Abstract
Cerebrospinal fluid (CSF) penetration and the pharmacokinetics of vancomycin were studied after continuous infusion (50 to 60 mg/kg of body weight/day after a loading dose of 15 mg/kg) in 13 mechanically ventilated patients hospitalized in an intensive care unit. Seven patients were treated for a sensitive bacterial meningitis and the other six patients, who had a severe concomitant neurologic disease with intracranial hypertension, were treated for various infections. Vancomycin CSF penetration was significantly higher (P < 0.05) in the meningitis group (serum/CSF ratio, 48%) than in the other group (serum/CSF ratio, 18%). Vancomycin pharmacokinetic parameters did not differ from those obtained with conventional dosing. No adverse effect was observed, in particular with regard to renal function.
Vancomycin has a slow bactericidal activity with a low MIC and a time-dependent activity with a limited penetration in cerebrospinal fluid (CSF) when administered by intermittent infusion over a period of at least 90 min every 8 or 12 h (5). Continuous infusion of vancomycin makes it possible to achieve a constant bactericidal level in blood and may also result in better CSF penetration (4). The benefit of prescribing continuous infusion has been reported for children (1) with postneurosurgical meningitis (2) and staphylococcus-resistant bone infections (3). The pharmacokinetics of antibiotics are modified in intensive care unit (ICU) patients due to the large daily fluid balance, acute changes in body weight, hypoalbuminemia, edema, and low hematocrit values. These modifications lead to marked changes in elimination half-life, volume of distribution, and clearance (12, 20, 25).
The aim of this study was to evaluate vancomycin penetration of CSF and its pharmacokinetics after administration by continuous infusion in ICU patients under mechanical ventilation in whom pharmacokinetic modifications could be expected.
After institutional approval and informed consent from a close relative were obtained, 13 consecutive patients of either sex, 25 to 58 years of age, hospitalized in the ICU and infected by vancomycin-sensitive bacteria were enrolled in the study. These patients underwent mechanical ventilation for acute respiratory failure. Exclusionary criteria included an age of <18 years, renal dysfunction, and an expected hospital stay of <72 h. Seven patients were treated for sensitive bacterial meningitis. Primary pathologies were head trauma (five cases) and medical coma (two cases). The other six patients were treated for various infections, and an external CSF shunt was already in place for the treatment of a primary pathology (severe neurologic disease with intracranial hypertension). Primary pathologies were medical coma (three cases), subarachnoid hemorrhage (two cases), and stroke (one case). The bacteria involved in infections were Staphylococcus epidermidis (six cases), Staphylococcus aureus (three cases), Streptococcus pneumoniae (two cases), Enterococcus faecalis (one case), and Corynebacterium (one case). The cases of S. epidermidis meningitis were related to neurosurgery or a CSF shunt system for treatment of intracranial hypertension following severe head trauma. These patients also had significant CSF pleiocytosis and low sugar levels.
In each patient, a loading dose of vancomycin of 15 mg/kg of body weight was administered for 2 h followed by a continuous infusion of 50 to 60 mg · kg−1 · day−1. Serum vancomycin levels were controlled daily to maintain values between 20 and 30 mg · liter−1. When required for treatment monitoring, CSF vancomycin levels were measured in samples obtained by lumbar puncture from seven patients with meningitis. For the other six patients, CSF was obtained from the shunt catheter to determine the vancomycin concentrations. Renal function was monitored daily by serum creatinine measurements.
In order to perform the pharmacokinetic analysis, serial blood samples were collected from an arterial-line catheter after the vancomycin infusion was stopped at the same time of day for all patients (6:00 p.m.). Samples were obtained on the first day at the end of the vancomycin infusion at 2:00, 2:30, 3:00, 4:00, 5:00, 6:00, and 8:00 p.m. and midnight. On the second day, they were obtained at 6:00 a.m., noon, 2:00 p.m., and midnight. On the third day, they were obtained at 6:00 a.m. and 2:00 p.m. Samples were centrifuged and serum was stored at −80°C until assay. Serum vancomycin concentrations were measured by a fluorescence polarization immunoassay (FLX system; Abbott Laboratories). Samples were assayed with quality control in each run. The coefficient of variation was between 1.5 and 2.9% for intraday variation and between 0.9 and 3.6% for interday variation. The limit of quantification was 0.60 mg · liter−1 (16). The CSF penetration of vancomycin was evaluated as follows: individual serum and CSF values were used to calculate the CSF/serum ratios, and then individual ratios were averaged. PharmK, a computer program developed for pharmacokinetic modeling of experimental data, was used (11). The program was written in “C” computer language based on the high-level user interface Macintosh operating system. An interactive algorithm based on the exponential stripping method was used for the initial parameter estimation. Nonlinear pharmacokinetic model fitting is based on the maximum-likelihood estimation method and was performed by the Levenberg-Marquardt method based on χ2 criteria (11). Pharmacokinetic analysis was performed for the first eight patients. Vancomycin concentrations in serum were plotted against time, and individual pharmacokinetic parameters were determined by a noncompartmental analysis. The β-phase elimination half-life, volume of distribution at steady state, and area under the serum concentration-time curve extrapolated to infinity were assessed by conventional methods (22).
Results are presented as means ± standard deviations (SD). Comparisons were made using the Mann-Whitney U test for unpaired data and the chi-square test. A P value of <0.05 was considered significant. On average, patients received 62 ± 17 mg of vancomycin · kg−1 · day−1 for 13 ± 7 days. The highest serum vancomycin levels ranged from 46.8 mg · liter−1 in patient 12 to 24.2 mg · liter−1 in patient 4, and the lowest levels ranged from 26.2 mg · liter−1 in patient 9 to 11.6 mg · liter−1 in patient 11. Patients with meningitis had maximal levels in CSF ranging from 5.7 to 19.0 mg · liter−1 (mean, 11.1 ± 4.9 mg · liter−1), with a mean serum/CSF ratio of 48%, while other patients had maximal levels in CSF ranging from 4.89 to 2.42 mg · liter−1 (mean, 3.45 ± 1.11 mg · liter−1), with a mean serum/CSF ratio of 18% (P < 0.05) (Table 1).
TABLE 1.
Vancomycin penetration in the CSF in patients with and without meningitis
| Patient category and no. | Serum vancomycin level (mg/liter)
|
Concn in CSF (mg/liter)
|
Concn in CSF/concn in serum
|
|||
|---|---|---|---|---|---|---|
| Highest | Lowest | Maximal | Minimal | Maximal | Minimal | |
| Patients with meningitis | ||||||
| 1 | 39.0 | 19.5 | 13.0 | 10.5 | 0.65 | 0.39 |
| 3 | 21.2 | 15.3 | 9.9 | 9.4 | 0.47 | 0.32 |
| 6 | 40.2 | 14.9 | 5.7 | 2.1 | 0.21 | 0.11 |
| 9 | 36.0 | 26.2 | 15.5 | 5.5 | 0.42 | 0.28 |
| 11 | 30.8 | 11.6 | 8.5 | 3.2 | 0.36 | 0.12 |
| 12 | 46.8 | 19.1 | 19.0 | 11.1 | 0.89 | 0.57 |
| 13 | 39.7 | 21.8 | 6.3 | 1.6 | 0.42 | 0.30 |
| Mean ± SD | 36.24 ± 8.19 | 18.45 ± 4.89 | 11.13 ± 4.92 | 6.20 ± 4.08 | 0.48 ± 0.22 | 0.29 ± 0.17 |
| Patients without meningitis | ||||||
| 2 | 36.1 | 18.6 | 4.9 | 3.7 | 0.21 | 0.21 |
| 4 | 24.2 | 14.6 | 2.4 | 2.4 | 0.20 | 0.15 |
| 5 | 28.5 | 15.0 | 4.7 | 4.7 | 0.18 | 0.17 |
| 7 | 34.5 | 17.1 | 3.1 | 3.1 | 0.24 | 0.11 |
| 8 | 24.3 | 16.4 | 3.1 | 3.1 | 0.20 | 0.13 |
| 10 | 34.1 | 24.9 | 2.4 | 2.4 | 0.07 | 0.10 |
| Mean ± SD | 30.28 ± 5.33 | 17.76 ± 3.78 | 3.45 ± 1.1a | 3.21 ± 0.87a | 0.18 ± 0.05a | 0.14 ± 0.04a |
P < 0.05 versus the mean for patients with meningitis.
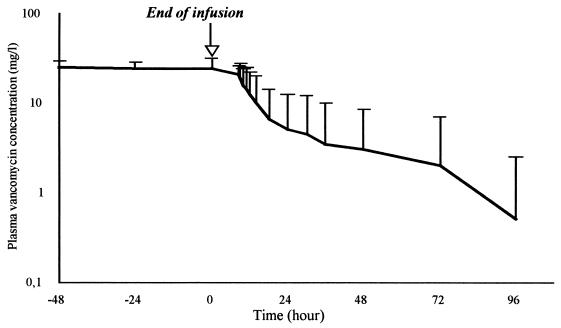
Pharmacokinetic parameters for vancomycin in serum were as follows: level in serum at the end of infusion, 22.6 ± 4.1 mg · liter−1; volume of distribution, 0.20 ± 0.05 liter · kg−1; elimination half-life, 6.9 ± 5.9 h; and clearance, 0.03 ± 0.02 liter · min−1. In one patient, the elimination half-life was 20 h without renal dysfunction. The decay of serum vancomycin levels after continuous infusion was stopped is shown in Fig. 1. Clinical and bacteriological success was achieved in all patients. No adverse effect was observed, particularly with regard to renal function. Serum creatinine was 67 ± 24 mmol · liter−1 on day 1 and 56 ± 19 mmol · liter−1 on the last day of treatment.
FIG. 1.
Plasma vancomycin levels after continuous infusion of the antibiotic was stopped. Vancomycin was administered for a mean duration of 13 ± 7 days at a dose of 62 ± 17 mg · kg−1 · day−1.
The principal results of this study were that high and bactericidal concentrations of continuous-infusion vancomycin (at a mean dose of 62 mg · kg−1 · day−1) could be achieved in the CSF of patients with meningitis. These concentrations were significantly higher than those obtained in patients without meningitis. Moreover, renal tolerance was excellent and clinical and bacteriological success was achieved in all treated patients. Vancomycin is widely used for different indications, with great success (7, 8, 13, 15, 21). It is recommended by the manufacturer that the drug be administered in two infusions (90 to 120 min) per day. However, the efficacy of vancomycin is in proportion not to the postinfusion peak concentration but to the length of time the drug concentration is higher than the MIC for the potential pathogens (17). Due to this time-dependent activity, administering vancomycin in divided doses at shorter intervals is a possibility (10), but administration by continuous infusion may be a better way to maximize the time above the MIC.
Clinical successes with administering vancomycin by continuous infusion have been reported for different infections: catheter infection, pneumonia, osteomyelitis, and septic arthritis (3, 6, 19, 24). Comparisons of patients treated by continuous infusion with those treated by conventional discontinuous infusion for severe methicillin-resistant staphylococcal infections showed that continuous infusion reduced the period of bacteremia in relation to infection (24, 25). For a comparable efficacy, continuous infusion was easier to adjust and more cost-efficient (14; M. Wysocki, F. Thomas, and M. Wolff, Letter, Lancet 345:646, 1995). Strausbaugh et al. demonstrated in an animal model of meningitis that vancomycin had a better penetration than penicillin M in inflammatory cerebrospinal tissue, with a CSF/serum ratio ranging from 4.5 to 19.4% (18). Stable vancomycin concentrations of 4 to 7 mg · liter−1 in the CSF were obtained in eight adult patients with postsurgery meningitis after 48 h of treatment with continuous infusion of vancomycin at a mean dose of 50 mg · kg−1 · day−1 (2). Our data also confirmed a better penetration in inflammatory meningeal tissue. The concentration ratio between CSF and serum was twice as high for the group of patients with meningitis as for the other group (48 versus 18%) (P < 0.05). The clinical and bacteriological efficacy of treatment by continuous infusion was high, since all of our patients recovered from their infections.
In the present study, the pharmacokinetic analysis demonstrated that at the steady state, clearance and elimination half-life were stable with a normal mean volume of distribution. Thus, the continuous infusion of vancomycin does not modify the pharmacokinetics of vancomycin in ICU patients (9).
With regard to tolerance, a comparative study did not indicate any difference between the two methods of infusion (23). We observed no adverse effect in spite of a high level of vancomycin in serum (mean concentration, 32 ± 8 mg · liter−1). Serum creatinine levels remained stable during the whole period of treatment for each patient.
The data from the present study strengthen the theoretical arguments in favor of the use of vancomycin by continuous infusion. This method of administration seems to be perfectly adapted to the pharmacokinetics of vancomycin. This antibiotic administered at a mean dose of 62 mg · kg−1 · day−1 to obtain stable concentrations of 25 to 30 mg · liter−1 in serum makes it possible to successfully treat bacterial meningitis. Levels in CSF of 6 to 19 mg · liter−1 can be achieved. Tolerance was excellent and no adverse effect was observed.
REFERENCES
- 1.Barois A, Estournet B, Moranne J B, Piliot J, Chabenat C, Bataille J. Infections ventriculaires à staphylocoque. Traitement par la vancomycine en perfusion continue. Presse Med. 1986;15:1805–1808. [PubMed] [Google Scholar]
- 2.Brinquin L, Rousseau J M, Boulesteix G, Diraison Y, Bonsignour J P. Vancomycine en perfusion continue dans les méningites à staphylocoques post-neurochirurgicales de l'adulte. Presse Med. 1993;22:1815–1817. [PubMed] [Google Scholar]
- 3.Desplaces N, Mamoudy P, Ducroquet F, Larrouturou P, Kitsis M D. Vancomycine en perfusion continue dans le traitement des infections ostéo-articulaires chroniques à staphylocoques multirésistants. Med Mal Infect. 1997;27:969–974. [Google Scholar]
- 4.Duffull S B, Begg E J, Chambers S T, Barclay M L. Efficacies of different vancomycin dosing regimens against Staphylococcus aureus determined with a dynamic in vitro model. Antimicrob Agents Chemother. 1994;38:2480–2482. doi: 10.1128/aac.38.10.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geraci J E, Heilman F R, Nichols D R, Wellman W E, Ross G T. Some laboratory and clinical experiences with a new antibiotic, vancomycin. Proc Staff Meet Mayo Clin. 1956;31:564–582. [PubMed] [Google Scholar]
- 6.Goulet O, Larchet M, Gaillard J L, Goulet V, Jan D, Revillon Y, Lortat-Jacob S, Descamps P, Ricour C. Catheter-related sepsis during long-term parenteral nutrition in paediatric gastroenterology patients: a study of 185 consecutive central venous catheters. Clin Nutr. 1990;9:73–78. doi: 10.1016/0261-5614(90)90056-x. [DOI] [PubMed] [Google Scholar]
- 7.Gump D W. Vancomycin for treatment of bacterial meningitis. Rev Infect Dis. 1981;3S:289–292. [PubMed] [Google Scholar]
- 8.Hawley H B, Gump D W. Vancomycin therapy of bacterial meningitis. Am J Dis Child. 1973;126:261–264. doi: 10.1001/archpedi.1973.02110190231025. [DOI] [PubMed] [Google Scholar]
- 9.James J K, Palmer S M, Levine D P, Rybak M J. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother. 1996;40:696–700. doi: 10.1128/aac.40.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine D P, Fromm B S, Reddy B R. Slow response to vancomycin or vancomycin plus rifampicin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 11.Lu D R, Mao F. An interactive program for pharmacokinetic modeling. J Pharm Sci. 1993;82:537–542. doi: 10.1002/jps.2600820521. [DOI] [PubMed] [Google Scholar]
- 12.Martin C, Mallet M N, Saux P, Bruguerolle B, Herat V, Gouin F. Tobramycin dosing in mechanically ventilated patients. Inaccuracy of a ‘rule of thumb’. J Antimicrob Chemother. 1988;22:505–511. doi: 10.1093/jac/22.4.505. [DOI] [PubMed] [Google Scholar]
- 13.Regnier B, Garaud J J, Wolff M, Rouveix F, Nkam M, Vachon F. La vancomycine seule et en association aux aminosides. In: Vachon F, Regnier B, editors. Problèmes actuels de pathologie infectieuse et de réanimation. Paris, France: Arnette; 1984. pp. 119–131. [Google Scholar]
- 14.Saunders N J. Why monitor peak vancomycin concentrations? Lancet. 1994;344:1748–1750. doi: 10.1016/s0140-6736(94)92890-8. [DOI] [PubMed] [Google Scholar]
- 15.Schaad U B, McCracken G H, Jr, Nelson J D. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr. 1980;96:119–126. doi: 10.1016/s0022-3476(80)80347-7. [DOI] [PubMed] [Google Scholar]
- 16.Schwenzer K S, Chao-Huei J W, Anhalt J P. Automated fluorescence polarization immunoassay for monitoring vancomycin. Ther Drug Monit. 1983;5:341–345. doi: 10.1097/00007691-198309000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Stratton C W, Liu C, Weeks L S. Activity of LY146032 compared with that of methicillin, cefazolin, cefamandole, cefuroxime, ciprofloxacin, and vancomycin against staphylococci as determined by kill-kinetic studies. Antimicrob Agents Chemother. 1987;31:1210–1215. doi: 10.1128/aac.31.8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strausbaugh L J, Murray T W, Sande M A. Comparative penetration of 6 antibiotics into the cerebrospinal fluid of rabbits with experimental staphylococcal meningitis. J Antimicrob Chemother. 1980;6:363–371. doi: 10.1093/jac/6.3.363. [DOI] [PubMed] [Google Scholar]
- 19.Thomas F, Michel P, Ravaud Y. Traitement d'infections à staphylocoque chez l'adulte par perfusion continue de vancomycine en monothérapie. Sem Hop Paris. 1988;64:215–218. [Google Scholar]
- 20.Van Dalen R, Tree T B. Pharmacokinetics of antibiotics in critically ill patients. Intensive Care Med. 1990;16:5235–5238. doi: 10.1007/BF01709707. [DOI] [PubMed] [Google Scholar]
- 21.Vichyanond P, Olson L C. Staphylococcal CNS infections treated with vancomycin and rifampicin. Arch Neurol. 1984;41:637–639. doi: 10.1001/archneur.1984.04210080045011. [DOI] [PubMed] [Google Scholar]
- 22.Wagner J G. Fundamentals of clinical pharmacokinetics. Hamilton, Ontario, Canada: Drug Intelligence Publications; 1975. pp. 217–231. [Google Scholar]
- 23.Wysocki M, Rauss A, Pean Y, Delatour F, Thomas F, Misset B, Cheval C, Similowski T, Timsit J F, Laisne M J, Mentec H, Lazard T, Mier L, Dreyfuss D, Tric L, Guerrini P, Briard C, Guérin C, Maury E. Continuous infusion of vancomycin for severe methicillin-resistant staphylococcal infections. Intensive Care Med. 1996;22:S325. [Google Scholar]
- 24.Wysocki M, Thomas F, Wolff M, Pean Y, Ravaud Y, Herman B. Comparison of continuous with discontinuous infusion of vancomycin in severe MRSA infections. J Antimicrob Chemother. 1995;35:352–354. doi: 10.1093/jac/35.2.352. [DOI] [PubMed] [Google Scholar]
- 25.Zaske D E, Cipolle R J, Strate R J. Gentamicin dosage requirements: wide interpatient variation in 242 surgery patients with normal renal function. Surgery. 1980;87:164–169. [PubMed] [Google Scholar]