Abstract
The pharmacokinetics of levofloxacin in serum and in skin blister fluid (SBF) was determined for 20 volunteers after a single 500-mg oral dose of levofloxacin. In addition, ex vivo bactericidal activity of SBF against Streptococcus pneumoniae and Staphylococcus aureus was studied. SBF containing levofloxacin and granulocytes killed 5.2 log of Streptococcus pneumoniae bacteria and 2.0 log of Staphylococcus aureus bacteria during a 6-h incubation.
Fluoroquinolones are rapidly acting and concentration-dependent bactericidal antibiotics that inhibit bacterial DNA gyrase (22). The earlier quinolones (e.g., norfloxacin and ciprofloxacin) are active mainly against gram-negative pathogens. The newer molecules retained their activity against gram-negative bacteria and exhibit improved activity against gram-positive bacteria and atypical pathogens, such as Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila (1, 5, 6, 16). Levofloxacin penetrates well into polymorphonuclear leukocytes (PMN), which can act as vehicles for transport and delivery of the active drug to sites of infection. Accumulation of the drug in PMN plays an important role in the treatment of intracellular pathogens (7, 10, 18).
In order to establish the tissue penetration of the drug, we analyzed the respective pharmacokinetic parameters of levofloxacin in serum and in the inflammatory fluid of skin blisters. In addition, we studied the ex vivo bactericidal activity of skin blister fluid (SBF) against two common clinical pathogens, Streptococcus pneumoniae and Staphylococcus aureus. Skin cantharide blisters were provoked in human volunteers, and SBF was sampled before, and at regular intervals after, a single oral dose of levofloxacin (12). The inflammatory exudate was incubated ex vivo with a clinical isolate of Streptococcus pneumoniae (serotype 3) and a methicillin-susceptible laboratory strain of Staphylococcus aureus (ATCC 29213). Time-kill curves were obtained by inoculation of 3 × 106 CFU/ml for studies with Streptococcus pneumoniae and by inoculation of 1.5 × 106 CFU/ml for studies with Staphylococcus aureus. The MICs and minimal bactericidal concentrations (MBCs) of levofloxacin for both test strains were established by a standard macrodilution assay in Mueller-Hinton broth (Becton Dickinson), with a final inoculum of approximately 5 × 105 CFU/ml, and incubated at 37°C for 24 h as described by the National Committee for Clinical Laboratory Standards (15). For in vitro testing, we used the commercially available levofloxacin (Tavanic) as an aqueous infusion solution (Hoechst Marion Roussel, Zurich, Switzerland). For the volunteer study, we used film-coated tablets (Tavanic) containing 512.46 mg of levofloxacin hemihydrate, corresponding to 500 mg of levofloxacin as an active ingredient (Hoechst Marion Roussel). Each volunteer received a single oral dose of 500 mg of this commercially available drug.
Approval for this study was obtained from the Ethics Committee of the University Hospitals. Twenty healthy male and female volunteers participated in the study. Pregnant or lactating women were excluded. After having given written informed consent, the volunteers gave a full medical history and underwent a physical examination. Skin blisters were induced 14 h prior to medication by applying eight plasters (1 by 1 cm) impregnated with 0.2% cantharidin ointment (Adler Pharmacy, Alf an der Mosel, Germany) to the forearm of each volunteer as previously described (12). Fourteen hours later, the plasters were removed. SBF was sampled by puncture of the blisters with a 22-gauge needle for ex vivo determination of the phagocytic-bactericidal activity against the test strain before (0 h) and at seven time points following (1, 2, 3, 5, 7, 9, and 24 h) drug administration, as previously described (25). The pharmacokinetic parameters were calculated from the serum and SBF samples of all 20 volunteers. In SBF from 10 subjects, the pharmacodynamic activity against Streptococcus pneumoniae was determined, while in SBF from the other 10 volunteers, that against Staphylococcus aureus was determined. One aliquot of the pooled SBF was analyzed without centrifugation, i.e., containing leukocytes, for the phagocytic-bactericidal assay ex vivo. A second aliquot was centrifuged at 12,000 × g for 3 min at 4°C before incubation with the test strain. A third aliquot was stocked after centrifugation for the pharmacokinetic measurements. Eight milliliters of venous blood was collected from an indwelling catheter for determination of serum levofloxacin concentrations at the following time points in relation to the time of drug administration: before (0 h) and 15, 30, 60 min, 2, 3, 5, 7, 9, and 24 h following drug intake. Blood samples were collected in plain tubes (Vacutainers), immediately cooled on ice, and then centrifuged at 12,000 × g for 3 min at 4°C. SBF samples were collected in Eppendorf tubes for determination of levofloxacin levels at the same time points as the blood samples, with the exception of the 15- and 30-min points. Serum and centrifuged SBF samples were stored in plastic tubes at −20°C until assayed.
Levofloxacin levels in serum and in SBF were determined by a validated agar diffusion microbioassay method, as previously used in our research laboratory. Escherichia coli V6311/65 (Hoechst Marion Roussel, Frankfurt am Main, Germany) was used as the test strain. The standard curve was performed in Hanks' balanced salt solution containing 40% decomplemented pooled serum. The curve was linear between 0.07 and 20 μg/ml. The limit of sensitivity of the assay was 0.05 μg/ml in both serum and SBF. The mean intra- and interassay coefficients of variation were less than 5%. Serum and SBF concentration-time data were analyzed using TopFit software (11). The weighting function was determined as follows: g(yi) = 1/yi, in which g(yi) is the weighted concentration and yi is the individual concentration as determined by the bioassay. The Akaike information criteria were used to discriminate among candidate models, and a two-compartment open distribution model was selected for the serum data. The zero-order absorption rate of levofloxacin into the serum compartment was assumed to be complete at the time to the peak concentration (Cmax) (3, 4). SBF concentration-time data were analyzed separately by a one-compartment distribution model with first-order absorption. The area under the concentration-time curve from time zero to 24 h (AUC0–24) was determined by the trapezoidal method. The area under the concentration-time curve from time zero to infinity (AUC0–∞) was calculated from the AUC0–24 and extrapolated from the terminal log-linear phase to infinity. The degree of penetration into the inflammatory exudate was determined from the ratio of the AUC0–∞ of the SBF to that of the serum. Apparent oral clearance (CL/F) was determined by dividing the dose by the AUC0–∞. The apparent volume of distribution (Varea/F) was calculated by dividing the CL/F by the elimination constant.
From each volunteer, SBF samples from five different time points (time zero and 1, 2, 3, and 5 h following a single dose of levofloxacin) were tested either centrifuged (i.e., without leukocytes) or uncentrifuged (i.e., with leukocytes). The phagocytic-bactericidal assay was miniaturized to a final volume of 100 μl, as previously described (12, 25). A medium containing 40% pooled normal human serum, 40% phosphate-buffered saline, and 20% Mueller-Hinton broth supported growth without autolysis of Streptococcus pneumoniae for at least 6 h. Each test tube contained 90 μl of medium or SBF and 10 μl of bacterial inoculum (3 × 105 Streptococcus pneumoniae CFU or 1.5 × 105 Staphylococcus aureus CFU). At each time point, four different mixtures were incubated in Eppendorf tubes as follows: (i) 90 μl of medium plus 10 μl of bacterial inoculum as a growth control, (ii) 90 μl of medium with the MBC of levofloxacin for Streptococcus pneumoniae and Staphylococcus aureus (1 and 0.3 μg/ml, respectively) plus 10 μl of bacterial inoculum as a drug control, (iii) 90 μl of uncentrifuged (complete) SBF plus 10 μl of bacterial inoculum to obtain a time-kill curve with leukocytes, and (iv) 90 μl of centrifuged SBF plus 10 μl of bacterial inoculum to obtain a time-kill curve without leukocytes. These four mixtures were incubated at a 35°C angle on a rotator (250 rpm) at 37°C. Before and 1 and 6 h after inoculation, tubes were vortexed, and 10-μl aliquots were sampled for quantitative culture after appropriate dilution in sterile water. Mean values and standard deviations (SD) are given for the demographic and pharmacokinetic data. The killing of test strains in SBF was compared by paired t tests.
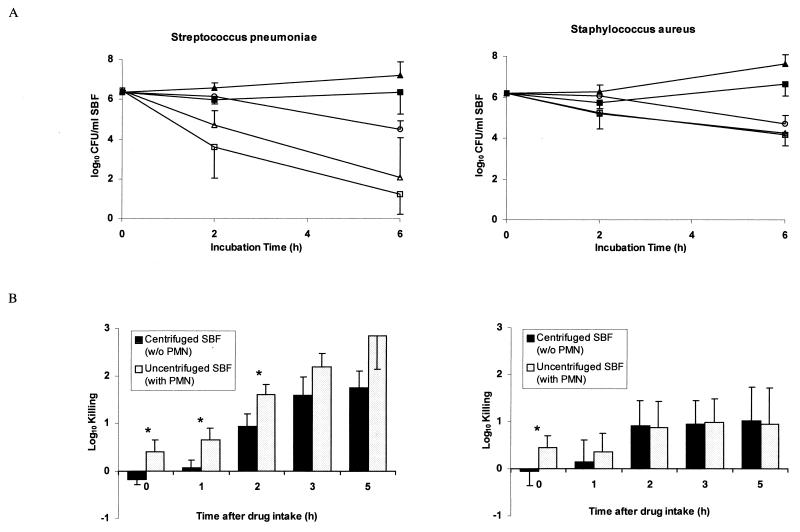
The MIC and MBC of levofloxacin for Streptococcus pneumoniae were 0.5 and 1.0 μg/ml, and for Staphylococcus aureus were 0.15 and 0.3 μg/ml, respectively. Twenty healthy subjects ranging in age from 21 to 50 years were enrolled (mean ± SD, 30.9 ± 9.5 years). Their weights ranged from 50 to 93 kg (mean ± SD, 70.5 ± 12.4 kg), and heights ranged from 160 to 193 cm (mean ± SD, 175 ± 11 cm). All subjects completed the study, and no adverse events were reported. The pharmacokinetic parameters of levofloxacin in serum and in SBF are summarized in Table 1. The mean elimination half-lives in serum and SBF were similar. Drug penetration into SBF was 124%. Figure 1A shows time-kill studies of Streptococcus pneumoniae and Staphylococcus aureus before and 5 h after intake of a 500 mg-tablet of levofloxacin. Each point corresponds to the mean value for all 10 volunteers in each study group. After 2 and 6 h of incubation, medium containing the MBC of levofloxacin showed 0.9- and 2.1-log killing of Streptococcus pneumoniae, respectively, and 0.5- and 1.6-log killing of Staphylococcus aureus, respectively. Centrifuged SBF (containing no PMN) drawn at the time of peak levofloxacin level in SBF showed 1.8- and 4.4-log killing of Streptococcus pneumoniae and 1.0- and 2.0-log killing of Staphylococcus aureus after 2 and 6 h of incubation, respectively. Killing of Streptococcus pneumoniae was further improved in the presence of PMN. However, no difference was observed in the killing of Staphylococcus aureus in the presence or absence of PMN. Figure 1B shows the killing of Streptococcus pneumoniae and Staphylococcus aureus, after 2 h of incubation, by uncentrifuged and centrifuged SBF harvested at different times after drug administration. It indicates the respective roles of the antimicrobial drug and the PMN in SBF. The killing of Streptococcus pneumoniae by levofloxacin in PMN-containing SBF samples was significantly better than that in centrifuged SBF samples (P < 0.05). In contrast, the killing of Staphylococcus aureus by levofloxacin was not improved in the presence of PMN at any time.
TABLE 1.
Pharmacokinetic parameters of levofloxacin in serum and SBF samples from 20 healthy volunteers following administration of a single 500-mg oral dose of levofloxacina
| Sample type | Cmax (μg/ml) | Tmax (h)b | t1/2β (h)c | AUC0–24 (μg · h/ml) | AUC0–∞ (μg · h/ml) | Lag time (h) | CL/F (ml/min) | Varea/F (liter)d |
|---|---|---|---|---|---|---|---|---|
| Serum | 6.92 ± 2.30 | 1.75 ± 0.64 | 8.10 ± 1.90 | 42.64 ± 9.08 | 47.55 ± 11.46 | 0.41 ± 0.30 | 180.8 ± 42.8 | 114.3 ± 37.7 |
| SBF | 3.61 ± 1.11 | 4.10 ± 1.56 | 9.21 ± 3.94 | 47.61 ± 12.62 | 58.91 ± 18.96 | 0.91 ± 0.36 | NAe | NA |
Values are means ± SD.
Tmax, time to maximum concentration of the drug.
t1/2β, elimination half-life.
Varea/F, apparent volume of distribution.
NA, not applicable.
FIG. 1.
(A) Median time-kill curves of Streptococcus pneumoniae and Staphylococcus aureus following incubation with centrifuged (▴) and uncentrifuged (■) SBF sampled before drug administration and following incubation with centrifuged (▵) and uncentrifuged (□) SBF sampled 5 h after drug intake. In addition, kill curves with medium containing the MBC of levofloxacin (○) are shown. Error bars indicate SD. (B) Killing of Streptococcus pneumoniae and Staphylococcus aureus 2 h after incubation with uncentrifuged and centrifuged SBF. Asterisks indicate statistically significant differences (P < 0.05). Error bars indicate SD.
The pharmacokinetic profile of levofloxacin has been evaluated in several studies (3, 4, 14, 20). Although levofloxacin accumulates minimally following a multiple-dose regimen, the pharmacokinetic parameters were not significantly different following the single-dose administration. Therefore, the pharmacokinetics of levofloxacin is predictable from the single-dose data (9). Further, it has been shown that a linear, two-compartment open model with first-order elimination best describes the disposition of levofloxacin. Our study confirms the rapid zero-order absorption of levofloxacin after oral administration (4). We showed that levofloxacin levels in serum and SBF exceeded the MICs for our test strains of Streptococcus pneumoniae and Staphylococcus aureus for 24 h following a single 500-mg oral dose. However, the killing activity of levofloxacin was shown to be best correlated with the ratio of its Cmax to the MIC for Staphylococcus aureus, and the AUC/MIC ratio may better correlate with microbiologic outcome when the Cmax/MIC ratio cannot be optimized (19). It is therefore clinically also important (8) that the penetration of levofloxacin into SBF was excellent, at 124%. This is similar to that of ciprofloxacin (103%) (2), sparfloxacin (117%) (13), clinafloxacin (93%) (23), and temafloxacin (105%) (17) and better than that of trovafloxacin (64%) (24). The fraction of the drug which is available for antimicrobial killing is determined by the drug concentration and the protein binding at the site of infection. SBF contains two-thirds of the serum protein level, with an identical distribution of the different types of proteins (25). Levofloxacin is only moderately bound by serum proteins (24 to 38%), and the degree of protein binding is not concentration dependent (5). In our study we used a microbiological assay measuring only the active fraction of the drug. This allowed a better comparison between the pharmacokinetic and pharmacodynamic analyses.
In the present study, we observed a strong and rapid bactericidal effect of levofloxacin against the investigated test strains. After a 6-h incubation, uncentrifuged SBF, containing levofloxacin and PMN, killed 5.2 log of Streptococcus pneumoniae bacteria and 2.0 log of Staphylococcus aureus bacteria. Part of the killing activity of SBF against Streptococcus pneumoniae was clearly due to the PMN, as shown by the improved killing by uncentrifuged SBF. In contrast, no additive killing effect of PMN with levofloxacin was observed in the killing of Staphylococcus aureus. This was unexpected, since the killing of Staphylococcus aureus in SBF not containing levofloxacin (predose sample) was identical to that of Streptococcus pneumoniae. This indicates that the improved killing of drug-damaged microorganisms by phagocytes occurred with Streptococcus pneumoniae but not with Staphylococcus aureus. This observation may explain why microorganisms persist during several days of adequate antimicrobial therapy in staphylococcal but not in pneumococcal infection (21).
In conclusion, levofloxacin exhibited excellent penetration into inflammatory fluid. We showed its potent bactericidal activity in a miniaturized phagocytic-bactericidal assay, particularly against Streptococcus pneumoniae but also against Staphylococcus aureus.
(These data were presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998, and the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999.)
Acknowledgments
This research was supported by an educational grant from Hoechst Marion Roussel.
REFERENCES
- 1.Baltch A L, Smith R P, Franke M A, Michelsen P B. Antibacterial effects of levofloxacin, erythromycin, and rifampin in a human monocyte system against Legionella pneumophila. Antimicrob Agents Chemother. 1998;42:3153–3156. doi: 10.1128/aac.42.12.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catchpole C, Andrews J M, Woodcock J, Wise R. The comparative pharmacokinetics and tissue penetration of single-dose ciprofloxacin 400 mg i.v. and 750 mg p.o. J Antimicrob Chemother. 1994;33:103–110. doi: 10.1093/jac/33.1.103. [DOI] [PubMed] [Google Scholar]
- 3.Chien S-C, Rogge M C, Gisclon L G, Curtin C, Wong F, Natarajan J, Williams R R, Fowler C L, Cheung W K, Chow A T. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–2260. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Child J, Mortiboy D, Andrews J M, Chow A T, Wise R. Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob Agents Chemother. 1995;39:2749–2751. doi: 10.1128/aac.39.12.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis R, Bryson H M. Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994;47:677–700. doi: 10.2165/00003495-199447040-00008. [DOI] [PubMed] [Google Scholar]
- 6.Donati M, Rumpianesi F, Marchetti F, Sambri V, Cevenini R. Comparative in-vitro activity of levofloxacin against Chlamydia spp. J Antimicrob Chemother. 1998;42:670–671. doi: 10.1093/jac/42.5.670. [DOI] [PubMed] [Google Scholar]
- 7.Donowitz G R. Tissue-directed antibiotics and intracellular parasites: complex interaction of phagocytes, pathogens, and drugs. Clin Infect Dis. 1994;19:926–930. doi: 10.1093/clinids/19.5.926. [DOI] [PubMed] [Google Scholar]
- 8.Drusano G L, Goldstein F W. Relevance of the Alexander Project: pharmacodynamic considerations. J Antimicrob Chemother. 1996;38(Suppl. A):141–154. doi: 10.1093/jac/38.suppl_a.141. [DOI] [PubMed] [Google Scholar]
- 9.Fish D N, Chow A T. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–119. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
- 10.Frank M O, Sullivan G W, Carper H T, Mandell G L. In vitro demonstration of transport and delivery of antibiotics by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1992;36:2584–2588. doi: 10.1128/aac.36.12.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinzel G, Woloszczak R, Thomann P. Topfit 2.0. Pharmacokinetic and pharmacodynamic data analysis system for the PC. Stuttgart, Germany: Gustav Fischer Publishers; 1993. [Google Scholar]
- 12.Hoogkamer J F W, Hesse W H, Sansano S, Zimmerli W. Pharmacodynamic activity of a cephalosporin, Ro 40-6890, in human skin blister fluid: antibiotic activity in concert with host defense mechanisms. Antimicrob Agents Chemother. 1993;37:2622–2627. doi: 10.1128/aac.37.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J H, Cooper M A, Andrews J M, Wise R. Pharmacokinetics and inflammatory fluid penetration of sparfloxacin. Antimicrob Agents Chemother. 1992;36:2444–2446. doi: 10.1128/aac.36.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee L-J, Hafkin B, Lee I-D, Hoh J, Dix R. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41:2196–2200. doi: 10.1128/aac.41.10.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. Approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 16.North D S, Fish D N, Redington J J. Levofloxacin, a second-generation fluoroquinolone. Pharmacotherapy. 1998;18:915–935. [PubMed] [Google Scholar]
- 17.Nye K, Shi Y G, Andrews J M, Ashby J P, Wise R. The in-vitro activity, pharmacokinetics and tissue penetration of temafloxacin. J Antimicrob Chemother. 1989;24:415–424. doi: 10.1093/jac/24.3.415. [DOI] [PubMed] [Google Scholar]
- 18.Pascual A, Garcia I, Perea E J. Uptake and intracellular activity of an optically active ofloxacin isomer in human neutrophils and tissue culture cells. Antimicrob Agents Chemother. 1990;34:277–280. doi: 10.1128/aac.34.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 20.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Wong F A, Corrado M. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother. 1998;42:1098–1104. doi: 10.1128/aac.42.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reymann M T, Holley H P J, Cobbs C G. Persistent bacteremia in staphylococcal endocarditis. Am J Med. 1978;65:729–737. doi: 10.1016/0002-9343(78)90790-8. [DOI] [PubMed] [Google Scholar]
- 22.Une T, Fujimoto T, Sato K, Osada Y. In vitro activity of DR-3355, an optically active ofloxacin. Antimicrob Agents Chemother. 1988;32:1336–1340. doi: 10.1128/aac.32.9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise R, Jones S, Das I, Andrews J M. Pharmacokinetics and inflammatory fluid penetration of clinafloxacin. Antimicrob Agents Chemother. 1998;42:428–430. doi: 10.1128/aac.42.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise R, Mortiboy D, Child J, Andrews J M. Pharmacokinetics and penetration into inflammatory fluid of trovafloxacin (CP-99,219) Antimicrob Agents Chemother. 1996;40:47–49. doi: 10.1128/aac.40.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerli W, Sansano S, Wittke B. Pharmacokinetics of cefetamet in plasma and skin blister fluid. Antimicrob Agents Chemother. 1996;40:102–104. doi: 10.1128/aac.40.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]