Abstract
Poorly differentiated thyroid cancer (PDC) and especially poorly differentiated areas (PDA) within follicular cell-derived differentiated thyroid cancer are ill-defined clinicopathological entities. We report our experience on their comparative prognostic outcomes This is a retrospective study of 61 patients (PDC = 29; PDA = 32) from Endocrine and Metabolic Surgery Department (2009 to 2017). Clinical and follow-up details are collected and digitally tabulated from departmental database. Gender ratio was M:F = 1:1.3 and 1:1.6. Mean age was 51 ± 12 years (16–76) and 54 ± 10.5 years (36–81) in PDA and PDC, respectively. Mean tumour size (4.6 ± 0.9 cm; 4.9 ± 1.2 cm), extrathyroidal invasion (59%; 73%) and regional lymphadenopathy were 50% and 55% in PDA and PDC, respectively. Total thyroidectomy was possible in 94% of PDA and in only 77% of PDC. Radioiodine ablation was utilised in 65% (PDA); 29% (PDC). With mean follow-up of 64 ± 23.5 months (12–103) in PDA and 37 ± 22 months (6–94) in PDC, nodal recurrence (PDC = 29%; PDA = 22%) and systemic metastasis was 41% in PDC (synchronous = 24%; metachronous = 17%); 19% in PDA (synchronous = 16%; metachronous = 3%). Five-year event-free survival (EFS) and overall survival (OS) was 90% and 93% in PDA, and 42% and 44% in PDC, respectively. Our study shows that PDA is a separate clinicopathological entity with significantly positive prognosis compared to PDC.
Keywords: Poorly differentiated thyroid cancer, Thyroglobulin, Radioiodine, Total thryroidectomy, Recurrence
Background
Ever since, the first description of poorly differentiated thyroid cancer (PDC) by Sakamoto [1], it has been evolving as a distinct clinicopathological entity. The widely held hypothesis justified by available literature is that the prognosis of PDC is intermediate between follicular differentiated thyroid cancer (DTC) and anaplastic or undifferentiated thyroid cancer [2–4]. Debate over its pathological definition and management continues due to its rarity and lack of prospective clinical trials or studies [4–7]. Adding to this existing conundrum on PDC is the pathological entity of poorly differentiated areas (PDA) interspersed within papillary thyroid cancer (PTC). Many case series and retrospective studies with varied inclusion criteria of poorly differentiated pathology within thyroid cancer have been reported globally [8, 9]. With further evolution of this entity and increasing knowledge, questions about the prognostic significance of PDA within PTC and its placement under the clinicopathological entity of PDC have arose. There were attempts to further categorise PDA as a separate entity from PDC [6–12]. To address this raging issue, we analysed our own experience of PDC versus PDA with specific emphasis on comparative prognosis and characterisation as separate clinicopathological entities.
Material and Methods
This is a retrospective study of a prospectively entered database spanning a period from 2009 to 2018. Out of 564 cases of thyroid cancer treated in our Endocrine Surgery Department, this study cohort included 76 cases with poorly differentiated pathology. Inclusion criteria was all thyroid cancer cases with histopathology of partial or florid poorly differentiated thyroid cancer (insular, trabecular, solid variants) either operated or diagnosed on trucut biospsy (in inoperable cases) with minimum follow-up of 1 year. Exclusion criteria are medullary thyroid cancer; other biologically aggressive variants of PTC such as tall cell, columnar cell, oncocytic, and diffuse sclerosing variants; cases operated elsewhere; those with incomplete records; and lost to follow-up cases. Finally, the study group included 61 cases as 15 were excluded because of incomplete information or lost to follow-up. This study complied with the international ethical norms of the Helsinki Declaration—Ethical Principles for Medical Research Involving Human Subjects, 2004 [13]. Informed consent was obtained from all the included members of the cohort.
Definitions and Standards Employed for This Study
TNM staging of AJCC 6th edition was applied to stage all cases [14].
AMES (age, metastasis, extrathyroidal invasion, tumour size) of Cady’s risk grouping was used [15].
WHO classification was used as a guide to define and classify pathological types. WHO definition of poorly differentiated thyroid cancer was employed [16].
Definition of poorly differentiated areas within papillary thyroid cancer (PTC) was presence of any foci of solid, trabecular or insular (STI) pathology in trucut or postoperative specimen. This subtype was termed as PDA in our study.
Treatment and Follow-up Strategy
It is our departmental protocol to subject the patients with thyroid cancer to total or near total thyroidectomy wherever feasible. Routine central compartment neck dissection and therapeutic lateral neck dissection were performed in our cases. Our policy is to routinely administer therapeutic RAI dose irrespective of RAI uptake for all PDC/PDA cases. Patient is started on suppressive thyroxine therapy with target TSH of <0.3 mIU/L. We consider PET positive, radioiodine negative, thyroglobulin (low or high) as recurrence and are considered for adjuvant radiotherapy or biological therapy or palliative chemotherapy. Follow-up is at 3 months, 6 months, 1 year and then yearly if there is no clinically and radiologically evident recurrence. No routine adjuvant external beam radiotherapy (EBRT) was administered.
Statistical Analysis
Statistical analysis was performed using IBM SPSS software. Recurrence-free survival and overall survival rates as an outcome of PDC versus PDA were estimated by Kaplan-Meier product limit estimate method, comparisons made by Log-rank test. Univariate and multivariate analysis were done using general linear model. P value of <0.05 was taken as statistically significant probability cutoff.
Results
Mean follow-up in PDA and PDC was 64 ± 23.5 months (12–103) in PDA and 37 ± 22 months (6–94), respectively. Mean age and gender ratio was 51 ± 12 years (16–76), M:F = 1:1.3; and 54 ± 10.5 years (36–81), 1:1.6 in PDA and PDC, respectively. Total thyroidectomy was possible in 96% of PDA and in only 80% of PDC. No cases of permanent hypoparathyroidism or recurrent laryngeal nerve palsy were noted in postoperative period. Frequency distribution of various operative procedures is detailed in Table 1. Regional lymphadenopathy was seen in 50% and 55% in PDA and PDC, respectively. The number of subjects according to TNM group staging was 39%, 19%, 23% and 18% in PDA and 10%, 19%, 23% and 48% in PDC, respectively. According to AMES risk stratification, 1.7:1 and 2.5:1 are the ratio of high risk versus low risk in PDA and PDC, respectively.
Table 1.
Operative details
| Surgical procedure | In PDA | In PDC |
|---|---|---|
| Thyroid surgery - | ||
| Total thyroidectomy | 30 (94%) | 22 (77%) |
| Near total thyroidectomy | 2 (6%) | 3 (10%) |
| Debulking | - | 4 (13%) |
| Surgery for lymphadenopathy | ||
| CCLND | 32 (100%) | 25 (86%) |
| MRND alone | 0 | 0 |
| CCLND + unilateral MRND | 4 | 5 |
| CCLND + bilateral MRND | 1 | 6 |
| Mediastinal dissection * | 2 | 8 |
| None ** | 0 | 4 |
| Other surgeries | ||
| Tracheal resection | 0 | 1 |
| Window tracheal resection | 0 | 2 |
| Shave excision | 2 | 6 |
| Metastatectomy | 1 | 3 |
*Percentage was not calculated for mediastinal level VI as they were found in addition to CCLN/MRND
**Lymph node dissection was possible in 4 cases (PDC) in which only tumour debulking was done
Histopathology was defined by presence of any STI component seen focally in 32 cases of PDA and predominantly (> 10% area) in 29 cases of PDC. Mean tumour size (4.6 ± 0.9 cm; 4.9 ± 1.2 cm), extrathyroidal invasion (59%; 73%) and radioiodine ablation were utilised in 65% (PDA) and 29% (PDC). Adjuvant EBRT was used in five cases of PDC and two cases of PDA. One PDC receive chemotherapy. All these 7 cases except one PDA case expired till last follow-up. Table 2 shows comparison of all clinicopathological variables and staging parameters between PDA and PDC, suggestive of an aggressive tumour biology in PDC vis-a-vis PDA. Details of both recurrence free survival and overall survival based on TNM and AMES risk groups are shown in Table 3.
Table 2.
Comparative significance of clinicopathological and survival variables
| Variable | PDA | PDC | P value |
|---|---|---|---|
| Age (in years) | 51 ± 12 | 54 ± 10.5 | 0.114 |
| Sex ratio (M:F) | 1:1.3 | 1:1.6 | 0.621 |
| Lymphadenopathy | 50% | 55% | 0.807 |
| Tumour size (in cm) | 4.6 ± 0.9 | 4.9 ± 1.2 | 0.09 |
| ETI | 59% | 73% | 0.03 |
| Metastasis | 14% | 41% | 0.009 |
| Recurrence rate | 25% | 46% | 0.02 |
| 5 year RFS | 90% | 42% | 0.09 |
| 5 year OS | 93% | 44% | 0.008 |
Table 3.
TNM and AMES wise survival
| Risk group | 5-year RFS in PDA | 5-year OS in PDA | 5-year RFS in PDC | 5-year OS in PDC |
|---|---|---|---|---|
| TNM | ||||
| < 55 years | ||||
| Stage I | 100% | 100% | 88% | |
| Stage II | 96% | 97% | 42% | |
| >55 years | ||||
|
Stage I Stage II Stage III Stage IV |
100% 96% 85% 82% |
100% 100% 89% 90% |
58% 45% 38% 28% |
62% 50% 45% 34% |
| AMES | ||||
| Low risk | 100% | 100% | 56% | 60% |
| High risk | 88% | 90% | 34% | 40% |
| Overall 5 year survival | 90% | 93% | 42% | 44% |
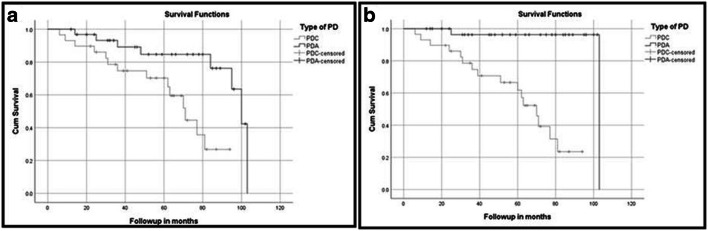
*This table shows the recurrence-free survival (RFS) and overall survival (OS) rates depicted by Kaplan-Meier/Log-rank curves in Fig. 1
Nodal recurrence (PDC = 29%; PDA = 22%) and systemic metastasis were 41% in PDC (synchronous = 24%; metachronous = 17%) and 19% in PDA (synchronous = 16%; metachronous = 3%). Five-year event-free survival (EFS) and overall survival (OS) were 90% and 93% in PDA, and 42% and 44% in PDC, respectively. Among AMES and TNM variables, only metastases affected EFS (P value = < 0.05) and none affected OS. Survival plots comparing RFS and OS between PDA and PDC are illustrated in Fig. 1. As shown in Table 4, age of patient, presence of distant metastasis and tumour size but not ETI had statistically significant effect on overall survival and recurrence-free survival. But, on multivariate analysis, the presence of metastases alone had statistically significant impact on the overall survival and recurrences. Finally, Table 5 shows the comparison of survival rates between our study and few other studies from literature.
Fig. 1.
a Recurrence-free survival comparison plot between PDA and PDC. b Overall free survival comparison plot between PDA and PDC
Table 4.
Univariate and multivariate analysis of prognostic factors between PDA and PDC
| Variable | UVA | MVA |
|---|---|---|
| Age | 0.03 | 0.08 |
| Sex | 0.912 | 0.756 |
| Tumour size | 0.01 | 0.09 |
| ETI | 0.07 | 0.988 |
| Metastases | 0.008 | 0.01 |
| Lymphadenopathy | 0.387 | 0.623 |
Table 5.
Comparison between various studies in literature
| Study group | N | 5-yr OS in PDA | 5-yr OS in PDC | 5-yr RFS in PDA | 5-yr RFS in PDC |
|---|---|---|---|---|---|
| Our study | 61 | 93% | 44% | 90% | 42% |
| Nishida * | 102 | 19.03 years | 9.15 years | 55% | 70% |
| Lai HW** | 82 | - | 72.2% | - | NM |
| Volante** | 183 | - | 85% | - | NM |
| Wreesman** | 12 | - | 70% | - | 51% |
| Sakamoto** | 35 | - | 65% | - | NM |
OS, overall survival rate; RFS, relapse-free survival rate; N, number of subjects
*OS is mentioned as mean survival period in this study
**PDA statistics are not applicable as it was not mentioned as separate entity in these studies
***NM = not mentioned in these studies
Discussion
PDC and especially PDA are ill-defined clinicopathological entities largely due to diverse definitions and rarity of disease leading to lack of consensus. Available literature and worldwide experience establishes PDC as an intermediate prognostic entity between DTC and ATC [2, 12–17]. At one end of spectrum, DTC is a largely indolent disease and ATC is a rapidly fatal disease at other end of spectrum in terms of prognosis. This biology holds true irrespective of extent and modality of treatment given. But reported prognosis varies within wide zone between that of DTC and ATC. This is partly due to wrong inclusion of either few biologically aggressive variants of DTC such as tall cell, columnar cell, oncocytic, diffuse sclerosing variants or undifferentiated ATC, which leads to erroneous prognostic results. Furthermore, PDA within DTC is a more confusing entity due to lack of uniform pathological criteria. In this study, we specifically tried to address the prognostic difference between PDC and PDA in based on our clinical experience and data. Furthermore, we attempt to provide a stringent definition en route establishing PDA and PDC as distinct clinicopathological entities.
Ever since Sakamoto proposed the term poorly differentiated carcinoma for this entity in 1983 [1], there has been intense effort to arrive at consensus on definition, categorisation, prognostication and treatment strategies between institutions from diverse geographies. Sakamoto used the definition of presence of solid, trabecular or scirrhous patterns in follicular origin thyroid cancer as PDC with prognosis intermediate between DTC and ATC. This seminal article was followed by other reports with various inclusion criteria and prognostic outcomes [5, 13, 14]. But the probable drawback of all these studies was inclusion of other high-grade subtypes of DTC. These defining criteria for PDC were difficult to utilise as they were not objective and relatively vague. To obviate this shortcoming, the World Health Organisation (WHO) in 2004 came up with a more objective definition of PDC—“follicular neoplasms that show limited evidence of structural follicular cell differentiation and occupy both morphologically and behaviorally an intermediate position between differentiated (follicular and papillary carcinomas) and undifferentiated (anaplastic) carcinomas”. This classification was intended to place various definitions of PDC that have been circulating in literature and diagnostic pathology reports with protean overlapping features and interpretations [16]. This was also intended to place all follicular derived thyroid cancers with high-grade features, but did not fit in to established high-risk categories of DTC (tall cell, columnar cell, Hurthle cell, etc.). Still, this definition was too broad and left scope for false inclusion of other subtypes of DTC. To further streamline this entity, a consensus meet was held at Turin in 2006, which gave a multi-tiered diagnostic algorithm for PDC—“any or all of STI features, absence of typical nuclear features of PTC, mitoses (≥3 mitoses per 10 hpf), convoluted nuclei, or necrosis”. This stringent Turin criteria proved to be most widely accepted definition in spite of room for errors in case of encapsulated lesions with high-grade features and extent of PD areas [18]. Though majority included STI as PD component, Nikiforov study showed that solid component should not be considered as PD pathology, but as a variant of DTC. Their experience suggested that solid pathology occurs primarily in children either as a result of radiation exposure or dietary iodine deficiency [19]. It rarely occurs in adults and appears to have similar prognosis of WDTC. But this observation was not replicated by other reports. Encapsulated DTC with high-grade features is a rarity. Thus, the only apparent chink in the armour of ideal definition of PDC appears to be extent of PD area. There have been attempts to give alternative definitions based on extent of PD areas. General rules for the description of thyroid cancer by Japanese society of thyroid surgery (JSTS) defines PDC as “cases having a poorly differentiated component, a lesion showing STI patterns, were separated from PTC and was an independent entity, even if only a slight amount of such a component is detected” [20]. There was a comparative outcome study of PDC based on the WHO and Sakamoto definitions. They reported that PDC (WHO) independently affected cause-specific survival, but PDC (Sakamoto) did not. That study concluded that PDC based on Sakamoto definition cannot be separated as independent histology, but only useful in predicted carcinoma recurrence and thus PDC (Sakamoto) be considered as a subtype of DTC [21, 22]. Another study also concluded that PDC (JSTS) should be defined as a subtype of DTC rather than as an independent entity [20]. To address this shortcoming of categorising PDC based on extent of PD areas, we conceived this comparative outcome study between less than 10% and more than 10% PD component PDC.
In the earlier studies, PDA was included in PDC with no distinction [1, 5]. Very few studies reported that PDA should be considered as separate entity due to better prognosis [10, 12]. Various studies have utilised diverse cutoff criteria ranging from presence of a tiny PD component (less than 10%) to more than 50% PD areas within a thyroid cancer pathology to bifurcate PDC and PDA [10, 23, 24]. But we opine that a large cutoff is a fallacious overestimate, as biologically any significant PD component in DTC may be tantamount to PDC and not PDA. We presume that lesser number of PDA cases versus PDC cases and inclusion of other high-grade variants of DTC (tall cell, columnar cell, Hurthle cell) might have resulted in statistically insignificant difference in many reports on PDC. We consider that a small focal area of PD within a predominant DTC pathology suffices to define PDA. Thus, we used 10% cutoff albeit arbitrary criterion to differentiate between PDA (< 10%/ focal) and PDC (> 10%/ predominant) it as PDA. First formal attempt of this distinction within PDC was reported by Nishida et al. [10], which used 10% cutoff and showed statistically significant difference in terms of outcome of PDC. This study showed statistically significant difference of 45 versus 30% relapses and 9.15 versus 19.03 years mean survival period between PDC and PDA, respectively. Similar observation was replicated in our study results. But the apparent drawback of Nishida study was inclusion of high-risk DTC cases within less than 10% PD component cases. Another recent study using 10% cutoff of PD component showed that the disease-specific survival, metastasis-free survival and relapse-free survival rates of PDC were significantly lower than that of patients with WDTC. But they included those with less than 10% PD component in DTC category [20]. On the contrary, we consider presence of any PD component with 10% cutoff to differentiate PDA from PDC. At the outset, this was the main inclusion criterion to define PDA apart from PDC and we also propose this new term PDA for any focal PD component less than 10% within DTC. As shown in Table 4, comparing prognostic outcomes of various studies with ours, only Nishida study matched closest to our design and criteria. But one striking difference between our study results from others except Nishida study is that survival rates in PDA and PDC are significantly different. It is apparent from Table 4 that the OS rate was lowest at 44% in our study compared to 65–85% in other reports. The apparent reason behind this wide difference is inclusion of supposedly PDA cases within PDC group leading to erroneously better survival rates in those studies. But based on our results, it is evident that survival rates are significantly better in PDA versus PDC, justifying their separation as distinct entities. We opine that PDA is a distinct clinicopathological entity between DTC and PDC. Moreover, as shown in our data, PDA appears to have an intermediate prognosis between DTC and PDC, but having comparable clinicopathological variables with PDC. Moreover, significant difference in tumour size, ETI, metastases rate and survival rates between PDA and PDC shows that they are biologically different.
Tsumori showed PDA at the site of tracheal invasion in ETI in >50% of DTC [25]. But in our study, pathologically, the sites of ETI were not always PD component. Even non-PD areas of DTC could lead to ETI of trachea, muscle etc., in more than 16/31 (50%) of cases. Furthermore, extent, depth and vascular invasion were lesser in PDA vis-a-vis PDC, enabling lesser debulking rates and better R0 resection rates. In spite of various surrogate markers such as age, ETI, genetic markers, PET, thyroglobulin and radioiodine avidity, we opine that the ultimate marker of prognostic outcome is tumour biology dictated by rapidity of growth, presence of distant metastasis and response to treatment. We speculate that comprehensive omics study (genomics, trascriptomics, proteomics, metabolomics) of tumour tissue correlated with prognostic outcome may in future help in predicting the phenotypes and tumour biology at clinical level in future.
Thus, studying PDA separately from PDC might help in better inter-institutional comparison of data, auditing and prognostication. Clear diagnosis based on stringent definition can also lead to lesser aggressive surgery, curative adjuvant RAI and follow-up protocols. The strengths of this study are clear distinction between PDA and PDC, comparison of various survival outcomes and first of its kind study from India. In spite of these benefits, we concede that our data is plagued by usual problem with any thyroid cancer studies, i.e. retrospective design, postoperative staging, shorter follow-up and no reliable preoperative prognostic markers necessitating individualised treatment on case to case basis. Thus, we need larger multicentric studies with longer follow-up from widely different geographical areas to establish and validate our findings.
Conclusions
PDA has clinically comparable age, gender ratio, aggressive locoregional disease, metastatic rate with PDC. But PDA has better radioiodine avidity and survival rates, thus distinguishing it as a separate clinicopathological entity with significantly positive prognosis compared to PDC.
Abbreviations
- PDC
Poorly differentiated thyroid cancer
- PDA
Poorly differentiated areas with DTC
- DTC
Follicular cell-derived differentiated thyroid cancer
- PTC
Papillary thyroid cancer
- ETI
Extrathyroidal invasion
- STI
Solid/trabecular/insular areas
- PD areas/ foci
Poorly differentiated foci
- EFS
Event-free survival
- OS
Overall survival
Author Contribution
Author 1: conceptualisation, main surgeon, manuscript writing, compilation of illustrations.
Author 2: manuscript writing and editing.
Author 3: manuscript writing, references.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ramakanth Bhargav Panchangam, Email: endoanswers@gmail.com.
Pradeep Puthenveetil, Email: pradeepputhenveetil@yahoo.com.
Sabaretnam Mayilvaganan, Email: drretnam@gmail.com.
References
- 1.Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983;52:1849–1855. doi: 10.1002/1097-0142(19831115)52:10<1849::AID-CNCR2820521015>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286–1295. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 3.Nambiar A, Pv S, Susheelan V, Kuriakose MA. The concepts in poorly differentiated carcinoma of the thyroid: a review article. J Surg Oncol. 2011;103:818–821. doi: 10.1002/jso.21803. [DOI] [PubMed] [Google Scholar]
- 4.Volante M, Papotti M. Poorly differentiated thyroid carcinoma: 5 years after the 2004 WHO classification of endocrine tumours. Endocr Pathol. 2010;21:1–6. doi: 10.1007/s12022-009-9100-4. [DOI] [PubMed] [Google Scholar]
- 5.LiVolsi VA, Baloch ZW. Predicting prognosis in thyroid carcinoma: can histology do it? Am J Surg Pathol. 2002;26:1064–1065. doi: 10.1097/00000478-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, Torchio B, Papotti MG. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer. 2004;100:950–957. doi: 10.1002/cncr.20087. [DOI] [PubMed] [Google Scholar]
- 7.Sobrinho-Simoes M, Sambade C, Fonseca E, Soares P. Poorly differentiated carcinomas of the thyroid gland: a review of the clinicopathologic features of a series of 28 cases of a heterogeneous, clinically aggressive group of thyroid tumors. Int J Surg Pathol. 2004;10:123–131. doi: 10.1177/106689690201000205. [DOI] [PubMed] [Google Scholar]
- 8.Carcangiu ML, Zampi G, Rosai J. Poorly differentiated (“insular”) thyroid carcinoma. Am J Surg Pathol. 1984;8:655–668. doi: 10.1097/00000478-198409000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Akslen LA, LiVolsi VA. Poorly differentiated thyroid carcinoma—it is important. Am J Surg Pathol. 2000;24:310–313. doi: 10.1097/00000478-200002000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Nishida T, Katayama S, Tsujimoto M, Nakamura J, Matsuda H. Clinicopathological significance of poorly differentiated thyroid carcinoma. Am J Surg Pathol. 1999;23:205–211. doi: 10.1097/00000478-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Collini P, Sampietro G, Rosai J, Pilotti S. Minimally invasive (encapsulated) follicular carcinoma of the thyroid gland is the low-risk counterpart of widely invasive follicular carcinoma but not of insular carcinoma. Virchows Arch. 2003;442:71–76. doi: 10.1007/s00428-002-0701-2. [DOI] [PubMed] [Google Scholar]
- 12.Ashfaq R, Vuitch F, Delgado R, Albores-Saavedra J. Papillary and follicular thyroid carcinomas with an insular component. Cancer. 1994;73:416–423. doi: 10.1002/1097-0142(19940115)73:2<416::AID-CNCR2820730229>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.World Medical Organization Declaration of Helsinki. BM J. 2004;313:1448–1449. [Google Scholar]
- 14.Döbert N, Menzel C, Oeschger S, Grünwald F. Differentiated thyroid carcinoma: the new UICC 6th edition TNM classification system in a retrospective analysis of 169 patients. AJCC Thyroid. 2004;14:65–70. doi: 10.1089/105072504322783867. [DOI] [PubMed] [Google Scholar]
- 15.Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419–425. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 16.Sobrinho-Simões M, Albores-Saavedra J, Tallini G, Santoro M, Volante M, Pilotti S et al. (2004) “Poorly differentiated carcinoma,” in World Health Organization Classification of Tumors: Pathology and Genetics of Tumours of Endocrine Organs, eds DeLellis RA, Lloyd RV, Heitz PU, Eng C. (Lyon: IARC Press) 73–76
- 17.Gregory Yu M, Rivera J, Jimeno C. Poorly differentiated thyroid carcinoma: 10-year experience in a Southeast Asian population. Endocrinol Metab (Seoul) 2017;32:288–295. doi: 10.3803/EnM.2017.32.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M, Sobrinho-Simoes M, Bussolati G, Rosai J. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31:1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Erickson LA, Nikiforova MN, Caudill CM, Lloyd RV. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior. Am J Surg Pathol. 2001;25:1478–1484. doi: 10.1097/00000478-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Hirokawa M, Kihara M, Takamura Y, Kobayashi K, Miyauchi A. Prognostic value of poorly differentiated carcinoma in Japanese Society of Thyroid Surgery in a series of papillary thyroid carcinoma patients: comparision with risk classification system in Kuma Hospital. Endocr J. 2012;59:817–821. doi: 10.1507/endocrj.EJ12-0175. [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Hirokawa M, Miyauchi A. Poorly differentiated carcinoma of the thyroid. Nihon Rinsho. 2007;65:1985–1990. [PubMed] [Google Scholar]
- 22.Ito Y, Hirokawa M, Fukushima M, Inoue H, Yabuta T, Kihara M. Prevalence and prognostic significance of poor differentiation and tall cell variant in papillary carcinoma in Japan. World J Surg. 2008;32:1535–1543. doi: 10.1007/s00268-007-9406-7. [DOI] [PubMed] [Google Scholar]
- 23.Higashino M, Ayani Y, Terada T, Kurisu Y, Hirose Y, Kawata R (2018) Clinical features of poorly differentiated thyroid papillary carcinoma. Auris Nasus Larynx So385-8146(18)30526-1 [DOI] [PubMed]
- 24.Volante M, Rapa I, Gandhi M, Bussolati G, Giachino D, Papotti M. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab. 2009;94:4735–4741. doi: 10.1210/jc.2009-1233. [DOI] [PubMed] [Google Scholar]
- 25.Tsumori T, Nakao K, Miyata M, Izukura M, Monden Y, Sakurai M, Kawashima Y, Nakahara K. Clinicopathologic study of thyroid carcinoma infiltrating the trachea. Cancer. 1985;56:2843–2848. doi: 10.1002/1097-0142(19851215)56:12<2843::AID-CNCR2820561221>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]