Abstract
Flowers, leaves, fruits and buds of Tropaeolum majus are used for ornamental, medicinal and food purposes. However, salt stress limits the development and productivity of T. majus due to biochemical, physiological and anatomical disturbances. Polyamine application is an alternative for mitigating the harmful effects of salt stress. Thus, the objective of this work was to evaluate the effects of spermine application in T. majus grown under salt stress. The experiment was carried out in a completely randomized design, in a 3 × 2 factorial scheme, with 0, 40 mM (moderate salt stress) and 80 mM (severe salt stress) NaCl, and 0 and 1 mM spermine, and with five replicates. Growth (plant height, stem diameter, number of leaves, number of flowers, number of buds, leaf dry mass, stem dry mass and flower dry mass), gas exchange (gs, A, E, Ci and WUE), relative water content, contents of free amino acids, phenolic compounds, reducing and non-reducing sugars, lipid peroxidation and enzymatic activities (CAT, POD and APX) were evaluated. Spermine application decreased the harmful effects of salt stress on the growth and gas exchange and increased flowering in T. majus. Furthermore, the relative water content of T. majus increased under severe salt stress conditions. Spermine application reduced the contents of total phenolic compounds, free amino acids, reducing sugars and non-reducing sugars on leaves of T. majus. Spermine application increased CAT and POD activities in plants under severe salt stress and POD and APX in plants under moderate salt stress.
Keywords: Salinity, Polyamine, Tropaeolaceae, nasturtium
Introduction
Major abiotic stresses include extreme temperature fluctuations, drought, salinity, and heavy metal contamination (He et al. 2018). Salinity is a specific abiotic stress that affects agricultural regions with arid and semi-arid conditions and those with inadequate irrigation systems. Salinity have affected approximately one billion hectares of arable land, and the problem is worsening owing to inadequate drainage and irrigation management (including irrigation with saline water), low rainfall, high evaporation, global warming (Ibrahim 2016; Ali et al. 2021), and soil characteristics (Saleem et al. 2021). High soil salinity is characterized by the concentration of soluble salts, mainly NaCl, which is used as a classifying parameter. Saline soils are defined as having electrical conductivity greater than 4 dS m-1 at 25 °C (approximately 40 mM NaCl), and they generate an osmotic pressure greater than 0.2 MPa (Munns and Tester 2008; Almeida et al. 2017).
Photosynthetic efficiency of plants is severly affected by salt stress due to restricted stomatal function and non-stomatal limitation. Owing to salt stress, chlorophyll is degraded, RuBisCO activity is inhibited, and membrane proteins in the photosynthetic apparatus are destroyed (Shu et al. 2013). Additionally, it reduces turgor pressure and water content in plant tissues, resulting in decreased growth and production (Jia et al. 2021).
Salinity can cause water deficit in plants due to the decrease in the osmotic potential of the soil, reducing the water absorption capacity of the roots. The primary response of plants to drought is stomatal closure (reduced stomatal conductance) due to disturbed water relations and local ABA synthesis (Pan et al. 2021). Stomatal closure decreases water loss through transpiration, which reduces CO2 absorption. When CO2 levels are low, ribulose-1,5-bisphosphate is oxygenated, resulting in higher production of H2O2 in peroxisomes of cells (Alharby et al. 2021). Under salt stress conditions, reactive oxygen species (ROS) accumulate, causing biochemical changes (Nxele et al. 2017). ROS produced in photosystem I (PSI) are scavenged by reactions as water-water cycle or Mehler-ascorbate peroxidase pathway (Ahanger and Agarwal 2017; Ahanger et al. 2017). Thus, the use of strategies to minimize the harmful effects of salt stress on plants is of crucial interest. The use of phytohormones, such as polyamines, can be an effective strategy to reduce these effects.
All living organisms, including bacteria, animals, and plants, contain polyamines, which are low-molecular-weight aliphatic polycations with extremely wide distributions (Liu et al. 2015). Polyamines are present in their free form in higher plants, mainly as putrescine, spermidine, spermine and cadaverine (Nahar et al. 2016). Free and conjugated polyamine levels are important for plant stress tolerance (Chen et al. 2019). Putrescine (Put), a compound with two amino groups, is a precursor of spermidine (Spd) and spermine (Spm) and plays a key role in polyamine biosynthesis (Pegg 2016). Put acts as a negative regulator, whereas Spm and Spd have positive effects on plant growth and developmental processes (Jia et al. 2021).
Polyamines are defence activators in higher plants. They have robust responses to adverse environmental conditions, and are associated with ROS homeostasis, where they activate antioxidant enzymes and scavenge ROS (Jia et al. 2021). The antioxidant defence machinery is a ROS scaveninging syste that protects plants from oxidative damage. A combination of enzymatic systems (such as SOD, CAT, APX, and POD) and non-enzymatic systems (such as ascorbate, glutathione, phenols, alkaloids, non-protein amino acids, and α-tocopherols) are employed to protect plant cells against damage caused by ROS (Nahar et al. 2016). Polyamines possibly play a protective role because of their ability to act as osmolytes or to stimulate the production and accumulation of other osmolytes, such as proline, glycine betaine, and GABA (Fang et al. 2020; Jankovska-Bortkevič et al. 2020). Spermine application facilitates ROS metabolism and photosynthesis, which improves plant growth and reduces the harmful effects of salt stress (Baniasadi et al. 2018). Spermine accumulation can activate a set of salt stress response genes making plants tolerant to stress (Marco et al. 2019). Spermine application alleviates salt stress in Oryza sativa(Paul and Roychoudhury 2017; Islam et al. 2020), Calendula officinalis (Baniasadi et al. 2018), and Vigna radiata (Nahar et al. 2017).
Nasturtium (Tropaeolum majus L. - Tropaeolaceae) is an edible, ornamental and medicinal plant, with showy flowers, single or double, reaching 2–3 m in length and 30–40 cm in height (Melo et al. 2018). This plant is native to the Andes and grows worldwide. It is one of the main edible flowers, with economic and social importance. T. majus flowers have a strong, spicy flavour and are abundantly used in salads, sauces, grilled dishes, and stuffed preparations (Koike et al. 2015). This plant also contains a wide variety of bioactive compounds, such as flavonoids (quercetin and isoquercitrin), fatty acids (oleic and linoleic), vitamin C and benzylthiocyanate (Bazylko et al. 2013). It can grow in both in gardens and in pots. Nasturtium grows in different environments; however, salinity can affect its growth (Bloem et al. 2014). Salinity of soil or irrigation water is common in several regions of the world, mainly in arid and semi-arid regions. Since spermine has been reported to alleviate the harmful effects of salt stress, this study aimed to evaluate the effects of spermine as a possible mitigation agent for salt stress in nasturtium.
Knowledge of the effects of salinity and polyamine application on the growth, gas exchange and biochemical potential of T. majus is scarce. Thus, the objective of this work was to evaluate the effects of spermine application in T. majus grown under salt stress.
Materials and methods
Experiment site and experimental design
The experiment was carried out in a greenhouse at the Department of Agronomy of the Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil. The minimum and maximum temperatures with humidity during the experiment were 15 ºC and 40%, 30 ºC and 86%, respectively. The experiment was distributed in a completely randomized design, in a 3 × 2 factorial scheme, with 0, 40 (moderate salt stress) and 80 (severe salt stress) mM NaCl, and 0 and 1 mM spermine with five replications.
Plant material
Seeds of T. majus (Assorted variety, semi-double blooms, produced by Feltrin Sementes – Farroupilha/RS) were sown in a 128-cell Styrofoam tray with a commercial substrate (Topstrato, EC = 0.5 ± 0.3 dS m− 1; pH = 5.8 ± 0.3). Seedlings were transplanted to pots containing 1.2 L of the same commercial substrate 12 days after planting. Spermine (Sigma-Aldrich) was diluted in deionized water and Tween 20 (Sigma-Aldrich) (0.05%) was used as a surfactant to increase plant absorption. Deionized water and Tween 20 (0.05%) were used as control. Plants were sprayed with approximately 10 mL of each solution or until completely wet. Spermine (Spm) applications were made every seven days for four weeks. The first Spm application was done on the first day of salt stress (saline irrigation), 20 days after planting (DAP).
The plants were irrigated daily, maintaining them at 80% pot capacity. The pots with the plants were weighed daily to calculate evapotranspiration and determine the amount of water to be applied (Girardi et al. 2016). The plants were fertigated once a week with 4 g L− 1 of 20-20-20 fertilizer + micronutrients (Peters Professional).
Variables analyzed
Plant growth
All data were collected 60 days after saline water irrigation commenced. Plant height (cm), stem diameter (mm), number of leaves (dimensionless), number of flowers (dimensionless), number of buds (dimensionless), leaves dry mass (g), stem dry mass (g) and flower dry mass (g) were evaluated.
Gas exchange
Gas exchange and chlorophyll indices were analyzed at 80 DAP. Gas exchange was assessed using an infrared gas analyzer (IRGA – model LCPro, ADC BioScientific Ltd.), and measurements were taken between 8 and 10 h on a specific day. Stomatal conductance (gs = mol H2O m− 2 s− 1), net photosynthesis (A = µmol CO2 m− 2 s− 1), transpiration (E = mmol H2O m− 2 s− 1), internal carbon concentration (Ci = µmol CO2 mol air− 1) and water use efficiency (WUE = µmol CO2/mmol H2O m− 2 s− 1) were evaluated.
Relative water content (RWC)
Ten discs (of 1 cm diameter) were removed from the first fully expanded leaves to determine the relative water content (RWC). After weighing and obtaining the fresh mass (FM), the leaf discs were immersed in deionized water for 3 h until they reached the turgid mass (TM). Afterwards, the discs were placed in an oven at 65 ºC for 48 h to obtain the dry mass (DM). The RWC was calculated using the formula: RWC(%)=[(FM-DM)/(TM-DM)] x 100.
Total phenolic compounds
Extracts used for sugar analysis were also examined for phenolic compounds. Gallic acid was used as a standard to determine the phenolic content according to Fu et al. (2010). Absorbance was determined using a spectrophotometer at 760 nm and the content was expressed in mg g− 1 fresh mass.
Free aminoacids
Total free amino acids content was measured from ethanol extracts using a method reported by Yemm et al. (1955), with glycine as a standard. Absorbance was determined using a spectrophotometer at 570 nm and the content was expressed in mM g− 1.
Reducing and non-reducing sugars
Soluble sugars were extracted from approximately 2 g of fresh ground leaves homogenized in 80% ethanol heated to 85 °C. The extract was centrifuged at 12,000 g for 8 min. The supernatant was collected and the precipitate was extracted with 80% ethanol. Sulfuric phenol method (Dubois et al. 1956) was used to determine the content of total soluble sugars (TSS) in the sample. The assay containing 0.25 mL of supernatant, 0.25 mL of 5% phenol and 1.25 mL of concentrated H2SO4 was incubated at 30 °C for 20 min. After cooling, absorbance was measured at 490 nm. The TSS content was expressed in percentages of TSS per leaves fresh mass, using sucrose as the standard.
The reducing sugar (RS) content was quantified using 3,5-dinitrosalicylic acid (DNS) method proposed by Gonçalves et al. (2010), with modifications. The supernatant (0.5 mL) was added to 0.5 mL of DNS reagent, and the tubes were heated in a water bath for 5 min. After cooling in an ice bath, 4 mL of water was added and absorbance was read at 540 nm. Fructose was used as the standard and the RS content was determined as %RS per fresh leaf mass. Non-reducing sugar content (NRS) was estimated by the difference between TSS and RS, and the results were expressed as %NRS per fresh mass.
Lipid peroxidation
Malondialdehyde (MDA) content was determined using thiobarbituric acid method described by Heath and Packer (1968). A leaf sample (0.2 g) was homogenized in 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 12,000 g for 15 min at 4 °C. Thereafter, 0.5 mL of the supernatant was mixed with 1.5 mL of 0.5% thiobarbituric acid (TBA) diluted in 20% TCA (w/v) and heated at 90 °C for 20 min in a water bath. Absorbance was measured using a spectrophotometer at 532 and 600 nm. MDA content was expressed as nmol g− 1 fresh mass.
Enzyme activity of the antioxidant system (CAT, POD and APX)
The enzyme extract was prepared by homogenizing 0.2 g of leaf in 2 mL extraction buffer containing 100 mM potassium phosphate (pH 7.0), 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% (w/v) polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 14,000 g for 15 min at 4 ºC. The supernatant was collected and used to determine catalase (CAT), peroxidase (POD) and ascorbate peroxidase (APX) activities.
The CAT activity was determined as described by Havir and McHale (1987). The reaction buffer contained 50 mM potassium phosphate (pH 7.0) and 12.5 mM H2O2. The reaction was initiated by adding enzymatic extract, and the activity was determined by a decrease in absorbance at 240 nm for 1 min. CAT activity was expressed as µmol min− 1 mg− 1 protein.
The POD activity was quantified according to the method proposed by Kar and Mishra (1976). The reaction buffer was prepared using 25 mM potassium phosphate (pH 6.5), 20 mM guaiacol, and 20 mM H2O2. The activity was determined by an increase in absorbance at 470 nm and expressed as µmol min− 1 mg− 1 protein. The APX activity was measured according to Nakano and Asada (1981). The reaction medium comprised 50 mM potassium phosphate (pH 7.8), 0.25 mM ascorbic acid and 0.3 mM H2O2. Activity was monitored by reducing absorbance at 290 nm for 1 min. The APX activity was expressed as µmol min− 1 mg− 1 protein. The protein content of the enzymatic extracts was determined according to the method proposed by Bradford (1976). Bovine serum albumin (BSA) was used to obtain the standard curve.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and, when significant (p ≤ 0.05), a comparison of means (Tukey’s test) was performed using ExpDes statistical package (Ferreira et al., 2018). Analysis of canonical correspondence with confidence ellipses (p ≤ 0.01) were performed to study the interrelationship between variables and factors using the candisc package (Friendly and Fox, 2017). Pearson’s correlation analysis was performed using the corrplot package (Wei and Simko, 2017). R statistical program (R Core Team, 2021) was used to perform the statistical analyses.
Results
Spermine application decreased the harmful effects of moderate salt stress and increased the plant height of T. majus. Spermine application reduced the negative impacts of moderate salt stress on stem diameter, leaf number and flower number compared to plants without stress and application of this phytohormone. Spermine application decreased the harmful effects of moderate salt stress on number of buds, stem dry mass and flower dry mass compared to plants without spermine application and under moderate salt stress. Spermine induced leaf and flower growth, both in plants without stress and those under moderate salt stress. Plant growth was diminished by severe salt stress (Fig. 1).
Fig. 1.
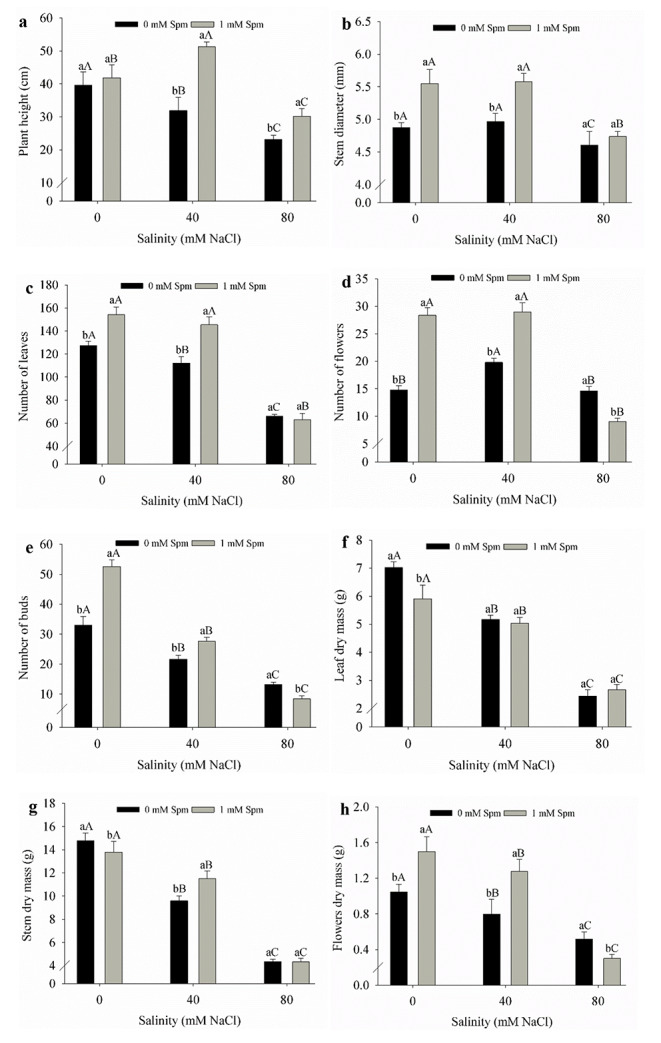
Plant height (a), stem diameter (b), number of leaves (c), number of flowers (d), number of buds (e), leaf dry mass (f), stem dry mass (g) and flower dry mass (h) of Tropaeolum majus under salt stress and spermine application. Means followed by the same lowercase letters do not differ for spermine and the same uppercase letters do not differ for salt stress by the Tukey test at 5%. n = 5
Spermine application reduced the harmful effects of moderate salt stress on stomatal conductance (gs) and transpiration (E) of T. majus. However, it did not reduce the effects of salt stress on net photosynthesis (A). Increase in salt stress decreased gs, A and E and increased internal carbon concentration (Ci). Spermine application decreased water use efficiency of plants under all the stress conditions, including the non-stressed plants. Spermine application decreased gs, A and E in plants under severe salt stress (Fig. 2).
Fig. 2.

Stomatal conductance (a), net photosynthesis (b), transpiration (c), internal carbon concentration (d) and water use efficiency (e) of Tropaeolum majus under salt stress and spermine application. Means followed by the same lowercase letters do not differ for spermine and the same uppercase letters do not differ for salt stress by the Tukey test at 5%. n = 5
Spermine application reduced the harmful effects of severe salt stress on the relative water content of T. majus. Spermine application decreased the contents of total phenolic compounds, free amino acids, reducing sugars and non-reducing sugars in plants under severe salt stress and in plants without stress. Spermine had no effect on the relative water content, total phenolic compounds and reducing sugars in plants under moderate salt stress. Increased salt stress decreased the contents of total phenolic compounds, reducing sugars, and non-reducing sugars (Fig. 3).
Fig. 3.

Relative water content (a), free amino acid content (b), total phenolic compounds (c), reducing sugars (d) and non-reducing (e) sugars of Tropaeolum majus under salt stress and spermine application. Means followed by the same lowercase letters do not differ for spermine and the same uppercase letters do not differ for salt stress by the Tukey test at 5%. n = 5
Spermine application decreased lipid peroxidation (MDA) of T. majus under moderate salt stress, but had no effect on plants under severe salt stress. Spermine application increased the activities of CAT and POD in plants under severe salt stress and of POD and APX in plants under moderate salt stress. Spermine application did not increase the APX activity of plants under severe salt stress. However, CAT activity was increased in the no-stressed plants. Moderate salt stress induced CAT activity but decreased POD and APX activities (Fig. 4).
Fig. 4.

Lipid peroxidation (a), catalase activity (b), peroxidase activity (c) and ascorbate peroxidase activity (d) of Tropaeolum majus under salt stress and spermine application. Means followed by the same lowercase letters do not differ for spermine and the same uppercase letters do not differ for salt stress by the Tukey test at 5%. n = 5
A canonical correspondence analysis with confidence ellipses were performed to study the interrelationship between the variables and factors. The plant height (PH), stem diameter (SD), number of leaves (NL) and number of flowers (NFl) had a strong relationship with spermine application in non-stressed plants (0P1) and in plants under moderate stress (40P1) (Fig. 5a). These results suggest that spermine attenuates the harmful effects of salt stress on these variables. The number of buds (NB), flower dry mass (FlDM), stem dry mass (SDM) and leaf dry mass (LDM) had a strong relationship with spermine application in no-stressed plants; however, plants under moderate stress that received spermine had stronger relationship with these variables than plants that did not receive spermine (Fig. 5b).
Fig. 5.
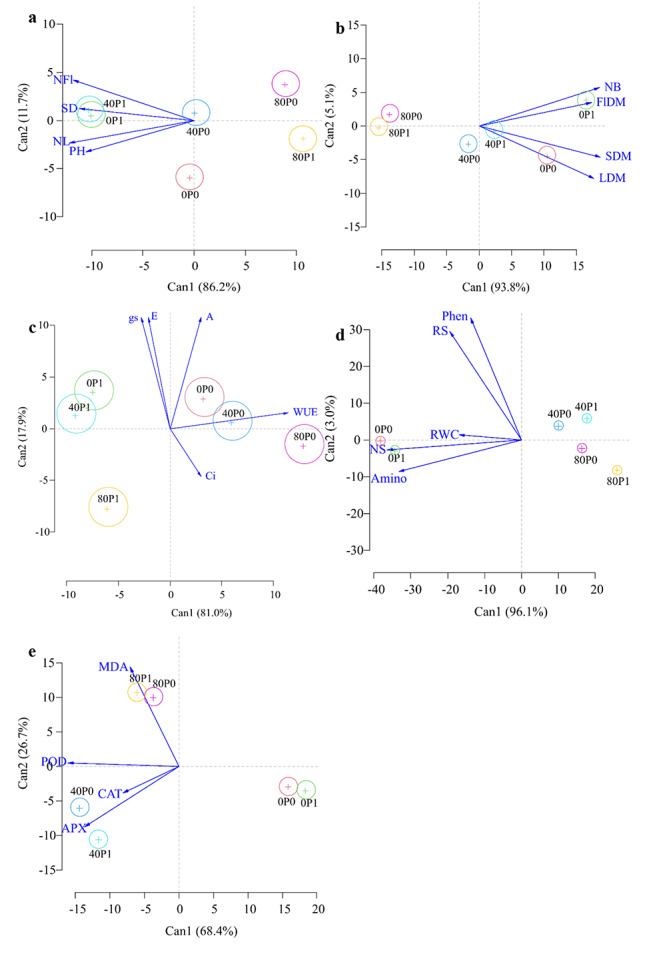
Canonical variables analysis of growth (a and b), gas exchange (c) and biochemistry (d and e) of Tropaeolum majus under salt stress and spermine application. LN = number of leaves, PH = plant height, SD = stem diameter, NFl = number of flowers, NB = number of buds, FlDM = flowers dry mass, SDM = stem dry mass, LDM = leaves dry mass, Phen = phenolic compounds, RWC = relative water content, RS = reducing sugars, NS = non-reducing sugars, Amino = amino acids, MDA = malondialdehyde, CAT = catalase, POD = peroxidase, APX = ascorbate peroxidase, P = spermine. n = 5
E, gs and A had a strong relationship with plants without salt stress and moderate stress, and spermine application (Fig. 5c). The contents of non-reducing sugars, amino acids and relative water content had a strong relationship with no-stressed plants both with and without spermine application (Fig. 5d). Reducing sugars and phenolic compounds had a strong relationship with plants under moderate salt stress and spermine application. The activities of POD and APX were more related to plants under moderate salt stress and spermine application, and CAT activity was more related to plants under moderate salt stress, but without spermine application (Fig. 5e). Lipid peroxidation was more related to plants under severe salt stress both with and without spermine application.
Gas exchanges (gs, A and E), sugar contents (RS and NS), amino acids (Ami), phenolic compounds (Phen) and relative water content (RWC) were positively correlated with the growth (LN, NFl, NB, PH, SD, SDM, LDM and FIDM) of T. majus plants (Fig. 6). CAT and APX activities were positively correlated with flower number (NFl) and stem diameter (SD). The activities of CAT, POD and APX enzymes were positively correlated. Lipid peroxidation was negatively correlated with all the variables, except internal carbon concentration (Ci).
Fig. 6.
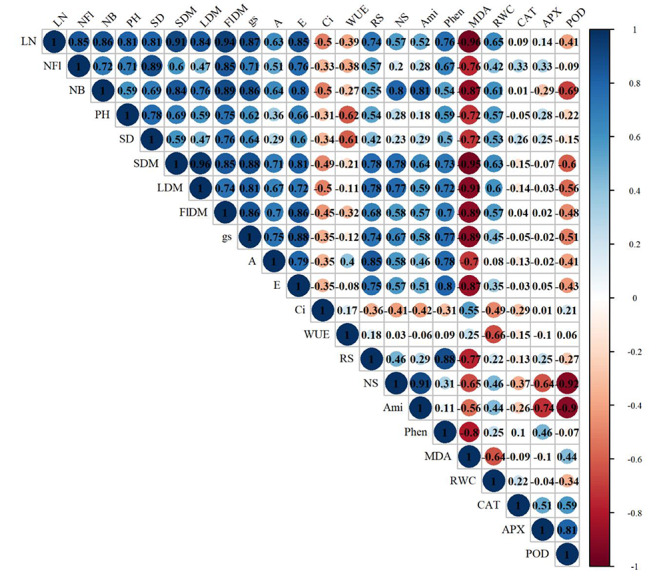
Pearson correlation of growth, gas exchange and biochemistry of Tropaeolum majus under salt stress and spermine application. LN = number of leaves, PH = plant height, SD = stem diameter, NFl = number of flowers, NB = number of buds, FlDM = dry mass of flowers, SDM = dry mass of stem, LDM = dry mass of stem, Phen = phenolic compounds, RWC = relative water content, RS = reducing sugars, NS = non-reducing sugars, Ami = amino acids, MDA = malondialdehyde, CAT = catalase, POD = peroxidase, APX = ascorbate peroxidase. n = 5
Discussion
The effects of spermine application on growth, physiological and biochemical changes of T. majus under salt stress had previously been unknown. This study reported how spermine mitigates the damage caused by salt stress on growth, gas exchange and biochemical changes of this plant.
In this study, spermine application reduced the harmful effects of moderate salt stress on growth of T. majus. This behavior is related to the role of polyamines in increasing cell division, photosynthetic efficiency, accumulation of assimilates, and in maintaining the antioxidant system of plants under salt stress (Baniasadi et al. 2018). Our results also showed that spermine increased the number of leaves and flowers of T. majus under both moderate salt stress and no-stressed plants. This may be related to the action of polyamines on bud differentiation (Chen et al. 2019) and, consequently, in the number of flowers. The role of polyamines in regulating flowering has been studied. A premature flowering phenotype of Arabidopsis thaliana has been correlated with a significant increase in putrescine levels (Molesini et al. 2015). Expression levels of GtSPDS and GtSPMS increased transiently during the vegetative to reproductive growth phases, and gene overexpression accelerated flowering, suggesting that these genes are involved in inflorescence induction in Gentiana triflora (Imamura et al. 2015). Salt stress negatively affects growth due to decreased turgor pressure and water content in plant tissues, leading to decreased growth rate (Chen et al. 2019; Paul and Roychoudhury 2019).
Our results demonstrated that spermine application decreased the harmful effects of salt stress on gas exchange of T. majus, as gs and E were higher in plants under moderate salt stress and spermine application and gs, A and E were higher in plants under severe salt stress and spermine application. Spermine application decreased the WUE of plants under all stress conditions. This behavior is related to the positive effects of spermine on photosynthetic efficiency under stress conditions, because of its acid neutralizing and antioxidant properties and membrane and cell wall stabilization (Shu et al. 2013). Spermine decreased the gs of T. majus plants under severe salt stress as a way of increasing tolerance to the water deficit caused by increased salinity to minimize water losses to the environment (Dias et al. 2021). This stomatal closure is related to a series of osmotic signalling mechanisms, that result in efflux of ions from guard cells, reducing their turgor and volume. This is signalled by increased abscisic acid (ABA) content in roots because of the decrease in soil water potential caused by increased salinity (Hsu et al. 2021). An increase in the growth of plants under stress after the application of phytohormones because of their impact on photosynthesis has been reported (Ahanger et al. 2020).
The increase in the relative water content of T. majus under severe salt stress and spermine application suggests that these plants suffered less maximum oxidative stress in terms of water loss. This is related to the action of this phytohormone on the water potential of plants (Tailor and Bhatla 2021) under stress, which regulates membrane transport by blocking through selective and non-selective ion channels (Wu et al. 2020). Effective reduction of cellular osmotic potential maintains plant cell metabolism and prevents dehydration damage induced by the water deficit caused by excess salts by accumulating more glycine betaine, soluble sugars and proline (Jia et al. 2021). Spermine application decreased the content of total phenolic compounds, free amino acids, reducing sugars and non-reducing sugars of plants under severe salt stress, possibly because of the action of this phytohormone in improving the tolerance of plants to stress. Regulation of osmolytes can be an effective mechanism for increasing plant tolerance to water stress induced by the salt accumulations in root zones, by maintaining adequate cell water for healthy metabolism (Alharby et al. 2021). Increase salt stress decreases the contents of total phenolic compounds, reducing sugars and non-reducing sugars because these compounds are a non-enzymatic pathways for ROS scavenging and osmotic potential regulation (Abdallah et al. 2016).
Lipid peroxidation determined from malondialdehyde (MDA) content is an indicator of stress-related biochemical changes in plants (Shah et al. 2021). Spermine decreases the MDA content of T. majus under moderate salt stress because it upregulates the antioxidant system and relieves oxidative stress through ROS scavenging (Ikbal et al. 2014; Nahar et al. 2016), resulting in lipid peroxidation. Furthermore, this behavior may be associated with the role of polyamines in interaction with anionic functional groups of membranes and proteins, in addition to influencing the stability and permeability of cell membranes by forming electrostatic bonds with groups of phospholipid heads (Sharma et al. 2021).
Exposure to salinity increases ROS production in plant cells (Pan et al. 2021). Plants use enzymes of the antioxidant system to scavenge ROS. This study revealed that spermineapplication increased the activities of CAT and POD in plants under severe salt stress and those of POD and APX under moderate salt stress. This behavior is associated with the action of polyamines in stimulating the activities of antioxidant enzymes to reduce the harmful effects of salt stress on the plants. Furthermore, polyamines form complexes with SOD, GPX and CAT, causing these enzymes to work more efficiently, than isolated enzymes (Li et al. 2014). Subsequently, this may mitigate of the harmful effects of stress. Spermine has been suggested to be signalling molecule that can activates antioxidant enzymes (Mitsuya et al. 2009). Polyamines up-regulate the activation of the nasturtium antioxidant system under salt stress. The mechanism involved can be due to this phytohormone plays a role in the modulation of ROS homeostasis, inhibiting the auto-oxidation of metals, such as Fe2+ and Cu2+, which impair supply of electrons for generation of ROS, act directly as antioxidants and scavenge ROS (Liu et al. 2015). Furthermore, this behavior is related to the role of polyamines in the regulation of antioxidant systems, along with changes in ROS production and redox status (Tanou et al. 2014). Osmotic and ionic imbalance caused by salt stress can create secondary stress in plants, including accumulation of toxic compounds such as ROS (hydroxyl radicals, hydrogen peroxide and superoxide anions) that accumulate in plant cells (Yang and Guo 2018). Spermidine application promotes activities of antioxidant enzymes torestore the integrity of photosynthetic apparatus in leaves of Carya illinoensis (Wu et al. 2020).
We observed, through canonical correspondence analysis, that spermine increases the growth and flowering of T. majus. This behavior is related to the role of this phytohormone in cell division, cell proliferation and differentiation, and morphological development (Zhang et al. 2014; Jia et al. 2021).
Gas exchange also had a strong relationship with plants that received spermine. This behavior is associated with the role of polyamines in maintaining photosynthetic performance, promoting RuBisCO activity and reducing carbohydrate accumulation to increase the photosynthetic rate, and act directly on the chloroplast thylakoid (Qian et al. 2021), allowing plants to better acclimatize to salt stress. Additionally, the levels of reducing sugars and phenolic compounds and the activities of POD and APX were more related to plants under spermine application, because of the action of this phytohormone in ROS scavenging, regulation of ROS homeostasis, and activation of the antioxidant enzyme machinery (Liu et al. 2019; Islam et al. 2020), thus facilitating the acclimatization to stress. Spermidine application increased CAT, SOD and POD activity of Gladiolus gandavensis under salt stress (Qian et al. 2021).
The positive correlation between gas exchange, sugar, amino acids, phenolic compounds and relative water content with the growth of T. majus is related to the vital role of osmoprotectors in improving the hyperosmolarity caused by salt stress and establishing cellular ionic homeostatic conditions (Saleem et al. 2021), improving gas exchange and consequently plant growth. An increase in MDA content decreased all variables because of the damage caused by ROS in response to increased salt stress. The positive correlation between enzymatic activities may be because POD and CAT decompose H2O2 into H2O (Shah et al. 2021). Spermine decreases MDA content under salt stress, stimulating protein synthesis or activating natural hormones to maintain membrane integrity (Qian et al. 2021).
Conclusions
Spermine application decreased the harmful effects of salt stress on growth and gas exchange and increased the flowering of T. majus. Furthermore, the relative water content of T. majus increased under severe salt stress conditions. However, spermine reduced the contents of total phenolic compounds, free amino acids, and reducing and non-reducing sugars. Spermine increased the activities of CAT and POD in plants under severe salt stress, and those of POD and APX in plants under moderate salt stress.
Acknowledgements
The authors wish to thank the Brazilian National Council for Scientific and Technological Development (CNPq - finance code 140636/2019-6), the Coordination for the Improvement of Higher Education Personnel (CAPES - finance code 001) and the Foundation for Research Support of the State of Minas Gerais (FAPEMIG). The authors wish to thank the David Michael Miller a native translator who corrected the language of this paper.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed Toshik Iarley da Silva, Marlon Gomes Dias, Nícolas Oliveira de Araújo, Mirelle Nayana de Sousa Santos and Renata Ranielly Pedroza Cruz. The first draft of the manuscript was corrected by Thiago Jardelino Dias, Wellington Souto Ribeiro, José Antonio Saraiva Grossi and José Geraldo Barbosa. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors thank the National Council for Scientifc and Technological Development (CNPq – finance code 140636/2019-6).
Available of data and material
All data generated or analyzed during this study will be provided upon request to the corresponding author.
Code Availability
Not applicable.
Declarations
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdallah SB, Aung B, Amyot L, Lalin I, Lachaal M, Karray-Bouraoui N, Hannoufam A. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol Plant. 2016;38:1–13. doi: 10.1007/s11738-016-2096-8. [DOI] [Google Scholar]
- Ahanger MA, Aziz U, Alsahli A, Alyemeni MN, Ahmad P. Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules. 2020;10(1):147. doi: 10.3390/biom10010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biology Plants. 2017;23(4):731–744. doi: 10.1007/s12298-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger MA, Agarwal RM. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol Biochem. 2017;115:449–460. doi: 10.1016/j.plaphy.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Alharby HF, Al-Zahrani HS, Alzahrani YM, Alsamadany H, Hakeem KR, Rady MM. Maize grain extract enriched with polyamines alleviates drought stress in triticum aestivum through up-regulation of the ascorbate–glutathione cycle, glyoxalase system, and polyamine gene expression. Agronomy. 2021;11:949. doi: 10.3390/agronomy11050949. [DOI] [Google Scholar]
- Ali M, Afzal A, Parveen A, Kamran M, Javed MR, Abbasi GH, Malik Z, Riaz M, Ahmad S, Chattha MS, Ali M, Ali Q, Uddin MZ, Rizwan M, Ali S. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol Biochem. 2021;158:208–218. doi: 10.1016/j.plaphy.2020.10.040. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Oliveira MM, Saibo NJ. Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet Mol Biology. 2017;40:326–345. doi: 10.1590/1678-4685-GMB-2016-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniasadi F, Saffari VR, Moud AAM. Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci Hort. 2018;234:312–317. doi: 10.1016/j.scienta.2018.02.069. [DOI] [Google Scholar]
- Bazylko A, Granica S, Filipek A, Piwowarski J, Stefańska J, Osińska E, Kiss AK. Comparison of antioxidant, anti-inflammatory, antimicrobial activity and chemical composition of aqueous and hydroethanolic extracts of the herb of Tropaeolum majus L. Ind Crops Prod. 2013;50:88–94. doi: 10.1016/j.indcrop.2013.07.003. [DOI] [Google Scholar]
- Bloem E, Haneklaus S, Kleinwächter M, Paulsen J, Schnug E, Selmar D. Stress-induced changes of bioactive compounds in Tropaeolum majus L. Ind Crops Prod. 2014;60:349–359. doi: 10.1016/j.indcrop.2014.06.040. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Frontiers in Plant Science, 9, 1945. 10.3389/fpls.2018.01945 [DOI] [PMC free article] [PubMed]
- Dias AS, Lima GSD, Gheyi HR, Melo ASD, Silva PCC, Soares LADA, Paiva FJS, Silva SSD. Effect of combined potassium-phosphorus fertilization on gas exchange, antioxidant activity and fruit production of West Indian cherry under salt stress. Arid Land Research and Management. 2021;35:1–18. doi: 10.1080/15324982.2021.1959464. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Biochem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Fang W, Qi F, Yin Y, Yang Z. Exogenous spermidine promotes γ-aminobutyric acid accumulation and alleviates the negative effect of NaCl stress in germinating soybean (Glycine max L.) Foods. 2020;9:267. doi: 10.3390/foods9030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Xu BT, Xu XR, Qin XS, Gan RY, Li HB. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules. 2010;15:8602–8617. doi: 10.3390/molecules15128602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi LB, Peiter MX, Bellé RA, Robaina AD, Torres RR, Kirchner JH, Ben LHB. Evapotranspiration and crop coefficients of potted Alstroemeria × Hybrida grown in greenhouse. Irriga. 2016;21(4):817–829. doi: 10.15809/irriga.2016v21n4p817-829. [DOI] [Google Scholar]
- Gonçalves M, Rodrigues-Jasso MR, Gomes N, Teixeira JA, Belo I. Adaptation of dinitrosalicylic acid method to microliter plates. Anal Methods. 2010;2(12):2046–2048. doi: 10.1039/c0ay00525h. [DOI] [Google Scholar]
- Havir EA, McHale NA. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987;84:450–455. doi: 10.1104/pp.84.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, He CQ, Ding NZ. Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front Plant Sci. 2018;9:1771. doi: 10.3389/fpls.2018.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives in Biochemistry and Biophysics. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hsu PK, Dubeaux G, Takahashi Y, Schroeder JI. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021;105(2):307–321. doi: 10.1111/tpj.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim EA. Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol. 2016;192:38–46. doi: 10.1016/j.jplph.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Ikbal FE, Hernández JA, Barba-Espín G, Koussa T, Aziz A, Faize M, Diaz-Vivancos P. Enhanced salt-induced antioxidative responses involve a contribution of polyamine biosynthesis in grapevine plants. J Plant Physiol. 2014;171:779–788. doi: 10.1016/j.jplph.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Imamura T, Fujita K, Tasaki K, Higuchi A, Takahashi H. Characterization of spermidine synthase and spermine synthase–The polyamine-synthetic enzymes that induce early flowering in Gentiana triflora. Bioch. Biochem Biophys Res Commun. 2015;463(4):781–786. doi: 10.1016/j.bbrc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Islam MA, Pang JH, Meng FW, Li YW, Ning XU, Chao YANG, Jun LIU. Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J Integr Agric. 2020;19(3):643–655. doi: 10.1016/S2095-3119(19)62705-X. [DOI] [Google Scholar]
- Jankovska-Bortkevič E, Gavelienė V, Šveikauskas V, Mockevičiūtė R, Jankauskienė J, Todorova D, Sergiev I, Jurkonienė S. Foliar application of polyamines modulates winter oilseed rape responses to increasing cold. Plants. 2020;9:179. doi: 10.3390/plants9020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T, Hou J, Iqbal MZ, Zhang Y, Cheng B, Feng H, Li Z, Liu L, Zhou J, Feng G, Nie G, Ma X, Liu W, Peng Y. Overexpression of the white clover TrSAMDC1 gene enhanced salt and drought resistance in Arabidopsis thaliana. Plant Physiol Biochem. 2021;165:147–160. doi: 10.1016/j.plaphy.2021.05.018. [DOI] [PubMed] [Google Scholar]
- Kar M, Mishra D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976;57:315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike A, Barreira JC, Barros L, Santos-Buelga C, Villavicencio AL, Ferreira IC. Irradiation as a novel approach to improve quality of Tropaeolum majus L. flowers: Benefits in phenolic profiles and antioxidant activity. Innov. Innovative Food Science & Emerging Technologies. 2015;30:138–144. doi: 10.1016/j.ifset.2015.04.009. [DOI] [Google Scholar]
- Li X, Gong B, Xu K. Interaction of nitric oxide and polyamines involves antioxidants and physiological strategies against chilling-induced oxidative damage in Zingiber officinale Roscoe. Sci Hort. 2014;170:237–248. doi: 10.1016/j.scienta.2014.03.026. [DOI] [Google Scholar]
- Liu JH, Wang W, Wu H, Gong X, Moriguchi T. Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci. 2015;6:827. doi: 10.3389/fpls.2015.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Arnold RJ, Yang SZ, Wu JY, Li ZH, Li Y, Cheng Y. Foliar application of exogenous polyamines to ameliorate drought-induced oxidative damage and physiological inhibition in Toona ciliata seedlings. Australian Forestry. 2019;82(3):139–150. doi: 10.1080/00049158.2019.1636349. [DOI] [Google Scholar]
- Marco F, Busó E, Lafuente T, Carrasco P. Spermine confers stress resilience by modulating abscisic acid biosynthesis and stress responses in Arabidopsis plants. Front Plant Sci. 2019;10:972. doi: 10.3389/fpls.2019.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo AC, Costa SCA, Castro AF, Souza ANV, Sato SW, Lívero FAR, Lourenço ELB, Baretta IP, Lovato ECW. Hydroethanolic extract of Tropaeolum majus promotes anxiolytic effects on rats. Revista Brasileira de Farmacognosia. 2018;28(5):589–593. doi: 10.1016/j.bjp.2018.06.006. [DOI] [Google Scholar]
- Mitsuya Y, Takahashi Y, Berberich T, Miyazaki A, Matsumura H, Takahashi H, Terauchi R, Kusano T. Spermine signaling plays a significant role in the defense response of Arabidopsis thaliana to cucumber mosaic virus. J Plant Physiol. 2009;166(6):626–643. doi: 10.1016/j.jplph.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Molesini B, Mennella G, Martini F, Francese G, Pandolfini T. Involvement of the putative N-acetylornithine deacetylase from Arabidopsis thaliana in flowering and fruit development. Plant Cell Physiol. 2015;56(6):1084–1096. doi: 10.1093/pcp/pcv030. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Mahmud JA, Suzuki T, Fujita M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma. 2017;254(1):445–460. doi: 10.1007/s00709-016-0965-z. [DOI] [PubMed] [Google Scholar]
- Nahar K, Hasanuzzaman M, Rahman A, Alam MM, Mahmud JA, Suzuki T, Fujita M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci. 2016;7:1104. doi: 10.3389/fpls.2016.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nxele X, Klein A, Ndimba BK. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. South Afr J Bot. 2017;108:261–266. doi: 10.1016/j.sajb.2016.11.003. [DOI] [Google Scholar]
- Pan T, Liu M, Kreslavski VD, Zharmukhamedov SK, Nie C, Yu M, Yu M, Kuznetsov VV, Allakhverdiev SI, Shabala S. Non-stomatal limitation of photosynthesis by soil salinity. Reviews in Environmental Science and Technology. 2021;51(8):791–825. doi: 10.1080/10643389.2020.1735231. [DOI] [Google Scholar]
- Paul S, Roychoudhury A. Seed priming with spermine and spermidine regulates the expression of diverse groups of abiotic stress-responsive genes during salinity stress in the seedlings of indica rice varieties. Plant Gene. 2017;11:124–132. doi: 10.1016/j.plgene.2017.04.004. [DOI] [Google Scholar]
- Paul S, Roychoudhury A. Transcript analysis of abscisic acid-inducible genes in response to different abiotic disturbances in two indica rice varieties. Theoretical and Experimental Plant Physiology. 2019;31(1):249–272. doi: 10.1007/s40626-018-0131-4. [DOI] [Google Scholar]
- Pegg AE. Functions of polyamines in mammals. J Biol Chem. 2016;291(29):14904–14912. doi: 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian R, Ma X, Zhang X, Hu Q, Liu H, Zheng J. Effect of exogenous spermidine on osmotic adjustment, antioxidant enzymes activity, and gene expression of Gladiolus gandavensis seedlings under salt stress. J Plant Growth Regul. 2021;40:1353–1367. doi: 10.1007/s00344-020-10198-x. [DOI] [Google Scholar]
- Saleem S, Mushtaq NU, Shah WH, Rasool A, Hakeem KR, Rehman RU. Morpho-physiological, biochemical and molecular adaptation of millets to abiotic stresses: a review. Phyton. 2021;90(5):1363. doi: 10.32604/phyton.2021.014826. [DOI] [Google Scholar]
- Shah AA, Yasin NA, Ahmed S, Abbas M, Abbasi GH. 4-Hydroxymelatonin alleviates nickel stress, improves physiochemical traits of Solanum melongena: Regulation of polyamine metabolism and antioxidative enzyme. Sci Hort. 2021;282:110036. doi: 10.1016/j.scienta.2021.110036. [DOI] [Google Scholar]
- Sharma K, Gupta S, Thokchom SD, Jangir P, Kapoor R. Arbuscular mycorrhiza-mediated regulation of polyamines and aquaporins during abiotic stress: deep insights on the recondite players. Front Plant Sci. 2021;12:1072. doi: 10.3389/fpls.2021.642101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu S, Yuan LY, Guo SR, Sun J, Yuan YH. Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem. 2013;63:209–216. doi: 10.1016/j.plaphy.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Tailor A, Bhatla SC. Polyamine homeostasis modulates plasma membrane-and tonoplast-associated aquaporin expression in etiolated salt-stressed sunflower (Helianthus annuus L.) seedlings. Protoplasma. 2021;258(3):661–672. doi: 10.1007/s00709-020-01589-8. [DOI] [PubMed] [Google Scholar]
- Tanou G, Ziogas V, Belghazi M, Christou A, Filippou P, Job D, Fotopoulos V, Molassiotis A. Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 2014;37(4):864–885. doi: 10.1111/pce.12204. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wang J, Yan D, Yuan H, Wang Y, He Y, Wang X, Li Z, Mei J, Hu M, Zhou T, Chong S, Zheng B. Exogenous spermidine improves salt tolerance of pecan-grafted seedlings via activating antioxidant system and inhibiting the enhancement of Na+/K+ ratio. Acta Physiol Plant. 2020;42(5):83. doi: 10.1007/s11738-020-03066-4. [DOI] [Google Scholar]
- Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018;217(2):523–539. doi: 10.1111/nph.14920. [DOI] [PubMed] [Google Scholar]
- Yemm EW, Cocking EC, Ricketts RE. The determination of amino-acids with ninhydrin. Analyst. 1955;80(948):209–214. doi: 10.1039/an9558000209. [DOI] [Google Scholar]
- Zhang GW, Xu SC, Hu QZ, Mao WH, Gong YM. Putrescine plays a positive role in salt-tolerance mechanisms by reducing oxidative damage in roots of vegetable soybean. J Integr Agric. 2014;13(2):349–357. doi: 10.1016/S2095-3119(13)60405-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study will be provided upon request to the corresponding author.
Not applicable.


