Abstract
Seed germination plays cardinal roles in seedling establishment and their successive growth. However, seed germination is retarded by far-red (FR) enrichment under low light stress, and the inhibitory signalling mechanism remains ambiguous. Our results indicated that low light treatment, both in the open and growth chamber conditions, inhibits rice seed germination by decreasing the gibberellin (GA) contents. To explore the mechanism of GA-deficiency under low light stress, differential expression profiling of GA-anabolic, -catabolic, ABA -anabolic, -catabolic, and SLR1 was investigated, revealing that expression of ABA- anabolic, GA-catabolic genes and SLR1 was upregulated with a simultaneous downregulation of ABA-catabolic and GA-anabolic genes under low light treatment. These results suggested that FR-induced GA inadequacy is resulted by upregulation of SLR1 and GA-catabolism genes consequently increase DELLA that further subsided GA-responses in the germinating rice seeds. Moreover, we provided evidence that FR-induced GA inadequacy demotes rice seed germination by decreasing amylase activity, eventually decreasing the carbohydrate solubilization in the germinating seeds. Finally, we suggest that under low light stress, due to a retarded conversion of phytochrome A to their bioactive form, the ABA-catabolic genes were eventually upregulated with a simultaneous downregulation of GA-anabolic genes. Consequently, a lower GA pool fails to leverage the GA-dependent DELLA degradation, further shutting down the expected GA responses that reduce germination efficiency under FR-enriched light.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-022-01167-7.
Keywords: Amylase, Gibberellic acid, Low light stress, Phytochrome A, Seed germination
Introduction
Light is the principal source of energy utilized by plants to maintain their growth and development. Upon perceiving light signals, various metabolic and developmental processes are orchestrated in the plants that regulate their growth phases (Jiao et al. 2007). A comprehensive transcriptome analysis of developing rice embryos and endosperm has helped us identify the genes feasibly involved in regulating seed development (Zhang et al. 2020). A genome-wide analysis of temporal and spatial gene expression patterns during seed development of rice has revealed a minimum of 43 F-box protein-encoding genes are directly regulated by the light (Jain et al. 2007). The quality and quantity of light reaching the soil or various absorbing organs of plant surface differ depending on multiple factors such as seasonal changes, atmospheric gases, pollutants, dense forest canopies, clouds, etc. An array of photo-biochemical systems in plants is responsible for recognizing changes in duration, direction, and spectral quality of light via photoreceptors which modulate various physiological processes by phytohormonal signaling pathways. Gibberellic acid (GA) and abscisic acid (ABA) are two primary phytohormones that antagonistically modulate seed germination by regulating GA and ABA-responsive functional proteins (Kim et al. 2008). GA promotes seed germination and breaks dormancy, while ABA induces dormancy during the process of seed maturation.
Small gene families encode phytochromes in higher plants, and primarily perceive red (R) and far-red (FR) wavelengths (Nayak et al. 2021). The complete genome sequences of Arabidopsis and rice have unveiled that Arabidopsis contains five phytochrome genes (Phy), PhyA to PhyE (Franklin and Quail 2010), whereas the rice genome possesses three phytochrome genes PhyA, PhyB, and PhyC (Panda et al. 2020). Over the last few decades, various researchers have revealed the distinct and cooperative functions of phytochromes A, B, and C in rice. Where phyA and phyB have been found to act redundantly to regulate de-etiolation under continuous red light (Rc), phyC was found to be involved in the photo-perception of continuous far-red signals (FRc) (Takano et al. 2005; Dey et al. 2021; Mohanty et al. 2021). Based on the radiant light energy, the response of phytochromes is very low fluence response (VLFR), low fluence response (LFR), or high irradiance response (HIR). Botto et al. (1996) reported the role of PhyA in the promotion of seed germination under canopy shade light in Arabidopsis. In recent studies, phytochrome A holoprotein (PhyA) has been reported to modulate seed germination and seedling establishment via very low fluence (VLFR) and low fluence response (LFR) modes in rice (Sineshchekov et al. 2020).
It is well known that germination of seeds in several crops is inhibited by canopy-induced low light stress, characterized by FR enrichment (Vaistij et al. 2018). Thus, the ecological significance of germination under dense canopies remains to be investigated. Interaction between phytochromes and phytohormones is becoming one of the significant issues in plant photo-physiology. Over the last few decades, various researchers have revealed the distinct and cooperative functions of phytochromes A, B, and C in rice. PhyA has been reported to regulate seed germination through the modulation of GA biosynthesis via phytochrome interacting factors (PIF), a subfamily of basic helix-loop-helix (bHLH) transcription factors that are characterized by the presence of AP domain, that binds to the light-activated configuration of phytochromes (Yan et al. 2020). Both phyA and phyB have been found to act redundantly to regulate de-etiolation under continuous red light (Rc), while phyC was found to be involved in the photo-perception of continuous far-red signals (FRc) (Takano et al. 2005). In preceding decades, an extensive analysis of rice databases has unveiled six PIF-like proteins in rice (PILs), OsPIL11-16 (Todaka et al. 2012; Cordeiro et al. 2016). Recent understanding of phytochrome-mediated cell signaling pathways has facilitated interpretations of many agriculturally significant attributes, such as photosynthesis, photorespiration, chlorophyll fluorescence, plant morphology, and grain characteristics in rice (Bose et al. 2018). For most agronomic and morphological traits, including germination rate and seedling vigor, information about PhyA-mediated regulatory pathways across phytohormones is minimal. Consequently, the signaling crosstalk between GA, PIF, and PhyA, central to seed germination regulation, in monocotyledonous cash crops like rice is poorly understood.
Ninety-five percent of global rice is cultivated during the Kharif season when LL stress prevails due to overcast skies (Panda 2014; Sekhar et al. 2019; Kumar et al. 2020). Fluctuating light intensity impairs rice production by 40–50% (Bardhan and Panigrahi 2018). Seed germination is reported to be repressed under FR enrichment condition (Piskurewicz et al. 2009). Previously, FLOWERING LOCUS T has been reported to act as the primary long day floral signal via a GA-dependent manner under fluctuating light conditions (Hisamatsu and King 2008). Furthermore, in a recent work, GA has been reported to improve seed germination and ornamental quality of Cyclamen species grown under short and long day conditions (Cornea-Cipcigan et al. 2020). Recently, in a series of seminal works, ABA and GA metabolism, regulatory signaling pathways, and their potential interactions with other phytohormones in the backdrop of shade stress have been reported (Banerjee and Roychoudhury 2019). In a study by Meng et al. (2016), Karrikins, a group of plant growth regulators, have been found to regulate soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. However, the underlying mechanisms that involve phytochrome-mediated elicitation of several phytohormones or how GA- and ABA-dependent signals are coordinated to repress germination under LL stress in rice remains unclear. To understand the impact of light intensity on seed germination and seedling phenotypic traits in rice and the possible role of PhyA in GA mediated regulation of this process, in the present study, we performed investigations in the following aspects: (i) to investigate the influence of low light on the germination rate and seedling phenotypic traits, (ii) to investigate the relationship between seed GA content and germination efficiency under low light stress, (iii) to investigate the amylase activities under low light stress and understand their correlation with the seed carbohydrate status, (iv) to investigate the differential expression of GA and ABA metabolic genes under low light stress to understand the crosstalk between phytohormones and seed germination in rice via the modulation of PhyA.
Materials and methods
Seed material
To infer the effects of low light (LL) stress on the germination efficiency of rice, LL-tolerant (Swarnaprabha) and susceptible (IR8) rice genotypes (Nayak et al. 1978) along with Akitakomachi (Wild Type: WT) and its phytochrome A mutant (phyA) were used in our study. The seeds of each genotype were germinated under open conditions in the Rabi and Kharif seasons of 2019 at ICAR-National Rice Research Institute (NRRI), Cuttack, Odisha, India. The seeds of the rice genotypes were germinated in the open; one set of each seed of these genotypes was subjected to low light (LL) treatment (75% of normal light) while the other set was raised under the open condition with 100% normal light intensity light (NL) which served as a control for Rabi and Kharif seasons of 2019. To further understand the interaction of phyA with the available light intensity and its role in the modulation of light-mediated seed germination, seedling phenotypic traits, and GA biosynthesis, the above four rice genotypes were germinated in Petri plates in the growth chamber of a climate-controlled greenhouse (Model:3500 G; Saveer Biotech, India) under continuous normal light (cNL: 1300 µmol m−2 s−), continuous low light (cLL1: 650 µmol m−2 s−1, and cLL2: 350 µmol m−2 s−1) and continuous dark (cDa) light regimes. The light intensities were measured with the help of a quantum radiometer (LI-1500 LICOR, USA).
Assessment of seed germination percentage under low light stress
Seeds were given dry heat treatment at 50 °C for 7 days to eliminate residual dormancy that might restrain the germination rate. 13–14% moisture content was maintained. Seeds with 2 mm of radicle or more were considered to be germinated. Seed germination percentage was calculated as described by Thakur et al. (2021).
Seed germination percentage (GP) = (Number of seeds germinated/total number of incubated seeds) X100.
Assessment of germination percentage(GP) under open conditions
Before sowing, the trays of 63 cm length, 55 cm breadth, and 9 cm depth were prepared by filling the soil. The well-matured, pest-free, and healthy seeds were directly soaked in a fungicidal suspension of Bavistin (1.0 g/l water solution) for 24 h. Afterward, the treated seeds were broadcasted uniformly in lines on well-prepared trays. Water was applied to maintain saturated conditions in the surface soil. Hundred seeds of each rice genotypes SP, IR8, WT, and phyA, were sown in the open to study GP. One set of each seed of these genotypes was subjected to low light (LL) treatment by using Agro shade nets (75% of normal light) matted on the wooden/bamboo frame (3.9mX3.9mX1.7 m) as mentioned in Section "Seed material". In contrast, the other set raised under the open condition with 100% normal light intensity light (NL) served as the control for Rabi and Kharif of 2019. GP was estimated at 48 (2nd day), 72 (3rd day), 96 (4th day), 120 (5th day), and 144 (6th day) hours of sowing. Photosynthetic active radiation (PAR) under both the light regimes was monitored using a radiometer (LI-1500 LICOR, USA) at different times of the day (9.00 am, 12.00 noon, and 4.00 pm of Indian Standard Time (IST), UTC + 05:30). Six replicas under each condition were maintained for accurate PAR measurement.
Assessment of germination percentage under growth chamber
Germination assessment was performed under in vitro conditions in the plant growth chamber after the primary dormancy was subdued via heat treatment at 50 °C for 7 days (Naredo et al. 1998). The seeds of SP, IR8, WT, and phyA were then sterilized in 2% (v/v) sodium hypochlorite solution with 0.1% (v/v) Tween 20 and rinsed with sterilized distilled water 4–5 times. 100 healthy unbroken seeds were immediately placed in the 110-mm Petri plates supplied with 25 mL of distilled water. For each genotype, four sets of Petri plates were arranged. In each set, six Petri plates were considered. The three sets of Petri plates were subjected to continuous normal light (cNL), two ranges of continuous low light (cLL1 and cLL2) and continuous dark (cDa) conditions in the growth chamber by maintaining incident light as 1300 µmol m−2 s−1for cNL, 650 µmol m−2 s−1 for cLL1 and 350 µmol m−2 s−1 for cLL2. For cDa condition, the Petri plates were wrapped with triple layers of aluminum foil and incubated in the dark. The light was obtained from Phillips TL8 W/33, and their intensities were standardized using the radiometer (LI-1500 LICOR, USA). A temperature of 28 °C with 70% relative humidity was maintained. The number of germinated seeds was expressed in percentage from 24 to 144 h of light treatments.
Estimation of mobilization efficiency
Mobilization efficiency (ME) in 15 germinating seedlings after the 144th hour of germination in all the biological replicates of SP, IR8, WT, and phyA germinated under open condition and growth chamber subjected to cNL, cLL1, cLL2, and cDa conditions was evaluated by the method of Perata et al. (1997) with the following formula:
Mobilization efficiency (ME) = (Dry weight of seedlings/Dry weight of cotyledon) X100.
Estimation of gibberellic acid content from seedling
The gibberellic acid (GA) content was measured following the technique described by Graham and Thomas (1961) from the 6-days old seedlings. A standard curve was plotted using known GA concentrations, and the endogenous gibberellic acid level was estimated by further plotting the absorbance values in the derived standard curve.
Determination of total starch
Grain starch content of 6 days incubated seedlings was estimated using α–amylase, amyloglucosidase, glucose oxidase plus peroxidase (GOPOD), and 4-aminoantipyrine reagents obtained from Megazyme (Total starch assay kit K-TSTA-100A, Megazyme International, Ireland Limited, Bray Business Park, Bray, Co, Wicklow, Ireland) as per Kumar et al. (2018).
Enzyme and reducing-sugar extraction
The total amylase and α-amylase activities along with reducing sugar content were estimated as per Cui et al. (2002). For crude extraction and determination of enzyme activity, all biochemical assays were performed in ice. During this process, fifteen wholesome 6th day germinated seedlings (cotyledons, juvenile roots, and shoots) were taken in a pre-chilled mortar and pestle and 3 ml of ice-cold Na2PO4 buffer (pH 7.0, 0.1 M) was added. The seeds were then homogenized and rinsed thoroughly with 3 ml of the previously used buffer. This is centrifuged at 10,000 g for 15 min at 4 °C. The supernatant was then collected as the crude extract to determine total amylase, α-amylase activity, and reducing sugar content.
Determination of total amylase activity (TAA)
The total amylase activity was then estimated calorimetrically by determining the reduction of the starch substrate as per Rood and Larsen (1988) with minor modifications. 0.3 ml of the crude extract was taken in a test tube. To this, 0.3 ml of 0.1 M Na-phosphate buffer (pH 7.0) and 0.5 ml of a 1% soluble-starch solution were added. The tube was then incubated for 10 min at 30 °C, and to this 0.5 ml of iodine solution was added. At 620 nm, optical density was then determined using a spectrophotometer for measuring the remaining starch (Thermo Scientific™Evolution-201-PC based). For the estimation of the starch in the crude extract, the above-explained procedure was followed with 0.5 ml of 0.1 M Na-phosphate buffer instead of the 0.5 ml of 1% soluble starch solution. Afterward, the amount of starch left behind in the reaction tube and the amount of starch in the crude extract was estimated by comparison with starch standards. The amount of hydrolyzed starch was then calculated as the integration of the added starch and the starch in the crude extract minus the remaining starch in the reaction tube. Total amylase activity was then expressed as the hydrolyzed soluble starch (mg) for 10 min.
Determination of α-amylase activity (AAA) and reducing sugar content (RSC)
The crude extract was first incubated for a while for 15 min at 70 °C to inactivate the β-amylase. The α-amylase activity was then estimated by measuring the total amount of reducing sugar in the sample as described by Yang and Zhang (1990). 0.3 ml of the above heat-treated extract was added to two differently labeled test tubes. To each of these tubes, 0.5 ml of 0.1 M Na-phosphate buffer (pH 6.5) was added. To the first tube, 0.5 ml of 1 M NaOH was added to inactivate the activity of α-amylase. Afterward, 0.5 ml of 1% soluble-starch solution was added to each of these two tubes, and then incubated at 40 °C for 20 min followed by immediate placing on ice. The reaction of the second tube was arrested by the addition of 0.5 ml of 1 M NaOH. This was followed by the addition of 0.3 ml of 1% 3,5-dinitro-salicylic acid to each tube. Both these tubes were then incubated in the boiling water for 5 min. Afterward, they were cooled, and absorbance (D) was measured at 520 nm using a spectrophotometer (Thermo Scientific™Evolution-201-PC based). The D at 520 nm in the first tube was used to estimate the reducing-sugar content (RSC) of the germinating seeds. The α-amylase activity was estimated as the difference in D at 520 nm between the two tubes.
Estimation of seedling phenotypes
To analyze the seedling phenotypes, seedling fresh weight (SFW), seedling dry weight (SDW), shoot length (SL), root length (RL), seedling height (SH), coleoptile length (CL), and hypocotyl length (HL) were measured from 15 days old seedlings after germination in all the biological replicates of SP, IR8, WT and phyA germinated under open conditions and growth chamber subjected to cNL, cLL1, cLL2, and cDa conditions.
Expression of genes involved in GA and ABA biosynthesis
Based on the regulatory roles of GA and ABA in the process of seed germination and seedling establishment (Vishal and Kumar 2018), we selected the most significant genes involved in their modulatory circuit (Tuan et al. 2018) and studied their expression analysis by qRT-PCR. Total RNA was extracted from the 6th-day seedlings of NL and LL germinated seeds from the open, and cNL, cLL1, cLL2, and cDa germinated seeds from the growth chamber of SP, IR8, WT, and phyA genotypes using 18 RNEasy Plant Mini Kit (Qiagen, USA) following the manufacturer’s protocol. First-strand cDNA synthesis was conducted using total DNase-treated RNA using primer script first-strand cDNA synthesis kit (Takara Clontech, Japan). Real-time polymerization chain reaction to estimate transcript levels of GA and ABA metabolism-related genes in the seedlings was performed in Eppendorf Master Cycler Real-Time PCR system (Eppendorf, USA), using SYBR Green master mix (Takara Clontech) to monitor double-stranded DNA synthesis. The qRT-PCR reaction was performed using gene-specific primer pairs (Supplementary Table S1) designed with PrimerQuest tool 30 (IDT). β-tubulin gene primer pair (FP: 50 ATGCGTGAGATTCTTCACATCC 30) and (RP:50 TGGGTACTCTTCACGGATCTTAG 30) was used for its amplification and was treated as the internal control.
Statistical analysis
The experiments were carried out with three biological as well as three technical replicates. The statistical analysis on the mean values of five randomly selected plants from each of the three replications for four rice genotypes (both under open and growth chamber conditions) was carried out on individual traits. The data of mean values for all the traits were analyzed for their variance following simple factorial RBD. Analysis of variance was done using the Indostat 7.5, along with the Tukey–Kramer method through Microsoft Excel 2019. The significance was tested by referring to the table given by Fisher (1936). The phenotypic correlations and principal component analysis (PCA) were estimated using PAST4.0 software (Hammer et al. 2001). Heatmap was also generated using Clustvis a web-based software to know the association among traits/parameters.
Results
Photosynthetic active radiation (PAR) monitoring
Measurement of PAR under low light and normal light intensity above the seedlings of all the studied genotypes was recorded (Fig. 1a and Supplementary Fig. 1) three times of the day, 9.00 am, 12.00 noon, and 4.00 pm of Indian Standard Time (IST), UTC + 05:30) to confirm the LL stress and NL intensity (Supplementary Table S2a). Approximately, 24.3% light reduction was observed under the low light condition (under Agroshade net) as compared to normal light in open condition. Similarly, to track the light intensity, PAR was recorded under cNL, cLL1, cLL2, and cDa conditions in the growth chamber (Supplementary Table S2b, Fig. 1b). Approximately, 24.99%, 49.79%, and 96.44% light reduction were observed under cLL1, cLL2, and cDa conditions, respectively as compared to normal light condition (cNL) in the growth chamber.
Fig. 1.
Photosynthetic active radiation (PAR) under normal light (NL) and low light (LL) regiments in open condition during Kharif and Rabi 2019 (a), and continuous normal light (cNL), continuous low lights (cLL1, cLL2) and continuous dark (cDa) in growth chamber condition (b). Each data point is the average of six replicates. Error bars represent SE. * = Mark above the bar represents the level of significance at < 0.05, ** at < 0.01 and *** = at < 0.001. Signifcance (value) is in comarision with respect normal light in filed condition (NL) and under growth chamber (cNL)
Assessment of seed germination percentage under low light stress
To understand the impact of LL stress on seed germination percentage, we germinated the seeds of SP, IR8, WT, and phyA under NL and LL conditions during Kharif and Rabi 2019, and compared their germination efficiency across light treatments and seasons. Further, to cross-check the possible role of phyA in the initiation of the various physiological processes that eventually promote the quiescent seeds to the stage of germination; we conducted a comparative analysis of the germination rate of the above genotypes under cNL, cLL1, cLL2, and cDa conditions in the growth chamber.
Seed germination percentage under open conditions
Germination percentage decreased significantly under LL stress in all the genotypes in both seasons. However, the magnitude of reduction was pronounced in phyA (Supplementary Table S3a, Supplementary Fig. 1). As compared to NL, the percent reduction in germination during Kharif in SP, IR8, WT and phyA were 42.88%, 45.93%, 53.54%, and 69.66% at 48 h; 37.42%, 37.22%, 41.67%, and 49.01% at 72 h; 33.30%, 33.25%, 40.50%, and 41.64% at 96 h; 13.80%, 12.75%, 14.41%, and 23.68% at 120 h; 10.61%, 10.59%, 12.35%, and 21.15% at 144 h of LL treatments, respectively. Similarly, as compared to NL, the reductions in germination were observed during Rabi in SP, IR8, WT and phyA at 48 h, 72 h, 96 h, 120 h and 144 h of LL treatments (Supplementary Table S3a, Fig. 2a). Both in Rabi and Kharif seasons, GP was significantly hampered in phyA than other tested genotypes, which was pronounced under LL than control (Supplementary Table S3a, Fig. 2a). This result indicates that reduction in the light intensity, due to seasonal consequences, negatively affects seed germination efficiency in rice.
Fig. 2.
Germination percentage in Swarnaprabha (SP), IR8, wild type (WT), and phytochrome A mutant (phyA) grown under normal light (NL) and low light (LL) in open conditions during Kharif and Rabi 2019 (a), and under continuous normal light (cNL), continuous low light (cLL1 and cLL2) and continuous dark (cDa) conditions in growth chamber (b). The sixth day germinated demonstrates the impact of fluctuating light intensity on seed germination attributes in rice. Each data point is the average of six replicates. Error bars represent SE. * = Mark above the bar represents the level of significance at < 0.05, ** at < 0.01 and *** = at < 0.001. Signifcance (value) is in comarision with respect normal light in field condition (NL) and under growth chamber (cNL)
Seed germination percentage under growth chamber
Germination percentage (GP) decreased significantly with a reduction in light intensity (Supplementary Table S3b, Supplementary Fig. 2). Among all the rice genotypes used in the experiment, phyA performed the poorest GP among all. GP decreased under cLL1 by 37.97%, 29.10%, 31.60%, 10.59%, 6.92% in SP and 49.06%, 31.95%, 41.43%, 10.80%, 13.30% in IR8 than cNL at 48 h, 72 h, 96 h, 120 h and 144 h of seed germination, respectively. Similarly, reductions were obserbed under cLL2 and cDa in SP and IR8 than cNL at different hrs of seed germination. Under cNL, GP in phyA decreased than WT by 8.38%, 25.18%, 42.55%, 46.93%, 37.73% at 48 h, 72 h, 96 h, 120 h and 144 h of seed germination, respectively. Similary, decreaments were observed under cLL1 and cLL2 at different hour of seed germination (Supplementary Table S3b, Fig. 2b). These results suggested that with the reduction in light intensity, seed germination rate decreased in rice.
Assessment of mobilization efficiency (ME) under low light stress
The percent of decrease of ME in SP, IR8, WT and phyA was 11.46%, 12.78%, 12.96% and 17.36%, respectively in Rabi, while 11.62%, 13.32%, 13.25% and 17.63%, respectively in Kharif under low light as compared to the normal light condition in the open (Supplementary Table S4a, Fig. 3a). Similarly, ME was significantly decreased in germinated seeds with the reduction in light intensity in the growth chamber. Decreases were found to be 11.55%, 22.55% and 34.55% in SP; 17.23%, 24.46%, 34.55% in IR8; 14.48%, 29.84% and 40.93% in WT, while 16.17%, 36.63%, and 50.69% in phyA, respectively under cLL1, cLL2 and cDA conditions as compared to normal light (Supplementary Table S4b, Fig. 3b).
Fig. 3.
Mobilization efficiency of 120 h incubated seeds of Swarnaprabha (SP), IR8, wild type (WT), and phytochrome A mutant (phyA) grown under normal light (NL) and low light (LL) in open conditions during Kharif and Rabi 2019 (a), and under continuous normal light (cNL), continuous low light (cLL1 and cLL2) and continuous dark (cDa) conditions in growth chamber (b). Each data point is the average of six replicates. Error bars represent SE. * = Mark above the bar represents the level of significance at < 0.05, ** at < 0.01 and *** = at < 0.001. Signifcance (value) is in comarision with respect to normal light in filed condition (NL) and under growth chamber (cNL)
Assessment of GA content in the seedlings during germination under low light stress
To investigate the impact of depleting light intensity on bioactive GA production, 6 days old seedlings from the open and growth chamber were assessed for endogenous GA content. In the open condition, GA content decreased under LL in all the genotypes. The percent reduction in GA content under LL than control in SP, IR8, WT, and phyA was 11.62%, 13.32%, 13.25%, and 17.63%, respectively in Kharif, while 11.46%, 12.78%, 12.96%, and 17.36%, respectively in Rabi (Supplementary Table S5a, Fig. 4a). A similar interdependence between GA production and light intensity was observed under the growth chamber. GA content decreased exponentially with a gradual reduction in light intensity from cLL1 to cDa. As compared to cNL, the percent reduction in GA content in SP, IR8, WT, and phyA were 3.96%, 7.32%, 10.48%, and 30.00%, respectively under cLL1; 7.10%, 9.42%, 10.77% and 35.42%, respectively under cLL2, while 41.64%, 43.40%, 84.29% and 145.28%, respectively under cDa (Supplementary Table S5b, Fig. 4b). This result indicated that bioactive GA in the germinating seeds is reduced under the FR-enriched environment in both the open and the growth chamber conditions, suggesting the role of light for GA production in germinating seeds.
Fig. 4.
Gibberellic acid (GA) content in the 6th day germinated seedlings determined from the cotyledons of Swarnaprabha (SP), IR8, wild type (WT), and phytochrome A mutant (phyA) under normal light (NL) and low light (LL) in open conditions during Kharif and Rabi 2019 (a), and under continuous normal light (cNL), continuous low light (cLL1 and cLL2) and continuous dark (cDa) conditions in growth chamber (b). Error bars represent SE. * = Mark above the bar represents the level of significance at < 0.05, ** at < 0.01 and *** = at < 0.001. Signifcance (value) is in comarision with respect normal light in filed condition (NL) and under growth chamber (cNL)
Assessment of starch content in the cotyledons during germination under low light stress
Starch content was estimated in 6 days incubated seeds, which was significantly higher in the seeds germinated under the light depleted environment both in open and growth chamber conditions. In the open condition, the percent increase in starch from the germinating seedlings of SP, IR8, WT, and phyA was 3.62%, 5.03%, 6.92%, and 11.66% in Rabi, while 4.47%, 4.53%, 5.58%, and 12.54% in Kharif, respectively under low light as compared to normal light condition (Supplementary Table S6a, Fig. 5a). Similarly, in the growth chamber condition, the starch accumulation percent was increased with the decrease in light intensity (3.52%, 5.79%, and 12.07% in SP; 5.06%, 13.06%, and 18.49% in IR8; 6.86%, 16.42% and 22.78% in WT, while 8.80%, 20.53%, and 24.89% in phyA, respectively under cLL1, cLL2 and cDA conditions) (Supplementary Table S6b, Fig. 5b). These results suggested that utilization of starch for embryonal growth increased under low light stress.
Fig. 5.
Carbohydrate contents and enzymatic solubilization in the 6th day germinated seedlings(juvenile root,shoot and cotyledons). The grain starch content and reducing sugar content were determined from the cotyledons of the 6th day germinated seedlings of Swarnaprabha (SP), IR8, wild type (WT), and phytochrome A mutant (phyA) germinated under under normal light (NL) and low light (LL) in open conditions (a, c, e, g), and under continuous normal light (cNL), continuous low light (cLL1 and cLL2) and continuous dark (cDa) conditions in growth chamber (b, d, f, h). The starch content (a, b), relative sugar (c, d), specific activity of α-amylase (AAA) (e, f) and total amylase (TAA) activities (g, h) were further determined under open (a, c, e, g) and growth chamber (b, d, f, h) conditions to understand the relationship between carbohydrate content and related solubilization activities in the seeds during germination under fluctuating light intensities. Each data point is the average of six replicates. Error bars represent SE. * = Mark above the bar represents the level of significance at < 0.05, ** at < 0.01 and *** = at < 0.001. Signifcance (value) is in comarision with respect normal light in filed condition (NL) and under growth chamber (cNL)
Assessment of carbohydrate solubilization in the cotyledons during germination under low light stress
To understand the impact of varying seasonal impacts and light intensities on the solubilization of carbohydrate reserves in the cotyledons during seed germination, 6th day germinated seedlings of SP, IR8, WT, and phyA from open conditions, subjected to NL and LL, along with growth chamber, subjected cNL, cLL1, cLL2, cDa, were evaluated for reducing sugar content (RSC), α-amylase activity (AAA) and total amylase activity (TAA) in their cotyledons.
Solubilisation of carbohydrate reserves in the cotyledons of seeds germinated under open conditions
Under open conditions, RSC, AAA, and TAA significantly decreased in all the tested genotypes under LL. The percent decrement in RSC in SP, IR8, WT, and phyA under LL than control (NL) were 3.29%, 8.12%, 9.06%, and 11.57%, respectively in Kharif; while 3.27%, 5.65%, 4.88%, and 8.20%, respectively in Rabi (Supplementary Table S6a, Fig. 5c). Similalry, decreaments were observed in TAA and AAA in SP, IR8, WT and phyA under LL than control both in Kharif and Rabi (Supplementary Table S6a, Fig. 5e, g). Under the LL environment, phyA showed a relatively significant decrease in AAA and TAA than WT by 43.30%, and 22.05%, respectively in Rabi, while 45.24% and 28.95%, respectively in Kharif. These results suggested that amylase activity in the germinating seeds is reduced with induced FR enrichment that consequently affected the carbohydrate solubilization necessary for embryonal growth.
Solubilization of carbohydrate reserves in the cotyledons of seeds germinated under growth chamber
Under the growth chamber, RSC, AAA, and TAA significantly decreased in all the tested genotypes with a decrease in light intensity, which was remarkably higher under cDa. In comparison to cNL, the percent decrement in RSC in SP, IR8, WT and phyA were 21.87%, 26.92%, 43.75%, and 45.00%, respectively under cLL1; 50.00%, 65.00%, 64.29%, and 81.25%, respectively under cLL2, while 95.00%, 83.33%, 126.97%, and 141.67%, respectively under cDa (Supplementary Table S6b, Fig. 5d). In comparison to cNL, the percent decrement in AAA and TAA in SP, IR8, WT and phyA under cLL1 were 9.80%, 10.20%, 13.04%, and 28.00%; 2.95%, 5.14%, 8.08%, and 17.97%, respectively. Similarly, under cLL2 and cDa, reduction were observed in AAA and TAA in SP, IR8, WT and phyA (Supplementary Table S6b, Fig. 5f, h). The reducing sugar content remarkably decreased with a reduction in light intensity. These results suggested that amylase activity reduced with the depletion in light intensity that further lowered starch solubilization, consequently lowering RSC content in the LL germinated seeds.
Assessment of seedling phenotype under low light stress
To understand the impact of varying seasonal conditions and light intensities on seedling phenotypes, 6th day germinated seedlings of SP, IR8, WT, and phyA from open subjected to NL and LL conditions along with subjected to cNL, cLL1, cLL2, cDa conditions from growth chamber were evaluated for their relative SFW, SDW, SL, RL, SH, and CL.
Phenotype of seedling grown under open conditions
Under open conditions, SFW, SDW, SL, RL, SH, and CL significantly increased in all the tested genotypes under LL. The percent increment in SFW, SDW, SL, RL, SH and CL under LL than control (NL) during Kharif were 6.86%, 9.14%, 12.65%, 20.15%, 17.40% and 43.53%, respectively in SP; 8.15%, 38.15%, 12.35%, 16.71%, 15.03% and 42.69%, respectively in IR8; 36.06%, 33.33%, 15.02%, 23.85%, 19.63% and 43.60% respectively in WT, while 42.38%, 42.72%, 16.56%, 27.15%, 22.01%, and 47.63%, respectively in phyA. Similarly, increases were oberseved in SFW, SDW, SL, RL, SH and CL under LL than control during Rabi in SP, IR8, WT and phyA (Supplementary Table S7a). Increment was more pronounced in phyA than WT in all these six phenotypic traits. These results suggested that suppression of photomorphogenesis under low light stress is affected more in phyA plants than other tested rice genotypes in this study.
Phenotype of seedling grown under growth chamber
Under the growth chamber, SFW, SDW, SL, RL, SH, and CL significantly increased in all the tested genotypes with a decrease in light intensity, which was remarkably higher under cDa. In comparison to cNL, the percent increment in SFW, SDW, SL, RL, SH and CL were 9.67%, 19.49%, 13.79%, 18.48%, 104.12%, and 16.78%, respectively in SP; 5.88%, 19.20%, 11.75%, 16.48%, 103.53%, and 14.70%, respectively in IR8; 30.91%, 32.63%, 16.77%, 23.89%, 117.25% and 20.79%, respectively in WT, while 31.84%, 58.94%, 19.05%, 26.65%, 118.26% and 23.24%, respectively in phyA under cLL1 treatment. Similar trends were observed under cLL2 and cDa treatments (Supplementary Table S7b). These results suggested that with the depletion in light intensity, suppression of seedling growth was affected.
Assessment of the differential expression of GA and ABA regulatory genes during germination under low light stress
Each cDNA preparation was normalized using β-tubulin as an internal control. Seed germination was significantly associated with the upregulation of GA and downregulation of ABA during the initiation of embryonal growth (Piskurewicz et al. 2008). To explore how fluctuating light intensity influences endogenous GA biosynthesis during seed germination, germinated seedlings from the open-grown under NL and LL conditions, while germinated seedlings from the growth chamber grown under cNL, cLL1, cLL2, and cDa conditions were analyzed for the expression of various GA and ABA metabolism genes. Value of less than 1 and more than 1 in rate change indicated the downregulation and upregulation, respectively in the expression of genes (Supplementary Table S8a, b).
Differential expression of GA and ABA regulatory genes during germination under low light stress under open conditions
The results showed that at 6 days after germination, GA anabolism genes such as OsGA3ox1 and OsGA3ox2 were downregulated (Fig. 6a), whereas GA catabolism genes such as OsGA2ox1 and OsGA2ox2 were upregulated (Fig. 6c) under gradual depletion in the light intensity. Inversely, SLR1, which is associated with negative regulation of bioactive GA biosynthesis (Fig. 6e) (Schwechheimer and Willige 2009), along with ABA anabolism genes such as OsNCED3, OsNCED4, and OsNCED5 were upregulated (Fig. 6g) with the decrease in light intensity (Supplementary Table S8a, b). In contrast, ABA catabolism-associated genes such as OsABA8ox2 and OsABA8ox3 were downregulated (Fig. 6i) with reducing light intensity. This result indicated that depletion in light intensity decreases seed germination via down-regulation of GA and upregulation of ABA-associated anabolism gene expression.
Fig. 6.
Expression pattern of gibberellic acid (GA) and abscisic acid (ABA) genes from the 6th day incubated seeds of Swarnaprabha (SP), IR8, wild type (WT), and phytochrome A mutant (phyA) under low light (LL) in open conditions a, c, e, g, i with respect to with respect normal light (NL), and under continuous low light (cLL1 and cLL2) and continuous dark (cDa) conditions in growth chamber b, d, f, h, j with respect to continuous normal light (cNL). GA metabolism genes, OsGA3ox1, OsGA3ox2, OsGA2ox1, OsGA2ox2 a, b, c, d and response regulation (SLR1) e, f along with ABA metabolism related genes, OsNCED3, OsNCED4, OsNCED5, OsABA8ox2, OsABA8ox3 g, h, i, j expression patterns were studied in the 6th day incubated seedlings under open conditions a, c, e, g, i and growth chamber b, d, f, h, j of Swarnaprabha (SP), IR8, wild type (WT), and phytochrome A mutant (phyA) grown under normal light (NL) and low light (LL) conditions in Kharif and Rabi 2019 and under continuous normal light (cNL), continuous low light (cLL1 and cLL2) and continuous dark (cDa) conditions. The expression level was determined by qRT-PCR. b- Tubulin was used as the internal standard. Each data point is the average of six replicates. Error bars represent SE. * = Mark above the bar represents the level of significance at < 0.05, ** at < 0.01 and *** = at < 0.001. Signifcance (value) is in comarision with respect normal light in filed condition (NL) and under growth chamber (cNL)
Differential expression of GA and ABA regulatory genes during germination under low light stress under growth chamber
A similar pattern of GA and ABA regulatory genes was recorded under the growth chamber condition. With the decrement in light intensity, GA-anabolic (Fig. 6b) and ABA-catabolic (Fig. 6j) genes were downregulated, whereas GA-catabolic (Fig. 6d), SLR1(Fig. 6f), and ABA-anabolic (Fig. 6h) genes were upregulated.
Analysis of variances (ANOVA), phenotypic correlation, principal component, and heat map analysis
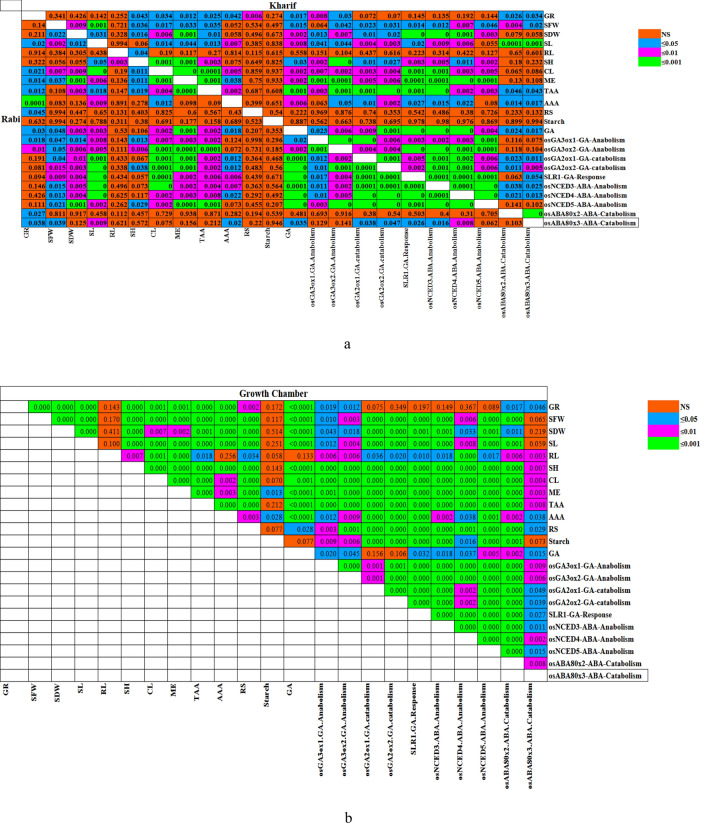
ANOVA revealed a highly significant mean sum of squares for all the 12 traits, suggesting the presence of sufficient variation among genotypes, seasons and light conditions for these traits. Highly significant variations were observed for treatment one (seasons) in nine out of 12 traits. Similarly, twelve, twelve, three, seven, twelve and three traits showed highly significant variations among tratments, namely, light conditions, genotypes, seasonxlight, lightxgenotype and seasonxlightxgenotype, respectivelly under open conditions (Supplementary Table S9a). Under the growth chamber, all the traits except for coleoptile length showed highly significant variations for treatments, light conditions and genotypes. For treatment three (lightxgenotype), 10 traits except for coleoptile length and total amylase activity showed significant variations (Supplementary Table S9b). The correlation and association analyses were carried out to establish a relationship between 23 different traits under different light situations. The 6th-day seed germination percentage (6SGP) exhibited a significantly positive association with height, coleoptile length, mobilization efficiency, total amylase activity, α-amylase activity, reducing sugar content, gibberellic acid content, GA-anabolic gene expression and ABA-catabolic gene expression. However, 6SGP was found to be non-significantly correlated with starch content, expression of GA-catabolic genes, SLR1, and ABA-anabolic genes both in Kharif and Rabi seasons (Fig. 7a, Supplementary Table S10 a, b). Under growth chamber condition, 6th-day seed germination percentage (6SGP) exhibited a significantly positive association with thirteen out of 23 traits (Fig. 7b, Supplementary Table S10 c). The principal component analysis was used to establish the patterns and interrelationships existing between the four genotypes with all the 23 traits (Supplementary Table S11 a, b, c). The first four principal components explained 85.17%, 96.13%, and 85.41% of total variations in Kharif season, Rabi season, and growth chamber, respectively with all the 23 traits. Hierarchical Heat map (Fig. 8a, b, c) displayed the degree of association between the rice genotypes and 23 traits. The variation in color pattern suggested the dominance of a particular trait associated with the genotypes. The darker maroon color depicts the most influential trait. Similarly, the lighter blue colors indicated a poor association of a particular trait with the respective genotype. Under all the three conditions (Kharif, Rabi, and growth chamber), phyA under both NL and LL showed the strongest association with mobilization efficiency.
Fig. 7.
Multicollenearity matrix showing phenotypic correlation for germination percentage, seedling phenotypes, and biochemical traits in seeds germinated under normal light (NL) and low light(LL) regiments in field condition (a) under growth chamber (b). SP-Swarnaprabha, WT-Wild type, PhyA-Phytochrome A mutant, GR-Germination rate at 6th day(%), SFW-Seedling fresh weight(gm), SDW-Seedling dry weight (gm), SL-Shoot length(cm), RL-Root length(cm), SH-Seedling height (cm), CE-Coleoptile length (cm), ME-Mobilization efficiency (%), TAA-Total amylase activity, Alpha amylase activity, RSC-Reducing sugar content, GA-Gibberlic acid content((µg/g FW)
Fig. 8.
Heat map showing the degree of association between rice genotypes (SP, IR8, WT and phyA) and 12 traits in Kharif 2019 (a), Rabi 2019, (b) and under growth chamber conditions (c). The columns represent different traits/ parameters, while the row represents genotypes under different light conditions. The variation in color pattern suggests the dominance of a particular trait associated with the genotypes. The dark (maroon) color depicting the most influential trait, while dark blue color indicated a poorest association of a particular trait with the respective genotype. The color scale is given on the top right side of the figure with values
Discussion
Seeds respond to various environmental conditions, such as light quality and quantity, to regulate their germination process. Depending on the spectral composition, type of photon fluence, and the physiological conditions of the seeds, the available irradiance can promote or inhibit germination (Shichijo et al. 2001). Low light stress resulting from canopy shading or fluctuating light availability generates an FR-enriched environment that has been previously reported to decrease seed germination (El-Keblawy and Gairola 2017). In this study, we explored the impact of the FR-enriched environment by reducing the PAR on the seed germination efficiency in rice via simulating the low light condition in the open through agro-net shading (≈ 24.3% shading) and under gradually lowered light intensities (cLL1 = 25%, cLL2 = 50%, and cDa = 95% shading) in growth chamber conditions. We then investigated the central role of GA in the solubilization of carbohydrate reserves in the cotyledon and its possible dependence on the available irradiance quality via the involvement of phytochrome A. Factorial ANOVA for all the 12 traits, including seasonal and light variations, was captured. These seasonal variations were significant for nine traits (including seed germination and GA content), indicating both seasons had different total light availability, which influenced rice germination rate. The variations were significant for all the 12 traits due to light conditions, indicating that low light imposed during the experimentation was significantly different from the normal light condition.
Our results demonstrated that low light stress negatively influences seed germination in rice (Fig. 2). Under the open condition, the GP was significantly lowered in Kharif than in Rabi when low light stress is naturally imposed due to the overcast skies. A similar result was observed in Arabidopsis by Vaistij et al. (2018). Previous reports suggested that phytochromes promote seed germination via the low-fluence response (LFR) and the very-low-fluence response (VLFR), which has a photon requirement of 1–1000 µmol m −2 s−1 and 0.1 to 1 µmol m −2 s−1 of R light, respectively (Sheerin and Hiltbrunner 2017). Therefore, an additional reduction of photon supply due to neighboring shade or environmental consequences such as cloudy overcast skies would generate a fluence deficiency, eventually affecting the rate of seed germination. Moreover, the promotion of seed germination by a VLFR requires a more substantial reduction in dormancy level, which is achieved when the seeds remain buried in the soil for extended periods (Botto et al. 1996). Eventually, there is little scope for the buried seeds in the seedbed to acclimatize in response to the inopportune photo-deficit condition to overcome dormancy and initiate cellular division at the embryonal axis.
Further to understand the phytohormone intervention in the regulation of seed germination under shade, we estimated the GA content of the germinated seedlings. Our data revealed a concomitant reduction in the GA content with the lowering of the PAR. Intriguingly, in addition, we found a positive correlation between seed germination rate and endogenous GA production in the seeds (Supplementary Table S10 a, b, c and Fig. 7). These results imply that low light stress may inhibit seed germination by decreasing the bioactive GA content in the incubated seeds. In higher plants, ABA and GA have been reported to antagonistically regulate seed dormancy and germination and respond to environmental cues, such as light, temperature, and abiotic stresses (Golldack et al. 2013). To further explore the role of GA in the regulation of embryonal growth under the light depleted environment, we studied the temporal expression profile of various GA and ABA metabolic genes via qRT-PCR (Fig. 6). Our results demonstrated that GA anabolic genes, i.e., GA3-oxidase genes (OsGA3ox1 and OsGA3ox2) were downregulated with a concomitant upregulation of GA catabolic genes, i.e., GA2-oxidase genes (OsGA2ox1 and OsGA2ox2), and SLR1 under low light stress. The fold-downregulation of GA-anabolic genes was remarkable in phyA than WT, SP, and IR8 incubated seeds both under in vivo and in vitro FR-enriched conditions. Additionally, we found a significant positive correlation between the expression of GA anabolic genes and percent germination in all the tested lines (Fig. 7, Supplementary Table S10). This data suggested that endogenous production of GA during germination favors embryonal growth, which is discouraged under low light stress. In Arabidopsis, light induces the GA3-oxidase genes, which encode enzymes that catalyze the final step in GA biosynthesis, such as GA9 and GA20 to GA4 and GA1; whereas GA catabolic gene encodes an enzyme that converts active GAs (GA4 and GA1) to inactive catabolites such as GA54 and GA8 (Yamauchi et al. 2007). This reciprocal regulation of GA metabolic genes results in a higher level of bioactive GA under normal light conditions (Oh et al. 2006). Therefore, we suggest that under low light stress, a relatively higher expression of GA catabolic genes might have played a central role in reducing bioactive GA otherwise required to encourage seed germination. Furthermore, we found a significant reduction in GA content in the low light germinated phyA seeds both in the open and growth chamber, which could be attributed to a relatively higher upregulation of GA-catabolic genes in them. This was further reflected in a sharp reduction in the percent germination of phyA seeds under low light stress than WT, suggesting the possible role of phyA in the biosynthesis of GA during seed germination. Toyomasu et al. (1998) reported the involvement of red light in promoting GA synthesis in lettuce seeds by inducing GA anabolic genes expression via phytochrome action. Additionally, we observed a positive correlation between the expression of SLR1 with GA catabolic genes (Fig. 7). A similar correlation was previously reported by Ueguchi-Tanaka et al. (2007). In rice, SLR1 encodes for DELLA protein that restrains GA responses (Salanenka et al. 2018). Furthermore, Cao et al. (2005) reported that a loss in the function of four DELLA genes in ga1-3 mutants encouraged seed germination in Arabidopsis, suggesting the role of DELLA proteins to act as integrators of environmental and endogenous cues to modulate seed germination. Previous reports have also identified several regulators of SAS acting downstream of the phytochrome photoreceptors, such as PHYTOCHROME-INTERACTING FACTORS (PIFs), which are believed to play a crucial role in moderating low light-induced rapid transcriptome reprogramming in plants (Hornitschek et al. 2012). Recently, PIFs have been reported as cardinal regulators of GA-mediated seed germination (Ravindran and Kumar 2019). Additionally, Oh et al. (2006) reported that under normal light conditions, the bioactive form of phytochromes destabilizes the PHYTOCHROME INTERACTING LIKE PROTEIN-5(PIL5) that consequently increased GA synthesis leading to DELLA degradation, ultimately triggering seed germination. Besides, DELLA has also been found to negatively regulate OsPIL14 activity, which positively regulates GA-anabolic gene expression, through a combinatorial strategy of sequestration and degradation of OsPIL14 in rice (Mo et al. 2020). Therefore, it is presumed that under LL stress, retardation of bioactive conversion of phytochrome might have played a crucial role in demoting GA synthesis leading to a lower seed germination rate. Our observations corroborated this hypothesis, which demonstrated a remarkable upregulation of SLR1, GA-catabolic genes, and ABA-anabolic genes in phyA than WT incubated seeds under low light conditions (Fig. 6). Furthermore, previous reports have suggested that GA and ABA antagonistically modulate seed germination (Shu et al. 2018). Additionally, we found a significant upregulation of ABA-anabolic genes (OsNCED3, OsNCED4, and OsNCED5) with a simultaneous downregulation of ABA-catabolic genes (OsABA8ox2 and OsABA8ox3) under low light stress. Our results demonstrated a considerable upregulation of ABA-anabolic genes in phyA incubated seeds under FR-enriched conditions, which was negatively correlated with seed germination percent. Recent reports have proposed interactions between the significant ABA signaling pathway and several other signaling factors in stress-responses and seed developmental processes (Nakashima and Yamaguchi-Shinozaki 2013). Kim et al. (2012) have unraveled a core signaling unit for ABA-responsive gene expression modulating seed germination and early seedling rice growth. Additionally, in a recent report, Qi et al. (2020) have shown that PIFs positively regulate the ABA signaling pathway during the seedling stage, specifically in a light-depleted environment. Therefore, it is expected that due to inefficient conversion of phytochromes to their bioactive forms under low light stress, the accumulation of PIFs increased, favoring the upregulation of ABA anabolic genes, consequently hampering the efficiency of seed germination. Xie et al. (2017) reported a similar increment in PIFs under the shade that was positively correlated with ABA biosynthesis in Arabidopsis. Thus, our data suggested that upregulation of ABA anabolic genes might have further sent positive signals for the downregulation of GA-anabolic genes decreasing seed germination rate under low light stress via a phytochrome-PIF dependent regulatory pathway.
In cereals, seed germination is dependent on the solubilization of storage carbohydrate reserves in the mature seeds that generate soluble sugars which act as the primary source of energy for the embryonic growth and seedling emergence (Beck and Ziegler 1989). Our data demonstrated a significant increment in starch content in the germinating seeds under FR-enriched conditions. α-Amylase is the central enzyme involved in starch mobilization; thus, α-amylase activity (AAA) significantly regulates seed germination (Karrer et al. 1993). To further evaluate the efficacy of starch solubilization, we estimated the amylase activities in the germinating seedlings and studied their interrelationship with the GA activity. In this context, we tested whether low light-induced bioactive GA deficiency reduces seed germination by decreasing AAA and TAA. We found a significant positive association between GA content, AAA, TAA, and seed germination (Supplementary Table S11, Fig. 8). Atzorn and Weiler (1983) reported a similar correlation in barley. In seeds of GA signaling repressor mutant slr1, a null mutant for DELLA, regardless of GA treatment, α-amylase gene expression levels were equivalent to those of GA-treated wild-type seeds (Yano et al. 2015). These previous studies confirm that GA plays a pivotal role in the regulation of AAA through transcriptional regulation in rice. Our results indicated that low light treatment significantly decreases the seed bioactive GA content, decreasing AAA and TAA. Additionally, we found a non-significant association between AAA, TAA, and grain starch content (Supplementary Table S11), suggesting a decrease in amylase activity reduces carbohydrate solubilization in the incubated seeds, inevitably affecting embryonal growth. This observation is in parallel to an increase in starch accumulation under light depleted environments, both in the open and growth chamber conditions, suggesting a decelerated rate of starch solubilization which is further corroborated by the reduction of mobilization efficiency in the germinating seeds under low light stress. Appleford et al. (2007) reported the GA-dependent positive regulation of α-amylase genes, α-Amy1 and α-Amy2, due to ectopic expression of PcGA2ox1 in wheat. Again, Yu (1999) reported that α-amylase genes were subdued by accumulated seed sugar via a feedback control mechanism in the embryo. The results of our current investigation and previous studies indicate that low light-induced GA inadequacy reduces α-amylase activity by down-regulating solubilization of seed carbohydrate reserves that negatively influence germination efficiency.
Our results demonstrated an enhanced seedling height under low light stress, suggesting a reduction in photomorphogenic suppression under the FR-enriched environment. Among all the tested genotypes, phyA had the highest increment in seedling shoot and root length under low light stress. Similarly, Roy et al. (2013) reported that under red light enriched environment (NL condition), suppression of photomorphogenesis is driven via a phytochrome A-mediated signaling. Additionally, we also found a significant increment in the coleoptile length of phyA seedlings incubated under an FR-enriched environment. Previously, physiological investigations of the phyA mutants at the early seedling stage have revealed that PhyA is accountable for inhibiting coleoptile elongation via VLFR response (Roy et al. 2013). We hypothesize that in the absence of phytochrome A, phyA lines under FR-enrichment maintained a higher PIF concentration in their nucleoplasm which further downregulates GA-anabolic genes that demotes amylase activities (AAA and TAA) necessary for starch solubilization (Fig. 9). Consequently, our data indicated that phytochrome A is primarily involved in suppressing red-light-dependent photomorphogenesis in rice.
Fig. 9.
Thematic presentation of regulation of phytochrome-GA mediated regulation of carbon solubilization under low light stress that reduces embryonal growth, further discouraging seed germination. Low light stress lowered the conversion of phytochromes to their biologically active form (Pfr) that increased the concentration of phytochrome interacting factors (PIF), consequently upregulating abscisic acid (ABA) anabolic genes (OsNCED3, OsNCED4, and OsNCED5) while downregulating gibberellic acid (GA) anabolic genes (OsGA3ox1, OsGA3ox2). An increased ABA favors seed dormancy while lowerd GA maintains a higher DELLA protein activity that also lowers GA response in the incubated seeds. Consequently, lower GA results in decreased α-amylase (AAA) and total amylase (TAA) activities that cause reduced starch solubulization, thus reducing sugar transportation to the embryonic growth and finally seed germination
Conclusion
The schedule of seed germination is crucial for the survival of the embryo and the seedling establishment. Our study provided evidence that an FR-enriched light environment retards seed germination in rice by reducing the bioactive GA as a consequence of a concomitant increased ABA content. Furthermore, bioactive GA inadequacy inhibits seed germination by reducing α-amylase and total amylase activity that reduced sugar transportation to the growing embryonic axis essential to initiate germination. A comparative expression profiling of GA and ABA metabolic genes further demonstrated complex crosstalk between the phytohormone and photoreceptors, which is dependent on the available light quality during the orchestrated events of germination (Fig. 9). Finally, we suggest that under low light stress, due to a retarded conversion of phytochrome A to their bioactive form, the PIF pool increases that eventually upregulates the ABA-catabolic genes with a simultaneous downregulation of GA-anabolic genes. Consequently, a lower bioactive GA pool fails to leverage the GA-dependent DELLA degradation, further shutting down the expected GA responses that reduce germination efficiency under FR-enriched light. The GA and ABA metabolic genes reported in this study constitute a good candidate gene cluster to understand the light governing signaling mechanism that is involved in the process of seedling establishment in rice. Therefore, this ideal characterization of the phytohormones and their molecular components associated with seed germination in the low light stress-associated interactive pathway would aid in the development of shade resilient rice varieties through biotechnological interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The financial assistance received from the Indian Council of Agricultural Research, New Delhi, India is gratefully acknowledged.
Author contributions
DP and SM equally contributed to work in conceptualization, design, Material preparation, data collection, writing and statistical analysis of the work. SD done the lab work and data entry. RPS helped in data curation and visualization. AK helped in Biochemical study. LB has done the Investigation, validation, reviewing, editing and finalization of the manuscript. MJB and BCT guided in whole manuscript preparation and the physiological work.
Declarations
Conflict of interest
The authors did not receive support from any organization for the submitted work. The authors have no competing interests to declare that are relevant to the content of this article. There is no conflict of interest among the authors regarding the authorship of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Darshan Panda and Soumya Mohanty have Contributed equally to the work
Contributor Information
Darshan Panda, Email: darshan.panda216@gmail.com.
Soumya Mohanty, Email: smmohanty.mohanty9@gmail.com.
Swagatika Das, Email: swagatikadas876@gmail.com.
Rameswar Prasad Sah, Email: ramesh.pbg@gmail.com.
Awadhesh Kumar, Email: awadh_iari@yahoo.com.
Lambodar Behera, Email: lbehera.publi2018@gmail.com, Email: lambodarjamujhadi@gmail.com.
Mirza Jaynul Baig, Email: mjbaigcrri@gmail.com.
Baishnab C. Tripathy, Email: baishnabtripathy@yahoo.com
References
- Appleford NE, Wilkinson MD, Ma Q, Evans DJ, Stone MC, Pearce SP, Powers SJ, Thomas SG, Jones HD, Phillips AL, Hedden P, Lenton JR. Decreased shoot stature and grain α-amylase activity following ectopic expression of a gibberellin 2-oxidase gene in transgenic wheat. J Exp Bot. 2007;58(12):3213–3226. doi: 10.1093/jxb/erm166. [DOI] [PubMed] [Google Scholar]
- Atzorn R, Weiler EW. The role of endogenous gibberellins in the formation of α-amylase by aleurone layers of germinating barley caryopses. Planta. 1983;159(4):289–299. doi: 10.1007/BF00393166. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Roychoudhury A (2019) The regulatory signaling of gibberellin metabolism and its crosstalk with phytohormones in response to plant abiotic stresses. Plant signaling molecules. Woodhead Publishing, pp 333–339
- Bardhan S, Panigrahi KCS (2018) Mechanisms involved in response to shade in rice. A review. In: Proceedings of the International Conference on Plant Developmental Biology. Cambridge Scholars Publishing, pp 3–15
- Beck E, Ziegler P. Biosynthesis and degradation of starch in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1989;40(1):95–117. [Google Scholar]
- Bose B, Kumar M, Singhal RK, Mondal S. Impact of seed priming on the modulation of physico-chemical and molecular processes during germination, growth, and development of crops. In: Amitava Rakshit A, Singh HB, editors. Advances in seed priming. Singapore: Springer; 2018. pp. 23–40. [Google Scholar]
- Botto JF, Sanchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110(2):439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Hussain A, Cheng H, Peng J. Loss of function of four DELLA genes leads to light-and gibberellin-independent seed germination in Arabidopsis. Planta. 2005;223(1):105–113. doi: 10.1007/s00425-005-0057-3. [DOI] [PubMed] [Google Scholar]
- Cordeiro AM, Figueiredo DD, Tepperman J, Borba AR, Lourenço T, Abreu IA, Ouwerkerk PBF, Quail PH, Oliveira MM, Saibo NJM. Rice phytochrome-interacting factor protein OsPIF14 represses OsDREB1B gene expression through an extended N-box and interacts preferentially with the active form of phytochrome B. Biochim Biophys Acta Gene Regul Mech. 2016;1859(2):393–404. doi: 10.1016/j.bbagrm.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Cipcigan M, Pamfil D, Sisea CR, Mărgăoan R. Gibberellic acid can improve seed germination and ornamental quality of selected cyclamen species grown under short and long days. Agronomy. 2020;10(4):516. [Google Scholar]
- Cui K, Peng S, Xing Y, Xu C, Yu S, Zhang Q. Molecular dissection of seedling-vigor and associated physiological traits in rice. Theor Appl Genet. 2002;105(5):745–753. doi: 10.1007/s00122-002-0908-2. [DOI] [PubMed] [Google Scholar]
- Dey P, Datta D, Pattnaik D, Dash D, Saha D, Panda D, Singhal RK. Plant perspectives to global climate changes. Cambridge: Academic Press; 2021. Physiological, biochemical, and molecular adaptation mechanisms of photosynthesis and respiration under challenging environments; pp. 79–100. [Google Scholar]
- El-Keblawy A, Gairola S. Dormancy regulating chemicals alleviate innate seed dormancy and promote germination of desert annuals. J Plant Growth Regul. 2017;36(2):300–311. [Google Scholar]
- Fisher RA. The use of multiple measurements in taxonomic problems. Ann Eugen. 1936;7:179–188. [Google Scholar]
- Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2010;61(1):11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golldack D, Li C, Mohan H, Probst N. Gibberellins and abscisic acid signal crosstalk: living and developing under unfavorable conditions. Plant Cell Rep. 2013;32(7):1007–1016. doi: 10.1007/s00299-013-1409-2. [DOI] [PubMed] [Google Scholar]
- Graham HD, Thomas LB. Rapid, simple colorimetric method for the determination of micro quantities of gibberellic acid. J Pharm Sci. 1961;50(1):44–48. doi: 10.1002/jps.2600500110. [DOI] [PubMed] [Google Scholar]
- Hammer O, Harper DA, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9. [Google Scholar]
- Hisamatsu T, King RW. The nature of floral signals in Arabidopsis. II. Roles for FLOWERING LOCUS T (FT) and gibberellin. J Exp Bot. 2008;59(14):3821–3829. doi: 10.1093/jxb/ern232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, Xenarios I, Fankhauser C. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signalling. Plant J. 2012;71:699–711. doi: 10.1111/j.1365-313X.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, Sharma P, Kapoor S, Tyagi AK, Khurana JP. F-box proteins in rice Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007;143(4):1467–1483. doi: 10.1104/pp.106.091900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8(3):217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- Karrer E, Chandler JM, Foolad MR, Rodriguez RL. Seedling vigor is correlated to the expression of one member of the rice α-amylase multigene family. Euphytica. 1993;66(3):163–169. [Google Scholar]
- Kim ST, Kang SY, Wang Y, Kim SG, Hwang DH, Kang KY. Analysis of embryonic proteome modulation by GA and ABA from germinating rice seeds. Proteomic. 2008;8(17):3577–3587. doi: 10.1002/pmic.200800183. [DOI] [PubMed] [Google Scholar]
- Kim H, Hwang H, Hong JW, Lee YN, Ahn IP, Yoon IS, Yoo SD, Lee S, Kim BG. A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth. J Exp Bot. 2012;63(2):1013–1024. doi: 10.1093/jxb/err338. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sahoo U, Baisakha B, Okpani OA, Ngangkham U, Parameswaran C, Basak N, Kumar G, Sharma SG. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J Cereal Sci. 2018;79:348–353. [Google Scholar]
- Kumar A, Panda D, Mohanty S, Biswal M, Dey P, Dash M, Sah RP, Kumar S, Baig MJ, Behera L. Role of sedoheptulose-1, 7 bisphosphatase in low light tolerance of rice (Oryza sativa L.) Physiol Mol Biol Plant. 2020;26:2465–2485. doi: 10.1007/s12298-020-00905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Chen F, Shuai H, Luo X, Ding J, Tang S, Yang W. Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. Sci Rep. 2016;6(1):1–12. doi: 10.1038/srep22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo W, Tang W, Jing Y, Bu Q, Lin R. PHYTOCHROME-INTERACTING FACTOR-LIKE14 and SLENDER RICE1 interaction controls seedling growth under salt stress. Plant Physiol. 2020;184(1):506–517. doi: 10.1104/pp.20.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S, Donde R, Das S, Panda D, Mishra B, Pradhan SK, Behera L. Utilization of genetic diversity and population structure to reveal prospective drought-tolerant donors in rice. Gene Rep. 2021;23:101151. [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013;32(7):959–970. doi: 10.1007/s00299-013-1418-1. [DOI] [PubMed] [Google Scholar]
- Naredo MEB, Juliano AB, Lu BR, De Guzman F, Jackson MT. Responses to seed dormancy-breaking treatments in rice species (Oryza sativa L.) Seed Sci Technol. 1998;26:675–690. [Google Scholar]
- Nayak SK, Janardhan KV, Murty KS. Photosynthetic efficiency of rice as influenced by light intensity and quality. Indian J Plant Physiol. 1978;21(1):48–52. [Google Scholar]
- Nayak L, Panda D, Dash GK, Lal MK, Swain P, Baig MJ, Kumar A. A chloroplast Glycolate catabolic pathway bypassing the endogenous photorespiratory cycle enhances photosynthesis, biomass and yield in rice (Oryza sativa L.) Plant Sci. 2021;314:111103. doi: 10.1016/j.plantsci.2021.111103. [DOI] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G. Light activates the degradation of PIL5 protein to promote seed germination through gibberelin in Arabidopsis. Plant J. 2006;47(1):124–139. doi: 10.1111/j.1365-313X.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- Panda D. Development of genetically engineered plant photoreceptors for generating crop plants with novel agronomic traits. Int J Genet Eng Biotechnol. 2014;5:93–102. [Google Scholar]
- Panda D, Biswal M, Mohanty S, Dey P, Swain A, Behera D, Baig MJ, Kumar A, Sah RP, Tripathy BC, Behera L. Contribution of phytochrome a in the regulation of sink capacity starch biosynthesis, grain quality, grain yield and related traits in rice. Plant Archiv. 2020;20(1):1179–1194. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. Mobilization of endosperm reserves in cereal seeds under anoxia. Annal Bot. 1997;79(1):49–56. [Google Scholar]
- Piskurewicz U, Jikumaru Y, Kinoshita N, Nambara E, Kamiya Y, Lopez-Molina L. The gibberellic acid signalling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell. 2008;20(10):2729–2745. doi: 10.1105/tpc.108.061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurewicz U, Turečková V, Lacombe E, Lopez-Molina L. Far-red light inhibits germination through DELLA-dependent stimulation of ABA synthesis and ABI3 activity. EMBO J. 2009;28(15):2259–2271. doi: 10.1038/emboj.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Liu S, Li C, Fu J, Jing Y, Cheng J, Li H, Zhang D, Wang X, Dong X, Han R, Li B, Zhang Y, Li Z, Terzaghi W, Song C-P, Lin R, Gong Z, Li J. PHYTOCHROME-INTERACTING FACTORS interact with the ABA receptors PYL8 and PYL9 to orchestrate ABA signaling in darkness. Mol Plant. 2020;13(3):414–430. doi: 10.1016/j.molp.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Ravindran P, Kumar PP. Regulation of seed germination: the involvement of multiple forces exerted via gibberellic acid signalling. Mol Plant. 2019;12(1):24–26. doi: 10.1016/j.molp.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Rood SB, Larsen KM. Gibberellins, amylase, and the onset of heterosis in maize seedlings. J Exp Bot. 1988;39(2):223–233. [Google Scholar]
- Roy A, Sahoo D, Tripathy BC. Involvement of phytochrome A in suppression of photomorphogenesis in rice seedling grown in red light. Plant Cell Environ. 2013;36(12):2120–2134. doi: 10.1111/pce.12099. [DOI] [PubMed] [Google Scholar]
- Salanenka Y, Verstraeten I, Löfke C, Tabata K, Naramoto S, Glanc M, Friml J. Gibberellin DELLA signalling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc Natl Acad Sci. 2018;115(14):3716–3721. doi: 10.1073/pnas.1721760115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Willige BC. Shedding light on gibberellic acid signaling. Curr Opin Plant Biol. 2009;12(1):57–62. doi: 10.1016/j.pbi.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Sekhar S, Panda D, Kumar J, Mohanty N, Biswal M, Baig MJ, Kumar A, Umakanta N, Samantaray S, Pradhan SK, Shaw BP, Swain P, Behera L. Comparative transcriptome profiling of low light tolerant and sensitive rice varieties induced by low light stress at active tillering stage. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-42170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin DJ, Hiltbrunner A. Molecular mechanisms and ecological function of far-red light signalling. Plant Cell Environ. 2017;40(11):2509–2529. doi: 10.1111/pce.12915. [DOI] [PubMed] [Google Scholar]
- Shichijo C, Katada K, Tanaka O, Hashimoto T. Phytochrome A-mediated inhibition of seed germination in tomato. Planta. 2001;213(5):764–769. doi: 10.1007/s004250100545. [DOI] [PubMed] [Google Scholar]
- Shu K, Zhou W, Chen F, Luo X, Yang W. Abscisic acid and gibberellin antagonistically mediate plant development and abiotic stress responses. Front Plant Sci. 2018;9:416. doi: 10.3389/fpls.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineshchekov V, Koppel L, Riemann M, Nick P. Phytochrome a and its functional manifestations in etiolated and far-red light-grown seedlings of the wild-type rice and its hebiba and cpm2 mutants deficient in the defence-related phytohormone jasmonic acid. Photochem Photobiol. 2020;97(2):335–342. doi: 10.1111/php.13340. [DOI] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Shinomura T. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell. 2005;17(12):3311–3325. doi: 10.1105/tpc.105.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S, Asthir B, Kaur G, Kalia A, Sharma A. Zinc oxide and titanium dioxide nanoparticles influence heat stress tolerance mediated by antioxidant defense system in wheat. Cereal Res Commun. 2021;21(7):1–12. [Google Scholar]
- Todaka D, Nakashima K, Maruyama K, Kidokoro S, Osakabe Y, Ito Y, Matsukura S, Fujita Y, Yoshiwara K, Ohme-Takagi M, Kojima M, Sakakibara H, Shinozaki K, Yamaguchi-Shinozaki K. Rice phytochrome-interacting factor-like protein OsPIL1 functions as a key regulator of internode elongation and induces a morphological response to drought stress. Proc Natl Acad Sci. 2012;109(39):15947–15952. doi: 10.1073/pnas.1207324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T, Kawaide H, Mitsuhashi W, Inoue Y, Kamiya Y. Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiol. 1998;118(4):1517–1523. doi: 10.1104/pp.118.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan PA, Kumar R, Rehal PK, Toora PK, Ayele BT. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front Plant Sci. 2018;9:668–672. doi: 10.3389/fpls.2018.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell. 2007;19(7):2140–2155. doi: 10.1105/tpc.106.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij FE, Barros-Galvão T, Cole AF, Gilday AD, He Z, Li Y, Harvey D, Larson TR, Graham IA. MOTHER-OF-FT-AND-TFL1 represses seed germination under far-red light by modulating phytohormone responses in Arabidopsis thaliana. Proc Natl Acad Sci. 2018;115(33):8442–8447. doi: 10.1073/pnas.1806460115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishal B, Kumar PP. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front Plant Sci. 2018;9:838. doi: 10.3389/fpls.2018.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Liu Y, Wang H, Ma X, Wang B, Wu G, Wang H. Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nat Commun. 2017;8(1):1–11. doi: 10.1038/s41467-017-00404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Takeda-Kamiya N, Hanada A, Ogawa M, Kuwahara A, Seo M, Yamaguchi S. Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiol. 2007;48(3):555–561. doi: 10.1093/pcp/pcm023. [DOI] [PubMed] [Google Scholar]
- Yan Y, Li C, Dong X, Li H, Zhang D, Zhou Y, Jiang B, Peng J, Qin X, Cheng J, Wang X, Song P, Qi l, Zheng Y, Li B, Terzaghi W, Yang S, Gou Y, Li J, MYB30 is a key negative regulator of Arabidopsis photomorphogenic development that promotes PIF4 and PIF5 protein accumulation in the light. Plant Cell. 2020;32(7):2196–2215. doi: 10.1105/tpc.19.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Zhang Q. Polymorphisms of α-amylase activity in barley landraces and cultivars from China. Euphytica. 1990;48(3):245–251. [Google Scholar]
- Yano K, Aya K, Hirano K, Ordonio RL, Ueguchi-Tanaka M, Matsuoka M. Comprehensive gene expression analysis of rice aleurone cells: probing the existence of an alternative gibberellin receptor. Plant Physiol. 2015;167(2):531–544. doi: 10.1104/pp.114.247940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SM. Cellular and genetic responses of plants to sugar starvation. Plant Physiol. 1999;121(3):687–693. doi: 10.1104/pp.121.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Tong H, Bai N, Liu M, Xiao T, Xue W. Phytohormone dynamics in developing endosperm influence rice grain shape and quality. J Int Plant Biol. 2020;62(10):1625–1637. doi: 10.1111/jipb.12927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.