Abstract
The low incidence of injury to the recurrent laryngeal nerve (RLN) and external branch of the superior laryngeal nerve (EBSLN) quoted in the literature is derived from expert series. The exact incidence of nerve injury of a thyroid surgeon will be revealed only if pre-operative and post-operative laryngoscopy is becoming routine practice. It is found that the injury rates are increased with routine post-operative laryngoscopy. Subjective voice change occurred in one third of patients all whom had normal vocal cord motion. Therefore, it is important to take written informed consent for voice change in addition to identification of both nerves and documenting it.
Keywords: Recurrent laryngeal nerve, External branch of superior laryngeal nerve, Nonrecurrent laryngeal nerve, Zuckerkandl tubercle, Voice change, Pre- and post-operative vocal cord assessment
Introduction
The challenging problem for the nineteenth century surgeon was the high mortality rate following total thyroidectomy, which was solved by the Nobel prize winning work (1908) by Theodor kocher, by his meticulous attention to the surgical anatomy and technique. At present, the most important concern is the post-operative voice change. Ideally in expert hands, the recurrent laryngeal nerve palsy (RLN) should be < 1%. The paralytic dysphonia and dysphagia are distressing for the patient and will lead on to litigation. The temporary RLN paralysis rate in a systematic review of 27 articles and 25,000 patients was found to be 9.8% with a range between 2.3 and 26% [1].
Incidence of recurrent laryngeal nerve (RLN) injury in experienced hands have been reported in the range of 1–2% [2]. The series with low incidence are treated in centers with large volume endocrine practice by expert surgeons. Even in the USA, 90% of thyroid surgery are done by general surgeons not focusing on speciality surgery, to the extent by surgeons doing less than 5 cases per year [3, 4]. According to a report, the incidence of RLN injury varied from 2 to 13% in general [5]. In a landmark JAMA editorial regarding post-thyroidectomy vocal cord paralysis, “A piece of mind. Moral wounds: Complicated complications,” it was found that 50% of the endocrine malpractice litigations were related to thyroid surgery of which 70% involved RLN injury in the USA in the period between 1985 and 1991 and the mean plantiff award varied from $1 to 2.5 million [6]. Claims however under-represent nerve injury and undoubtedly many more patients sustain avoidable injury. All cases of hoarseness of voice need not be due to RLN injury due to surgery.There are other causes for hoarness of voice like endotracheal tube–associated vocal cord/laryngeal injury, regional causes like strap muscle denervation, and regional scarring [7]. Perithyroidal neural plexuses inervating the pharyngeal and laryngeal structures will also result in dysphonia and dysphagia. Similarly, a normal voice after thyroidectomy does not mean intact laryngeal nerve.
In this review, we will be dealing with the anatomy of RLN and external branch of superior laryngeal nerve (EBSLN), its variations, its physiology and function, causes for damage, and prevention. We will also emphasize certain golden rules to be followed to prevent injury during operation. Although most attention has been paid to the RLN in recent years, considerable emphasis has also been rightly placed on the need to avoid damage to the EBSLN during thyroidectomy. An understanding of the anatomy and function of the nerve is an essential pre-requisite for thyroid surgery.
History of Laryngeal Nerve and Thyroid Surgery
For over a thousand years, any operative attack on the thyroid gland was considered breathtaking heroics, fraught with danger to the patient and almost certain bereavement for the family. Thyroidectomy placed a cloud of professional butchery over the head of the surgeon who might be tempted to indulge himself. As late as 1850, the French Academy of Medicine proscribed any attempt to remove the thyroid surgically. Only 106 bonafide thyroidectomies were recorded by mid-nineteenth century with a mortality rate of 40%, mostly from exsanguination and sepsis.
The RLN was probably first described by Galen as early as the second century, but their relevance to the operation of thyroidectomy remained hidden. Vesalius in the sixteenth century provided anatomical drawings of RLN and superior laryngeal nerve [8]. In 1872, Kocher performed his first thyroidectomy and his technique was later on adopted by Halstead. Kocher was a careful, skillful academician and his meticulous attention to detail became the norm. For Kocher’s remarkable contributions to thyroid surgery in 1909, he was awarded the Nobel prize. In 1889, his mortality rate was 2.4% and at the end of the century, his mortality rate was only 0.18% [9]. Other great surgical pioneers such as Wolfler and Billroth recognized the risk of injury to these nerves. William Stewart Halstead wrote the operative story of goiter titled, “The authors operation” [10] (Fig. 1).
Fig. 1.

Theodor Kocher(1841–1917)
Anatomy of RLN
The left RLN nerve arises from the vagus nerve and loops around the aortic arch behind the ligamentum arteriosum, ascending obliquely in the tracheoesophageal groove towards the thyroid gland. The right recurrent laryngeal nerve arises from the vagus nerve anterior to the first portion of the subclavian artery and passes around it, ascending obliquely to the right of the trachea behind common carotid artery. Thus, left RLN has a longer course compared to the right RLN.
Both recurrent laryngeal nerves enter the larynx on either side behind the inferior cornu of the thyroid cartilage. The nerve has extremely variable relationship to the inferior thyroid artery and its branches, usually dividing itself into two or more branches, before coursing backwards to enter the larynx (extralaryngeal branching). Up to six such divisions have been described. Each branch has abductor and adductor fibers. The anterior branch is the largest. Th nerve is at risk at this region. It is this variability of course, propensity for branching and relationship to the thyroid gland, inferior thyroid artery, and adjacent fascial layers which render the nerve liable to damage at surgery. The right RLN enters the base of the neck more laterally than the left side because of its course around the subclavian artery. It also tends to travel in a more oblique course. The nerves on both sides form an angle of 15°–30° relative to the trachea [11]. The average diameter of RLN is 2 mm and the average length from the takeoff from the vagus to its entry point is 8.5 cm on the right side and 10 cm on the left side [12]. The vulnerability is more on the right side (Fig. 2).
Fig. 2.

RLN identified pre-operatively
The Nerve Artery Relationship
The nerve may pass superficially (25%), deep (50%), and between the inferior thyroid artery branches (25%) [13]. This causes fixation of the nerve in the arterial fork resulting in one of the high-risk areas for nerve injury.
Fascial Relationship of the RLN
In the lower third of its course, the nerve is covered by fairly thin fascia and areolar tissue. In the middle and upper 3rd, the nerve has a crucial relation to the fascial layer, namely the suspensory ligament of Berry. In the upper third, the nerve passes deep to the ligament of Berry with a curving and looping course before entering the larynx. In 30% of cases, the RLN passes through the ligament of Berry itself. The nerve may lie superficial to the Berry ligament sometimes [13].
Relationship of RLN to Thyroid Gland
The nerve never pierces the actual thyroid capsule or the gland itself. But it is not uncommon for tunneling to occur owing to the irregular growth of thyroid nodules, malignancy, and enlarged metastatic nodes.
Importance of Zuckerkandl Tubercle (the Forgotten Tubercle)
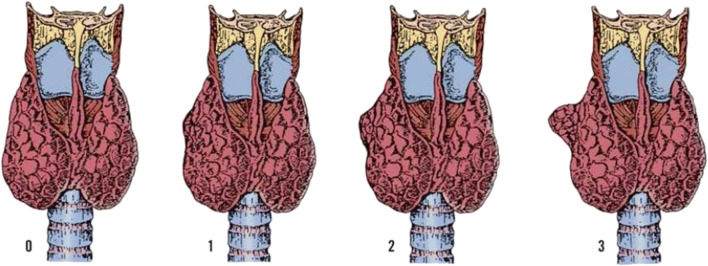
This tubercle was described by Zuckerkandl in 1902 and it was forgotten for 80 years. Tubercle of Zukerkandl is the site of fusion of median thyroid process with ultimobranchial body (Fig. 3). It is nothing but an enlargement of the lateral edge of the thyroid lobes. It is found in 14–55% of cases near the ligament of Berry [14, 15]. The RLN is constantly related to the tubercle and has been called an “arrow pointing to the RLN” [16]. Now we know the importance of this embryological remnant in thyroidectomy. It is important to note that the RLN lies in a fissure medial to the tubercle [16]. In addition, if it is left over in thyroidectomy, it will produce recurrence. There are 3 grades of the tubercle: grade 0, unrecognizable; grade 1, thickening of lateral edge; grade 2, smaller than 1 cm; and grade 3, larger than 1 cm [16] (Figs. 4 and 5).
Fig. 3.
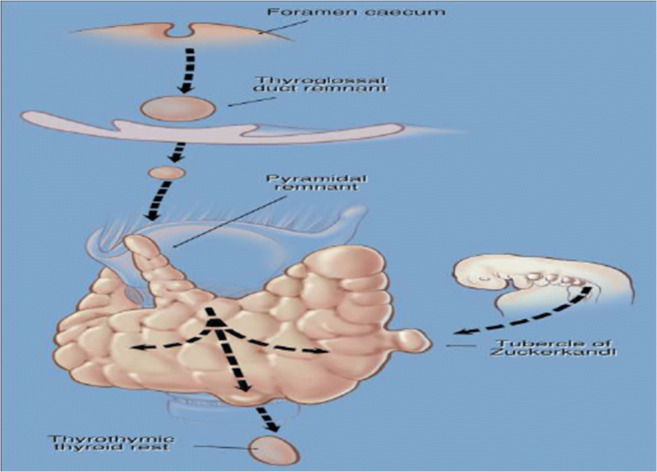
Developmental descend of thyroid and fusion of median thyroid process with ultimobranchial body
Fig. 4.
Three grades of Zukerkandl tubercle
Fig. 5.
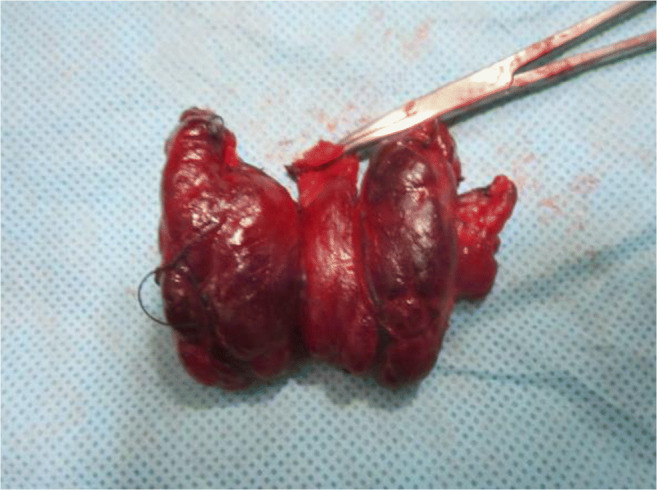
Total thyroidectomy specimen showing Zukerkandl tubercle grade 3 on right side
The nerve is accompanied by the inferior laryngeal artery which is seen posterior to the nerve in the Berry ligament region. A small branch from the artery will cross the nerve to enter the thyroid gland. Bleeding vessel here should not be clamped or coagulated without identifying the nerve and hence it becomes a vulnerable area. Medial retraction of the lobe makes the nerve vulnerable again. It is preferable to retract the lobe upwards after dissection of the lower pole.
RLN Supply
The RLN supplies all the intrinsic muscles of larynx except cricothyroid. The posterior cricoarytenoid muscle and lateral cricoarytenoid muscle abduct and adduct the vocal cords respectively. The thyroarytenoid muscles relax the vocal cords. The closure of the intercartilaginous portion of the rima glottidis is done by the transverse and oblique arytenoid muscles. The vocalis muscle is responsible for relaxing the posterior vocal ligament and tensing the anterior vocal ligament [17].
Galen’s Loop (Galen’s Anastomosis)
The posterior branch of the recurrent laryngeal nerve just before or just at the laryngeal entry point forms an anastomotic connection on the posterior surface of the posterior cricoarytenoid muscle (PCA) and interarytenoid muscle with the distal posterior branch of the internal division of the superior laryngeal nerve. This communication is called Galen’s anastomosis. This connection may be a single trunk or a double trunk or a plexus. This may be considered the primary sensory contribution of RLN to Galen’s anastomosis.
Identification of RLN
Discipline of Identifying the Nerve
The frequency of RLN injury after thyroid surgery should be below 1%, although achieved rates reflect the operating surgeons’ experience, and are greater in re-explorations and in operations for cancer. Its susceptibility to injury during ligation, transection, clamping, and use of diathermy are important to remember. Identifying the RLN at every procedure is associated with lower morbidity rates than operating with the nerve unseen. In addition, identification produces familiarity with the structure of the nerve. Prioleau considered it to be a dangerous practice to expose the nerve and stated, “It is an axiom in thyroid surgery that a RLN seen is injured.” This “ostrich philosophy” is no more acceptable. Crile thought that the nerve is more sensitive to exposure than peripheral nerve. Lahey in 1938 advocated routine exposure of RLN at operation in order to avoid nerve injury quoting an incidence of less than 0.3% [18]. If the RLN is injured when identified, it is more likely to be a transient palsy and recovery will soon follow. In the present consumer era, not only do we need to identify the nerve, it must be documented in the operation notes.
The RLN can be identified in the lower part of the neck in the triangle described by Lore and Riddle separately.
Triangle of Lore
The medial border of the triangle of Lore is made up of the trachea/esophagus, medial edge of the retracted strap muscles laterally and superior base by the lower edge of the retracted thyroid’s inferior pole [13].
Riddell’s Triangle
This is bounded laterally by the common carotid artery above by the inferior thyroid artery and medially by recurrent laryngeal nerve [19].
There Are 3 Surgical Approaches for RLN Identifications
Lateral Approach
The superior and inferior poles are initially dissected and the entire lobe is retracted gently medially. The inferior parathyroid (PT) is dissected off the inferior pole. The nerve is not uncovered inferiorly. This approach is ideal for routine thyroidectomy. RLN-inferior thyroid artery crossing is a landmark. Extralaryngeal branching is at this level. It is important to remember nonrecurrence of the nerve on the right side [13].
The Inferior Approach
Here the nerve is identified in the triangle of Lore, the apex of which is formed by the thoracic inlet inferiorly, trachea medially, medial edge of retracted strap muscles laterally, and lower edge of the inferior pole forming the base superiorly. This is suitable for revision thyroidectomy and large cervical goiters [13].
Superior Approach
Here we identify the nerve at the ligament of Berry region at the laryngeal entry point. This method is technically more challenging. Ligament of Berry is fibrous and bleeds easily. Inferior cornu of the thyroid cartilage is a landmark. It is useful for large and substernal goiters and when other approaches fail [13].
Backdoor Approach for Recurrent Goiter
This approach is used for identification of nerve in reoperations through a previously undisturbed plane on the medial aspect of the sternocleidomastoid muscle. The anterior border of sternomastoid is mobilized lateral to the strap muscles and reflected laterally. The omohyoid muscle and the sternohyoid muscles are exposed, as they fan out over the carotid sheath. Inferior to omohyoid muscle, the stenohyoid muscle is reflected medially and the carotid sheath is identified which is retracted laterally. This exposes the paratracheal space lateral to the inferior pole of thyroid. The RLN is usually found in the trachea-esophageal groove. On the right side, the nerve ascends from lateral to medial [20–22].
Remember the 3 points of the highest risk for RLN damage (Fig. 6).
Ligament of Berry
During ligation of branches of inferior thyroid artery
At the thoracic inlet
Fig. 6.
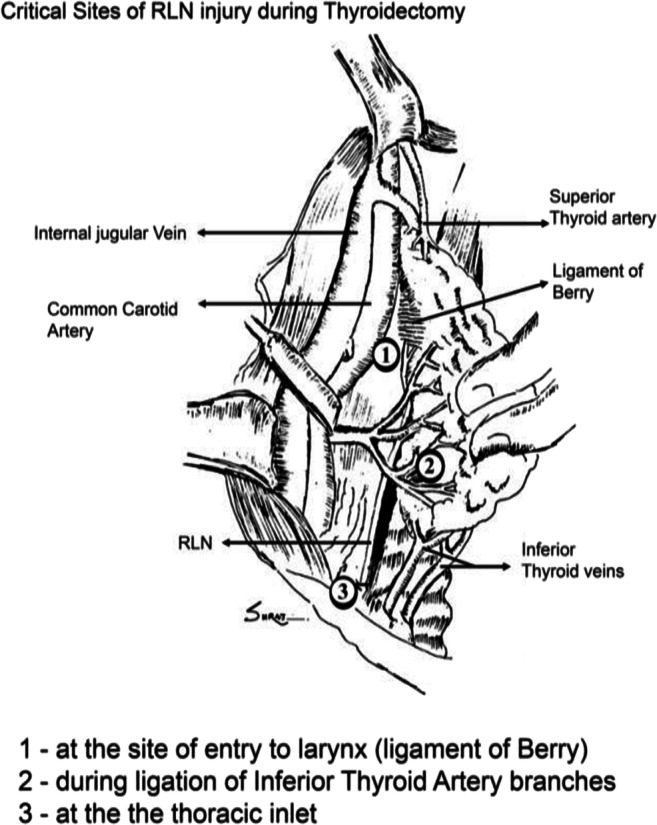
Critical sites of injury. Taken from Clinical Surgery Pearls by Dr. R Dayananda Babu 3rd Edition
Recurrent Laryngeal Nerve Paralysis
Complete unilateral RLN palsy causes the vocal cord to be immobile between the inspiratory and phonation positions—the so-called paramedian position. The opposite cord is able to swing across the midline to compensate during phonation, and therefore, though initially the voice may be hoarse, it will soon recover with a susceptibility to fatigue towards the end of the day. It rarely causes breathing difficulties except during extreme physical activity giving rise to audible stridor. Contracture and fibrosis of the cord may occur afterwards, producing airway problems.
If both RLN and ESLN are damaged, the cord will be held in an abducted inspiratory position. Although the airway is satisfactory, the voice would be husky. Hoarseness later improves after a period of weeks or months as the fibrosis draws the cord closer to the midline.
When the internal branch of the SLN is damaged in association with injury to other laryngeal nerves, there will be sensory loss and will be prone to aspiration and spillage of liquids into the larynx.
Bilateral RLN damage is a clinical catastrophe and both cords are fixed in the paramedian position. Initially, airway may be adequate, but once the atrophy and fibrosis of the cord set in, the cords move closer together resulting in difficulty in breathing with stridor and marked limitation on physical exertion. A tracheostomy is frequently necessary for bilateral RLN damage even though the voice continues to improve.
Why RLN Palsy Leads onto Median or Paramedian Position of Vocal Cords?
The most accepted theory is one of Semon’s law, which states that in all progressive organic lesions, the abductor fibers of the nerve which are phylogenetically newer are more susceptible and thus the first to be paralyzed compared to adductor fibers [23]. The other theory is of Wagner and Grossman which states that the cricothyroid muscle which receives innervation from superior laryngeal nerve keeps the cord in paramedian position due to its adductor function [24].
Nonrecurrent Laryngeal Nerve
Nonrecurrent laryngeal nerve is a rare embryologically derived variant found in 0.25–0.99% of patients mostly found on the right side and extremely rare on the left side (0.004%) [25, 26]. Nonrecurrent laryngeal nerve on the right side is associated with an aberrant right subclavian artery (86.7%) [25, 27]. Dissection along the fascial spaces transversely between the carotid sheath and larynx will reveal this nerve. It was first described by Steadman in 1823 on the right side [28]. Being an extreme rarity, it is unexpected in thyroid surgery. However, if recurrent laryngeal is not seen in position, the presence of nonrecurrent nerve should be suspected, and adequate knowledge of the anatomy and types of nonrecurrent laryngeal nerves should be kept in mind. There are three types of nonrecurrent laryngeal nerve (NRLN). They are as follows:
Type 1: This nerve directly arises from vagus and travels with the superior thyroid pedicle vessels.
Type 2A: This nerve travels transversely parallel to the trunk of the inferior thyroid artery, but it is superficial to the artery.
Type 2B: Similar to Type 2A but deep to or between the branches of the inferior thyroid artery [29].
Meticulous dissection in the tracheoesophageal groove longitudinally will normally reveal the RLN. If the RLN is not found, one should suspect NRLN and dissect transversely along the fascial spaces between the carotid sheath and the larynx will reveal the presence of NRLN.
External Branch of Superior Laryngeal Nerve (Nerve of Amelita Galli-Curci) (Fig. 7)
Fig. 7.

Amelita Galli-Curci
Anatomy of EBSLN
Although most attention has been paid to the RLN in recent years, considerable emphasis has also been rightly placed on the need to avoid damage to this nerve during thyroidectomy. The superior laryngeal nerve separates from the vagus at the base of the skull at the level of cornu of hyoid. It divides into two branches: The larger internal branch penetrates the thyrohyoid membrane which is sensory to superior part of larynx. The external branch of the superior laryngeal nerve travels along the lateral surface of the inferior constrictor. It then descends anteriorly and medially entering the cricothyroid. The external branch of the superior laryngeal nerve (EBSLN) is the motor nerve to the cricothyroid muscle which produces tension of the vocal cords. The muscle contraction slides the upper laryngeal cartilage, i.e., thyroid cartilage forwards relative to lower laryngeal cartilage which is the cricoid cartilage and that is how it adjusts the vocal fold length and tension. It is responsible for the production of high-pitched voice sounds (high note nerve) and projection of voice.
Identification and Preservation of EBSLN
In all thyroidectomies including substernal goiter, the dissection begins in the upper pole. Identify the Reeve space which is an avascular plane between the upper pole of thyroid and cricothyroid muscle. The nerve is located in the sternothyroid laryngeal triangle bounded medially by the inferior constrictor muscle and cricothyroid muscle anteriorly by sternothyroid muscle and laterally by superior thyroid pole [30].
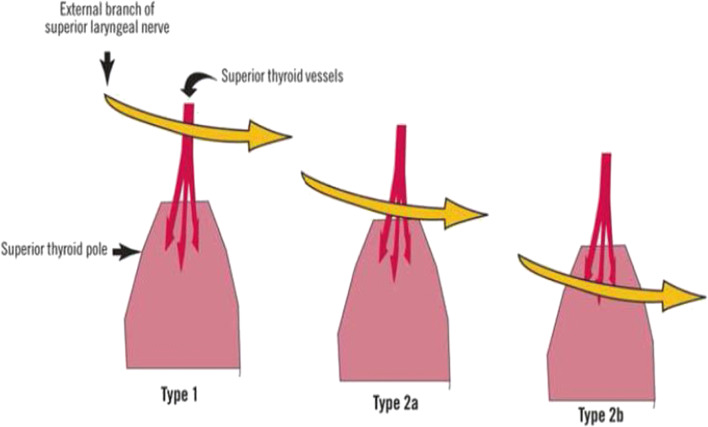
Cernea Classification for EBSLN (Fig. 8)
Fig. 8.
Cernea classification
In 1992, Claudio R. Cernea proposed the following surgical anatomical classification of the EBSNL [31].
Type 1: nerve crossing the superior thyroid vessels 1 or more centimeter above a horizontal plane passing through the upper border of the superior thyroid pole.
Type 2a: nerve crossing the vessel less than 1 cm above the plane passing through the superior thyroid pole.
Type 2b: nerve below the plane of the upper border of the superior thyroid pole.
Fourth type: Kierner added a 4th type where EBSLN runs dorsally to the superior thyroid pedicle. This anatomical relationship was seen 13% of their dissections [32].
Type 1 nerve was the most common (60–68%) in Cernea series. Type 2b has the highest risk of iatrogenic damage and in Cernea series, it was 14% in clinical setting [31].
Trauma to EBSLN results in inability to lengthen the vocal fold and to create a high-pitch sound and vocal projection. The clinical presentation is quite subtle in many patients with increased tendency for vocal fatigue and decreased pitch range being the most common symptom. For the singer or the professional voice user, paralysis of EBSLN may be career threatening by the loss of the upper register of the voice. Most surgeons are familiar with the story of the famous soprano of the world, Amelita Galli-Curci who underwent a thyroidectomy under local anesthesia for a 170-g goiter with identification and preservation of recurrent laryngeal nerve. During the surgery, her surgeon was speaking to her continuously to be sure that the RLN is safe. However, her voice became permanently hoarse and she had to give up singing. Since that time, EBSLN is known as “the nerve of Amelita Galli-Curci” [33]. An interesting fact is that few surgeons make an effort to identify this nerve because the clinical presentation is very subtle.
Identification and Preservation of EBSLN
Expose the sternothyroid laryngeal triangle (space of Reeve) [34]. Partial incision of sternothyroid muscle may help. Open up the space between the cricothyroid muscle and upper pole of the thyroid gland after giving strong downward and outward traction. The nerve should be sought more or less transversely between the superior thyroid artery and the pharyngeal constrictor or cricothyroid muscle. It is safer to do dissection from medial to lateral side. The muscular branches of the superior thyroid artery to the pharyngeal constrictor and cricothyroid may bleed. There is risk of nerve injury during cauterization as the nerve slips under these muscles. The superior thyroid vessels divide into three branches embracing the upper pole of thyroid: two are seen anteriorly and one dorsally. Gentle traction of the thyroid lobe caudally will give better view as described by Loré Jr. et al. [35]. Ligate the branches of the artery individually placing the ligature as caudally as possible. Detection of EBSLN injury is objectively done by electromyography of cricothyroid muscle by placing an electrode percutaneously in the muscle and asking the patient to produce a high tone [36, 37].
Laryngoscopy Findings of EBSLN Palsy
The paralyzed cord will be at a lower level, it will be hyperemic, and bowing of vocal cord will be present. The glottic chink is oblique (rotation of posterior commissure to paralyzed side). Injury rate varies in different series from 12 to 28% [36].
Intraoperative Nerve Monitoring
Anatomical integrity of the nerve does not always translate into functional integrity; therefore, the need for intraoperative nerve monitoring is recommended by specialist centers. Intraoperative nerve monitoring (IONM) involves two components: stimulation of the nerve via a probe and a method to measure a response to stimulation. There are several methods available for RLN monitoring. They are palpation of the posterior cricoarytenoid muscle twitch, needle electrode placement in vocalis or thyroarytenoid muscle, postcricoid electrodes placed in the hypopharynx, or use of an endotracheal tube with integrated and exposed wire electrodes which are in contact with vocal cords [38].
Endotracheal-based surface electrodes are the most commonly used method. Both intermittent and continuous stimulation methods are available. The intermittent IONM (I-IONM) helps to trace the nerve and to prognosticate functionality when the damage has happened. The problem with I-IONM is that damage to the nerve can occur in between two stimulations. The continuous IONM (C-IONM) can overcome this problem and in this method, the ipsilateral vagus is stimulated by a specifically designed stimulating electrode which continuously stimulates the nerve by automatic periodic stimulation (APS) [39]. It is real time and abnormal signal transmission alerts the surgeon before the irreversible damage [38, 40]. The recording part involves the endotracheal tube with four pre-fashioned surface EMG recording electrodes. Two for each vocalis muscle. The stimulation part includes a stimulator probe which may be monopolar or bipolar. The reference electrode is placed more distally over the shoulder or sternum to minimize stimulus artifact. The laryngeal twitch is palpated with a finger behind the posterior lamina of the cricoid cartilage during ipsilateral vagal stimulation. The presence of twitch indicates the contraction of posterior cricoarytenoid muscle and functioning of RLN. Presence of a signal from vagus at a higher point and absence at a caudal point suggests nerve damage. This can be done for EBSLN also. The advantage of C-IONM is attributed to identifying nontransection injuries like traction, thermal damage, compression, and clamping [38, 41]. The drawbacks of IONM are technical issues requiring additional staff, a long learning curve, and increase in operating time [42–44]. The routine use of IONM may help to shorten the learning curve.
The discussion with the anesthesiologist is important for the success of IONM because the endotracheal tube electrodes must be abutting the vocal cords. The signal transduction can be interfered with lubricants and lignocaine jelly. The endotracheal tube should be well secured at the exit from the mouth to avoid positional changes throughout the case. The monitoring involves EMG response and therefore the neuromuscular blockade must be avoided as far as possible.
Pre-operative Vocal Cord Assessment
Lack of informed consent for voice change is another reason for litigation. An analysis of 30 cases retrieved from US database showed that 7 out of 9 patients with RLN injuries claimed a lack of informed consent [6]. The importance of knowing invasive disease, i.e., T4, allows for aggressive surgical planning in carcinoma thyroid which is possible with a pre-operative laryngoscopy and counseling. In addition, many patients undergoing surgery for benign disease are having idiopathic vocal cord paralysis, and therefore, it is important to do laryngoscopy and document it [45]. The 2003 British Association of Thyroid and Endocrine Surgeons’ audit showed that inspite of recommendation, pre-operative laryngoscopy was done only in 70% of patients [7]. Current NCCN guidelines recommend vocal cord examination in all patients undergoing thyroid cancer surgery.
Post-operative Vocal Cord Assessment
The 2003 British Association of Endocrine Surgeons’ audit showed that post-operative vocal cord assesment was performed only in 4.1% of patients after first surgery and 7% of patients after reoperative surgery [7]. The postoperative rate of vocal cord paralysis doubles with routine laryngeal examination (1–6 weeks post-operatively) as compared with post-operative hoarseness [46].
All hoarsness of voice need not be due to RLN injury by surgery. Similarly, a normal voice after thyroidectomy does not mean intact laryngeal nerve. Clinical evaluation of the voice without laryngosocpic examination lacks sensitivity in detection of paralysis. Up to 1/3rd of patients with paralysis may be asymptomatic [47]. Similarly, subjective voice changes occur in 1/3rd of patients all of whom had normal vocal cord motion post-operatively [48]. The other causes of hoarsness apart from nerve paralysis are endotracheal tube–associated vocal cord injury, strap muscle denervation, and regional scarring [7]. The larynx is central in modern thyroid surgery, and therefore, post-operative laryngoscopic examination allows a surgeon to accurately note his own RLN palsy rate.
Voice changes are common and cannot be distinguished from nerve injury without laryngeal examination. Pre- and post-operative laryngoscopy should become part of the assessment of patients undergoing thyroidectomy.
Prevention of Damage to the Laryngeal Nerve
Discipline of identifying the RLN and the external branch of superior laryngeal nerve during thyroid surgery.
Do not mass ligate the superior pole of the thyroid gland. Identify the EBSLN in the space of Reeves. Ligate the individual branches of the superior thyroid artery after skeletonizing the artery as caudally as possible.
Remember the critical sites of greatest risk for injury to the RLN.
Do not use electrocoagulation anywhere near RLN and EBSLN. It is not widely appreciated how much tissue injury can occur within 2 cm of electrocoagulated area.
Suction damage is another reason for injury—do not keep the suction tip close to the RLN.
Mass ligation of the lower pole is inadvisable—it is safer to ligate the lower pole veins individually so that the injury to the lower 3rd of RLN can be avoided.
Never give traction to the laryngeal nerve.
Summary
The identification of RLN and EBSLN are gold standard in thyroidectomy. “In the early days it takes much self-discipline to force oneself to search for the nerve, but the anatomical knowledge gained so painfully will stand the surgeon and his patients in good stead.”
If the nerve is injured during identification, it is likely to be transient. It is important to take informed consent for voice change in all cases of thyroidectomy. Routine pre-operative and post-operative indirect laryngoscopy should become part of the assessment of all patients undergoing thyroidectomy.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Attached separately.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jeannon J-P, Orabi AA, Bruch GA, Abdalsalam HA, Simo R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract. 2009;63(4):624–629. doi: 10.1111/j.1742-1241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 2.Zakaria HM, Al Awad NA, Al Kreedes AS, Al-Mulhim AMA, Al-Sharway MA, Hadi MA, et al. Recurrent laryngeal nerve injury in thyroid surgery. Oman Med J. 2011;26(1):34–38. doi: 10.5001/omj.2011.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228(3):320–330. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saunders BD, Wainess RM, Dimick JB, Doherty GM, Upchurch GR, Gauger PG. Who performs endocrine operations in the United States? Surgery. 2003;134(6):924–931. doi: 10.1016/S0039-6060(03)00420-3. [DOI] [PubMed] [Google Scholar]
- 5.Djohan RS, Rodriguez HE, Connolly MM, Childers SJ. Intraoperative monitoring of recurrent laryngeal nerve function. Am Surg. 2000;66(6):595–597. [PubMed] [Google Scholar]
- 6.Munch S, deKryger L. A piece of my mind. Moral wounds: complicated complications. JAMA. 2001;285(9):1131–1132. doi: 10.1001/jama.285.9.1131. [DOI] [PubMed] [Google Scholar]
- 7.Mihai R, Randolph GW. Thyroid surgery, voice and the laryngeal examination—time for increased awareness and accurate evaluation. World J Endocr Surg. 2009;1(1):1–5. doi: 10.5005/jp-journals-10002-1001. [DOI] [Google Scholar]
- 8.Kaplan EL, Salti GI, Roncella M, Fulton N, Kadowaki M. History of the recurrent laryngeal nerve: from Galen to Lahey. World J Surg. 2009;33(3):386–393. doi: 10.1007/s00268-008-9798-z. [DOI] [PubMed] [Google Scholar]
- 9.Kocher T. Ueber ein drittes tausend Kropfexstirpationen. Arch f klin Chir. 1906;79:786–791. [Google Scholar]
- 10.Halsted WS (1920) The operative story of goiter. Johns Hopkins Hos Rep 19:71–257
- 11.Shindo ML, Wu JC, Park EE. Surgical anatomy of the recurrent laryngeal nerve revisited. Otolaryngol neck Surg. 2005;133(4):514–519. doi: 10.1016/j.otohns.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Sepulveda A, Sastre N, Chousleb A. Topographic anatomy of the recurrent laryngeal nerve. J Reconstr Microsurg. 1996;12(01):5–10. doi: 10.1055/s-2007-1006445. [DOI] [PubMed] [Google Scholar]
- 13.Randolph GW (2003) Surgical anatomy of the recurrent laryngeal nerve. Surg Thyroid Parathyr Gland 300–302
- 14.Sadler GP, Clark OH. Thyroid and parathyroid. In: Schwartz SI, Shires GT, Spencer FC, editors. Principles of surgery. New York: McGraw-Hill; 1999. pp. 1395–1470. [Google Scholar]
- 15.Mirilas P, Skandalakis JE. Zuckerkandl’s tubercle: Hannibal ad Portas. J Am Coll Surg. 2003;196(5):796–801. doi: 10.1016/S1072-7515(02)01831-8. [DOI] [PubMed] [Google Scholar]
- 16.Pelizzo MR, Toniato A, Gemo G. Zuckerkandl’s tuberculum: an arrow pointing to the recurrent laryngeal nerve (constant anatomical landmark) J Am Coll Surg. 1998;187(3):333–336. doi: 10.1016/S1072-7515(98)00160-4. [DOI] [PubMed] [Google Scholar]
- 17.Bliss RD, Gauger PG, Delbridge LW. Surgeon’s approach to the thyroid gland: surgical anatomy and the importance of technique. World J Surg. 2000;24(8):891–897. doi: 10.1007/s002680010173. [DOI] [PubMed] [Google Scholar]
- 18.Hannan SA. The magnificent seven: a history of modern thyroid surgery. Int J Surg. 2006;4(3):187–191. doi: 10.1016/j.ijsu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Riddell V. Thyroidectomy: prevention of bilateral recurrent nerve palsy, results of identification of the nerve over 23 consecutive years (1946-69) with a description of an additional safety measure. Br J Surg. 1970;57(1):1–11. doi: 10.1002/bjs.1800570102. [DOI] [PubMed] [Google Scholar]
- 20.El-Erian AM, El-Raouf AA, Nabeel I, El-Kholy M (2016) Lateral approach to attack superior thyroid vascular pedicle eliminates the need for strap muscles cutting during thyroidectomy
- 21.Singaporewalla RM, Tan BC, Rao AD (2008) The lateral “backdoor” approach to open thyroid surgery: a comparative study. Asian J Surg 41(4):384–388. 10.1016/j.asjsur.2017.05.003 [DOI] [PubMed]
- 22.Oertli D, Udelsman R (2007) Surgery of the thyroid and parathyroid glands. Springer
- 23.Kirchner JA (1996) Semon’s law a century later. J Laryngol Otol 1982;96(7):645–57. Available from: https://www.cambridge.org/core/article/semons-law-a-century-later/F11D4C392EE271E7037DCDDFD695E7E2 [DOI] [PubMed]
- 24.Blitzer A, Jahn AF, Keidar A (1996) Semon’s law revisited: an electromyographic analysis of laryngeal synkinesis. Ann Otol Rhinol Laryngol 105(10):764–9. Available from: 10.1177/000348949610501002 [DOI] [PubMed]
- 25.Uludag M, Isgor A, Yetkin G, Citgez B (2009) Anatomic variations of the non-recurrent inferior laryngeal nerve. BMJ Case Rep 2009:bcr1020081107. Available from: http://casereports.bmj.com/content/2009/bcr.10.2008.1107.abstract [DOI] [PMC free article] [PubMed]
- 26.Defechereux T, Albert V, Alexandre J, Bonnet P, Hamoir E, Meurisse M. The inferior non recurrent laryngeal nerve: a major surgical risk during thyroidectomy. Acta Chir Belg. 2000;100:62–67. [PubMed] [Google Scholar]
- 27.Henry BM, Sanna S, Graves MJ, Vikse J, Sanna B, Tomaszewska IM, Tubbs RS, Walocha JA, Tomaszewski KA (2017) The non-recurrent laryngeal nerve: a meta-analysis and clinical considerations. PeerJ 5:e3012–e3012. Available from: https://pubmed.ncbi.nlm.nih.gov/28344898 [DOI] [PMC free article] [PubMed]
- 28.Wang Y, Ji Q, Li D, Wu Y, Zhu Y, Huang C, Shen Q, Wang Z, Zhang L, Sun T. Preoperative CT diagnosis of right nonrecurrent inferior laryngeal nerve. Head Neck. 2011;33(2):232–238. doi: 10.1002/hed.21434. [DOI] [PubMed] [Google Scholar]
- 29.Rathnakar P, Shetty K. Nonrecurrent laryngeal nerve: a rare entity. J Heal Allied Sci NU. 2012;2(01):42–44. doi: 10.1055/s-0040-1703556. [DOI] [Google Scholar]
- 30.Moosman DA, DeWeese MS. The external laryngeal nerve as related to thyroidectomy. Surg Gynecol Obstet. 1968;127(5):1011–1016. [PubMed] [Google Scholar]
- 31.Cernea CR, Ferraz AR, Nishio S, Dutra AJ, Hojaij FC, dos Santos LR. Surgical anatomy of the external branch of the superior laryngeal nerve. Head Neck. 1992;14(5):380–383. doi: 10.1002/hed.2880140507. [DOI] [PubMed] [Google Scholar]
- 32.Kierner AC, Aigner M, Burian M (1998) The external branch of the superior laryngeal nerve: its topographical anatomy as related to surgery of the neck. Arch Otolaryngol Neck Surg 124(3):301–3. Available from: 10.1001/archotol.124.3.301 [DOI] [PubMed]
- 33.Kark AE, Kissin MW, Auerbach R, Meikle M. Voice changes after thyroidectomy: role of the external laryngeal nerve. Br Med J (Clin Res Ed) 1984;289(6456):1412–1415. doi: 10.1136/bmj.289.6456.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aina EN, Hisham AN. External laryngeal nerve in thyroid surgery: recognition and surgical implications. ANZ J Surg. 2001;71(4):212–214. doi: 10.1046/j.1440-1622.2001.02078.x. [DOI] [PubMed] [Google Scholar]
- 35.Loré JMJ, Kokocharov SI, Kaufman S, Richmond A, Sundquist N. Thirty-eight-year evaluation of a surgical technique to protect the external branch of the superior laryngeal nerve during thyroidectomy. Ann Otol Rhinol Laryngol. 1998;107(12):1015–1022. doi: 10.1177/000348949810701204. [DOI] [PubMed] [Google Scholar]
- 36.Cernea CR, Ferraz AR, Furlani J, Monteiro S, Nishio S, Hojaij FC, Dutra A, Jr, Marques LA, Pontes PAL, Bevilacqua RG. Identification of the external branch of the superior laryngeal nerve during thyroidectomy. Am J Surg. 1992;164(6):634–639. doi: 10.1016/S0002-9610(05)80723-8. [DOI] [PubMed] [Google Scholar]
- 37.Teitelbaum BJ, Wenig BL (1995) Superior laryngeal nerve injury from thyroid surgery. Head Neck 17(1):36–40. Available from: 10.1002/hed.2880170108 [DOI] [PubMed]
- 38.Randolph GW, Dralle H, Abdullah H, Barczynski M, Bellantone R, Brauckhoff M, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121(Suppl):S1–S16. doi: 10.1002/lary.21119. [DOI] [PubMed] [Google Scholar]
- 39.Schneider R, Machens A, Sekulla C, Lorenz K, Elwerr M, Dralle H (2020) Superiority of continuous over intermittent intraoperative nerve monitoring in preventing vocal cord palsy. Br J Surg. 10.1002/bjs.11901 [DOI] [PubMed]
- 40.Schneider R, Randolph GW, Dionigi G, Wu C-W, Barczynski M, Chiang F-Y, al-Quaryshi Z, Angelos P, Brauckhoff K, Cernea CR, Chaplin J, Cheetham J, Davies L, Goretzki PE, Hartl D, Kamani D, Kandil E, Kyriazidis N, Liddy W, Orloff L, Scharpf J, Serpell J, Shin JJ, Sinclair CF, Singer MC, Snyder SK, Tolley NS, van Slycke S, Volpi E, Witterick I, Wong RJ, Woodson G, Zafereo M, Dralle H. International neural monitoring study group guideline 2018 part I: staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope. 2018;128(Suppl):S1–S17. doi: 10.1002/lary.27359. [DOI] [PubMed] [Google Scholar]
- 41.Dionigi G, Wu C-W, Kim HY, Rausei S, Boni L, Chiang F-Y. Severity of recurrent laryngeal nerve injuries in thyroid surgery. World J Surg. 2016;40(6):1373–1381. doi: 10.1007/s00268-016-3415-3. [DOI] [PubMed] [Google Scholar]
- 42.Alesina PF, Hinrichs J, Meier B, Cho EY, Bolli M, Walz MK. Intraoperative neuromonitoring for surgical training in thyroid surgery: its routine use allows a safe operation instead of lack of experienced mentoring. World J Surg. 2014;38(3):592–598. doi: 10.1007/s00268-013-2372-3. [DOI] [PubMed] [Google Scholar]
- 43.Sturgeon C, Sturgeon T, Angelos P. Neuromonitoring in thyroid surgery: attitudes, usage patterns, and predictors of use among endocrine surgeons. World J Surg. 2009;33(3):417–425. doi: 10.1007/s00268-008-9724-4. [DOI] [PubMed] [Google Scholar]
- 44.Al-Qurayshi Z, Kandil E, Randolph GW. Cost-effectiveness of intraoperative nerve monitoring in avoidance of bilateral recurrent laryngeal nerve injury in patients undergoing total thyroidectomy. Br J Surg. 2017;104(11):1523–1531. doi: 10.1002/bjs.10582. [DOI] [PubMed] [Google Scholar]
- 45.Fenton JE, Timon CI, McShane DP. Recurrent laryngeal nerve palsy secondary to benign thyroid disease. J Laryngol Otol. 1994;108(10):878–880. doi: 10.1017/S0022215100128385. [DOI] [PubMed] [Google Scholar]
- 46.Bergenfelz A, Jansson S, Kristoffersson A, Mårtensson H, Reihnér E, Wallin G, Lausen I. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbeck's Arch Surg. 2008;393(5):667–673. doi: 10.1007/s00423-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 47.Sittel C, Stennert E, Thumfart WF, Dapunt U, Eckel HE. Prognostic value of laryngeal electromyography in vocal fold paralysis. Arch Otolaryngol Head Neck Surg. 2001;127(2):155–160. doi: 10.1001/archotol.127.2.155. [DOI] [PubMed] [Google Scholar]
- 48.de Pedro NI, Fae A, Vartanian JG, Barros APB, Correia LM, Toledo RN, et al. Voice and vocal self-assessment after thyroidectomy. Head Neck. 2006;28(12):1106–1114. doi: 10.1002/hed.20480. [DOI] [PubMed] [Google Scholar]