontogeny. Clin Pharmacol Ther. 2003; 74(4):312–325. [DOI] [PubMed] [Google Scholar]
- 27.Bodé S, Dreyer M, Greisen G Gastric emptying and small intestinal transit time in preterm infants: A scintigraphic method. J Pediatr Gastroenterol Nutr. 2004; 39(4):378–382. [DOI] [PubMed] [Google Scholar]
- 28.Vreugdenhil G, Sinaasappel M, Bouquet J A comparative study of the mouth to caecum transit time in children and adults using a weight adapted lactulose dose. Acta Paediatr Scand. 1986; 75(3):483–488. [DOI] [PubMed] [Google Scholar]
- 29.Valentin J Basic anatomical and physiological data for use in radiological protection: Reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. Icrp publication 89. Ann ICRP. 2002; 32(3-4):5–265. [PubMed] [Google Scholar]
- 30.Maharaj AR, Edginton AN. Examining small intestinal transit time as a function of age: Is there evidence to support age-dependent differences among children? Drug Metab Dispos. 2016; 44(7):1080–1089. [DOI] [PubMed] [Google Scholar]
- 31.Murphy GM, Signer E Bile acid metabolism in infants and children. Gut. 1974; 15(2):151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suchy FJ, Balistreri WF, Heubi JE, Searcy JE, Levin RS. Physiologic cholestasis: Elevation of the primary serum bile acid concentrations in normal infants. Gastroenterology. 1981; 80(5 pt 1):1037–1041. [PubMed] [Google Scholar]
- 33.Challacombe DN, Edkins S, Brown GA. Duodenal bile acids in infancy. Arch Dis Child. 1975; 50(11):837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavy US,M; Davidson M;. Role of bile acids in fat absorption in low birth weights infants. Pediatr Res. 1971; 5387. [Google Scholar]
- 35.Kearns GL, Bradley JS, Jacobs RF, Capparelli EV, James LP, Johnson KM, Abdel-Rahman SM. Single dose pharmacokinetics of pleconaril in neonates. Pediatric pharmacology research unit network. Pediatr Infect Dis J. 2000; 19(9):833–839. [DOI] [PubMed] [Google Scholar]
- 36.Forsyth JS, Donnet L, Ross PE. A study of the relationship between bile salts, bile salt-stimulated lipase, and free fatty acids in breast milk: Normal infants and those with breast milk jaundice. J Pediatr Gastroenterol Nutr. 1990; 11(2):205–210. [DOI] [PubMed] [Google Scholar]
- 37.Forsyth JS, Ross PE, Bouchier IA. Bile salts in breast milk. Eur J Pediatr. 1983; 140(2):126–127. [DOI] [PubMed] [Google Scholar]
- 38.Kimura A, Mahara R, Inoue T, Nomura Y, Murai T, Kurosawa T, Tohma M, Noguchi K, Hoshiyama A, Fujisawa T, Kato H Profile of urinary bile acids in infants and children: Developmental pattern of excretion of unsaturated ketonic bile acids and 7beta-hydroxylated bile acids. Pediatr Res. 1999; 45(4 Pt 1):603–609. [DOI] [PubMed] [Google Scholar]
- 39.Nishiura H, Kimura A, Yamato Y, Aoki K, Inokuchi T, Kurosawa T, Matsuishi T Developmental pattern of urinary bile acid profile in preterm infants. Pediatr Int. 2010; 52(1):44–50. [DOI] [PubMed] [Google Scholar]
- 40.Klotz U Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid. Clin Pharmacokinet. 1985; 10(4):285–302. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, Crnkovic CM, Won KJ, Yang X, Lee JR, Orjala J, Lee H, Jeong H Commensal gut bacteria convert the immunosuppressant tacrolimus to less potent metabolites. Drug Metab Dispos. 2019; 47(3):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linday L, Dobkin JF, Wang TC, Butler VP Jr., Saha JR, Lindenbaum J Digoxin inactivation by the gut flora in infancy and childhood. Pediatrics. 1987; 79(4):544–548. [PubMed] [Google Scholar]
- 43.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019; 570(7762):462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Javdan B, Lopez JG, Chankhamjon P, Lee YJ, Hull R, Wu Q, Wang X, Chatterjee S, Donia MS. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020; 181(7):1661–1679.e1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarmusch AK, Vrbanac A, Momper JD, Ma JD, Alhaja M, Liyanage M, Knight R, Dorrestein PC, Tsunoda SM. Enhanced characterization of drug metabolism and the influence of the intestinal microbiome: A pharmacokinetic, microbiome, and untargeted metabolomics study. Clin Transl Sci. 2020; 13(5):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann-Kogadeeva M, Zimmermann M, Goodman AL. Insights from pharmacokinetic models of host-microbiome drug metabolism. Gut Microbes. 2020; 11(3):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couto N, Al-Majdoub ZM, Gibson S, Davies PJ, Achour B Quantitative proteomics of clinically relevant drug-metabolizing enzymes and drug transporters and their intercorrelations in the human small intestine. 2020; 48(4):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome p450 "pie". Drug Metab Dispos. 2006; 34(5):880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson TN, Tanner MS, Taylor CJ, Tucker GT. Enterocytic cyp3a4 in a paediatric population: Developmental changes and the effect of coeliac disease and cystic fibrosis. Br J Clin Pharmacol. 2001; 51(5):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen YT, Trzoss L, Yang D, Yan B Ontogenic expression of human carboxylesterase-2 and cytochrome p450 3a4 in liver and duodenum: Postnatal surge and organ-dependent regulation. Toxicology. 2015; 33055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fakhoury M, Litalien C, Medard Y, Cavé H, Ezzahir N, Peuchmaur M, Jacqz-Aigrain E Localization and mrna expression of cyp3a and p-glycoprotein in human duodenum as a function of age. Drug Metab Dispos. 2005; 33(11):1603–1607. [DOI] [PubMed] [Google Scholar]
- 52.Brussee JM, Yu H, Krekels EHJ. First-pass cyp3a-mediated metabolism of midazolam in the gut wall and liver in preterm neonates. 2018; 7(6):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ståhlberg MR, Hietanen E, Mäki M Mucosal biotransformation rates in the small intestine of children. Gut. 1988; 29(8):1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT. Up-regulation of glutathione s-transferase activity in enterocytes of young children. Drug Metab Dispos. 1999; 27(12):1466–1469. [PubMed] [Google Scholar]
- 55.Lodish HB,A; Zipursky Sl;. Molecular cell biology. New York: W.H.Freeman; 2000. [Google Scholar]
- 56.Hardikar W, Ananthanarayanan M, Suchy FJ. Differential ontogenic regulation of basolateral and canalicular bile acid transport proteins in rat liver. J Biol Chem. 1995; 270(35):20841–20846. [DOI] [PubMed] [Google Scholar]
- 57.Konieczna A, Erdösová B, Lichnovská R, Jandl M, Cížková K, Ehrmann J Differential expression of abc transporters (mdr1, mrp1, bcrp) in developing human embryos. J Mol Histol. 2011; 42(6):567–574. [DOI] [PubMed] [Google Scholar]
- 58.Mizuno T, Fukuda T, Masuda S, Uemoto S, Matsubara K, Inui K, Vinks AA. Developmental trajectory of intestinal mdr1/abcb1 mrna expression in children. Br J Clin Pharmacol. 2014; 77(5):910–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mooij MG, Schwarz UI, De Koning BA, Leeder JS, Gaedigk R, Samsom JN, Spaans E, Van Goudoever JB, Tibboel D, Kim RB, De Wildt SN. Ontogeny of human hepatic and intestinal transporter gene expression during childhood: Age matters. Drug Metab Dispos. 2014; 42(8):1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson TN, Thomson M Intestinal metabolism and transport of drugs in children: The effects of age and disease. J Pediatr Gastroenterol Nutr. 2008; 47(1):3–10. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan JE, Keefe D, Zhou Y, Satlin L, Fang H, Yan JH. Pharmacokinetics, safety profile, and efficacy of aliskiren in pediatric patients with hypertension. Clin Pediatr (Phila). 2013; 52(7):599–607. [DOI] [PubMed] [Google Scholar]
- 62.Sonnier M, Cresteil T Delayed ontogenesis of cyp1a2 in the human liver. Eur J Biochem. 1998; 251(3):893–898. [DOI] [PubMed] [Google Scholar]
- 63.Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, Meibohm B, Nigam SK, Rieder M, De Wildt SN. Human ontogeny of drug transporters: Review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther. 2015; 98(3):266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keiser M, Kaltheuner L, Wildberg C, Müller J, Grube M, Partecke LI, Heidecke CD, Oswald S The organic anion-transporting peptide 2b1 is localized in the basolateral membrane of the human jejunum and caco-2 monolayers. J Pharm Sci. 2017; 106(9):2657–2663. [DOI] [PubMed] [Google Scholar]
- 65.Mooij MG, De Koning BE, Lindenbergh-Kortleve DJ, Simons-Oosterhuis Y, Van Groen BD, Tibboel D, Samsom JN, De Wildt SN. Human intestinal pept1 transporter expression and localization in preterm and term infants. Drug Metab Dispos. 2016; 44(7):1014–1019. [DOI] [PubMed] [Google Scholar]
- 66.Groneberg DA, Döring F, Eynott PR, Fischer A, Daniel H Intestinal peptide transport: Ex vivo uptake studies and localization of peptide carrier pept1. Am J Physiol Gastrointest Liver Physiol. 2001; 281(3):G697–704. [DOI] [PubMed] [Google Scholar]
- 67.Cheung KWK, Van Groen BD, Burckart GJ, Zhang L, De Wildt SN, Huang SM. Incorporating ontogeny in physiologically based pharmacokinetic modeling to improve pediatric drug development: What we know about developmental changes in membrane transporters. J Clin Pharmacol. 2019; 59 Suppl 1S56–s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson TN, Bonner JJ, Tucker GT, Turner DB, Jamei M Development and applications of a physiologically-based model of paediatric oral drug absorption. Eur J Pharm Sci. 2018; 11557–67. [DOI] [PubMed] [Google Scholar]
- 69.Kearns GL, Abdel-Rahman SM, Blumer JL, Reed MD, James LP, Jacobs RF, Bradley JA, Welshman IR, Jungbluth GL, Stalker DJ. Single dose pharmacokinetics of linezolid in infants and children. Pediatr Infect Dis J. 2000; 19(12):1178–1184. [DOI] [PubMed] [Google Scholar]
- 70.Siber GR, Echeverria P, Smith AL, Paisley JW, Smith DH. Pharmacokinetics of gentamicin in children and adults. J Infect Dis. 1975; 132(6):637–651. [DOI] [PubMed] [Google Scholar]
- 71.Moore RD, Smith CR, Lietman PS. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984; 149(3):443–448. [DOI] [PubMed] [Google Scholar]
- 72.Van Donge T, Pfister M, Bielicki J, Csajka C, Rodieux F, Van Den Anker J, Fuchs A Quantitative analysis of gentamicin exposure in neonates and infants calls into question its current dosing recommendations. Antimicrob Agents Chemother. 2018; 62(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brodersen R, Robertson A Ceftriaxone binding to human serum albumin: Competition with bilirubin. Mol Pharmacol. 1989; 36(3):478–483. [PubMed] [Google Scholar]
- 74.Kanakoudi F, Drossou V, Tzimouli V, Diamanti E, Konstantinidis T, Germenis A, Kremenopoulos G Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin Chem. 1995; 41(4):605–608. [PubMed] [Google Scholar]
- 75.Kingston HG, Kendrick A, Sommer KM, Olsen GD, Downes H Binding of thiopental in neonatal serum. Anesthesiology. 1990; 72(3):428–431. [DOI] [PubMed] [Google Scholar]
- 76.Meistelman C, Benhamou D, Barre J, Levron JC, Mahe V, Mazoit X, Ecoffey C Effects of age on plasma protein binding of sufentanil. Anesthesiology. 1990; 72(3):470–473. [DOI] [PubMed] [Google Scholar]
- 77.Sethi PK, White CA, Cummings BS, Hines RN. Ontogeny of plasma proteins, albumin and binding of diazepam, cyclosporine, and deltamethrin. 2016; 79(3):409–415. [DOI] [PubMed] [Google Scholar]
- 78.Matoth Y, Zaizov R, Varsano I Postnatal changes in some red cell parameters. Acta Paediatr Scand. 1971; 60(3):317–323. [DOI] [PubMed] [Google Scholar]
- 79.Proytcheva MA. Issues in neonatal cellular analysis. Am J Clin Pathol. 2009; 131(4):560–573. [DOI] [PubMed] [Google Scholar]
- 80.Grover A, Benet LZ. Effects of drug transporters on volume of distribution. Aaps j. 2009; 11(2):250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hahn D, Emoto C, Vinks AA, Fukuda T Developmental changes in hepatic organic cation transporter oct1 protein expression from neonates to children. Drug Metab Dispos. 2017; 45(1):23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prasad B, Gaedigk A, Vrana M, Gaedigk R, Leeder JS, Salphati L, Chu X, Xiao G, Hop C, Evers R, Gan L, Unadkat JD. Ontogeny of hepatic drug transporters as quantified by lc-ms/ms proteomics. Clin Pharmacol Ther. 2016; 100(4):362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomson MM, Hines RN, Schuetz EG, Meibohm B Expression patterns of organic anion transporting polypeptides 1b1 and 1b3 protein in human pediatric liver. Drug Metab Dispos. 2016; 44(7):999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fukuda T, Chidambaran V, Mizuno T, Venkatasubramanian R, Ngamprasertwong P, Olbrecht V, Esslinger HR, Vinks AA, Sadhasivam S Oct1 genetic variants influence the pharmacokinetics of morphine in children. Pharmacogenomics. 2013; 14(10):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagner JB, Abdel-Rahman S, Van Haandel L, Gaedigk A, Gaedigk R, Raghuveer G, Kauffman R, Leeder JS. Impact of slco1b1 genotype on pediatric simvastatin acid pharmacokinetics. J Clin Pharmacol. 2018; 58(6):823–833. [DOI] [PubMed] [Google Scholar]
- 86.Wagner JB, Abdel-Rahman S, Gaedigk R, Gaedigk A, Raghuveer G, Staggs VS, Kauffman R, Van Haandel L, Leeder JS. Impact of genetic variation on pravastatin systemic exposure in pediatric hypercholesterolemia. Clin Pharmacol Ther. 2019; 105(6):1501–1512. [DOI] [PubMed] [Google Scholar]
- 87.Wagner JB, Abdel-Rahman S, Gaedigk A, Gaedigk R, Raghuveer G, Staggs VS, Van Haandel L, Leeder JS. Impact of slco1b1 genetic variation on rosuvastatin systemic exposure in pediatric hypercholesterolemia. Clin Transl Sci. 2020; 13(3):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao C, Liao MZ, Han LW, Thummel KE, Mao Q Hepatic transport of 25-hydroxyvitamin d(3) conjugates: A mechanism of 25-hydroxyvitamin d(3) delivery to the intestinal tract. Drug Metab Dispos. 2018; 46(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki H, Sawada Y, Sugiyama Y, Iga T, Hanano M, Spector R Transport of imipenem, a novel carbapenem antibiotic, in the rat central nervous system. J Pharmacol Exp Ther. 1989; 250(3):979–984. [PubMed] [Google Scholar]
- 90.Jacobs RF, Kearns GL, Brown AL, Longee DC. Cerebrospinal fluid penetration of imipenem and cilastatin (primaxin) in children with central nervous system infections. Antimicrob Agents Chemother. 1986; 29(4):670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nicolas JM, De Lange ECM. Mind the gaps: Ontogeny of human brain p-gp and its impact on drug toxicity. Aaps j. 2019; 21(4):67. [DOI] [PubMed] [Google Scholar]
- 92.Roberts EG, Shulkin BL. Technical issues in performing pet studies in pediatric patients. J Nucl Med Technol. 2004; 32(1):5–9; quiz 10-11. [PubMed] [Google Scholar]
- 93.Verscheijden LFM, Van Hattem AC, Pertijs J, De Jongh CA, Verdijk RM. Developmental patterns in human blood-brain barrier and blood-cerebrospinal fluid barrier abc drug transporter expression. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. Abc transporter (p-gp/abcb1, mrp1/abcc1, bcrp/abcg2) expression in the developing human cns. Neuropediatrics. 2008; 39(4):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lam J, Baello S, Iqbal M, Kelly LE, Shannon PT, Chitayat D, Matthews SG, Koren G The ontogeny of p-glycoprotein in the developing human blood-brain barrier: Implication for opioid toxicity in neonates. Pediatr Res. 2015; 78(4):417–421. [DOI] [PubMed] [Google Scholar]
- 96.Bouwmeester NJ, Van Den Anker JN, Hop WC, Anand KJ, Tibboel D Age- and therapy-related effects on morphine requirements and plasma concentrations of morphine and its metabolites in postoperative infants. Br J Anaesth. 2003; 90(5):642–652. [DOI] [PubMed] [Google Scholar]
- 97.Koren G, Butt W, Chinyanga H, Soldin S, Tan YK, Pape K Postoperative morphine infusion in newborn infants: Assessment of disposition characteristics and safety. J Pediatr. 1985; 107(6):963–967. [DOI] [PubMed] [Google Scholar]
- 98.Way WL, Costley EC, Leongway E Respiratory sensitivity of the newborn infant to meperidine and morphine. Clin Pharmacol Ther. 1965; 6454–461. [DOI] [PubMed] [Google Scholar]
- 99.Bhatt DK, Mehrotra A, Gaedigk A, Chapa R, Basit A, Zhang H, Choudhari P, Boberg M, Pearce RE, Gaedigk R, Broeckel U, Leeder JS, Prasad B Age- and genotype-dependent variability in the protein abundance and activity of six major uridine diphosphate-glucuronosyltransferases in human liver. Clin Pharmacol Ther. 2019; 105(1):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hahn D, Emoto C, Euteneuer JC, Mizuno T, Vinks AA, Fukuda T Influence of oct1 ontogeny and genetic variation on morphine disposition in critically ill neonates: Lessons from pbpk modeling and clinical study. Clin Pharmacol Ther. 2019; 105(3):761–768. [DOI] [PubMed] [Google Scholar]
- 101.Emoto C, Johnson TN, Neuhoff S, Hahn D, Vinks AA, Fukuda T Pbpk model of morphine incorporating developmental changes in hepatic oct1 and ugt2b7 proteins to explain the variability in clearances in neonates and small infants. CPT Pharmacometrics Syst Pharmacol. 2018; 7(7):464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krekels EH, Tibboel D, De Wildt SN, Ceelie I, Dahan A, Van Dijk M, Danhof M, Knibbe CA. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet. 2014; 53(6):553–563. [DOI] [PubMed] [Google Scholar]
- 103.Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov. 2005; 4(10):825–833. [DOI] [PubMed] [Google Scholar]
- 104.Hines RN. Developmental expression of drug metabolizing enzymes: Impact on disposition in neonates and young children. Int J Pharm. 2013; 452(1-2):3–7. [DOI] [PubMed] [Google Scholar]
- 105.Allegaert K, Van Den Anker J Ontogeny of phase i metabolism of drugs. J Clin Pharmacol. 2019; 59 Suppl 1S33–s41. [DOI] [PubMed] [Google Scholar]
- 106.Badée J, Qiu N, Collier AC, Takahashi RH, Forrest WF, Parrott N, Schmidt S, Fowler S Characterization of the ontogeny of hepatic udp-glucuronosyltransferase enzymes based on glucuronidation activity measured in human liver microsomes. J Clin Pharmacol. 2019; 59 Suppl 1S42–s55. [DOI] [PubMed] [Google Scholar]
- 107.Salem F, Johnson TN, Abduljalil K, Tucker GT, Rostami-Hodjegan A A re-evaluation and validation of ontogeny functions for cytochrome p450 1a2 and 3a4 based on in vivo data. Clin Pharmacokinet. 2014; 53(7):625–636. [DOI] [PubMed] [Google Scholar]
- 108.Salem F, Johnson TN, Barter ZE, Leeder JS, Rostami-Hodjegan A Age related changes in fractional elimination pathways for drugs: Assessing the impact of variable ontogeny on metabolic drug-drug interactions. J Clin Pharmacol. 2013; 53(8):857–865. [DOI] [PubMed] [Google Scholar]
- 109.Upreti VV, Wahlstrom JL. Meta-analysis of hepatic cytochrome p450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2016; 56(3):266–283. [DOI] [PubMed] [Google Scholar]
- 110.Riddick DS, Ding X, Wolf CR, Porter TD, Pandey AV, Zhang QY, Gu J, Finn RD, Ronseaux S, Mclaughlin LA, Henderson CJ, Zou L, Flück CE. Nadph-cytochrome p450 oxidoreductase: Roles in physiology, pharmacology, and toxicology. Drug Metab Dispos. 2013; 41(1):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Le Guennec JC, Billon B Delay in caffeine elimination in breast-fed infants. Pediatrics. 1987; 79(2):264–268. [PubMed] [Google Scholar]
- 112.Blake MJ, Abdel-Rahman SM, Pearce RE, Leeder JS, Kearns GL. Effect of diet on the development of drug metabolism by cytochrome p-450 enzymes in healthy infants. Pediatr Res. 2006; 60(6):717–723. [DOI] [PubMed] [Google Scholar]
- 113.Pearce RE, Gaedigk R, Twist GP, Dai H, Riffel AK, Leeder JS, Gaedigk A Developmental expression of cyp2b6: A comprehensive analysis of mrna expression, protein content and bupropion hydroxylase activity and the impact of genetic variation. Drug Metab Dispos. 2016; 44(7):948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E Human hepatic cyp2b6 developmental expression: The impact of age and genotype. Biochem Pharmacol. 2009; 78(2):184–190. [DOI] [PubMed] [Google Scholar]
- 115.Salem AH, Fletcher CV, Brundage RC. Pharmacometric characterization of efavirenz developmental pharmacokinetics and pharmacogenetics in hiv-infected children. Antimicrob Agents Chemother. 2014; 58(1):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allegaert K, Van Den Anker JN, Verbesselt R, De Hoon J, Vanhole C, Tibboel D, Devlieger H O-demethylation of tramadol in the first months of life. Eur J Clin Pharmacol. 2005; 61(11):837–842. [DOI] [PubMed] [Google Scholar]
- 117.Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, James LP, Wilson JT, Kearns GL, Leeder JS. Ontogeny of dextromethorphan o- and n-demethylation in the first year of life. Clin Pharmacol Ther. 2007; 81(4):510–516. [DOI] [PubMed] [Google Scholar]
- 118.Allegaert K, Van Schaik RH, Vermeersch S, Verbesselt R, Cossey V, Vanhole C, Van Fessem M, De Hoon J, Van Den Anker JN. Postmenstrual age and cyp2d6 polymorphisms determine tramadol o-demethylation in critically ill neonates and infants. Pediatr Res. 2008; 63(6):674–679. [DOI] [PubMed] [Google Scholar]
- 119.Allegaert K, Rochette A, Veyckemans F Developmental pharmacology of tramadol during infancy: Ontogeny, pharmacogenetics and elimination clearance. Paediatr Anaesth. 2011; 21(3):266–273. [DOI] [PubMed] [Google Scholar]
- 120.T'jollyn H, Snoeys J, Vermeulen A, Michelet R, Cuyckens F, Mannens G, Van Peer A, Annaert P, Allegaert K, Van Bocxlaer J, Boussery K Physiologically based pharmacokinetic predictions of tramadol exposure throughout pediatric life: An analysis of the different clearance contributors with emphasis on cyp2d6 maturation. Aaps j. 2015; 17(6):1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klein K, Zanger UM. Pharmacogenomics of cytochrome p450 3a4: Recent progress toward the "missing heritability" problem. Front Genet. 2013; 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sadler NC, Nandhikonda P, Webb-Robertson BJ, Ansong C, Anderson LN, Smith JN, Corley RA, Wright AT. Hepatic cytochrome p450 activity, abundance, and expression throughout human development. Drug Metab Dispos. 2016; 44(7):984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ. Developmental expression of the major human hepatic cyp3a enzymes. J Pharmacol Exp Ther. 2003; 307(2):573–582. [DOI] [PubMed] [Google Scholar]
- 124.Sim SC, Edwards RJ, Boobis AR, Ingelman-Sundberg M Cyp3a7 protein expression is high in a fraction of adult human livers and partially associated with the cyp3a7*1c allele. Pharmacogenet Genomics. 2005; 15(9):625–631. [DOI] [PubMed] [Google Scholar]
- 125.Williams JA, Ring BJ, Cantrell VE, Jones DR, Eckstein J, Ruterbories K, Hamman MA, Hall SD, Wrighton SA. Comparative metabolic capabilities of cyp3a4, cyp3a5, and cyp3a7. Drug Metab Dispos. 2002; 30(8):883–891. [DOI] [PubMed] [Google Scholar]
- 126.Vyhlidal CA, Gaedigk R, Leeder JS. Nuclear receptor expression in fetal and pediatric liver: Correlation with cyp3a expression. Drug Metab Dispos. 2006; 34(1):131–137. [DOI] [PubMed] [Google Scholar]
- 127.Vyhlidal CA, Bi C, Ye SQ, Leeder JS. Dynamics of cytosine methylation in the proximal promoters of cyp3a4 and cyp3a7 in pediatric and prenatal livers. Drug Metab Dispos. 2016; 44(7):1020–1026. [DOI] [PubMed] [Google Scholar]
- 128.Boberg M, Vrana M, Mehrotra A, Pearce RE, Gaedigk A, Bhatt DK, Leeder JS, Prasad B Age-dependent absolute abundance of hepatic carboxylesterases (ces1 and ces2) by lc-ms/ms proteomics: Application to pbpk modeling of oseltamivir in vivo pharmacokinetics in infants. Drug Metab Dispos. 2017; 45(2):216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hines RN, Simpson PM, Mccarver DG. Age-dependent human hepatic carboxylesterase 1 (ces1) and carboxylesterase 2 (ces2) postnatal ontogeny. Drug Metab Dispos. 2016; 44(7):959–966. [DOI] [PubMed] [Google Scholar]
- 130.Shi D, Yang D, Prinssen EP, Davies BE, Yan B Surge in expression of carboxylesterase 1 during the post-neonatal stage enables a rapid gain of the capacity to activate the anti-influenza prodrug oseltamivir. J Infect Dis. 2011; 203(7):937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang X, Morris SM, Gearhart JM, Ruark CD, Paule MG, Slikker W Jr., Mattison DR, Vitiello B, Twaddle NC, Doerge DR, Young JF, Fisher JW. Development of a physiologically based model to describe the pharmacokinetics of methylphenidate in juvenile and adult humans and nonhuman primates. PLoS One. 2014; 9(9):e106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cashman JR, Xiong YN, Xu L, Janowsky A N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): Role in bioactivation and detoxication. J Pharmacol Exp Ther. 1999; 288(3):1251–1260. [PubMed] [Google Scholar]
- 133.Koukouritaki SB, Simpson P, Yeung CK, Rettie AE, Hines RN. Human hepatic flavin-containing monooxygenases 1 (fmo1) and 3 (fmo3) developmental expression. Pediatr Res. 2002; 51(2):236–243. [DOI] [PubMed] [Google Scholar]
- 134.Xu M, Bhatt DK, Yeung CK, Claw KG, Chaudhry AS, Gaedigk A, Pearce RE, Broeckel U, Gaedigk R, Nickerson DA, Schuetz E, Rettie AE, Leeder JS, Thummel KE, Prasad B Genetic and nongenetic factors associated with protein abundance of flavin-containing monooxygenase 3 in human liver. J Pharmacol Exp Ther. 2017; 363(2):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tayama Y, Miyake K, Sugihara K, Kitamura S, Kobayashi M, Morita S, Ohta S, Kihira K Developmental changes of aldehyde oxidase activity in young japanese children. Clin Pharmacol Ther. 2007; 81(4):567–572. [DOI] [PubMed] [Google Scholar]
- 136.Tayama Y, Sugihara K, Sanoh S, Miyake K, Kitamura S, Ohta S Developmental changes of aldehyde oxidase activity and protein expression in human liver cytosol. Drug Metab Pharmacokinet. 2012; 27(5):543–547. [DOI] [PubMed] [Google Scholar]
- 137.Bhatt DK, Gaedigk A, Pearce RE, Leeder JS, Prasad B Age-dependent protein abundance of cytosolic alcohol and aldehyde dehydrogenases in human liver. Drug Metab Dispos. 2017; 45(9):1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miyagi SJ, Collier AC. The development of udp-glucuronosyltransferases 1a1 and 1a6 in the pediatric liver. Drug Metab Dispos. 2011; 39(5):912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhatt DK, Basit A, Zhang H, Gaedigk A, Lee SB, Claw KG, Mehrotra A, Chaudhry AS, Pearce RE, Gaedigk R, Broeckel U, Thornton TA, Nickerson DA, Schuetz EG, Amory JK, Leeder JS, Prasad B Hepatic abundance and activity of androgen- and drug-metabolizing enzyme ugt2b17 are associated with genotype, age, and sex. Drug Metab Dispos. 2018; 46(6):888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Richard K, Hume R, Kaptein E, Stanley EL, Visser TJ, Coughtrie MW. Sulfation of thyroid hormone and dopamine during human development: Ontogeny of phenol sulfotransferases and arylsulfatase in liver, lung, and brain. J Clin Endocrinol Metab. 2001; 86(6):2734–2742. [DOI] [PubMed] [Google Scholar]
- 141.Barker EV, Hume R, Hallas A, Coughtrie WH. Dehydroepiandrosterone sulfotransferase in the developing human fetus: Quantitative biochemical and immunological characterization of the hepatic, renal, and adrenal enzymes. Endocrinology. 1994; 134(2):982–989. [DOI] [PubMed] [Google Scholar]
- 142.Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab. 2002; 87(12):5760–5768. [DOI] [PubMed] [Google Scholar]
- 143.Adjei AA, Gaedigk A, Simon SD, Weinshilboum RM, Leeder JS. Interindividual variability in acetaminophen sulfation by human fetal liver: Implications for pharmacogenetic investigations of drug-induced birth defects. Birth Defects Res A Clin Mol Teratol. 2008; 82(3):155–165. [DOI] [PubMed] [Google Scholar]
- 144.Ladumor MK, Bhatt DK, Gaedigk A, Sharma S, Thakur A, Pearce RE, Leeder JS, Bolger MB, Singh S, Prasad B Ontogeny of hepatic sulfotransferases and prediction of age-dependent fractional contribution of sulfation in acetaminophen metabolism. 2019; 47(8):818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levy G, Khanna NN, Soda DM, Tsuzuki O, Stern L Pharmacokinetics of acetaminophen in the human neonate: Formation of acetaminophen glucuronide and sulfate in relation to plasma bilirubin concentration and d-glucaric acid excretion. Pediatrics. 1975; 55(6):818–825. [PubMed] [Google Scholar]
- 146.Jiang XL, Zhao P, Barrett JS, Lesko LJ, Schmidt S Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacometrics Syst Pharmacol. 2013; 2(10):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Allocati N, Masulli M, Di Ilio C, Federici L Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018; 7(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mccarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: Phase ii conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002; 300(2):361–366. [DOI] [PubMed] [Google Scholar]
- 149.Strange RC, Howie AF, Hume R, Matharoo B, Bell J, Hiley C, Jones P, Beckett GJ. The development expression of alpha-, mu- and pi-class glutathione s-transferases in human liver. Biochim Biophys Acta. 1989; 993(2-3):186–190. [DOI] [PubMed] [Google Scholar]
- 150.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, Van Velzen D Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and cavalieri principle. Lab Invest. 1991; 64(6):777–784. [PubMed] [Google Scholar]
- 151.Chen N, Aleksa K, Woodland C, Rieder M, Koren G Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006; 21(2):160–168. [DOI] [PubMed] [Google Scholar]
- 152.Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. 2004; 7(1):17–25. [DOI] [PubMed] [Google Scholar]
- 153.Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, Hoy WE, Bertram JF, Black MJ. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011; 22(7):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spitzer A 1978. Renal physiology and functional development. In: EDELMAN C (ed.) Pediatric kidney disease. Boston: Little, Brown and Company. [Google Scholar]
- 155.Mccrory W 1978. Embryonic development and prenatal maturation of the kidney. In: EDELMAN C (ed.) Pediatric kidney disease. Boston: Little, Brown and Company. [Google Scholar]
- 156.Fetterman GH, Shuplock NA, Philipp FJ, Gregg HS. The growth and maturation of human glomeruli and proximal convolutions from term to adulthood: Studies by microdissection. Pediatrics. 1965; 35601–619. [PubMed] [Google Scholar]
- 157.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ, Holford NH. Human renal function maturation: A quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009; 24(1):67–76. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr. 1984; 104(6):849–854. [DOI] [PubMed] [Google Scholar]
- 159.Saxén H, Hoppu K, Pohjavuori M Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin Pharmacol Ther. 1993; 54(3):269–277. [DOI] [PubMed] [Google Scholar]
- 160.De Cock RF, Allegaert K, Brussee JM, Sherwin CM, Mulla H, De Hoog M, Van Den Anker JN, Danhof M, Knibbe CA. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: Towards a semi-physiological function for maturation in glomerular filtration. Pharm Res. 2014; 31(10):2643–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, De Hoog M, Van Den Anker JN, Danhof M, Knibbe CA. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet. 2012; 51(2):105–117. [DOI] [PubMed] [Google Scholar]
- 162.Zhao W, Biran V, Jacqz-Aigrain E Amikacin maturation model as a marker of renal maturation to predict glomerular filtration rate and vancomycin clearance in neonates. Clin Pharmacokinet. 2013; 52(12):1127–1134. [DOI] [PubMed] [Google Scholar]
- 163.Rubin MI, Bruck E, Rapoport M, Snively M, Mckay H, Baumler A Maturation of renal function in childhood: Clearance studies. J Clin Invest. 1949; 28(5 Pt 2):1144–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003; 349(12):1157–1167. [DOI] [PubMed] [Google Scholar]
- 165.Cheung KWK, Van Groen BD, Spaans E, Van Borselen MD, De Bruijn A, Simons-Oosterhuis Y, Tibboel D, Samsom JN, Verdijk RM, Smeets B, Zhang L, Huang SM, Giacomini KM, De Wildt SN. A comprehensive analysis of ontogeny of renal drug transporters: Mrna analyses, quantitative proteomics, and localization. Clin Pharmacol Ther. 2019; 106(5):1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cristea S, Krekels EHJ, Rostami-Hodjegan A, Allegaert K, Knibbe CaJ. The influence of drug properties and ontogeny of transporters on pediatric renal clearance through glomerular filtration and active secretion: A simulation-based study. Aaps j. 2020; 22(4):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boyer JL. Bile formation and secretion. Compr Physiol. 2013; 3(3):1035–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Van Groen BD, Van De Steeg E, Mooij MG, Van Lipzig MMH, De Koning BaE, Verdijk RM, Wortelboer HM, Gaedigk R, Bi C, Leeder JS, Van Schaik RHN, Van Rosmalen J, Tibboel D, Vaes WH, De Wildt SN. Proteomics of human liver membrane transporters: A focus on fetuses and newborn infants. Eur J Pharm Sci. 2018; 124217–227. [DOI] [PubMed] [Google Scholar]
- 169.Dawson PA, Lan T, Rao A Bile acid transporters. J Lipid Res. 2009; 50(12):2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Verscheijden LFM, Koenderink JB, Johnson TN, De Wildt SN, Russel FGM. Physiologically-based pharmacokinetic models for children: Starting to reach maturation? Pharmacol Ther. 2020; 211107541. [DOI] [PubMed] [Google Scholar]
- 171.Johnson TN, Abduljalil K, Nicolas JM, Muglia P, Chanteux H, Nicolai J, Gillent E, Cornet M, Sciberras D Use of a physiologically based pharmacokinetic-pharmacodynamic model for initial dose prediction and escalation during a paediatric clinical trial. Br J Clin Pharmacol. 2020. [DOI] [PubMed] [Google Scholar]
- 172.Krekels EH, Neely M, Panoilia E, Tibboel D, Capparelli E, Danhof M, Mirochnick M, Knibbe CA. From pediatric covariate model to semiphysiological function for maturation: Part i-extrapolation of a covariate model from morphine to zidovudine. CPT Pharmacometrics Syst Pharmacol. 2012; 1(10):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Adiwidjaja J, Boddy AV, Mclachlan AJ. Implementation of a physiologically based pharmacokinetic modeling approach to guide optimal dosing regimens for imatinib and potential drug interactions in paediatrics. Front Pharmacol. 2019; 101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang X, Yang Y, Grimstein M, Fan J, Grillo JA, Huang SM, Zhu H, Wang Y Application of pbpk modeling and simulation for regulatory decision making and its impact on us prescribing information: An update on the 2018-2019 submissions to the us fda's office of clinical pharmacology. J Clin Pharmacol. 2020; 60 Suppl 1S160-s178. [DOI] [PubMed] [Google Scholar]
- 175.Calvier EaM, Nguyen TT, Johnson TN, Rostami-Hodjegan A, Tibboel D, Krekels EHJ, Knibbe CaJ. Can population modelling principles be used to identify key pbpk parameters for paediatric clearance predictions? An innovative application of optimal design theory. Pharm Res. 2018; 35(11):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Emoto C, Johnson TN, Hahn D, Christians U, Alloway RR, Vinks AA, Fukuda T A theoretical physiologically-based pharmacokinetic approach to ascertain covariates explaining the large interpatient variability in tacrolimus disposition. CPT Pharmacometrics Syst Pharmacol. 2019; 8(5):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Combes FP, Einolf HJ, Coello N, Heimbach T, He H, Grosch K Model-informed drug development for everolimus dosing selection in pediatric infant patients. CPT Pharmacometrics Syst Pharmacol. 2020; 9(4):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A Changes in liver volume from birth to adulthood: A meta-analysis. Liver Transpl. 2005; 11(12):1481–1493. [DOI] [PubMed] [Google Scholar]
- 179.Barter ZE, Chowdry JE, Harlow JR, Snawder JE, Lipscomb JC, Rostami-Hodjegan A Covariation of human microsomal protein per gram of liver with age: Absence of influence of operator and sample storage may justify interlaboratory data pooling. Drug Metab Dispos. 2008; 36(12):2405–2409. [DOI] [PubMed] [Google Scholar]
- 180.Liu T, Lewis TR, Moore JN, Kraft WK, Gauda EB, Sartori D, Moody DE, Gobburu JVS, Ivaturi V Could postnatal age-related uridine diphosphate glucuronic acid be a rate-limiting factor in the metabolism of morphine during the first week of life? CPT Pharmacometrics Syst Pharmacol. 2019; 8(7):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lewis T, Van Haandel L, Scott A, Leeder JS. Intensive and prolonged urine collection in preterm infants reveals three distinct indomethacin metabolic patterns: Potential implications for drug dosing. Pediatr Res. 2018; 84(3):325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smits A, Annaert P, Van Cruchten S, Allegaert K A physiology-based pharmacokinetic framework to support drug development and dose precision during therapeutic hypothermia in neonates. Front Pharmacol. 2020; 11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tay-Sontheimer J, Shireman LM, Beyer RP, Senn T, Witten D, Pearce RE, Gaedigk A, Gana Fomban CL, Lutz JD, Isoherranen N, Thummel KE, Fiehn O, Leeder JS, Lin YS. Detection of an endogenous urinary biomarker associated with cyp2d6 activity using global metabolomics. Pharmacogenomics. 2014; 15(16):1947–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Achour B, Al-Majdoub ZM, Grybos-Gajniak A, Lea K, Kilford P, Zhang M, Knight D, Barber J, Schageman J, Rostami-Hodjegan A Liquid biopsy enables quantification of the abundance and interindividual variability of hepatic enzymes and transporters. Clin Pharmacol Ther. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Darwich AS, Polasek TM, Aronson JK, Ogungbenro K, Wright DFB, Achour B, Reny JL, Daali Y, Eiermann B, Cook J, Lesko L, Mclachlan AJ, Rostami-Hodjegan A Model-informed precision dosing: Background, requirements, validation, implementation, and forward trajectory of individualizing drug therapy. Annu Rev Pharmacol Toxicol. 2021; 61225–245. [DOI] [PubMed] [Google Scholar]
- 186.Hosey-Cojocari CM, Bi C, Yan Y, Leeder JS (2018) Variability in the growth of type 2 diabetic children compared to center for disaease control and prevention references. American Society for Clinical Pharmacology and Therapeutics Annual Meeting, 2018 Orlando, FL. [Google Scholar]
- 187.Sanathanan LP, Peck CC. The randomized concentration-controlled trial: An evaluation of its sample size efficiency. Control Clin Trials. 1991; 12(6):780–794. [DOI] [PubMed] [Google Scholar]
- 188.Karlsson KE, Grahnén A, Karlsson MO, Jonsson EN. Randomized exposure-controlled trials; impact of randomization and analysis strategies. Br J Clin Pharmacol. 2007; 64(3):266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ladumor MK, Thakur A, Sharma S, Rachapally A, Mishra S, Bobe P, Rao VK, Pammi P, Kangne H, Levi D, Balhara A, Ghandikota S, Joshi A, Nautiyal V, Prasad B, Singh S A repository of protein abundance data of drug metabolizing enzymes and transporters for applications in physiologically based pharmacokinetic (pbpk) modelling and simulation. Sci Rep. 2019; 9(1):9709. [DOI] [PMC free article] [PubMed] [Google Scholar]