Abstract
Papillary thyroid carcinoma (PTC) is the most common malignant tumour of the thyroid and it is often found in association with Hashimoto’s thyroiditis (HT). This concomitance is still under debate. The aim of this study is to investigate the influence of Hashimoto’s thyroiditis in patients with papillary thyroid carcinoma. Two thousand two hundred eighteen patients underwent thyroidectomy in our department between January 2015 and January 2020. Of these, 435 patients had surgery for papillary thyroid carcinoma and form the basis of our studies. The association between PTC and HT was found in 180 patients (41.4%), mostly represented in the female group (78.9%), with a lower median age than patients with PTC without HT. In comparison to patients with PTC alone, the PTC-HT group had less invasive and smaller tumours, as well as less lymph node involvement. Moreover, tumours of patients with PTC-HT were diagnosed earlier. Our data showed that Hashimoto’s thyroiditis may be considered a protective factor when PTC develops. Furthermore, we concluded that patients with PTC and HT had a better prognosis and a lower risk of recurrence than those that did not have HT.
Keywords: Papillary thyroid carcinoma, Hashimoto’s thyroiditis, Thyroid surgery, Endocrine surgery
Introduction
Thyroid cancer is the most common endocrine malignancy, and its incidence rate has increased worldwide [1, 2]. Papillary thyroid carcinoma (PTC) is the main histological type, accounting for about 80% of cases, and it occurs more frequently in women, with a prevalence ratio of 4.0:1 [3, 4].
Hashimoto’s thyroiditis (HT), also called “chronic lymphocytic thyroiditis,” is part of the spectrum of autoimmune thyroid diseases, in which thyroid cells are destroyed by a cellular autoimmune process and locally released cytokines [5]. This disease is the most common cause of hypothyroidism, with an incidence of approximately 1 case/1,000 people/year and a prevalence of 8 cases per 1,000 [6]. Hypothyroidism could also develop after a possible transient state of euthyroidism and/or hyperthyroidism.
The association between HT and PTC was first described by Dailey et al. in 1955, and since then, many studies have investigated this relationship with conflicting results [4, 7, 8]. Some authors suggested that thyroiditis can be considered a precancerous lesion, while other studies reported thyroiditis as being a protective factor decreasing tumour aggressiveness [9–12]. In any case, although the relationship between thyroiditis and cancer is not clear, the simultaneous presence of the two diseases is not rare. Guidelines in clinical practice may be necessary in order to screen and follow up patients with thyroiditis who have a high risk of developing papillary thyroid carcinoma.
This retrospective study was held in order to understand the extent of the relationship with HT, the influence on the tumour stage and the recurrence rate in patients with and without HT. All our patients who underwent thyroidectomy for papillary thyroid carcinoma were included in the study.
Materials and Methods
The study subjects were all those patients who underwent total thyroidectomy for a thyroid tumour in our department from January 2015 to January 2020. Patients who had previous thyroid surgery were excluded from the study. Demographic and clinical data were retrospectively entered into a computerised endocrine surgery registry, which contains data on all thyroid surgery patients.
All patients were given a physical examination. An ultrasound-guided fine needle aspiration biopsy (FNAB) of the thyroid gland (US) was performed, as well as the determination of free thyroid hormones (T3 and T4) and a thyroid stimulating hormone (TSH) test. Thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb) were only tested in patients diagnosed with HT.
Surgical procedures, consisting of a total thyroidectomy with central lymph node dissection (level 6), were carried out at our institute on every patient with a pre-operative diagnosis of suspected cancer. The same equipment was used.
Postsurgical diagnosis of HT was defined as the diffuse lymphoplasmacytic infiltrate of the parenchyma and the presence of lymphoid follicles covered in many areas by Hurthle cells obtained from a review of the pathologic slides.
All patients were followed up clinically by an endocrinologist and an oncologist every 6 months during the first year and then once a year, up to 60 months. Postoperative surveillance included clinical examination, blood test (including TSH, TPOAb, TgAb and thyroglobulin) and ultrasound imaging of the neck.
Statistical analysis—the presence of a relationship between PTC with and without HT and TNM staging, to determine the extent of cancer and lymph node involvement, was analysed using the Pearson chi-square significance test or Fisher’s exact test.
Results
Two thousand two hundred eighteen patients underwent thyroid surgery in our department between January 2015 and January 2020. Only cases of thyroid carcinoma were considered, and histological examinations reported the diagnosis of papillary thyroid carcinoma in 435 cases (86.5%). Other histologic thyroid tumours were not included in our study: follicular thyroid carcinoma was found in 43 cases (8.5%), medullary thyroid carcinoma was found in 18 cases (3.6%), and anaplastic thyroid carcinoma was found in 7 cases (1.4%).
Those patients with papillary thyroid carcinoma included 338 females (77.8%) and 97 males (22.4%), whose median age was 50.1 years (range 15–80 years).
At pathology, one hundred and eighty patients had Hashimoto’s thyroiditis together with PTC (PTC-HT group, 41.4%). In this group of patients, the average age at the time of thyroid surgery was 40 years (range: 15–63 years old) and included 142 females (78.9%) and 38 males (21.1%). The female:male ratio was similar in the two groups (F:M = 3.7:1 v. F:M = 3.3:71, respectively, p = n.s).
Sore throats and fever were present in the clinical history of 140 patients (32.1%); 17 patients (3.9%) showed dysphonia and compressive symptoms.
The diagnostic procedure in 278 patients (64%) began with the discovery of an incidental neck palpable mass or a hypoechoic nodule during a neck ultrasonographic examination.
All patients had FNAB under echographic guidance for suspected nodules. Cytological reports suggested that 72% of all patients had malignancies, with TIR 4 and TIR 5 nodules being considered malignant nodules. Patients with a solitary nodule resulting in doubtful cytological reports (TIR3B) and suspect demographic features were also included.
HT diagnosis was established preoperatively in 170 cases (94.4%), taking into account serum antithyroglobulin and antithyroid peroxidase antibody measurements (which resulted above normal levels), clinical features and US features; Following an endocrinologist’s assessment, 113 patients were receiving L-T4 therapy for hypothyroidism, while 57 patients did not require any therapy because TSH, fT3 and fT4 were all in range. Pathologist studies were used to diagnose HT in 10 patients after surgery.
The interval between the diagnosis of thyroiditis and thyroid surgery for cancer was 3.1 years (range: 3 months to 14 years).
As regards surgical treatment, all patients underwent total thyroidectomy and central lymphadenectomy. Unilateral latero-cervical lymphadenectomy was performed in 17 patients and bilateral in 3 patients.
In the PTC-only group, 150 patients had stage I disease (58.9%), 7 patients stage II (2.9%), 75 patients stage III (29.3%) and 23 patients stage IV (8.9%). In the PTC-HT group, 135 patients had stage I disease (75%), 8 patients stage II (4.2%) and 37 patients stage III (20.8%), with no patients having stage IV disease. Statistical analyses showed a significant result when comparing the incidence of stage IV (p < 0.05, chi-square test) (part A in Table 1).
Table 1.
Statistical analyses of TNM staging, size tumours and lymph nodes metastases between PTC-HT group and PTC-only group
| PTC-only group | PTC-HT group | Statistical analyses | |
|---|---|---|---|
| A | |||
| Stage I | 150 pts (58.9%) | 135 pts (75%) | p value = 1.5 |
| Stage II | 7 pts (2.9%) | 8 pts (4.2%) | p value = 2 |
| Stage III | 75 pts (29.3%) | 37 pts (20.8%) | p value = 0.8 |
| Stage IV | 23 pts (8.9%) | 0% | p value = 0.012 (p value < 0.05, chi-square test) |
| B | |||
| T < 2 cm | 210 pts (82.4%) | 150 pts (83.3%) | p value = 1 |
| 2 cm < T < 4 cm | 15 pts (5.8%) | 16 pts (8.4%) | p value = 0.8 |
| T > 4 cm | 7 pts (2.9%) | 14 pts (8.3%) | p value = 0.1 |
| Extrathyroidal extension | 23 pts (8.9%) | 0% | p value = 0.01 (p value < 0.05, chi-square test) |
| C | |||
| N0 | 165 pts (64.7%) | 143 pts (79.2%) | p value = 1 |
| N1 | 90 pts (35.3%) | 37 pts (20.8%) | p value = 0.02 (p value < 0.05, Fisher’s exact test) |
Moreover, in the PTC-only group, 210 patients had a tumour size < 2 cm (T1, 82.4%), 15 patients a tumour between 2 and 4 cm (T2, 5.8%) and 7 patients with lesions > 4 cm (T3, 2.9%). Finally, 23 patients showed aggressive tumours with rapid growth and the involvement of close organs (T4, 8.9%). In the PTC-HT group, 150 patients (83.3%) had a T1 tumour, 16 patients (8.4%) a T2 tumour and 14 patients (8.3%) had a T3 tumour (p < 0.05, chi-square test) (part B in Table 1). There was also a different incidence of presentation of central lymph node metastases between the two groups: central lymph node metastases had a lower frequency in the PTC-HT group compared to the PTC-only group (20.8% v. 35.3%, p < 0.05, Fisher’s exact test) (part C in Table 1).
All of the patients in both groups were alive after an average of 3 years of follow-up (min. 6 months to max. 300 months). However, the disease recurred in 15 patients (6.8%) in the form of latero-cervical metastases: 3 of these patients belonged to the PTC-HT group and 12 patients to the PTC-only group (p = n.s., Fisher’s exact test).
Discussion
PTC is the most prevalent thyroid cancer (80–90%) [1–4]. As a result of the improvement in diagnostic tools, its incidence rate over all thyroid cancers has increased by an average of 4.5% per year [1]. Many conditions have been considered predisposing factors for PTC, including ionising radiation, oral contraceptive use, late menarche and chromosomal rearrangements [13–17].
The co-occurrence of HT and PTC remains an active area of research, with conflicting data reported. It is still not known today whether autoimmune disease predisposes the development of cancer, or whether carcinogenesis induces the autoimmune response (Fig. 1).
Fig. 1.
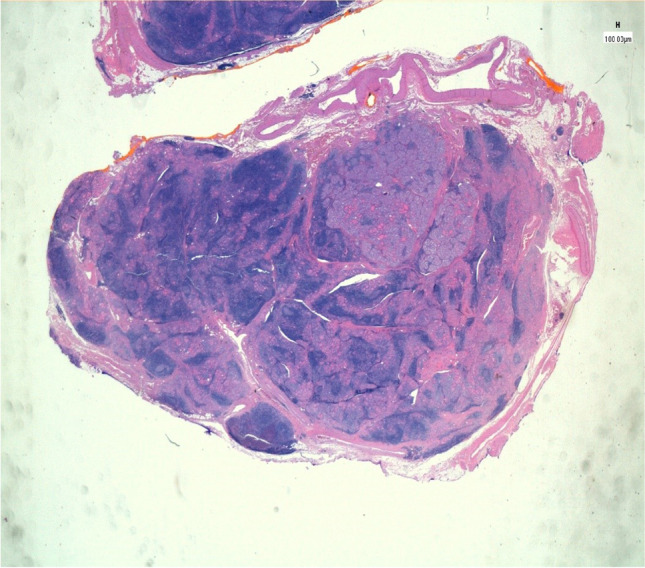
The proliferation focus sharply standing out against the surrounding thyroid parenchyma, which shows a rich follicular lymphoid infiltrate
Our study attempts to analyse the possible significant relationship between these two entities and the impact of HT in the prognosis of PTC. The incidence of HT in our large case series of PTC patients reached 41.4%, finding 180 patients (out of 435) with a histological diagnosis of HT associated with the presence of a PTC (Fig. 2).
Fig. 2.
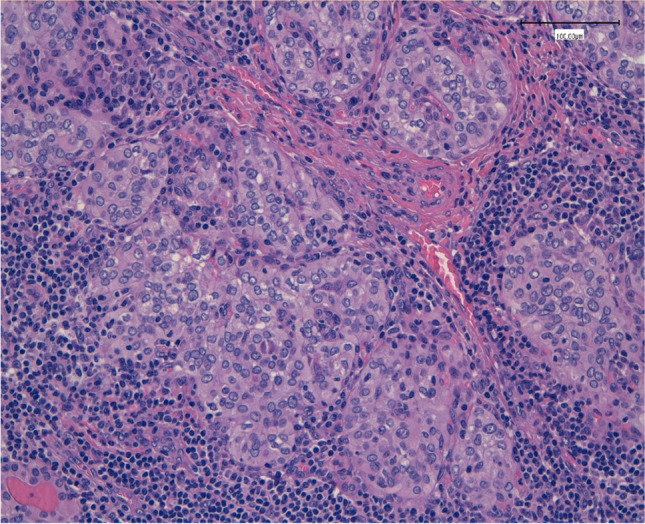
The lymphoid infiltrates penetrating among the papillary carcinoma aggregates
A frequency of HT in approximately 23% of PTC cases is reported in the literature (range from 5 to 85%) [7]. The wide range of incidence could be explained with the different HT diagnostic criteria: many authors considered the histology report, while others also included the value of autoantibodies.
The role of thyroiditis is doubtful. Many authors suggested that thyroiditis could contribute to the development of cancer in different ways. First, hypothyroidism in HT causes higher TSH levels, which have a trophic effect on follicular cells derived from thyroid cancers [8, 10, 11]. Moreover, McLeod et al. reported that elevated TSH levels are associated with an increased risk of thyroid cancer and other studies reported that levothyroxine treatment, resulting in the development of thyroid “autonomy” by reducing TSH levels, may slow cancer progression [18, 19]. However, Paparodis et al. recently demonstrated that TSH serum elevation is not linked to the development of carcinogenesis in patients with chronic thyroid autoimmunity [20]. The TSH value in our series was in the normal range, and 113 patients were receiving levothyroxine therapy for hypothyroidism for endocrinology indications. We, therefore, considered this data to be irrelevant and excluded it from our analysis.
Other factors that could explain the relationship between HT and PTC is the hyperplasia of the residual normal follicular epithelium, which could overgrow and develop into cancer, or the immunodepressive state induced by HT. It has also been established that HT causes a proinflammatory autoimmune state that can lead to thyroid carcinoma, due to cellular damages caused by reactive alterations in stromal cells [21].
Many researchers have studied biomolecular markers to try and explain the link between PTC and HT. These include the RET/PTC rearrangement as reported by Wirtschafter et al., p63 expression in patients with HT and PTC which is negative in normal thyroid and the BRAF V600E mutation [22, 23]. All these theories potentially provide an explanation for the strict relationship between PTC and HT, but they are still not considered significant.
In our series, we noted that PTC patients with HT were mostly female (78.9%). In addition, we found that HT patients had a younger average age of PTC onset than non-HT patients (40 years v. 50.1 years). A further factor that may explain it is the annual tests that HT patients are advised to have, which include lab tests, clinical examinations and US.
Furthermore, while comparing TNM staging in PTC patients with and without HT, we noted that the HT group had no patients with stage IV cancer, whereas the PTC-only group had 8.9%. In fact, patients in the PTC-only group had tumours that were at a more advanced stage. The significance of this relationship was validated by statistical analyses (p value < 0.05).
We also found that PTC patients with HT were associated with a low rate of central lymph node metastases (20.8% v. 35.3%, p value < 0.05). Our findings were corroborated by Kim et al. and other studies, which suggest that HT with PTC could protect against central lymph node metastases (Table 1) [24–26].
The literature reports the possibility of TPOAb and TgAb being involved in the destruction of tumour cells expressing thyroid-specific antigens in PTC, thus slowing down the diffusion of the cancer and the possibility of recurrence [24, 25]. The numbers of infiltrating lymphocytes contain many cytotoxic T cells that inhibit tumour cell growth and metastases [27]. These findings, combined with the strict surveillance, could explain why PTC-HT tumours have a lower TNM staging and why the average age of diagnosis is younger.
Conclusion
Many studies have demonstrated that patients with PTC and HT have a better prognosis [26, 28–30]. Because the prognosis of PTC is excellent, in our experience, the difference in overall survival is not remarkable. The difficulty lies in analysing the difference in survival between patients with PTC and patients with PTC and HT. Another reason why we were unable to detect a difference in survival is because our study’s follow-up was short. We also found that patients with PTC and HT had a lower recurrence than PTC patients without HT (3 patients v. 12 patients, respectively).
In our opinion, Hashimoto’s thyroiditis, irrespective of the role that it can have in the development of cancer, should be considered a potential protective factor.
The problem of the coexistence of HT and PTC should be minimised, but not neglected: what is important is a proper diagnostic procedure.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Semenciw R, Ugnat AM, Mao Y. Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer. 2001;85(9):1335–1339. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimm O. Thyroid cancer. Cancer Lett. 2001;163(2):143–156. doi: 10.1016/S0304-3835(00)00697-2. [DOI] [PubMed] [Google Scholar]
- 4.Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150(1):49–52. doi: 10.1016/j.jss.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mincer, D. L., & Jialal, I. (2019). Hashimoto thyroiditis. In StatPearls [Internet]. StatPearls Publishing. [PubMed]
- 6.Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13(4–5):391–397. doi: 10.1016/j.autrev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70(2):291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- 8.Resende de Paiva C, Grønhøj C, Feldt-Rasmussen U, von Buchwald C. Association between Hashimoto’s thyroiditis and thyroid cancer in 64,628 patients. Front Oncol. 2017;7:53. doi: 10.3389/fonc.2017.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto’s thyroiditis. Eur Surg Res. 2010;45(3–4):333–337. doi: 10.1159/000320954. [DOI] [PubMed] [Google Scholar]
- 10.Fiore E, Latrofa F, Vitti P. Iodine, thyroid autoimmunity and cancer. Eur Thyroid J. 2015;4(1):26–35. doi: 10.1159/000371741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrell E, Heffron C, Murphy M, O'Leary G, Sheahan P. Impact of lymphocytic thyroiditis on incidence of pathological incidental thyroid carcinoma. Head Neck. 2017;39(1):122–127. doi: 10.1002/hed.24544. [DOI] [PubMed] [Google Scholar]
- 12.Jackson D, Handelsman RS, Farrá JC, Lew JI. Increased incidental thyroid cancer in patients with subclinical chronic lymphocytic thyroiditis. J Surg Res. 2020;245:115–118. doi: 10.1016/j.jss.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Williams ED, Abrosimov A, Bogdanova T, Demidchik EP, Ito M, LiVolsi V, Tsyb AF. Thyroid carcinoma after Chernobyl latent period, morphology and aggressiveness. Br J Cancer. 2004;90(11):2219–2224. doi: 10.1038/sj.bjc.6601860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, Wingren G. A pooled analysis of case-control studies of thyroid cancer¶ II. Menstrual and reproductive factors. Cancer Causes Control. 1999;10(2):143–155. doi: 10.1023/A:1008880429862. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi S, Preston-Martin S, Dal Maso L, Negri E, La Vecchia C, Mack WJ, Jin F. A pooled analysis of case–control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control. 1999;10(6):583–595. doi: 10.1023/A:1008907227706. [DOI] [PubMed] [Google Scholar]
- 16.Musholt TJ, Musholt PB, Petrich T, Oetting G, Knapp WH, Klempnauer J. Familial papillary thyroid carcinoma: genetics, criteria for diagnosis, clinical features, and surgical treatment. World J Surg. 2000;24(11):1409–1417. doi: 10.1007/s002680010233. [DOI] [PubMed] [Google Scholar]
- 17.Soveid M, Monabbati A, Sooratchi L, Dahti S. The effect of iodine prophylaxis on the frequency of thyroiditis and thyroid tumors in Southwest, Iran. Saudi Med J. 2007;28(7):1034. [PubMed] [Google Scholar]
- 18.Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168(3):343–349. doi: 10.1530/EJE-12-0903. [DOI] [PubMed] [Google Scholar]
- 19.McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. 2012;97(8):2682–2692. doi: 10.1210/jc.2012-1083. [DOI] [PubMed] [Google Scholar]
- 20.Paparodis RD, Bantouna D, Karvounis E, Imam S, Jaume JC. Higher TSH is not associated with thyroid cancer risk in the presence of thyroid autoimmunity. J Clin Endocrinol Metab. 2020;105(7):dgaa237. doi: 10.1210/clinem/dgaa237. [DOI] [PubMed] [Google Scholar]
- 21.Tamimi DM. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int J Surg Pathol. 2002;10(2):141–146. doi: 10.1177/106689690201000207. [DOI] [PubMed] [Google Scholar]
- 22.Wirtschafter A, Schmidt R, Rosen D, Kundu N, Santoro M, Fusco A, Rothstein JL. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis. Laryngoscope. 1997;107(1):95–100. doi: 10.1097/00005537-199701000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Unger P, Ewart M, Wang BY, Gan LI, Kohtz DS, Burstein DE. Expression of p63 in papillary thyroid carcinoma and in Hashimoto’s thyroiditis: a pathobiologic link? Hum Pathol. 2003;34(8):764–769. doi: 10.1016/S0046-8177(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim SS, Lee BJ, Lee JC, Kim SJ, Jeon YK, Kim MR, Kim IJ. Coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma: the influence of lymph node metastasis. Head Neck. 2011;33(9):1272–1277. doi: 10.1002/hed.21594. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2014;140(6):1021–1026. doi: 10.1007/s00432-014-1629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn D, Heo SJ, Park JH, Kim JH, Sohn JH, Park JY, Park J. Clinical relationship between Hashimoto's thyroiditis and papillary thyroid cancer. Acta Oncol. 2011;50(8):1228–1234. doi: 10.3109/0284186X.2011.602109. [DOI] [PubMed] [Google Scholar]
- 27.Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, Park CS. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci. 2012;27(8):883–889. doi: 10.3346/jkms.2012.27.8.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab. 2013;98(6):2409–2414. doi: 10.1210/jc.2013-1309. [DOI] [PubMed] [Google Scholar]
- 29.Marotta V, Sciammarella C, Chiofalo MG, Gambardella C, Bellevicine C, Grasso M, Troncone G. Hashimoto’s thyroiditis predicts outcome in intrathyroidal papillary thyroid cancer. Endocr Relat Cancer. 2017;24(9):485–493. doi: 10.1530/ERC-17-0085. [DOI] [PubMed] [Google Scholar]
- 30.Toniato A, Boschin I, Casara D, Mazzarotto R, Rubello D, Pelizzo M. Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol. 2008;15(5):1518–1522. doi: 10.1245/s10434-008-9859-4. [DOI] [PubMed] [Google Scholar]


