Abstract
Ornate dog tick, Dermacentor reticulatus is an important vector of Babesia canis, and Rickettsia spp. and other pathogens of veterinary and public health interest. The current study is the first to investigate the long-term changes in prevalence of these pathogens in expanding tick populations in Central Europe. Molecular techniques (PCR, sequencing) were applied for the detection of pathogen DNA in adult (n = 2497) and juvenile ticks (1096 larvae and 410 nymphs). DNA of Rickettsia spp. was identified in 35% of adults and 12.6% of juvenile ticks. DNA of B. canis was detected in 3% of adult ticks and only in ticks from the Eastern region (regional prevalence 6%). As previously, no B. canis-positive ticks were found in Western Poland, including ticks from Wrocław area (n = 298). DNA of B. canis was identified in 0.33% of juvenile ticks (in 3 pools of larvae and 2 nymphs) from the Eastern region. In the current study we confirmed high occurrence of R. raoultii in adults ticks from all four zones and relatively high prevalence of B. canis in the Eastern population of D. reticulatus, corresponding well with high incidence of canine babesiosis in this area of Poland. Finally, we confirmed R. raoultii and B. canis infection in all life stages of D. reticulatus ticks.
Subject terms: Environmental sciences, Risk factors
Introduction
Vector-borne diseases constitute a serious problem of medical and veterinary importance1,2. Among canine tick-borne diseases, canine babesiosis, caused by apicomplexan parasite Babesia canis is of the greatest significance in central and eastern Europe3. Ornate dog tick, Dermacentor reticulatus is a main if not the only vector of B. canis4.
Recent study on the distribution of canine babesiosis in Poland in 2018 and occurrence of adult ticks in endemic areas and areas historically free of this tick species (2016–2018) revealed great discrepancy both in distribution of babesiosis cases and tick abundance, with ‘hot spot’ in Central and Eastern Poland5. The present study aimed to investigate the prevalence of B. canis in questing adult ticks collected from these regions and to find the link with distribution of babesiosis in Poland. Furthermore, D. reticulatus ticks are example of fast-spreading tick species and these dynamic changes in geographical range may be accompanied by changes in prevalence of pathogens in ticks. As we have previously determined prevalence of B. canis and Rickettsia spp. in ticks collected in years 2012–2014 from different regions of Poland6, the present study provides the update on prevalence of these pathogens in previously examined regions/sites and let us compare the long-term changes in infection frequency.
It is worth to underline, that in the previous studies B. canis was not detected in adult questing ticks from Western Poland, neither in juvenile ticks collected from rodents6–8 despite sporadic occurrence of canine babesiosis in this region5. In the current study we attempted to collect and examine a significant number of questing D. reticulatus ticks from Western Poland, including ticks collected in Wrocław area, in location with confirmed babesiosis cases in dogs.
Juvenile D. reticulatus ticks are heavily understudied in comparison to adult ticks, due to their hidden nidiculous style of life1. One of the aims of our current study was to examine a significant number of larvae and nymphs collected from rodents in the Eastern region of D. reticulatus occurrence for the presence of B. canis and Rickettsia spp. DNA and to compare prevalence of these pathogens between adult and juvenile ticks from the same sites.
Transovarial transmission of B. canis in D. reticulatus is believed to constitute the main route of transmission in vector population enabling persistence of parasite in certain tick populations9–11. Despite this belief, only few studies have confirmed the occurrence of B. canis in larvae and nymphs of ornate dog tick12. We have confirmed transovarial transmission of B. canis from infected females collected from dogs to eggs and larvae hatched in laboratory condition11. In present study we searched for Babesia spp. in juvenile ticks obtained from rodents, mainly voles. Detection of B. canis DNA in larvae and nymphs feeding on rodents (main reservoir of B. microti) would prove the efficiency of vertical transmission of B. canis. We hypothesize that an effective transovarial and transstadial transmission of B. canis in ticks are the main cause for its persistence in D. reticulatus population in B. canis hyperendemic areas in Central and Eastern Poland5,13.
Dermacentor reticulatus ticks are also important vectors of bacteria form the genus Rickettsia14,15. Rickettsia spp. transmitted by D. reticulatus are etiological agents of TIBOLA/DEBONEL (Tick-Borne Lymphadenopathy/Dermacentor-Borne-Necrosis-Erythema-Lymphadenopathy) group of diseases16. The first case of TIBOLA/DEBONEL in Poland was recorded in in 201117. In our previous study (2012–2014), similar to B. canis, prevalence of Rickettsia spp. in D. reticulatus markedly differed in different regions of Poland6. Interestingly, the pattern was reverted, with much higher prevalence of Rickettsia raoultii in ticks collected from the Western endemic zone of D. reticulatus occurrence in comparison to Central and Eastern Poland6. The present study enabled the comparison of Rickettsia prevalence and distribution between two study periods and between adult and juvenile ticks collected from the Eastern endemic areas.
To summarize, the aims of the present study were: (1) to determine and compare the prevalence of B. canis and Rickettsia spp. in questing adult D. reticulatus ticks collected from different regions of Poland; (2) to determine the long-term trends in prevalence between different ticks populations; (3) to determine and compare prevalence of infection in juvenile D. reticulatus ticks in comparison to adult ticks from the same areas.
Materials and methods
Adult ticks
In the main study, adult questing ticks were collected in three-year period, 2016–2018, including 864 ticks from 2016, 877 from 2017 and 756 from 2018.
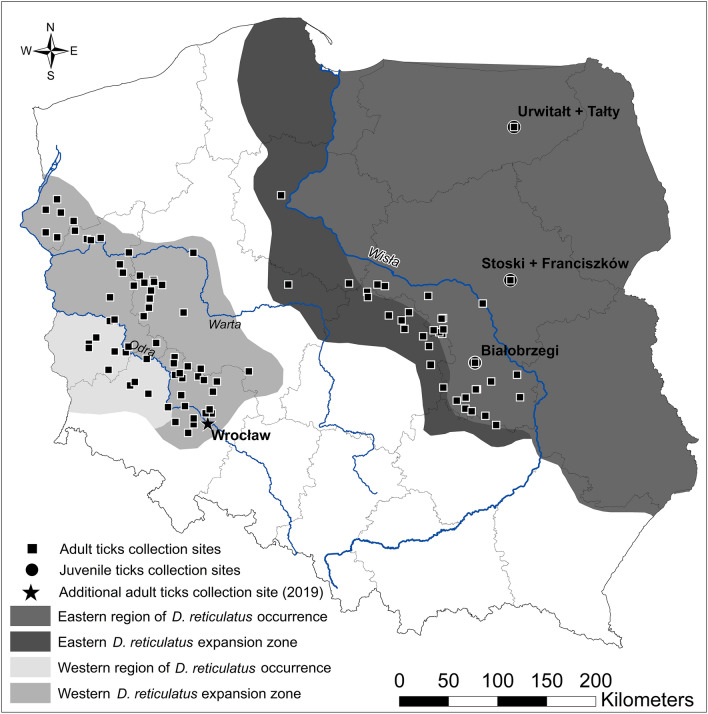
In the main study, 2497 adult ticks (1446 females and 1051 males) were examined for pathogen occurrence: 1264 ticks from the Eastern region of D. reticulatus occurrence (1142 from the eastern endemic area and 122 from the eastern expansion zone) and 1233 ticks from the Western region of D. reticulatus occurrence (635 from the western endemic area and 598 from the western expansion zone) (zones marked in Fig. 1, following Dwużnik-Szarek et al.5 ).
Figure 1.
Map of endemic areas and expansion zones for adult questing Dermacentor reticulatus ticks (Dwużnik-Szarek et al. 2021) and sites of the collection of juvenile ticks. The map was designed using ArcGIS (ESRI) version 10.8.1 software (institutional licence purchased by the University of Warsaw, Warsaw, Poland). Briefly, each georeferenced location of tick collection (listed in Dwużnik-Szarek et al.5) was projected as a point type .shp layer and then used as the raw data for spatial analyses. A radius buffer was calculated for each point, allowing to interpolate a range of occurrence of the tick. The base layer consisted of contour map of Poland: country borders and largest administrative units (voivodeships).
All procedures of adult D. reticulatus collection were described in details in Dwużnik-Szarek et al.5.
Additionally, 298 questing adult D. reticulatus (152 females, 146 males) collected in Wrocław area in 2019, located within the city where cases of canine babesiosis were recorded (Kiewra, unpublished), were also examined.
Larvae and nymphs collected from rodents
Altogether, 1096 larvae in 150 pools and 410 nymphs processed individually, were examined for Rickettsia spp. and B. canis. Description of collection of juvenile feeding ticks is provided in Dwużnik-Szarek et al.8 Briefly, rodents were trapped in habitats preferable for D. reticulatus (meadows, fallow lands and wetlands, etc.) in four sites in the Eastern region of D. reticulatus occurrence, endemic for D. reticulatus5,8 (Fig. 1): three sites in Mazovia voivodeship: Stoski + Franciszków (analyzed together as they are situated in close proximity of a few kilometers and represent similar open submerged habitat) and Białobrzegi and one site in Warmia-Mazuria voivodeship- Urwitałt (coordinates in Dwużnik-Szarek et al.5). Additionally, data on Babesia and Rickettsia occurrence in juvenile D. reticulatus collected from Białobrzegi and Urwitałt + Tałty (these sites were also analyzed together, for their close proximity and habitat similarity [mixed forest])12 was included in statistical analyses of prevalence.
DNA isolation
Genomic DNA was extracted from ticks (larvae, nymphs, adults) using the Genomic Mini AX Tissue Spin kit (A&A Biotechnology, Gdańsk, Poland) in accordance with the manufacturer's protocol.
Adult D. reticulatus collected from the environment were processed individually. SPEX SamplePrep Freezer/Mill 6875D (Laboratory Equipment for Sample Preparation & Handling, Rickmansworth, Great Britain) was used to prepare the tick tissue homogenate for the subsequent extraction. This equipment enables the complete homogenization of 24 samples in the temperature of liquid nitrogen (− 195.8 °C) within 2 min. During one cycle, tick was placed in cryogenic vial and frozen in liquid nitrogen. Then it was pulverized into a homogeneous mixture with a magnetically driven impactor moved at great speed from one end to the other end of the vial. After the cycle was completed and the samples were removed from the grinder, the content of the vials was rinsed and transferred into 1.5 ml Eppendorf tubes with LSU buffer (Genomic Mini AX Tissue Spin kit, A&A Biotechnology, Gdańsk, Poland).
Juvenile ticks were drained of ethanol and then homogenized with a Tissue Grinder Mixy Professional homogenizer (Nippon Genetics Europe, Düren, Germany). From each rodent a maximum of 50 larvae (5 pools, up to 10 larvae in one pool) and 5 nymphs (processed individually) were tested.
Pathogen detection
For detection of Rickettsia spp., primers Cs409 (5′-CCTATGGCTATTATGCTTGC-3′) and Rp1258 (5′-ATTGCAAAAAGTACAGTGAACA-3′) were used for amplification of a 750 bp fragment of the citrate synthase (gltA) gene18 as follows: initial denaturation in 95 °C for 5 min, 40 cycles of denaturation at a temperature of 95 °C for 30 s, 45 s of primer annealing in 59 °C, and elongation in 65 °C for 1 min19.
For molecular screening of Babesia spp., primers CRYPTO R (5'-GCTTGATCCTTCTGCAGGTTCACCTAC-3') and CRYPTO F (5'-AACCTGGTTGATCCTGCCAGT-3') were used to amplify ~ 1200 bp fragment of 18S rDNA in the first step of nested-PCR reaction. In second reaction primers BabGR2 (5'-CCAAAGACTTTGATTTCTCTC-3') and BabGF2 (5'-GYYTTGTAATTGGAATGATGG-3') amplified ~ 550 bp fragment of 18S rDNA20. Reaction conditions were as described in Tołkacz et al.21. For species-specific detection of B. canis, primers BcCOX1nR (5'-GGCCCTGTTCGGTATTGCAT-3') and BcCOX1nF (5'-CCATTTTGTTCTTTCAATTGGTGC-3') were used to amplify ~ 328 bp fragment of mitochondrial cytochrome c oxidase subunit 1 (cox1) gene22. Reaction conditions were as follows: 94 °C for 5 min, followed by 40 cycles at 94 °C for 20 s, 59 °C for 30 s, 72 °C for 45 s and final elongation at 72 °C for 7 minutes22. DNA of B. canis was used as positive control, negative controls were performed with 2 μl of sterile water in the absence of template DNA. PCR products were visualized on 1.5% agarose gel stained with Midori Green Stain (Nippon Genetics Europe, Düren, Germany).
Selected PCR products were sequenced by a private company (Genomed, Warsaw, Poland). Sequence alignments were carried out using BLAST-NCBI. Molecular phylogenetic analyses were performed in Molecular Evolutionary Genetics Analysis (MEGA) X open access software (https://www.megasoftware.net/) using Maximum Likelihood method of tree-construction. The evolutionary model was chosen with accordance to the data (following implemented model test in MEGA X) and bootstrapped over 1000 randomly generated sample trees.
Statistical analysis
Minimum Infection Rate (MIR) was calculated for pools of larvae; if a sample was positive it was assumed that only one tick specimen in the pool was infected19. Analyses regarding larvae were conducted on MIR.
For the analysis of prevalence (% PCR-positive ticks), we applied maximum likelihood techniques based on log linear analysis of contingency tables in the IBM SPSS Statistics: PS IMAGO PRO Academic v.7 (institutional licence purchased by the University of Warsaw, Warsaw, Poland)5,8.
For adult ticks factors such a tick sex (two levels: male, female), region of D. reticulatus occurrence (two levels: the Western region, the Eastern region,), zones (four levels: western endemic zone, western expansion zone, eastern endemic zone, western expansion zone), season (two levels: spring, autumn), year (three levels: 2016, 2017, 2018) were used in models with the presence or absence of pathogen DNA (B. canis, Rickettsia spp.) considered as a binary factor (0, 1). In case of juvenile D. reticulatus ticks, sites (three levels: Białobrzegi, Franciszków + Stoski, Urwitałt + Tałty) and tick stage (larva or nymph) were used in models with the presence or absence of pathogen DNA (B. canis, Rickettsia spp.) considered as a binary factor (0, 1).
For each level of analysis in turn, beginning with the most complex model, involving all possible main effects and interactions, those combinations that did not contribute significantly to explaining variation in the data were eliminated in a stepwise fashion beginning with the highest level interaction (backward selection procedure). A minimum sufficient model was then obtained, for which the likelihood ratio of χ2 was not significant, indicating that the model was sufficient in explaining the data5,8. Values of P < 0.05 were considered as significant.
Ethics approval
All of the procedures (trapping and handling of free-living rodents) were conducted with the approval of the First Warsaw Local Ethics Committee for Animal Experimentation in Poland (ethical license number: 706/2015), according to the principles governing experimental conditions and care of laboratory animals required by the European Union and the Polish Law on Animal Protection. Following collection of ticks, animals were released at the point of capture.
Results
Adult questing Dermacentor reticulatus
In total, 36.1% of examined ticks were infected with at least one pathogen. In the Western region of D. reticulatus occurrence (western endemic area + western expansion zone) 33.5% of ticks were infected, in the Eastern region (eastern endemic area + eastern expansion zone) 38.7% were infected (pathogen presence/absence × region of D. reticulatus occurrence: χ21 = 7.30, P = 0.007).
Babesia canis was detected in adult ticks with total prevalence of 3.0% (74/2497) and as we suspected, DNA of B. canis was detected only in ticks from the Eastern region of D. reticulatus occurrence (Babesia presence/absence × region of D. reticulatus occurrence: χ21 = 105.81, P < 0.0001), with regional prevalence of 5.9% (Table 1). No B. canis-positive ticks were found among 298 ticks collected from Wrocław area.
Table 1.
Prevalence of B. canis and R. raoultii in D. reticulatus ticks in four zones.
| Region (no. of ticks) | Babesia canis | 95%Cl | Rickettsia raoultii | 95%Cl | Zones | Babesia canis | 95%Cl | Rickettsia raoultii | 95%Cl |
|---|---|---|---|---|---|---|---|---|---|
| Eastern (1264) | 5.9% (74/1264) | 4.7–7.3 | 35.9% (454/1246) | 33.3–38.6 | Eastern endemic zone (1142) | 6.1% (70/1142) | 4.9–7.6 | 35.7% (408/1142) | 33.0–38.5 |
| Eastern expansion zone (122) | 3.3% (4/122) | 1.1–7.6 | 37.7% (46/122) | 29.5–46.5 | |||||
| Western (1233) | 0 | nc | 33.5% (413/1233) | 30.9–36.2 | Western endemic zone (635) | 0 | nc | 30.7% (195/635) | 27.2–34.4 |
| Western expansion zone (598) | 0 | nc | 36.5% (218/598) | 32.7–40.4 |
Nc not calculated.
Slight difference in prevalence of B. canis was also observed between two eastern zones with about 6% of infected ticks in the eastern endemic area in comparison to about 3% in eastern expansion zone (not significant; NS) (Table 1).
Additionally, higher percentage of B. canis-positive ticks was noted in spring than in autumn (6.8% [95 Cl%: 5.1–8.8] vs 4.7% [95 Cl%: 3.2–6.7], respectively) (Babesia presence/absence × season: χ21 = 23.60, P < 0.001). The highest prevalence of B. canis was noted in 2018 (8.5% [5.9–11.9%]), followed by 2017 (5.6% [3.9–7.9%]) and 2016 (4.0% [2.4–6.2%]) (Babesia presence/absence × year of tick collection: χ22 = 6.99, P = 0.03). Tick sex had no effect on prevalence of B. canis (NS).
DNA of Rickettsia spp. was detected in 34.7% of total ticks. Prevalence of Rickettsia spp. was similar in the Eastern and Western region of D. reticulatus occurrence (Table 1, NS). There were some minor differences in percentage of PCR-positive ticks between four zones. Higher prevalence of Rickettsia was detected in ticks collected in the both expansion zones in comparison to the endemic regions (Table 1, NS). Season of tick collection and tick sex had no effect on prevalence of Rickettsia (NS). Interestingly, we detected significant differences in prevalence of this pathogen between years of tick collection. The highest prevalence, 39.4% [36.1–42.6%] was noticed in 2016 followed by 35.8% [32.7–39.0%] in 2017 and 28.2% [25.1–31.5%] in 2018 (Rickettsia presence/absence × year: χ22 = 23.26, P < 0.001).
Co-infections of Babesia and Rickettsia could be recorded only in ticks collected in the Eastern region of the D. reticulatus occurrence, and only 3.2% (40/1264) of ticks from this region carried two pathogens (co-infection × region of D. reticulatus occurrence: χ21 = 59.15, P < 0.0001).
Juvenile Dermacentor reticulatus
Total prevalence of pathogens in juvenile D. reticulatus, including MIR, was 12.6% [11.1–14.3%]. Among instars, 8.7% [7.1–10.3%] of larvae and 23.4% [19.5–27.6%] of nymphs were positive for at least one pathogen (pathogen presence/absence × tick stage: χ21 = 53.04 P < 0.001). Total prevalence by site (L + N) was the highest in Urwitałt + Tałty, Masuria—19.2% [15.6–23.3%], followed by Stoski + Franciszków in Mazovia—16.2% [10.4–23.7%], with the lowest value in Białobrzegi (Mazovia)—9.6% [7.8–11.5%] (pathogen presence/absence × site: χ22 = 24.38, P < 0.001).
Rickettsia spp.
12.6% [11.0–14.3%] of examined juvenile D. reticulatus ticks were Rickettsia-positive. Prevalence of Rickettsia was more than twice higher in nymphs compared to larvae (22.9% [19.1–27.2%] vs 8.7% [MIR] [7.1–10.4%], respectively) (Rickettsia present/absence × tick stage: χ21 = 50.1, P < 0.001).
Among sites, the highest prevalence of this pathogen was detected in juvenile ticks in Urwitałt + Tałty, 19.0% [15.4–23.0%], followed by Stoski + Franciszków, 16.2% [10.4–23.2%] and Białobrzegi, 9.5% [7.8–11.4%] (Rickettsia presence/absence × site: χ22 = 24.03, P < 0.001).
Babesia canis
DNA of B. canis was identified in 0.33% [0.13–0.73%] of instars, with 0.27% [0.08–0.73%] in larvae (3 positive pools collected from Microtus oeconomus, Myodes glareolus and Apodemus agrarius in Białobrzegi) and in 0.49% [0.10–0.53%] of nymphs (one positive nymph collected from M. oeconomus in Białobrzegi, one nymph removed from M. glareolus, Urwitałt) (NS). DNA of B. canis was detected in juvenile ticks from Białobrzegi and Urwitałt + Tałty but not in much lower number of instars originated from Stoski and Franciszków (NS) (Table 2).
Table 2.
Comparison of B. canis prevalence between juvenile and questing adult ticks D. reticulatus in endemic sites.
| Site | Larvae | 95% Cl | Nymphs | 95% Cl | Adults | 95% Cl |
|---|---|---|---|---|---|---|
| Białobrzegi | 0.35% (3/859) | 0.1–0.9 | 0.81% (1/124) | 0.09–3.7 | 1.85% (1/54) | 0.2–8.3 |
| Stoski* | 0% (0/52) | 0 | 0% (0/65) | 0 | 3.28% (8/244) | 1.6–6.1 |
| Urwitałt** | 0% (0/185) | 0 | 0.45% (1/221) | 0.05–2.1 | 5.56% (8/144) | 2.7–10.2 |
| Total | 0.27% (3/1096) | 0.08–0.7 | 0.49% (2/410) | 0.10–0.53 | 3.85% (17/442) | 2.3–5.6 |
*Stoski + Franciszków together for juvenile tick stages.
**Urwitałt + Tałty together for juvenile tick stages.
Comparison of B. canis prevalence between juvenile ticks and questing adult ticks collected from endemic areas
442 adult ticks from three endemic sites, Urwitałt, Białobrzegi and Stoski were examined for B. canis presence. DNA of B. canis was detected in all tick stages (in larvae, nymph and adult ticks) in Białobrzegi site (Table 2). Although there were some differences in prevalence between sites, they were insignificant (NS; Table 2).
Molecular identification of pathogen species/genotypes
Rickettsia spp.
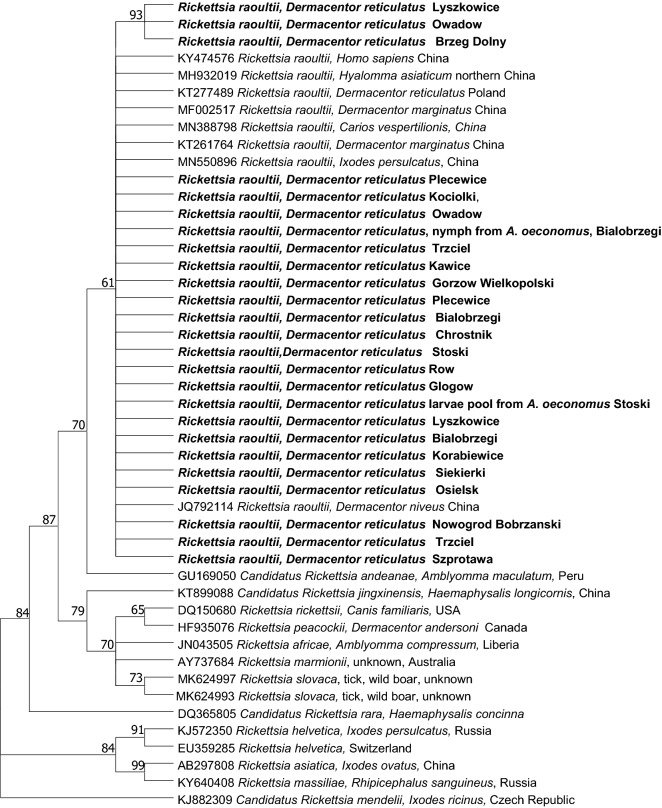
42 of 867 Rickettsia-positive PCR products were sequenced. Among these, 38 were obtained from adult questing ticks (14 originated from eastern endemic area, four from eastern expansion zone, nine from western endemic area and 11 from western expansion zone). Four sequences were derived from juvenile D. reticulatus ticks: three sequences were obtained from larvae (Stoski) and one from a nymph (Białobrzegi). All obtained sequences displayed the highest identity (99.83–100%) to R. raoultii (GenBank accession numbers: MN388798 and MN550896). The phylogenetic tree, incorporating 25 sequences obtained in this study and 22 reference sequences from GenBank, is presented in Fig. 2. The tree topology showed that sequences obtained from examined ticks clustered on the one separate branch, as expected from BLAST analysis, constituting the R. raoultii clade.
Figure 2.
Molecular phylogenetic analysis of a 750 bp fragment of the citrate synthase (gltA) gene of Rickettsia raoultii. The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano model. The tree with the highest log likelihood (− 1924.77) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.3308)). This analysis involved 58 nucleotide sequences. There were a total of 703 positions in the final dataset.
Babesia spp.
Among 74 PCR products from adult D. reticulatus ticks, 31 Babesia-positive samples were sequenced. Sequences representing cox1 gene (n = 26) showed high similarity (in range 99.7–100%) to the sequence of B. canis derived from red fox from Poland (MN147867) and B. canis derived from a dog, USA (KC207822). Three sequences were derived from ticks collected in the eastern expansion zone and 22 from the eastern endemic region of D. reticulatus occurrence, Masovian voivodeship and one from Urwitałt, Warmia-Mazuria voivodeship. A representative tree for cox1 sequences (ten sequences derived from this study and 11 reference sequences from GenBank), obtained using the Maximum Likelihood method and Hasegawa-Kishino-Yano model is presented in Fig. 3. Our sequences (GenBank accession numbers OL549270- OL549279) clustered on one separate branch with the other two B. canis cox1 gene sequences deposited in GenBank.
Figure 3.
Molecular phylogenetic analysis of cox1 of Babesia spp. (328 bp). The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano model. The tree with the highest log likelihood (− 1413.72) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 21 nucleotide sequences. There were a total of 236 positions in the final dataset.
Five PCR products of Babesia detected in adult ticks, representing 550 bp-fragment of 18S rDNA, were sequenced successfully. Representative sequence was deposited in GenBank under accession number MZ363934 and showed 99.8% identity (488/489) to B. canis derived from red fox (Poland), domestic dog (China) and D. reticulatus tick (Kazakhstan) (GenBank accession numbers MN134074, MK571831 and MK070118, respectively).
Additionally, two of three PCR products of Babesia-positive larvae (cox1 fragment) were sequenced successfully. Both samples were essentially identical (identity above 99%) to the sequence of B. canis, MN147867, from red fox, Poland.
Discussion
Our current study allowed to monitor long-term dynamic of prevalence of two main pathogens vectored by D. reticulatus in endemic areas and zones of expansion in Central Europe. The main finding is stable great difference in prevalence of B. canis between the Western and Eastern populations of D. reticulatus. Higher prevalence of B. canis was also recorded in both endemic areas in comparison to both expansion zones. Additionally, there was the association between the occurrence of B. canis in adult questing ticks and juvenile stages in the eastern endemic sites of D. reticulatus presence. Interestingly, marked changes were observed in R. raoultii prevalence in ticks from different regions.
In the current study we found B. canis-positive ticks again only in the Eastern region of D. reticulatus occurrence with relatively high (about 6%) prevalence in the eastern endemic zone and half lower (3.3%) prevalence in the eastern expansion zone. The highest prevalence was noted in 2018 (8.5%). Similar pattern was found in our previous study6, with 8% prevalence in the Masovian endemic zone and almost 5% prevalence in the eastern expansion zone, west of the Vistula River. This finding supports successful maintenance/circulation of B. canis in the Eastern D. reticulatus population. Stable high prevalence of B. canis observed in central and eastern Poland (including capitol city Warsaw) over the period of six years is also reflected in high number of canine babesiosis cases (n = 1532) and incidence of babesiosis (53/1000 dogs), noted in that region in 2018, and accompanied by relatively high fatality rate of 2.5%5. Furthermore, in some localities in this region, incidence reached up to 250 cases/1000 dogs in 2018, thus confirming maintenance of hyperendemic region for canine babesiosis5,13,23. It is worth to underline, that in the current study the highest prevalence of B. canis was observed in Urwitałt, in Warmia-Mazuria voivodeship, one of the oldest area known as endemic for D. reticulatus5,24,25. Babesia canis was also identified in numerous recent studies on adult D. reticulatus collected in Eastern and NE Poland with prevalence in range 0.6–7.3%26–29.
In agreement with our previous study, DNA of B. canis wasn’t detected in ticks collected from Western Poland, neither in current nor in other studies6,7. We haven’t found B. canis DNA in ticks collected from Wrocław area, despite occurrence of canine babesiosis in this city5. However, the annual number and incidence of canine babesiosis cases in several veterinary clinics from the area of Western Poland were extremely low in 2018- a total 19 cases/year and 0.4/1000 dogs, respectively, in comparison with central and eastern Poland and suggest very low local prevalence in ticks (< 0.1%) or very focal occurrence of infected D. reticulatus ticks. Focal occurrence of B. canis-infected ticks was reported earlier in Switzerland30 and the UK31.
Recently, high percentage of B. canis- positive Ixodes ricinus ticks was detected near Poznań32. However, these results require more investigations, as this high prevalence is not reflected in the number/incidence of babesiosis cases in this locality5 and is not confirmed by other studies on I. ricinus ticks4.
Rickettsia raoultii was the most common pathogen in both adult and juvenile ticks. Bacteria from this genus are known as intracellular endosymbionts of various invertebrates, including ticks33. Prevalence of R. raoultii in adult ticks in the current study was higher in comparison to data from Austria (14.9%)34, Romania (18%)35, Slovakia (22.3–27%)36 and Ukraine (28%)37. Prevalence was lower than prevalence of Rickettsia observed four years earlier, during our previous study (44.1%)6 and in other studies in Poland (in range 41–44%)26,38. In our previous study significant differences in prevalence of Rickettsia between western and eastern populations were observed6. Prevalence of Rickettsia was by 10% higher in the Western population of D. reticulatus than in the Eastern one. In the present study prevalence was similar across four zones and two regions. However, in previous study (2012–2014) great majority of ticks (n = 1993) originated from the Eastern region of D. reticulatus occurrence and only 592 from the Western one, but in the current study (2016–2018) the number of examined ticks from both regions was similar (1264 vs 1233). Interestingly, although DNA of R. raoultii was identified in larvae and nymphs of D. reticulatus, the pattern was typical for tick-borne pathogens acquired externally (by feeding) and transmitted transstadially, with growing prevalence from larvae, through nymphs to adults.
Despite high prevalence of these bacteria in D. reticulatus population, the impact of R. raoultii on tick fitness or feeding process has yet to be elucidated39,40. For humans many Rickettsia species, including R. raoultii, are considered as pathogenic41. Prevalence of rickettsioses determined by indirect immunofluorescent assay (IFA) in Poland in years 2006–2012 reached 2.7%42. In North-Eastern Poland, where both I. ricinus and D. reticulatus ticks are abundant, presence of anti-Rickettsia IgG antibodies was confirmed in 51% of foresters and 27% of farmers43. Although registration of rickettsioses cases is obligatory in Poland, less than half of detected cases are likely reported42.
In current study, we made the effort to sample both adult and juvenile ticks from the sites endemic for D. reticulatus and B. canis. Babesia canis was identified in juvenile ticks, three larval pools and two nymphs, from one of this endemic sites with prevalence below 1%, while prevalence in adult ticks ranged from 2 to 5.6%. In Białobrzegi and Stoski sites only recently classified as endemic5, prevalence was much lower than in old endemic sites. But in Białobrzegi, B. canis was detected in every tick stage. In agreement with our results, Dunaj et al.28 reported B. canis infection in nine nymphs of D. reticulatus from the eastern endemic region. Detection of DNA of B. canis in partially engorged small specimens might have been negatively affected by the presence of other babesiae (i.e. B. microti) or other parasites (i.e. Hepatozoon) in blood meal taken by instars12. Identification of B. canis in larvae and nymphs collected from rodents supports the predicted routes of circulation of this piroplasm in endemic D. reticulatus population through transovarial and transstadial transmission. The main animal reservoir of B. canis in Poland constitute domestic dogs, as these parasites were rarely recorded in free-living canids, red foxes and grey wolves5,13,22,23,44–46.
Conclusions
In the present study we determined the prevalence of B. canis and Rickettsia spp. in questing adult D. reticulatus ticks collected from different regions in Poland. Furthermore, we confirmed high occurrence of R. raoultii in adult ticks from all four zones and relatively high prevalence of B. canis in the Eastern population of D. reticulatus, corresponding well with high incidence of canine babesiosis in this area of Poland. Interestingly, no B. canis-positve ticks were found again in Western Poland, including Wrocław area. Finally, we confirmed R. raoultii and B. canis infection in all life stages of D. reticulatus ticks.
Acknowledgements
The study was funded by the National Science Centre (NCN) Sonata Bis grant no. 2014/14/E/NZ7/00153 (AB).
Author contributions
Conceptualization: D.D.S., A.B.; Funding acquisition: A.B.; Investigation and data acquisition: D.D.S., E.J.M., D.K., A.C., A.R., A.B.; Data analysis: D.D.S., A.R., A.B.; Writing—original draft: D.D.S., A.B.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Földvári G, Široky P, Szekeresm S, Majoros G, Sprong H. Dermacentor reticulatus: A vector on the rise. Parasitess Vectors. 2016;9:314. doi: 10.1186/s13071-016-1599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray SA, Estrada-Peña A, Zintl A. Vectors of babesiosis. Ann. Rev. Entomol. 2019;64:149–165. doi: 10.1146/annurev-ento-011118-111932. [DOI] [PubMed] [Google Scholar]
- 3.Solano-Gallego L, Sainz A, Roura X, Estrada-Pena A, Guadalupe M. A review of canine babesiosis: The European perspective. Parasites Vectors. 2016;9:336. doi: 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajer A, Dwużnik-Szarek D. The specificity of Babesia-tick vector interactions: Recent advances and pitfalls in molecular and field studies. Parasites Vectors. 2021;14:507. doi: 10.1186/s13071-021-05019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwużnik-Szarek D, et al. A monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasites Vectors. 2021;14:267. doi: 10.1186/s13071-021-04758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mierzejewska EJ, Pawełczyk A, Radkowski M, Welc-Falęciak R, Bajer A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasites Vectors. 2015;8:490. doi: 10.1186/s13071-015-1099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krol N, et al. Dermacentor reticulatus (Fabricius, 1794) and Babesia canis (Piana et Galli-Valerio, 1895) as the parasites of companion animals (dogs and cats) in the Wrocław area, south-western Poland. Ann. Parasitol. 2016;62:125–130. doi: 10.17420/ap6202.44. [DOI] [PubMed] [Google Scholar]
- 8.Dwużnik-Szarek D, Mierzejewska EJ, Bajer A. Occurrence of juvenile Dermacentor reticulatus ticks in three regions in Poland: The final evidence of the conquest. Parasites Vectors. 2021;14:536. doi: 10.1186/s13071-021-05039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonenshine DE, Mather TN. Ecological Dynamics of Tick-Borne Zoonoses. Oxford University Press; 1994. p. 49. [Google Scholar]
- 10.Uilenberg G. Babesia: A historical overview. Vet. Parasitol. 2006;138:3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Mierzejewska EJ, Dwużnik D, Bajer A. Molecular study of transovarial transmission of Babesia canis in the Dermacentor reticulatus tick. Ann. Agric. Environ. Med. 2018;25:669–671. doi: 10.26444/aaem/94673. [DOI] [PubMed] [Google Scholar]
- 12.Dwużnik D, et al. The role of juvenile Dermacentor reticulatus ticks as vectors of microorganisms and the problem of 'meal contamination'. Exp. Appl. Acarol. 2019;78:181–202. doi: 10.1007/s10493-019-00380-6. [DOI] [PubMed] [Google Scholar]
- 13.Bajer A, et al. The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland. Vet. Parasitol. 2014;202:276–286. doi: 10.1016/j.vetpar.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Földvári G, Rigó K, Lakos A. Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn. Microbiol. Infect. Dis. 2013;76:387–389. doi: 10.1016/j.diagmicrobio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Vozmediano A, et al. Dermacentor marginatus and Dermacentor reticulatus, and their infection by SFG rickettsiae and Francisella-like endosymbionts, in mountain and periurban habitats of Northwestern Italy. Vet. Sci. 2020;7:157. doi: 10.3390/vetsci7040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portillo A, Santibáñez S, García-Álvarez L, Palomar AM, Oteo JA. Rickettsioses in Europe. Microbes Infect. 2015;17:834–838. doi: 10.1016/j.micinf.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Chmielewski T, Rudzka D, Fiecek B, Maczka I, Tylewska-Wierzbanowska S. Case of TIBOLA/DEBONEL (tick—borne lymphadenopathy/Dermacentor spp.—borne necrosis—erythema—lymphadenopathy) in Poland. Przegl. Epidemiol. 2011;65:583–586. [PubMed] [Google Scholar]
- 18.Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 1997;47:252–261. doi: 10.1099/00207713-47-2-252. [DOI] [PubMed] [Google Scholar]
- 19.Kowalec M, et al. Rickettsiales occurrence and co-occurrence in Ixodes ricinus ticks in natural and urban areas. Microb. Ecol. 2019;77:890–904. doi: 10.1007/s00248-018-1269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnet S, Jouglin M, L’Hostis M, Chauvin A. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg. Infect. Dis. 2007;13:1208–1210. doi: 10.3201/eid1308.061560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tołkacz K. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae) Parasites Vectors. 2017;10:66. doi: 10.1186/s13071-017-2007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mierzejewska EJ, et al. The role of the red fox (Vulpes vulpes) as a reservoir of the haemoparasites Babesia spp. and Hepatozoon canis and its association with the tick, Dermacentor reticulatus. Ticks Tick Borne Dis. 2021;12:101551. doi: 10.1016/j.ttbdis.2020.101551. [DOI] [PubMed] [Google Scholar]
- 23.Bajer A, Mierzejewska EJ, Rodo A, Welc-Falęciak R. The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland. Part 2: Occurrence and control of babesiosis in a sled dog kennel during a 13-year-long period. Vet. Parasitol. 2014;202:234–240. doi: 10.1016/j.vetpar.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Mierzejewska EJ, Estrada-Peña A, Alsarraf M, Kowalec M, Bajer A. Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick Borne Dis. 2016;7:94–106. doi: 10.1016/j.ttbdis.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Kubiak K, et al. Dermacentor reticulatus ticks (Acari: Ixodidae) distribution in north-eastern Poland: An endemic area of tick-borne diseases. Exp. Appl. Acarol. 2018;75:289–298. doi: 10.1007/s10493-018-0274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zając V, et al. Prevalence of infections and co-infections with 6 pathogens of Dermacentor reticulatus ticks collected in eastern Poland. Ann. Agric. Eviron. Med. 2017;24:26–32. doi: 10.5604/12321966.1233893. [DOI] [PubMed] [Google Scholar]
- 27.Wójcik-Fatla A, Zając V, Sawczyn A, Cisak E, Dutkiewicz J. Babesia spp. in questing ticks from eastern Poland: Prevalence and species diversity. Parasitol. Res. 2015;114:3111–3116. doi: 10.1007/s00436-015-4529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunaj J, Trzeszczkowski A, Moniuszko-Malinowska A, Rutkowski K, Pancewicz S. Assessment of tick-borne pathogens presence in Dermacentor reticulatus ticks in north-eastern Poland. Adv. Med. Sci. 2021;66:113–118. doi: 10.1016/j.advms.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Grochowska A, et al. Detection of Borrelia burgdorferi s.l., Anaplasma phagocytophilum and Babesia spp. in Dermacentor reticulatus ticks found within the city of Białystok, Poland—first data. Exp. Appl. Acarol. 2021;85:63–73. doi: 10.1007/s10493-021-00655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaarschmidt D, et al. Questing Dermacentor reticulatus harbouring Babesia canis DNA associated with outbreaks of canine babesiosis in the Swiss Midlands. Ticks Tick Borne Dis. 2013;4:334–340. doi: 10.1016/j.ttbdis.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 31.de Marco MDMF, et al. Emergence of Babesia canis in southern England. Parasites Vectors. 2017;10:241. doi: 10.1186/s13071-017-2178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberska J, et al. Prevalence of Babesia canis DNA in Ixodes ricinus ticks collected in forest and urban ecosystems in west-central Poland. Ticks Tick Borne Dis. 2021;12:101786. doi: 10.1016/j.ttbdis.2021.101786. [DOI] [PubMed] [Google Scholar]
- 33.Nováková M, Šmajs D. Rickettsial endosymbionts of ticks. Ticks Tick Borne Pathog. 2018 doi: 10.5772/intechopen.80767. [DOI] [Google Scholar]
- 34.Špitalská E, Stefanidesova K, Kocianova K, Bolid V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp. Appl. Acarol. 2012;57:189–197. doi: 10.1007/s10493-012-9539-8. [DOI] [PubMed] [Google Scholar]
- 35.Duscher GG, et al. First report of Rickettsia raoultii in field collected Dermacentor reticulatus ticks from Austria. Ticks Tick Borne Dis. 2016;7:720–722. doi: 10.1016/j.ttbdis.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Levytska VA, et al. Detection of pathogens in ixodid ticks collected from animals and vegetation in five regions of Ukraine. Ticks Tick Borne Dis. 2021;12:101586. doi: 10.1016/j.ttbdis.2020.101586. [DOI] [PubMed] [Google Scholar]
- 37.Ionita M, et al. Molecular detection of Rickettsia conorii and other zoonotic spotted fever group rickettsiae in ticks, Romania. Ticks Tick Borne Dis. 2016;7:150–153. doi: 10.1016/j.ttbdis.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Stańczak J, Biernat B, Racewicz M, Zalewska M, Matyjasek A. Prevalence of different Rickettsia spp. in Ixodes ricinus and Dermacentor reticulatus ticks (Acari: Ixodidae) in north-eastern Poland. Ticks Tick Borne Dis. 2018;9:427–434. doi: 10.1016/j.ttbdis.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Bonnet SI, Binetruy F, Hernández-Jarguín AM, Duron O. The tick microbiome: Why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell. Infect. Microbiol. 2017;7:236. doi: 10.3389/fcimb.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YK, et al. The bacterial microbiome of field-collected Dermacentor marginatus and Dermacentor reticulatus from Slovakia. Parasites Vectors. 2019;12:325. doi: 10.1186/s13071-019-3582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, et al. Isolation and identification of Rickettsia raoultii in human cases: A surveillance study in 3 medical centers in China. Clin. Infect. Dis. 2018;66:1109–1115. doi: 10.1093/cid/cix917. [DOI] [PubMed] [Google Scholar]
- 42.Maczka I, Roguska U, Tylewska-Wierzbanowska S. Prevalence of rickettsioses in Poland in 2006–2012. Przegl. Epidemiol. 2013;67:721–723. [PubMed] [Google Scholar]
- 43.Borawski K, et al. Prevalence of spotted fever group Rickettsia in north-eastern Poland. Infect. Dis. 2019;51:810–814. doi: 10.1080/23744235.2019.1660800. [DOI] [PubMed] [Google Scholar]
- 44.Zygner W, Górski P, Wędrychowicz H. Detection of the DNA of Borrelia afzelii, Anaplasma phagocytophilum and Babesia canis in blood samples from dogs in Warsaw. Vet. Rec. 2009;164:465–467. doi: 10.1136/vr.164.15.465. [DOI] [PubMed] [Google Scholar]
- 45.Adaszek Ł, Martinez AC, Winiarczyk S. The factors affecting the distribution of babesiosis in dogs in Poland. Vet. Parasitol. 2011;181:160–165. doi: 10.1016/j.vetpar.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 46.Karbowiak G, Hapunik J, Miniuk M. The case of babesiosis in farmed wolf (Canis lupus L) Wiad. Parazytol. 2008;54:237–243. [PubMed] [Google Scholar]