Abstract
Endoplasmic reticulum (ER) stress has been linked to anesthesia-induced neurotoxicity, but melatonin seems to play a protective role against ER stress. Synchronized Caenorhabditis elegans were exposed to isoflurane during the developmental period; melatonin treatment was used to evaluate its role in preventing the defective unfolded protein response (UPR) and ER-associated protein degradation (ERAD). The induced expression of hsp-4::GFP by isoflurane was attenuated in the isoflurane-melatonin group. Isoflurane upregulated the expression of ire-1, whereas melatonin did not induce ire-1 expression in C. elegans even after isoflurane exposure. With luzindole treatment, the effect of melatonin on the level of ire-1 was significantly attenuated. The reduced expression of sel-1, sel-11, cdc-48.1, and cdc-48.2 due to isoflurane was restored by melatonin, although not up to the level of the control group. The amount of polyubiquitinated proteins was increased in the isoflurane group; however, melatonin suppressed its accumulation, which was significantly inhibited by a proteasome inhibitor, MG132. The chemotaxis index of the isoflurane-melatonin group was improved compared with the isoflurane group. Melatonin may be a potential preventive molecule against defective UPR and ERAD caused by repeated anesthesia exposure. The ire-1 branch of the UPR and ERAD pathways can be the target of melatonin to reduce anesthesia-induced ER stress.
Subject terms: Neuroscience, Medical research, Molecular medicine
Introduction
Anesthetic agents have undeniably been used very safely during the last half of the century. Nevertheless, they are currently being associated with previously unrecognized neurotoxic effects that manifest at an advanced age or after multiple exposures. Over the past decade, volatile anesthetic agents have been reported to trigger apoptotic neuronal degeneration and learning deficits in neonatal animals and primates1–4. Therefore, a better understanding of anesthesia-induced neurodegeneration and determination of its importance in human medicine have become imperative. Additionally, understanding the anesthetic mechanism is an obligation in order to circumvent its toxic effects in vulnerable patients.
The crucial role of endoplasmic reticulum (ER) stress has previously been reported with regard to anesthesia-induced neurodegeneration5–8. Isoflurane has been shown to cause ryanodine receptor-associated ER stress, resulting in caspase-3 activation, and eventually neurotoxicity5. Sevoflurane-mediated ER stress was found to be associated with hippocampal injury6. ER stress is characterized by the accumulation of misfolded proteins. It activates the unfolded protein response (UPR), which is comprised of three branches: the ribonuclease inositol-requiring proteins-1 (IRE-1), the protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor-6 (ATF-6)9. ER-associated protein degradation (ERAD) is a major pathway associated with the translocation of misfolded proteins from ER lumen into the cytosol for subsequent ubiquitination, which allows them to be degraded by proteasomes9. The UPR and ERAD are well conserved between mammals and C. elegans10. Recently, we found ER stress and UPR to be increased and ERAD interrupted by repeated isoflurane exposure, thereby causing the accumulation of ubiquitinated proteins in C. elegans11.
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized primarily by the pineal gland in mammals and regulates the circadian rhythm. Previous studies have reported melatonin to have anti-oxidative properties12,13 and free-radical scavenging effects14. In addition, its protective action against ER stress through the activation of the ERAD pathway had been proposed earlier15. In this study, we aimed to investigate whether melatonin can modulate the UPR and ERAD pathways induced by repeated anesthesia, and prevent the accumulation of ubiquitinated proteins in C. elegans.
Results
Effect of melatonin on ER stress
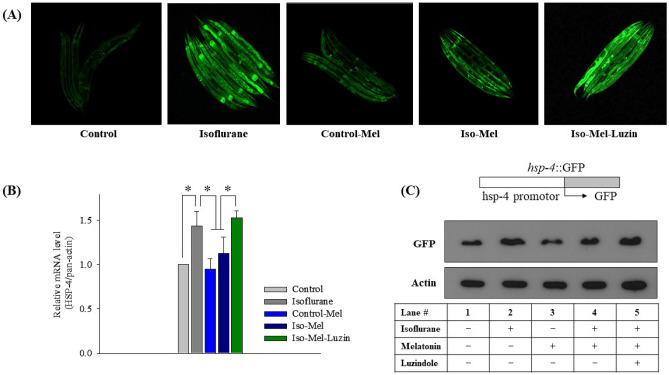
In C. elegans, hsp-4 was monitored to evaluate ER stress, it being homologous to binding immunoglobulin protein (BiP) in humans. The expression pattern of hsp-4::GFP is shown in Fig. 1A. The induced expression of hsp-4::GFP by isoflurane was attenuated in the isoflurane-melatonin group. A melatonin receptor antagonist, luzindole, was added during the isoflurane exposure period, and it abolished the effects of melatonin on hsp-4::GFP. The changes of hsp-4 gene level and GFP protein in each group were validated by real time-PCR (Fig. 1B) and western blot of GFP (Fig. 1C), respectively.
Figure 1.
Phsp-4::GFP expression in young adults after repeated isoflurane exposure with or without concurrent melatonin or luzindole treatment. (A) Phsp-4::GFP expression. Phsp-4::GFP expression was increased significantly after isoflurane exposure, while it was decreased with concurrent melatonin treatment. The inhibitory effect of melatonin on Phsp-4::GFP expression was significantly suppressed by luzindole. The experiments measuring GFP were performed 5 times and 5–10 worms per condition were monitored in each experiment. (B) Expression of the hsp-4 gene. All batches included three plates in each group, and the same assay was performed three times. (C) Schematic diagram of the hsp-4::GFP reporter construction and western blot for GFP expression. Western blot analysis was performed by using equal amounts of protein lysate prepared from approximately 500 worms. Error bar, standard deviation; *P < 0.05 vs. the control group; †P < 0.05 vs. the control-melatonin group; ‡P < 0.05 vs. the isoflurane-melatonin group.
ERUPR and ER-associated protein degradation by melatonin
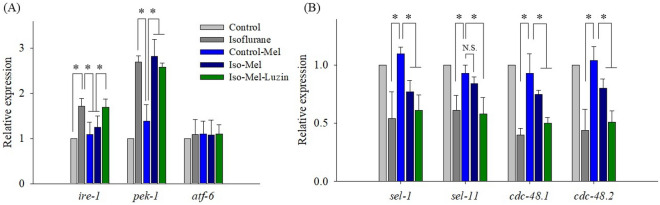
To determine the effects of melatonin on the regulation of UPR, representative UPR genes, ire-1, pek-1, and atf-6 of C. elegans (corresponding to human IRE1, PERK, and ATF6) were studied by real-time PCR.
Isoflurane upregulated the expression of ire-1 (P < 0.001), whereas melatonin did not induce ire-1 expression in C. elegans even after isoflurane exposure (P = 0.156 vs. the control group). When luzindole was added, it significantly attenuated the effect of melatonin on the level of ire-1 (Fig. 2A). Although pek-1 was enhanced by isoflurane (P < 0.001), melatonin or luzindole had no effect on its level (P < 0.001 vs. the control group and P = 0.508 vs. the isoflurane group). Moreover, the expression of atf-6 was not affected by isoflurane, melatonin, or luzindole.
Figure 2.
Expression of genes related to the unfolded protein response and endoplasmic reticulum-associated protein degradation pathways. (A) Increased ire-1 expression followed by repeated isoflurane exposure was suppressed by melatonin treatment. Luzindole attenuated the effect of melatonin on the level of ire-1. (B) The expression of sel-1, sel-11, cdc-48.1, and cdc-48.2 was significantly decreased after repeated isoflurane exposure. While the differences in sel-1, cdc-48.1, and cdc-48.2 expression were still significant between the control-melatonin and the isoflurane-melatonin groups, sel-11 expression was comparable between the two groups. All batches included three plates in each group, and the same assay was performed three times. Error bar, standard deviation; *P < 0.05; N.S., not significant.
Figure 2B shows the relative expression of four genes related with the ERAD pathway. Isoflurane downregulated sel-1, sel-11, cdc-48.1, and cdc-48.2 expression significantly, while melatonin alone had no effect on them. Reduced expression of sel-1, sel-11, cdc-48.1, and cdc-48.2 caused by isoflurane was restored by melatonin, although not up to the level of the control group. The most protective effect of melatonin was observed in sel-11. While the differences of sel-1, cdc-48.1, and cdc-48.2 expression were still significant between the control-melatonin and the isoflurane-melatonin group, sel-11 expression did not decrease significantly by combined exposure of isoflurane and melatonin. However, luzindole treatment significantly eliminated the effect of melatonin on sel-1, sel-11, cdc-48.1, and cdc-48.2 expression.
Accumulation of polyubiquitinated proteins by melatonin
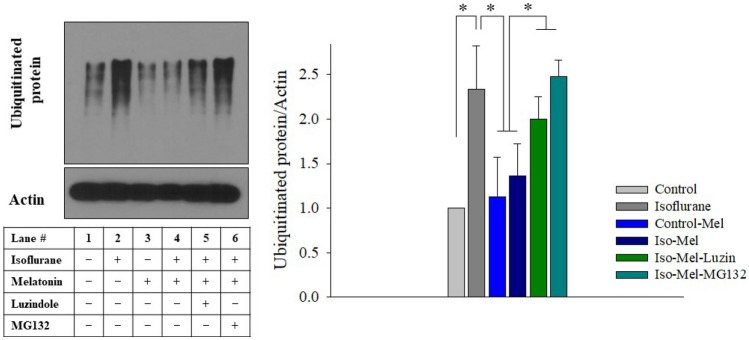
When the ERAD pathway is disturbed by isoflurane, unfolded or misfolded proteins can accumulate in the ER or cytoplasm. Misfolded proteins are ubiquitinated in the cytoplasm before their removal by proteasome for degradation. The amount of polyubiquitinated proteins was increased in the isoflurane group (P < 0.001), but melatonin suppressed its accumulation (P = 0.040 vs. the control group) (Fig. 3). The inhibitory effects of melatonin on the accumulation of ubiquitinated proteins were significantly suppressed by luzindole and a proteasome inhibitor, MG132.
Figure 3.
Western blot for ubiquitinated proteins. Higher levels of polyubiquitinated protein were observed in the isoflurane group, which was attenuated by melatonin treatment. The inhibitory effects of melatonin on the accumulation of ubiquitinated proteins were significantly suppressed by luzindole and a proteasome inhibitor, MG132. All batches included three plates in each group and the same assay was performed five times. Error bar, standard deviation. *P < 0.05.
Effect of melatonin on chemotaxis index by isoflurane
The chemotaxis index was decreased significantly due to repeated isoflurane exposure, as previously reported (P < 0.001)11,16. When C. elegans was grown in NGM plates containing melatonin, the chemotaxis index was not significantly affected (P = 0.624). The chemotaxis index of the isoflurane-melatonin group was better than that of the isoflurane group (P < 0.001); however, it was still significantly lower than that of the control group (P = 0.007) (Fig. 4).
Figure 4.
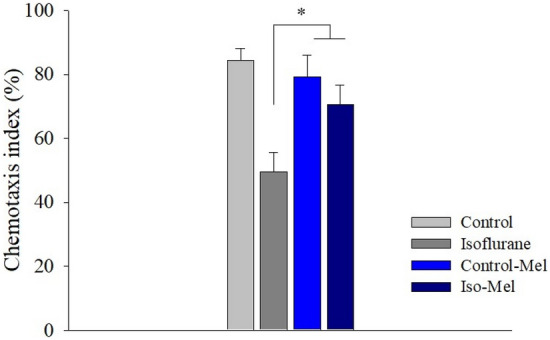
The chemotaxis index following the melatonin treatment. When C. elegans was not treated with melatonin, the chemotaxis index decreased significantly following isoflurane exposure. Melatonin treatment during the isoflurane exposure period could restore the chemotaxis index. *P < 0.05.
Discussion
Various studies have demonstrated that prolonged ER stress is related to neurodegenerative diseases, such as Parkinson’s disease or Alzheimer’s disease; neuronal apoptosis and neuroinflammation are caused by a persistent and excessive stress response17,18. Inhaled anesthetics have been reported to cause ER stress, which may be related to anesthesia-induced neurotoxicity (AIN)6,7,19,20. In our previous report, we found that repeated inhalation anesthetics could cause significant ER stress with UPR and ERAD pathway disruption followed by ubiquitinated protein accumulation in a C. elegans model11.
The role of melatonin in the prevention or treatment of PD or AD has been discussed earlier21,22. However, whether melatonin modulated ER stress and ERAD pathway in AIN was not clear. To the best our knowledge, this study is first to show that melatonin modulates the expression of ire-1 and prevents the depletion of ERAD-related genes in isoflurane-exposed C. elegans. Specifically, the accumulation of polyubiquitinated proteins was reduced by melatonin.
Melatonin significantly attenuated the isoflurane-induced upregulation of hsp-4, which is the worm homologue of BiP. In C. elegans, hsp-4 acts as an important sensor of ER stress and is a key upstream component of UPR23. When ER stress is triggered, hsp-4 is dissociated from the luminal domain of ire-1 or pek-1 and binds to the unfolded proteins. Consequently, the ire-1 and pek-1 branches of UPR initiate their role in managing ER stress24,25. Of these two genes, melatonin modulated the expression of ire-1 only. Accordingly, we proposed that the ire-1 pathway, one of the UPR signaling pathways, might play an important role in melatonin-related neuroprotection in C. elegans under anesthesia-induced ER stress. However, it remains unclear whether melatonin might directly reduce the hsp-4 or ire-1 expression. IRE1 phosphorylation and subsequent XBP1 splicing in anesthesia-induced neurodegeneration, and melatonin’s protective role should be evaluated in further studies.
Misfolded proteins are usually retrotranslocated to the cytosol, where the ones that are ubiquitinated are degraded by the proteasome26. The ERAD complex formed by SEL1L and HRD1 is conserved in mammals, and is known to be related with neurodegenerative diseases such as Parkinson’s or Alzheimer’s disease27–32. In C. elegans, their orthologs, sel-1 and sel-11, were affected by repeated isoflurane exposure11. In addition, p97 is known to expedite the degradation of misfolded proteins. The central component of the ubiquitin–proteasome system is p97, which guides protein substrates to the 26S proteasome for degradation33. C. elegans uniquely possesses two p97 homologues, namely cdc-48.1 and cdc-48.2, both depleted by repeated isoflurane exposure11.
Melatonin treatment prevented the significant depletion of these four ERAD-related genes after repeated isoflurane exposure, even though they did not reach the level of the control group. Interestingly, the expression of sel-11 only did not show a significant difference after isoflurane exposure in melatonin-treated worms. Together with ire-1 in UPR, sel-11 was the gene that benefited the most from the protection of the melatonin treatment in C. elegans. Previously, the close relationship between IRE1 and HRD1 was reported in humans, where ER stress induces HRD1 expression through the IRE1 pathway to maintain homeostasis34,35. Treatment with melatonin also reduced the levels of polyubiquitinated proteins by maintaining ERAD function. Furthermore, a proteasome inhibitor, MG132, significantly suppressed the melatonin effect on ubiquitinated protein accumulation under ER stress. Thus, under ER stress, melatonin might regulate ubiquitinated protein levels through the ERAD system.
Evidence has been found of the accumulation of polyubiquitinated proteins in neurodegenerative diseases36–38. The effects of melatonin have previously been examined in order to identify potential therapeutic or preventive agents in several neurodegenerative diseases. For example, the pathological signature of Alzheimer’s disease includes the deposits of Aβ plaques and neurofibrillary tangles, which promote neuronal degeneration; melatonin treatment was found to enhance the clearance of Aβ in Alzheimer’s disease transgenic mice39–41. Secondly, the accumulation of aggregated mutant α-synuclein leads to the formation of intracellular inclusions called Lewy bodies, which are the major hallmark of Parkinson’s disease42. Melatonin could attenuate the expression of α- synuclein in the dopaminergic pathway and protect neurons from α-synuclein-induced cytotoxicity43,44. The results led to the hypothesis that melatonin could specifically reduce the accumulation of abnormal protein aggregation via the activation of ERAD. Unfortunately, we cannot reveal the characteristics of accumulated polyubiquitinated proteins yet. Tao et al. reported that multiple exposure to inhalation anesthetics induces Tau phosphorylation and cognitive impairment in mice45. In addition, several commonly used anesthetics might increase Aβ accumulation in animal models46. Given the limited profiles about the accumulated pathological proteins due to anesthetic agents, targeted protein analysis is warranted in the future.
Melatonin shows an effect through the G protein-mediated MT1, MT2, or MT3 receptors, and it regulates neural activities through MT1 receptors in C. elegans47. In this study, luzindole significantly suppressed the effects of melatonin on hsp-4, ire-1, sel-11, and ubiquitinated protein accumulation. Thus, we speculate that melatonin may play a protective role in AIN via MT1 receptors. Additional research is required on the details of molecular and genetic mechanisms of melatonin in defective UPR and ERAD caused by repeated anesthesia exposure.
In a C. elegans model, AIN was proved by a chemotaxis assay. We have previously reported that repeated isoflurane exposure decreases the chemotaxis index16,48, correlated with ER stress and ERAD abnormalities11. Based on what has been discussed above, melatonin seemed to prevent AIN in C. elegans, which was proved by the recovered chemotaxis index even after repeated isoflurane exposure.
In conclusion, our results suggest that melatonin may be a potential preventive molecule against defective UPR and ERAD caused by repeated anesthesia exposure. The ire-1 branch of the UPR and ERAD pathways could be the target of melatonin to reduce the anesthesia-induced ER stress. Further studies are required to unravel how melatonin acts in mammal models and how the cascade may be related to AIN.
Methods
Caenorhabditis elegans strains, wild-type N2 (WB Cat# WBStrain00000001, RRID: WBStrain00000001) and zcls4[hsp-4::GFP]V (WB Cat# WBStrain00034065, RRID: WBStrain00034065), and an Escherichia coli strain (OP50) were purchased from the Caenorhabditis Genetics Center (Minneapolis, MN, USA) and maintained at 20 °C as per the regular protocol described in our previous report16. All experiments were performed at 20 °C, unless indicated otherwise. Molecular biology chemicals were obtained from MERCK KOREA (Seoul, South Korea).
Melatonin preparation, anesthesia exposure, and chemotaxis assay
Synchronized worms were divided into four groups, namely control, isoflurane, control-melatonin, and isoflurane-melatonin groups. The isoflurane and isoflurane-melatonin groups were exposed to isoflurane four times, at the first (L1), second (L2), third (L3), and fourth (L4) larval stages. The worms were anesthetized using 99.9% effective dose of isoflurane, which had been determined by our previous experiment. The duration of each exposure was 1 h, and the interval between each anesthesia was 3 to 4 h.
The worms of control-melatonin and isoflurane-melatonin groups were maintained in the melatonin-added nutrient growth medium (NGM) with OP50. Melatonin was diluted in the NGM to have the final concentrations of 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, and 1000 nM. As a melatonin receptor inhibitor and a proteasome inhibitor, 300 μM luzindole or 50 μM MG132 was respectively added to the medium during the anesthetic exposure47,49.
In order to evaluate the behavioral effect due to repeated anesthesia exposure, a chemotaxis assay was performed as described previously16. Briefly, when synchronized worms became young adults, they were washed in S-basal medium and the worm pellet was located at the center of the chemotaxis plate. The number of worms found in each attractant or control site was counted, and the chemotaxis index was calculated according to the following formula: (number of worms at attractant site − number of worms at control site)/total number of worms × 100. The chemotaxis assay was performed with each melatonin concentration (Fig. S1) and 100 nM melatonin was chosen and used in subsequent experiments.
Fluorescence imaging
Adult-stage worms, of the zcls4[hsp-4::GFP]V strain, were immobilized using 0.5 M sodium azide and mounted on an agar pad. Green fluorescence protein (GFP) expression was visualized using a ZEISS LSM 710 confocal microscope system (Oberkochen, Germany). Based on GFP expression in the control group, that of other three groups was quantified.
RNA isolation and real-time polymerase chain reaction (PCR)
Total RNA was isolated from the adult worms of each group and RNA quality was assessed by measuring the absorbance ratio at 260–280 nm and at 260–230 nm. cDNA was synthesized using Maxima H minus first strand cDNA synthesis kit (THERMO FISHER SCIENTIFIC, Waltham, MA, USA). Real-time PCR was then performed using each primer, cDNA, and POWER SYBR GREEN PCR MASTER MIX (BIOSYSTEMS, Waltham, MA, USA). Primer sequences are listed in Table 1. Gene expression levels were normalized to pan-actin, and the ratio of expression was compared across the four groups.
Table 1.
Forward and reverse primer sequences for real-time PCR.
| hsp-4 | Forward | CGTGGCAAACGCGTACTGTGATGAAGGAGC |
| Reverse | CAGTTCATCATGATCCTCCGATTGCTCCTC | |
| ire-1 | Forward | ACAATGGCTAGTCAGCGAGG |
| Reverse | CTTCTGGAGCAATCCAGCCA | |
| pek-1 | Forward | TGACATTGACACCGACGAGG |
| Reverse | TGCCCGATGACCTTCTTGAC | |
| atf-6 | Forward | ATCGTTGCTCCTGCCTAGTG |
| Reverse | TCAATTGGCCAGTCCCTGTC | |
| sel-1 | Forward | GTGGACGAGGGCTCAATCAA |
| Reverse | AATGCATCGGCACTTCCTGA | |
| sel-11 | Forward | GCGTCTTCCACACCAACAAC |
| Reverse | CCTAGAAGACGTGCTAGGCG | |
| cdc-48.1 | Forward | TGCTCACAATGTGGTTCGGA |
| Reverse | GAACAACACGCAAGGAGCAG | |
| cdc-48.2 | Forward | GAGAAGCGTATCGTCTCGCA |
| Reverse | TTAGTAGCGGCGATCACGAC | |
| pan-actin | Forward | TCGGTATGGGACAGAAGGAC |
| Reverse | CATCCCAGTTGGTGACGATA |
Western blot analysis
Worms were lysed in cold RIPA lysis buffer (BRA0500, BIOMAX; 25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 1% sodium dodecyl sulfate) containing 100 mM iodoacetamide, and the worm lysates were quantified using a PIERCE BCA PROTEIN ASSAY KIT (THERMO FISHER SCIENTIFIC). Equal volumes of protein lysates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membrane was blocked by 5% skim milk in a Tris-buffered saline with 0.1% Tween 20 (20 mM Tris, 500 mM sodium chloride, and 0.1% Tween 20, pH 7.5), and incubated for 1 h at 20 °C with primary anti-ubiquitin antibody (1:1000; ABCAM Cat# ab7254, RRID:AB_305802) and anti-actin antibody (1:5000; ABCAM Cat# ab14128, RRID:AB_300931), followed by horseradish peroxidase-conjugated secondary antibody (SANTA CRUZ BIOTECHNOLOGY Cat# sc-516102, RRID:AB_2687626). Signal was detected with a chemiluminescence kit (DG-W250, DoGen, Korea).
Statistics
Data are expressed as the mean ± standard deviation, unless specified otherwise. Student’s t-test or one-way analysis of variance with Bonferroni t-test for post-hoc analysis was performed, as appropriate. All batches contained three plates in each group, and each plate included approximately 50–100 worms. The same assay was performed three times, unless otherwise stated. The SPSS software (ver. 21; IBM Co., Armonk, NY, USA) was used for statistical analyses and a P value < 0.05 was considered significant.
Supplementary Information
Acknowledgements
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2017R1A2B1011917).
Author contributions
H-J.S. and H-S.N. designed and directed the project. H-J.S., H.K., and J.Y. carried out the experiment. B.-W.K. and S.-H.D. verified the analytical methods and performed the analysis. H-J.S. wrote the manuscript with support from S-H.D. and H-S.N. All authors discussed the results and contributed to the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are only available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-09853-y.
References
- 1.Clausen NG, Hansen TG, Disma N. Anesthesia neurotoxicity in the developing brain: Basic studies relevant for neonatal or perinatal medicine. Clin. Perinatol. 2019;46:647–656. doi: 10.1016/j.clp.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Vutskits L, Davidson A. Update on developmental anesthesia neurotoxicity. Curr. Opin. Anaesthesio.l. 2017;30:337–342. doi: 10.1097/ACO.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 3.Coleman K, et al. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2017;126:74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noguchi KK, et al. Isoflurane exposure for three hours triggers apoptotic cell death in neonatal macaque brain. Br. J. Anaesth. 2017;119:524–531. doi: 10.1093/bja/aex123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, et al. Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br. J. Anaesth. 2014;113:695–707. doi: 10.1093/bja/aeu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu G, et al. Endoplasmic reticulum stress mediates distinct impacts of sevoflurane on different subfields of immature hippocampus. J. Neurochem. 2017;142:272–285. doi: 10.1111/jnc.14057. [DOI] [PubMed] [Google Scholar]
- 7.Komita M, Jin H, Aoe T. The effect of endoplasmic reticulum stress on neurotoxicity caused by inhaled anesthetics. Anesth. Analg. 2013;117:1197–1204. doi: 10.1213/ANE.0b013e3182a74773. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Gong M, Yan M, Zhang X. Sevoflurane induces endoplasmic reticulum stress mediated apoptosis in hippocampal neurons of aging rats. PLoS ONE. 2013;8:e57870. doi: 10.1371/journal.pone.0057870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read A, Schroder M. the unfolded protein response: An overview. Biology. 2021;10:384. doi: 10.3390/biology10050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SK. Endoplasmic reticulum homeostasis and stress responses in Caenorhabditis elegans. Prog. Mol. Subcell Biol. 2021;59:279–303. doi: 10.1007/978-3-030-67696-4_13. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Shin HJ, Do SH, Na HS. Role of unfolded protein response and endoplasmic reticulum-associated degradation by repeated exposure to inhalation anesthetics in Caenorhabditis elegans. Int. J. Med. Sci. 2021;18:2890–2896. doi: 10.7150/ijms.58043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez C, et al. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 13.Crespo I, et al. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J. Pineal Res. 2010;49:193–200. doi: 10.1111/j.1600-079X.2010.00787.x. [DOI] [PubMed] [Google Scholar]
- 14.Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 2013;54:245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 15.Choi SI, et al. Melatonin reduces endoplasmic reticulum stress and corneal dystrophy-associated TGFBIp through activation of endoplasmic reticulum-associated protein degradation. J. Pineal Res. 2017;63:e12426. doi: 10.1111/jpi.12426. [DOI] [PubMed] [Google Scholar]
- 16.Do SH, Lee SY, Na HS. The effect of repeated isoflurane exposure on serine synthesis pathway during the developmental period in Caenorhabditis elegans. Neurotoxicology. 2019;71:132–137. doi: 10.1016/j.neuro.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Colla E. Linking the endoplasmic reticulum to Parkinson's disease and alpha-synucleinopathy. Front. Neurosci. 2019;13:560. doi: 10.3389/fnins.2019.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerakis Y, Hetz C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer's disease. FEBS J. 2018;285:995–1011. doi: 10.1111/febs.14332. [DOI] [PubMed] [Google Scholar]
- 19.Seo EH, et al. Impact of general anaesthesia on endoplasmic reticulum stress: Propofol vs. isoflurane. Int. J. Med. Sci. 2019;16:1287–1294. doi: 10.7150/ijms.36265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B, Ou G, Chen Y, Zhang J. Inhibition of protein tyrosine phosphatase 1B protects against sevoflurane-induced neurotoxicity mediated by ER stress in developing brain. Brain Res. Bull. 2019;146:28–39. doi: 10.1016/j.brainresbull.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan V, et al. Therapeutic potential of melatonin and its analogs in Parkinson's disease: Focus on sleep and neuroprotection. Ther. Adv. Neurol. Disord. 2011;4:297–317. doi: 10.1177/1756285611406166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, et al. Melatonin in Alzheimer's disease. Int. J. Mol. Sci. 2013;14:14575–14593. doi: 10.3390/ijms140714575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapulkin WJ, Hiester BG, Link CD. Compensatory regulation among ER chaperones in C. elegans. FEBS Lett. 2005;579:3063–3068. doi: 10.1016/j.febslet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 24.Safra M, Ben-Hamo S, Kenyon C, Henis-Korenblit S. The ire-1 ER stress-response pathway is required for normal secretory-protein metabolism in C. elegans. J. Cell Sci. 2013;126:4136–4146. doi: 10.1242/jcs.123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson CE, Kinkel S, Kim DH. Physiological IRE-1-XBP-1 and PEK-1 signaling in Caenorhabditis elegans larval development and immunity. PLoS Genet. 2011;7:e1002391. doi: 10.1371/journal.pgen.1002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amm I, Sommer T, Wolf DH. Protein quality control and elimination of protein waste: The role of the ubiquitin-proteasome system. Biochim. Biophys. Acta. 2014;1843:182–196. doi: 10.1016/j.bbamcr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Omura T, Kaneko M, Okuma Y, Matsubara K, Nomura Y. Endoplasmic reticulum stress and Parkinson's disease: The role of HRD1 in averting apoptosis in neurodegenerative disease. Oxid. Med. Cell Longev. 2013;2013:239854. doi: 10.1155/2013/239854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omura T, et al. Ubiquitin ligase HMG-CoA reductase degradation 1 (HRD1) prevents cell death in a cellular model of Parkinson's disease. Biochem. Biophys. Res. Commun. 2018;506:516–521. doi: 10.1016/j.bbrc.2018.10.094. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko M, et al. Loss of HRD1-mediated protein degradation causes amyloid precursor protein accumulation and amyloid-beta generation. J. Neurosci. 2010;30:3924–3932. doi: 10.1523/JNEUROSCI.2422-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito R, et al. Effects of oxidative stress on the solubility of HRD1, a ubiquitin ligase implicated in Alzheimer's disease. PLoS ONE. 2014;9:e94576. doi: 10.1371/journal.pone.0094576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko M, Okuma Y, Nomura Y. Molecular approaches to the treatment, prophylaxis, and diagnosis of Alzheimer's disease: Possible involvement of HRD1, a novel molecule related to endoplasmic reticulum stress, in Alzheimer's disease. J. Pharmacol. Sci. 2012;118:325–330. doi: 10.1254/jphs.11r11fm. [DOI] [PubMed] [Google Scholar]
- 32.Saltini G, et al. A novel polymorphism in SEL1L confers susceptibility to Alzheimer's disease. Neurosci. Lett. 2006;398:53–58. doi: 10.1016/j.neulet.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Kloppsteck P, Ewens CA, Forster A, Zhang X, Freemont PS. Regulation of p97 in the ubiquitin-proteasome system by the UBX protein-family. Biochim. Biophys Acta. 2012;1823:125–129. doi: 10.1016/j.bbamcr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko M, Ishiguro M, Niinuma Y, Uesugi M, Nomura Y. Human HRD1 protects against ER stress-induced apoptosis through ER-associated degradation. FEBS Lett. 2002;532:147–152. doi: 10.1016/s0014-5793(02)03660-8. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, et al. Human HRD1 promoter carries a functional unfolded protein response element to which XBP1 but not ATF6 directly binds. J. Biochem. 2008;144:477–486. doi: 10.1093/jb/mvn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 38.Favit A, Grimaldi M, Alkon DL. Prevention of beta-amyloid neurotoxicity by blockade of the ubiquitin-proteasome proteolytic pathway. J. Neurochem. 2000;75:1258–1263. doi: 10.1046/j.1471-4159.2000.0751258.x. [DOI] [PubMed] [Google Scholar]
- 39.Pappolla MA, et al. Melatonin treatment enhances abeta lymphatic clearance in a transgenic mouse model of amyloidosis. Curr. Alzheimer Res. 2018;15:637–642. doi: 10.2174/1567205015666180411092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hossain MF, et al. Melatonin in Alzheimer's disease: A latent endogenous regulator of neurogenesis to mitigate Alzheimer's neuropathology. Mol. Neurobiol. 2019;56:8255–8276. doi: 10.1007/s12035-019-01660-3. [DOI] [PubMed] [Google Scholar]
- 41.Spinedi E, Cardinali DP. Neuroendocrine-metabolic dysfunction and sleep disturbances in neurodegenerative disorders: Focus on Alzheimer's disease and melatonin. Neuroendocrinology. 2019;108:354–364. doi: 10.1159/000494889. [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson's disease: Molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 43.Sae-Ung K, Ueda K, Govitrapong P, Phansuwan-Pujito P. Melatonin reduces the expression of alpha-synuclein in the dopamine containing neuronal regions of amphetamine-treated postnatal rats. J. Pineal Res. 2012;52:128–137. doi: 10.1111/j.1600-079X.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- 44.Ono K, et al. Effect of melatonin on alpha-synuclein self-assembly and cytotoxicity. Neurobiol. Aging. 2012;33:2172–2185. doi: 10.1016/j.neurobiolaging.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Tao G, et al. Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3beta activation in young mice. Anesthesiology. 2014;121:510–527. doi: 10.1097/ALN.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Z, Xu Z. General anesthetics and beta-amyloid protein. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;47:140–146. doi: 10.1016/j.pnpbp.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka D, Furusawa K, Kameyama K, Okamoto H, Doi M. Melatonin signaling regulates locomotion behavior and homeostatic states through distinct receptor pathways in Caenorhabditis elegans. Neuropharmacology. 2007;53:157–168. doi: 10.1016/j.neuropharm.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Na HS, et al. The genetics of isoflurane-induced developmental neurotoxicity. Neurotoxicol. Teratol. 2017;60:40–49. doi: 10.1016/j.ntt.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nyamsuren O, Faggionato D, Loch W, Schulze E, Baumeister R. A mutation in CHN-1/CHIP suppresses muscle degeneration in Caenorhabditis elegans. Dev. Biol. 2007;312:193–202. doi: 10.1016/j.ydbio.2007.09.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are only available from the corresponding author on reasonable request.