Abstract
Lymph node metastasis is a considerable variable influencing postoperative American Thyroid Association (ATA) risk stratification in pediatric differentiated thyroid cancer (DTC). The primary aim of this study was to ascertain the factors predicting nodal metastasis and describe the outcomes in relation to the ATA risk. Patients 18 years or younger operated between December 2005 and December 2019 were analyzed. Demographic, clinicopathological, treatment, and outcome data were recorded. Factors associated with nodal metastasis were assessed by univariate and multivariate regression analysis. Patients were stratified into low-, intermediate-, and high-risk as per the pediatric ATA guidelines. A total of 86 patients (43% male; median [IQR] age, 12 (10–14) years) underwent surgery during the study period. Lymph node metastases were present in 70 (82.4%) patients involving the lateral (8%) and central compartment (4.7%) alone and both (88.6%) compartments. Extrathyroid extension (ETE) was present in 65%; 35%, minimal; and 30%, extensive. On univariate analysis, nodal metastasis was more frequent in male patients, multifocal tumor, lymphovascular invasion, and ETE. On multivariate analysis, only ETE was predictive of nodal disease with an odds ratio of 8. Minimal and extensive ETEs were both significantly associated with lymph node metastases when compared to the absence of ETE. The 5-year disease-free survival was 100%, 95.7%, and 66% in the low-, intermediate-, and high-risk groups respectively (p < 0.0001). Pediatric DTCs have an exceptionally high incidence of lymph node metastasis. ETE is the single most important predictor of nodal disease. The ATA pediatric risk stratification is useful in predicting clinical outcomes.
Keywords: Differentiated thyroid cancer, Pediatric, Lymph node metastases, Treatment outcome, Risk stratification
Background
Childhood differentiated thyroid cancer (DTC) is a rare disease. In India, the reported incidence of childhood thyroid cancers is between 3 and 7% of all thyroid cancers [1]. In the Surveillance, Epidemiology, and End Results (SEER) database, they represent 1.8% of all thyroid malignancies [2]. Childhood DTC is more likely to have lymph node (LN) disease, extrathyroid extension (ETE), and pulmonary metastasis [3, 4]. Despite this, pediatric DTC has a more favorable prognosis, better progression-free survival in metastatic disease and disease that remains responsive to radioactive iodine (RAI) for prolonged periods [4]. Notwithstanding these major variances, recurrences are more frequent in children than in adults [5]. The presence of LN metastases remains a significant factor in recurrence [6, 7]. Additionally, the American Thyroid Association (ATA) risk stratification for management of DTC in children relies significantly on information gained from nodal dissection to offer adjuvant RAI therapy without much consideration to the characteristics of the thyroid tumor [8].
Since very few studies have focused on identifying the factors associated with nodal disease in pediatric DTC [9–11], the present study was aimed at determining the factors influencing LN metastasis. We also evaluated the outcomes according to the ATA risk stratification.
Methods
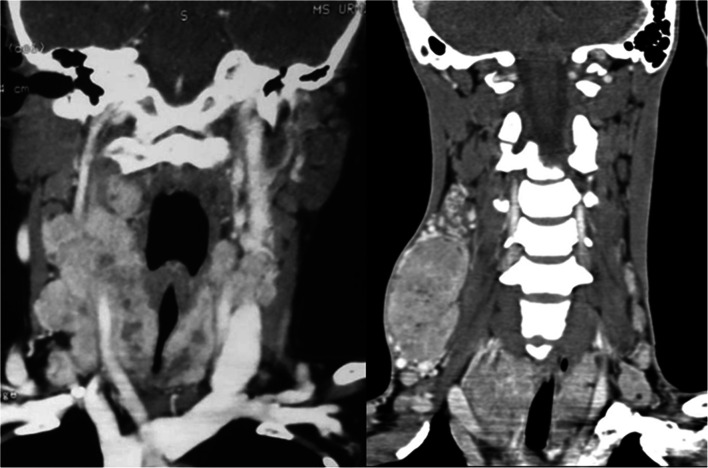
A retrospective analysis of a prospectively maintained database of pediatric patients with DTC operated between December 2005 and December 2019 was performed. Inclusion criteria for this study were as follows: patients with histology of DTC aged less than or equal to 18 years and availability of comprehensive medical records. Exclusion criteria included recurrent disease, medullary thyroid cancer, and other benign or malignant thyroid lesions. All patients with suspected or proven DTC were assessed and treated as per standard prevailing guidelines by a multidisciplinary team. Minimum preoperative investigations included thyroid function tests, an ultrasound examination of the neck, fine needle aspiration cytology (FNAC), and chest X-ray. An ultrasound evaluation included scanning the thyroid for nodule size and focality. Central and lateral neck compartments were thoroughly examined for suspicious nodes. FNAC was reported according to the Bethesda system of reporting thyroid cytology [12]. Vocal cord assessment using Hopkins rod telescope was performed. Computerized tomography (CT) was ordered selectively for patients with bulky nodal disease, retrosternal extension, suspected invasion of the trachea or esophagus, and those presenting with cord palsy (Fig. 1). Synchronous metastases were defined as distant metastases confirmed prior to surgery, during first, or within 6 months after RAI treatment. Metachronous metastases were defined as distant metastases more than 6 months after RAI treatment.
Fig. 1.
Coronal contrast-enhanced computed tomography images showing bilateral lateral lymph node metastases along with extrathyroid extension and narrowing of the trachea from the thyroid lesion involving both lobes
The operative procedure of choice in treatment-naïve patients was total thyroidectomy, and hemithyroidectomy was offered only when preoperative FNAC was not conclusive for cancer (Bethesda III/IV). Completion thyroidectomy was performed for those operated elsewhere when residual disease was present in the neck or if RAI scan or treatment was indicated. Therapeutic compartmental nodal dissection was performed for preoperative or intraoperatively detected nodes. The central compartment was exposed in all patients and inspected for the presence of grossly detectable LN. In their presence, prophylactic central compartment dissection on the side of the thyroid lesion was performed irrespectively of the size of the LN. The lateral compartment was dissected when central compartment LN was positive or preoperative ultrasound detected lateral neck disease.
Pathologic variables recorded were tumor histology, size, focality, ETE, lymphovascular invasion (LVI), LN involvement, and extranodal extension (ENE). Single lesions within the gland were classified as unifocal and 2 or more as multifocal tumors. The tumor with the largest dimensions was taken as the primary tumor. ETE was defined as tumor infiltration beyond the thyroid capsule. It was classified as minimal when detected only microscopically and gross when extensive invasion into the soft tissue, larynx, pharynx, esophagus, or recurrent laryngeal nerve was present. Cervical LN metastases were categorized into no LN metastasis (N0), central LN metastasis (N1a), unilateral lateral LN metastases (unilateral N1b disease), and bilateral lateral LN metastases (bilateral N1b disease). Cervical LN were also classified based on the number of metastatic LNs into N0/Nx, LN metastases (N1) with <5 metastatic LNs, N1 with 6–10 metastatic LNs, and N1 with >10 metastatic LNs. All tumors were classified after surgery following the 8th American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) system [13]. Patients were stratified into low, intermediate, or high risk based on the ATA pediatric risk stratification system [8]. The cutoff point for extensive N1b metastases was adopted as more than 10 metastatic lateral cervical LNs or large metastatic LN (>3 cm) [14, 15].
Diagnostic RAI whole-body scan was performed following total thyroidectomy within 12 weeks of surgery. RAI therapy was administered for cervical uptake not amenable to surgery and distant metastasis. TSH suppression was maintained using thyroxine supplementation.
Follow-up was performed with an ultrasound of the neck, serum thyroglobulin (Tg) with anti-thyroglobulin antibody (TgAb) assays, and RAI scans when indicated. The interval of follow-up visits was at a minimum of 6 months after completion of treatment and extended thereafter to yearly intervals based on response to previous RAI therapy. The patients were considered to be in complete remission (CR) at final follow-up if they had a suppressed serum Tg <1 ng/mL, no detectable TgAb, and no structural evidence of disease. Patients with Tg values >1 ng/mL or stimulated Tg values >2 ng/mL, any evidence of disease on imaging (ultrasonography, CT, or RAI scan), or biopsy-proven disease (cytology or histology) were considered having a persistent disease. Patients with only elevated Tg values were classified as having a biochemical persistent disease in the absence of structural disease on imaging. A recurrence was defined as new biochemical or structural disease that was detected following any period of CR.
Data were recorded in the IBM SPSS platform and analyzed using the SPSS version 25. For the various continuous variables, means, medians, and an interquartile range (IQR) were calculated and comparisons were performed using the Student “t” test for means when variables were normally distributed and the Mann-Whitney U test for medians when the data were not normally distributed. For the categorical variables, proportions were noted and compared using the chi-square test. Overall survival (OS) was calculated from the date of diagnosis to date of death from any cause or date of the last follow-up. Disease-free survival (DFS) was calculated from the date of diagnosis to the date of the first recurrence or progression of disease in metastatic patients or the date of the last follow-up. Survivals were calculated using the Kaplan-Meier method. The correlation of continuous and categorical variables to a categorical dependent variable was measured using odds ratio (OR) modeled from a logistic regression analysis. Results were considered significant with p values of ≤0.05.
Results
A total of 86 pediatric DTC were operated on during the study period. The baseline characteristics of the study patients are presented in Table 1. The median age of the patients was 12 years (IQR, 10 to 14 years). Most patients were females (56%). Total thyroidectomy was performed in 83 (96.5%) patients including a completion thyroidectomy following hemithyroidectomy or incomplete removal at earlier surgery elsewhere in 14 (16.3%) patients and hemithyroidectomy in 3 (3.5%) patients. The majority of the patients had classical PTC (69.8%) followed by follicular variant of PTC (25.6%).
Table 1.
Baseline clinicopathological characteristics of study patients
| Characteristic | N = 86 (%) |
|---|---|
| Age (years) | |
| Median | 12 |
| < 10 | 23 |
| 11–14 | 47 |
| 15–18 | 16 |
| Gender | |
| Male | 37 |
| Female | 49 |
| Tumor size (cm) | |
| < 1 | 8 (9.3) |
| 1.1–2 | 22 (25.6) |
| 2.1–4 | 40 (46.5) |
| > 4.1 | 16 (18.6) |
| Histology | |
| PTC | 60 (69.8) |
| Follicular variant | 22 (25.6) |
| Hurthle cell variant | 2 (2.3) |
| Hurthle cell carcinoma | 1 (1.2) |
| FTC | 1 (1.2) |
| Tumor focality | |
| Multifocal | 40 (46.5) |
| Unifocal | 46 (53.5) |
| ETE | |
| Minimal | 30 (35) |
| Extensive | 26 (30.2) |
| Absent | 30 (35) |
| LVI | |
| Yes | 42 (48.8) |
| No | 38 (44.2) |
| Unknown | 6 (7) |
| T stage | |
| T1 | 27 (31.4) |
| T2 | 26 (30.2) |
| T3a | 7 (8.1) |
| T3b | 10 (11.6) |
| T4a | 12 (14) |
| T4b | 4 (4.7) |
| N stage | |
| N0 | 16 (18.6) |
| N1a unilateral | 4 (4.7) |
| N1a bilateral | 1 (1.2) |
| N1b unilateral | 23 (26.7) |
| N1b bilateral | 42 (48.8) |
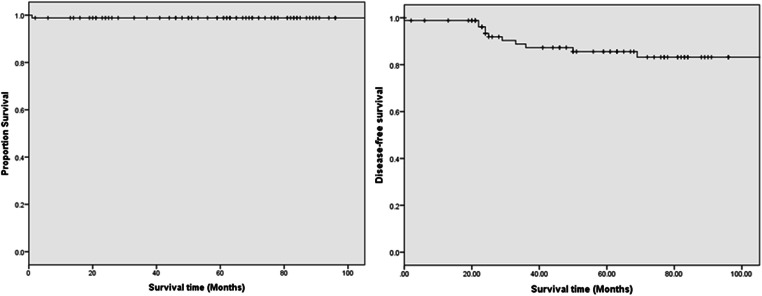
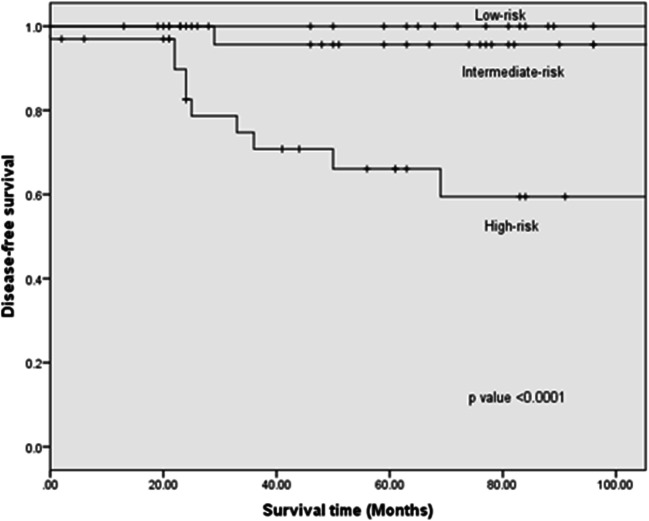
Clinically enlarged LNs were present in 53 (61.6%) patients preoperatively. Central compartment clearance alone was performed for 4 patients (4.6%), and combined central and lateral neck dissection was performed for 76 patients (88.3%). Postoperative pathological LN metastasis was confirmed in 70 (81.4%) patients including lateral LN metastases in 7 (8.1%) patients and central LN metastases in 4 (4.7%) patients; both compartments were involved in 59 (88.6%) patients. The median number of positive LN was 3 (IQR: 0–7) in the central compartment and 5.5 (IQR: 1–10) in the lateral compartment. The number of positive LN was < 5 in 14 (16.3%), 6–10 in 12 (14%), and > 10 in 44 (51.2%) patients. Further ENE was found in 61 (70.9%) patients. Synchronous distant metastases which included pulmonary metastases in all cases were seen in 20 patients of which 5 were apparent on CT chest prior to surgery. Therapeutic RAI was performed in 78 (90.7%) with 25 (32%) receiving more than one treatment. The median follow-up of the entire cohort was 69 months (IQR, 46‑90 months). Structural persistent disease was observed in 6 (7%) patients and 5 (5.8%) patients developed recurrent disease including 3 with distant metastases and one each with thyroid bed and regional LN recurrence. Median DFS was 61 months (IQR, 24.5‑88.5 months). Only one mortality secondary to extensive pulmonary metastases was recorded. At the last follow-up, 9 patients were alive with disease while 76 patients are in CR. The 5-year OS was 98.8% and DFS was 85.6% (Fig. 2). According to the ATA pediatric risk classification, 20 patients (23.3%) were in the low-risk group, 32 patients (37.2%) were in the intermediate-risk group, and 34 patients (39.5%) were in the high-risk group. The 5-year DFS was 100% in the low-risk group, 95.7% in the intermediate-risk group, and 66% in the high-risk group (p < 0.0001; Fig. 3).
Fig. 2.
Overall and disease-free survival curves
Fig. 3.
Disease-free survival according to the American Thyroid Association risk stratification
Factor Influencing Nodal Metastasis
In univariate analysis (Table 2), male sex, multifocal tumor, LVI, and ETE were found to be significantly associated with pathologic LN metastasis. However, age and tumor size were not found to have a bearing on the nodal disease. Both minimal (p = <0.0001) and extensive (p = <0.0001) ETEs were significantly associated with LN metastases. On multivariate analysis, only ETE was found to be a predictor of LN metastasis with an OR of 8. Gender, multifocality, and LVI lost their predictive value in multivariate modeling.
Table 2.
Univariate and multivariate regression model for factors associated with lymph node metastasis
| OR(95%CI) | p value | Adjusted OR (95%CI) | p value | |
|---|---|---|---|---|
| Age | ||||
| <12 | 1.0 | 1.0 | ||
| > = 12 | 0.56 (0.16–1.93) | 0.36 | 0.834 (0.16–4.50) | 0.83 |
| Gender | ||||
| Males | 1.0 | 1.0 | ||
| Females | 0.14 (0.03–0.67) | 0.014 | 0.41 (0.05–3.38) | 0.41 |
| Lesion | ||||
| Single | 1.0 | 1.0 | ||
| Multifocal | 4.86 (1.27–18.55) | 0.021 | 3.57 (0.57–22.20) | 0.17 |
| Tumor size | ||||
| <=4 | 1.0 | 1.0 | ||
| >4 | 1.75 (0.35–8.61) | 0.49 | 0.80 (0.08–7.93) | 0.85 |
| ETE | ||||
| Yes | 23.62 (4.85–115.06) | <0.001 | 8.08 (1.44–45.42) | 0.018 |
| No | 1.0 | 1.0 | ||
| LVI | ||||
| Yes | 16.70 (2.04–136.94) | 0.009 | 6.39 (0.62–65.66) | 0.12 |
| No | 1.0 | |||
Discussion
In the present study, we report a higher proportion of patients with ETE (65%) in contrast to 31–58% reported in previous studies [9, 11, 15]. In the SEER database, ETE was the most important factor that influenced nodal metastasis with an OR of 7.28 along with small contributions from multifocality and size of the primary tumor [9]. For our patients, ETE was the sole factor that predicted LN metastasis with an OR of 8. We found even minimal ETE associated with increased risk of LN metastases. In a study by Jain et al., over 94% of patients with microscopic ETE had positive LN [10]. In a study on adult cohorts of DTC, microscopic ETE had twice the odds for nodal disease as compared to no ETE [16]. Even though the AJCC 8th edition disregards microscopic ETE in risk stratification, based on our results, as well as others, we propose the inclusion of minimal ETE in the pediatric ATA risk stratification [10, 13, 16]. If ETE could be accurately detected on preoperative ultrasound, the surgeon can be more informed about the need for prophylactic nodal dissection. Equivalently, ETE assumes enormous importance when detected pathologically as it predicts residual disease in neck nodes if nodal clearance was not executed and these patients should be subjected to RAI scanning.
Contrary to many other studies, our results demonstrate that age, gender, tumor size, and focality assumed very little importance when ETE is also accounted for. The size of the primary tumor is used as one of the criteria to offer prophylactic central compartment dissection. Size larger than 4 cm is associated with increased LN metastasis in some studies [17, 18]. However, these cutoffs are derived from adult literature, and in the same studies, 36% of tumors less than 4 cm had nodal metastasis [18]. Since the volume of pediatric thyroid itself is smaller, this size cutoff may not be a valid extrapolation. Kim et al. reported a serial increase in odds of nodal disease with an increase in size in pediatric DTC [9]. In our study, tumors larger than 4 cm had non-significantly higher nodal metastasis, and in the multivariate regression analysis, the size of the tumor did not contribute to the prediction of nodal positivity.
Multifocality is a risk factor for disease recurrence and is considered a high-risk feature for LN metastasis. In one study, multifocality was associated with nearly twice the odds for the nodal disease [9]. In our patients, multifocal tumors had a higher incidence of positive LN however focality of the tumor did not contribute to predicting nodal disease when adjusted for other confounders.
There is conflicting data regarding the association of age in childhood DTC with LN metastases. The largest study comes from Belarus where younger children had a higher incidence of nodal disease and lung metastasis [18]. However, 92% were exposed to radiation during the Chernobyl disaster, and thus, their findings are not generalizable. We could not detect any association of the nodal disease with age. Even though children less than 12 years had a higher incidence of nodal metastasis, this was not statistically significant. From the SEER database, sex was not a predictor of nodal metastasis even though there is a suggestion from adult literature that male patients suffer from more aggressive disease and fare worse [9]. We had a significantly higher proportion of male children with nodal disease compared to females, but on multivariate regression, gender yielded its predictive value.
The inherent limitations of a retrospective study design apply to the present study. Moreover, the cohort belongs to a tertiary referral center and results may not be generalizable in the community setting. The very high incidence of node positivity translated into a modest number of patients in the node-negative group which made the comparisons have large confidence intervals. Thus, factors that have not assumed significance in our study may in fact become relevant in a larger dataset. Lastly, follow-up duration is relatively short when the natural history of thyroid cancers is acknowledged. However, since our primary aim was to explore the factors associated with nodal metastasis, these drawbacks do not critically affect our endpoint.
Conclusions
Pediatric DTCs have an exceptionally high incidence of LN metastasis, and the majority of these nodes are associated with ENE. Male sex, multifocal disease, LVI, and ETE were all associated with increased chances of LN metastasis while ETE was the single most significant predictor of nodal metastasis in pediatric DTCs. When ETE is detected preoperatively, prophylactic nodal dissection should be performed, and when detected postoperatively, RAI should be offered if not for other reasons as there is a high likelihood of residual neck disease. The ATA risk stratification is useful for predicting the risk for persistent or recurrent disease in pediatric DTC.
Availability of Data and Material
All data and material are available within the manuscript.
Code Availability
Not applicable.
Declarations
Ethics Approval
Institutional Ethics Committee approval and waiver of consent were taken.
Consent to Participate
Consent waiver was taken.
Consent for Publication
Consent was taken.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar A, Bal CS. Differentiated thyroid cancer. Indian J Pediatr. 2003;70:707–713. doi: 10.1007/BF02724312. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) SEER Cancer statistics review, 1975–2010
- 3.Biko J, Reiners C, Kreissl MC, Verburg FA, Demidchik Y, Drozd V. Favourable course of disease after incomplete remission on (131)I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. Eur J Nucl Med Mol Imaging. 2011;38:651–655. doi: 10.1007/s00259-010-1669-9. [DOI] [PubMed] [Google Scholar]
- 4.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156:167–172. doi: 10.1016/j.jss.2009.03.098. [DOI] [PubMed] [Google Scholar]
- 5.Wada N, Sugino K, Mimura T, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Nakayama H, Hirakawa S, Yukawa N, Rino Y, Masuda M, Ito K. Treatment strategy of papillary thyroid carcinoma in children and adolescents: clinical significance of the initial nodal manifestation. Ann Surg Oncol. 2009;16:3442–3449. doi: 10.1245/s10434-009-0673-4. [DOI] [PubMed] [Google Scholar]
- 6.Wada N, Masudo K, Nakayama H, Suganuma N, Matsuzu K, Hirakawa S, Rino Y, Masuda M, Imada T. Clinical outcomes in older or younger patients with papillary thyroid carcinoma: impact of lymphadenopathy and patient age. Eur J Surg Oncol. 2008;34:202–207. doi: 10.1016/j.ejso.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Palmer BA, Zarroug AE, Poley RN, Kollars JP, Moir CR. Papillary thyroid carcinoma in children: risk factors and complications of disease recurrence. J Pediatr Surg. 2005;40:1284–1288. doi: 10.1016/j.jpedsurg.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S, American Thyroid Association Guidelines Task Force Management guidelines for children with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer. Thyroid. 2015;25:716–759. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Sun Z, Adam MA, Adibe OO, Rice HE, Roman SA, Tracy ET. Predictors of nodal metastasis in pediatric differentiated thyroid cancer. J Pediatr Surg. 2017;52:120–123. doi: 10.1016/j.jpedsurg.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Jain NK, Mostoufi-Moab S, Hawkes CP, Nelson ND, Surrey LF, Jones ZS, Adzick NS, Kazahaya K, Bauer AJ. Extrathyroidal extension is an important predictor of regional lymph node metastasis in pediatric differentiated thyroid cancer. Thyroid. 2020;30:1037–1043. doi: 10.1089/thy.2019.0229. [DOI] [PubMed] [Google Scholar]
- 11.Spinelli C, Tognetti F, Strambi S, Morganti R, Massimino M, Collini P. Cervical lymph node metastases of papillary thyroid carcinoma, in the central and lateral compartments, in children and adolescents: predictive factors. World J Surg. 2018;42:2444–2453. doi: 10.1007/s00268-018-4487-z. [DOI] [PubMed] [Google Scholar]
- 12.Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27:1341–1346. doi: 10.1089/thy.2017.0500. [DOI] [PubMed] [Google Scholar]
- 13.Amin MB (2017) Editor-in-chief, AJCC Cancer staging system, 8th edition
- 14.Sung TY, Jeon MJ, Lee YH, Lee YM, Kwon H, Yoon JH, Chung KW, Kim WG, Song DE, Hong SJ. Initial and dynamic risk stratification of pediatric patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2017;102:793–800. doi: 10.1210/jc.2016-2666. [DOI] [PubMed] [Google Scholar]
- 15.Jeon MJ, Kim YN, Sung TY, Hong SJ, Cho YY, Kim TY, Shong YK, Kim WB, Kim SW, Chung JH, Kim TH, Kim WG. Practical initial risk stratification based on lymph node metastases in pediatric and adolescent differentiated thyroid cancer. Thyroid. 2018;28:193–200. doi: 10.1089/thy.2017.0214. [DOI] [PubMed] [Google Scholar]
- 16.Kim JW, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, Kim SY. Extent of extrathyroidal extension as a significant predictor of nodal metastasis and Extranodal extension in patients with papillary thyroid carcinoma. Ann Surg Oncol. 2017;24:460–468. doi: 10.1245/s10434-016-5594-4. [DOI] [PubMed] [Google Scholar]
- 17.Powers PA, Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Francis GL. Tumor size and extent of disease at diagnosis predict the response to initial therapy for papillary thyroid carcinoma in children and adolescents. J Pediatr Endocrinol Metab. 2003;16:693–702. doi: 10.1515/jpem.2003.16.5.693. [DOI] [PubMed] [Google Scholar]
- 18.Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, Yamashita S. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243:525–532. doi: 10.1097/01.sla.0000205977.74806.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available within the manuscript.
Not applicable.