Abstract
Background & Aims:
Digital health technologies may be useful tools in the management of chronic diseases. We performed a systematic review of digital health interventions in the management of patients with inflammatory bowel diseases (IBD) and evaluated its impact on 1) disease activity monitoring, 2) treatment adherence, 3) quality of life measures, and/or 4) healthcare utilization.
Methods:
Through a systematic review of multiple databases through August 31, 2020, we identified randomized controlled trials (RCTs) in patients with IBD comparing digital health technologies vs. standard-of-care for clinical management and monitoring, and reporting impact on IBD disease activity, treatment adherence, quality of life, and/or healthcare utilization or cost-effectiveness. We performed critical qualitative synthesis of the evidence supporting digital health interventions in patients with IBD, and rated certainty of evidence using GRADE.
Results:
Overall, 14 RCTs were included (median, 98 patients; range, 34–909 patients; follow-up <12 months) and compared web-based interventions, mobile applications, and different telemedicine platforms to standard-of-care clinic-based encounters. Though overall disease activity and risk of relapse was comparable between digital health technologies and standard-of-care (very low certainty of evidence), digital health interventions were associated with lower rate of healthcare utilization and health care costs (low certainty of evidence). Digital health interventions did not significantly improve patients’ quality of life and treatment adherence compared with standard-of-care (very low certainty of evidence). Trials may have intrinsic selection bias due to nature of digital interventions.
Conclusions:
Digital health technologies may be effective in decreasing healthcare utilization and costs, though may not offer advantage in reducing risk of relapse, quality of life and improving treatment adherence in patients with IBD. These techniques may offer value-based care for population health management.
Keywords: eHealth, health records, telemedicine, Crohn’s disease, population health
INTRODUCTION
Inflammatory bowel diseases (IBD) are lifelong conditions associated with significant morbidity, high burden of healthcare utilization, decrease worked productivity and disability.(1, 2) Care for IBD is chronic, complex and associated with significant healthcare costs. In the United States, annual US healthcare spending on IBD has increased from $6.4 billion in 1996 to $25.4 billion in 2016, corresponding to a per patient increase in spending from $5714 to $14,033.(3) Approximately, 56% of total healthcare spending is attributed to inpatient and emergency department visits while pharmaceutical costs accounted for 20%. Given the chronic and unpredictable nature of IBD with high frequency of unplanned healthcare utilization and increasing use of expensive, targeted, and disease-modifying therapies, population health management strategies, which involve coordination of care at a population level to improve outcomes and effectively manage clinical and financial risks, are important for effective and efficient care management.(4) Traditional structured clinic-based encounters where providers and patients have limited time and resources are frequently episodic and reactive with significant variability in quality of care, whereas effective population health management requires proactive, planned, and individualized long-term chronic care management.(5–8) Digital health technologies can overcome the physical and time limitations of traditional face-to-face encounters through remote monitoring of disease activity, increasing access to healthcare providers via mobile technologies, and enhance patient participation through individualized alerts and action plans.(8)
With the rapid technological advances, digital health tools and telemedicine (defined as diagnosis, treatment and monitoring of disease by means of the internet, mobile phone applications and wearable devices) can potentially provide an opportunity for high-value care, by improving efficiency in healthcare delivery.(9) Multiple trials on the development and implementation of multi-dimensional digital health technologies and telemedicine have variably impacted clinical outcomes, remote disease monitoring, IBD-related quality of life (QoL) and healthcare utilization.(10, 11)
Hence, to evaluate components and impact of digital health interventions on key clinical and healthcare-related outcomes compared to standard-of-care (SoC), we performed a systemic review of randomized controlled trials (RCTs) on digital health technologies in patients with IBD.
MATERIALS AND METHODS
We performed this systematic review according to the guidelines as prescribed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Appendix, PRISMA checklist), and the process followed an a priori established protocol.
Selection criteria
We included RCTs in (A) adult and/or pediatric patients with IBD, comparing (B) digital health interventions (e.g., web-based, telemedicine, mobile telephone applications) with (C) SoC clinical management, and (D) evaluating the impact of these interventions on clinical outcomes, QoL measures, disease monitoring, treatment adherence, and/or healthcare utilization. We excluded non-randomized studies and single-arm, pre-post cohort studies.
Search strategy
We performed a systematic review from inception to August 31, 2020 using a structured systematic literature search of multiple electronic databases: MEDLINE (PubMed), EMBASE, and Cochrane Library, without any language restrictions. The search strategy involved combining controlled vocabulary with keywords and MeSH terms for telemedicine (which includes mobile health, mHealth, telehealth, eHealth) combined with inflammatory bowel diseases (Supplementary Appendix, search strategy). Two authors (IM, SS) independently reviewed the title and abstract of studies identified in the search to exclude studies that did not meet the inclusion criteria as set forth a priori to address the research question of interest. After reviewing the title and abstracts of potential studies, we examined the remaining articles’ full text to determine whether the study included relevant information. Next, the bibliographies of selected articles and review articles on the topic were manually searched for additional inclusion in our systematic review. Lastly, a manual search of conference proceedings of major gastroenterology conferences between 2016–2020 (Digestive Diseases Week, American College of Gastroenterology annual meeting, Crohn’s and Colitis Congress, European Crohn’s and Colitis Organization annual meeting) was reviewed to identify additional studies published only in abstract form. Disagreements regarding inclusion/exclusion of studies were resolved by a third author (NHN).
Data extraction
We used a standardized case report form to collect data on the following: 1) study characteristics: primary author and time period of study or year of publication, location of the population studied, patient population (adults or children), single or multicenter, number of groups in the trials, number of study participants, and attrition rates (defined as the number patients either lost to follow-up, dropped out of the study, and/or did not follow study protocol); 2) patient characteristics: IBD subtype, age, proportion of males, proportion of smokers, proportion with CD, disease duration, medications at time of enrollment; 3) digital health technologies: telemedicine, web-based, mobile applications, including specific patient- and provider-facing components of interventions; and 4) outcome measurements collected and organized by themes: disease activity indices, QoL measures, treatment adherence, healthcare utilization (e.g., hospitalizations, surgeries, emergency room (ER) visits, office visits, procedures, telephone encounters) and cost-effectiveness. Additionally, we sought to describe the components of digital health interventions, and their relative impact on clinical outcomes, by the phase of care (pre-visit planning, pre- and post-medication administration), whether digital health intervention was administered to supplement or replace SoC face-to-face encounters, active or passive engagement of healthcare providers, and focus of the interventions (patient- or provider-centered).
Quality assessment of included studies
We evaluated the studies for risk of bias using Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2).(12) RoB2 includes domains of bias focusing on different aspects of trial design, conduct, and reporting with a series of signaling questions within each domain aimed at evaluating the salient features of the trial that may be at risk of bias. Based on answers to the signaling questions, a judgement can be rendered as ‘Low’ or ‘High’ risk of bias or ‘Some concerns.’ Guidance on the use of this tool and additional information can be found elsewhere.(12)
Synthesis and analysis of included studies
Since the studies included multi-dimensional interventions, different measurement tools to evaluate a range of clinical outcomes, we opted to synthesize the evidence qualitatively, and refrained from performing a meta-analysis. We summarized findings by themes and in the context of a common clinical outcome (e.g., monitoring/assessing disease activity, evaluating QoL, treatment adherence, and healthcare utilization). We reported certainty of evidence using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach.
We also summarized findings by the type of digital health interventions – web-based (e.g., secure messaging using web portal), patient portal, bidirectional communication using mobile technologies (e.g., secure text messaging, applications for self-reporting of symptoms), and/or combination. Where data was available, we attempted to qualitatively evaluate the influence of the intensity/frequency and workflow of digital health technologies on the intended outcomes.
RESULTS
Based on our search strategy, we obtained 1015 articles for screening. After applying our inclusion and exclusion criteria, a total of 14 RCTs were included in this systematic review.(11, 13–25) Figure 1 details the study selection flowsheet. Table 1 provides an overview of the studies included in the review, and Supplementary Table 1 details intervention, outcomes and measurement tools in these studies. From the fourteen studies, five studies were based in the United States while the rest were in Europe.(16, 17, 21, 24, 25). Five studies included children or adolescents only.(13–15, 20, 24). Most studies had follow-up periods of 12 months or less.(11, 13, 16–25). The control arm in all studies were patients who received standard of care, traditional healthcare delivery systems as prevalent at the time and place in which the studies were conducted.
Figure 1.
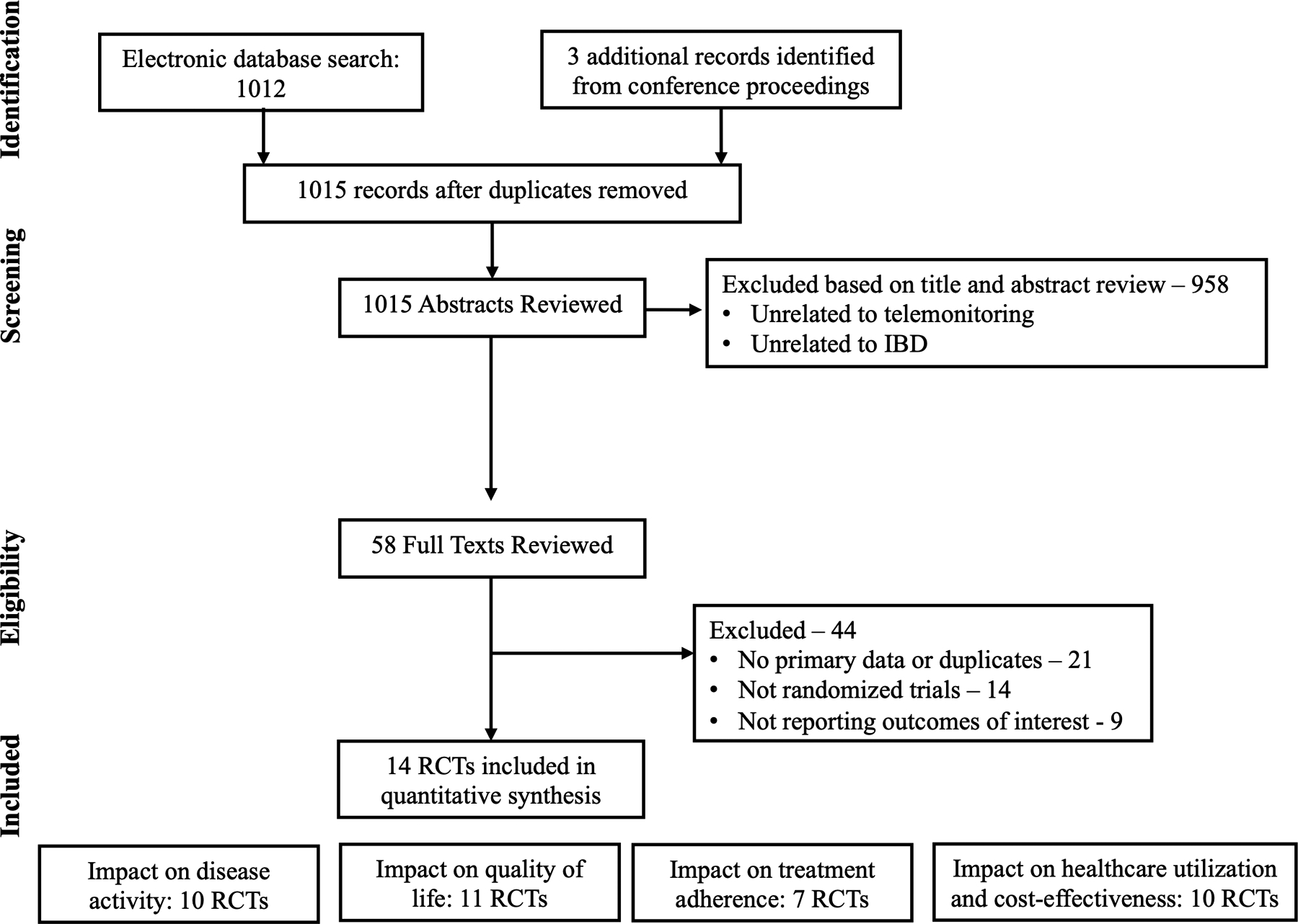
Study selection flowsheet
Table 1.
Randomized controlled trials comparing digital health technologies vs. traditional clinical encounters in monitoring and managing patients with IBD
| Study ID | Author, year | Study, location, patient population, centers | Number of groups, number of study participants, and attrition rates | Outcomes and measurement tools | Intervention, control, and face-to-face clinic visits |
|---|---|---|---|---|---|
| 1 | Cross R, 2019 | RCT, United States, adults, multicenter |
3 groups:
1. TELE-IBD weekly (W) 2. TELE IBD every other week (EOW) 3. Control 348 enrolled: 117 control, 115 EoW, and 116 W Attrition rates: 8.5% control, 13.9% EoW, and 19% W |
Disease activity: HBI for CD and SCCAI for UC/indeterminate patients QoL: IBQ questionnaire Utilization of health care resources: reviewed EMR for healthcare utilization (hospitalizations, surgery, emergency department and office visits, procedures, intravenous therapeutics, and telephone and electronic encounters) |
Intervention: web- and mobile-based (secure messaging to patients from web-portal) – TELE-IBD system. Bidirectional communication between patients and healthcare providers via text messages only. Patients would receive individualized alerts and action plans based on responses. Control: standard of care following evidence-based guidelines Both the intervention and control groups underwent study visits at baseline, 6 and 12 months in addition to their routine clinic visits. |
| 2 | Cross R, 2012 | RCT, United States, adults, multicenter |
2 groups: 1. UCHAT – comprised of a home unit, decision support server, and a web-based clinician portal 2. Best available care (BAC) 167 eligible → 113 refused to participate BAC 22, UCHAT 25 Attrition rates: 15% BAC, 32% UCHAT |
Disease activity: Seo index QoL: IBDQ questionnaire Treatment Adherence: Morisky Medication Adherence Score |
Intervention: web-based (decision support server and a clinician portal) and home unit (netbook computer and electronic scale). Patients answered questions about their symptoms, treatment side effects or adherence, and received IBD-related education using the home unit, which occurred weekly. Control: standard of care following evidence-based guidelines All patients underwent clinic visits every 4 months. |
| 3 | De Jong, 2017 | RCT, Netherlands, adults, multicenter |
2 groups:
1. Web-based (myIBDcoach) 2. Control 3000 patients asked to participate → 2091 excluded (41% did not return invitation letter and 12% declined participation) Telemedicine 465, Control 444 Did not report attrition rate. |
Disease activity/outcomes: number of flares as measured by Monitor IBD at Home (MIAH) questionnaire QoL: SIBDQ questionnaire Treatment adherence: Morisky Medication Adherence Scale Healthcare utilization: IBD-related hospitalizations, emergency room visits, surgeries, and/or corticosteroid use Others: self-efficacy, diagnosis and treatment related knowledge, smoking behaviors, and number of outpatient visits |
Intervention: Web-based system (myIBDcoach) that monitors and registers questions for disease activity, treatment use, treatment adherence, treatment satisfaction, side effects. questions on factors affecting disease, patient reported outcomes (PRO) on quality of life and work productivity. At least one routine outpatient clinic visit per year with as-needed visits based on alarm symptoms identified through myIBDcoach. Control: standard of care following local protocol. Patients received routine clinic visits. |
| 4 | Elkjaer M, 2010 | RCT, Denmark and Ireland, adults, multicenter |
2 groups:
1. Web (Constant-Care) (W) 2. Control (C) Denmark: Web 105, Control 106 485 pts with UC invited→ 50% no response and 2% denied → 233 pts randomized (117 web + 116 control) → 105 web + 106 control at baseline → 89 web + 97 control at 12-month follow-up Web: 10 never attended baseline; 4 refused participation after baseline; others no longer qualified Control: 8 never attended baseline; 3 refused to participate after baseline; others no longer qualified Ireland: Web 51, Control 41 100 pts randomized (52 web + 48 control) → 5 excluded (age limit) → 51 web + 41 control at baseline → 40 web + 38 control at 12 months follow-up |
Disease activity: SCCAI QoL: SIBDQ Others: Compliance questionnaire (CQ); Satisfaction questionnaire (SQ); knowledge base regarding IBD, medication, diet, and complications; anxiety and depression; and generic health survey with SF-36 for Denmark and SF-12 for Ireland |
Intervention: web-group received disease specific education and self-treatment via http://www.constant-care.dk. All web-patients and their relatives were educated on IBD-related topics (recognizing a flare, disease course, surgeries, etc.) as part of their enrollment. If patients had a relapse, they were asked to log on weekly for a total of 4 weeks (otherwise once a month if they were in remission) and could be treated acutely for 4 weeks with 4 grams daily of 5-aminosalicylic acid (ASA). Additional treatment with topical 5-ASA and/or prednisolone was individualized. Control: standard of care with routine appointments. All patients had visits at baseline, 6 months and at 12 months. |
| 5 | Krier M, 2011 | RCT, United States, adults, single center |
2 groups:
1. Remote telemedicine encounter (TE) 2. face-to-face standard encounter Remote telemedicine encounter 15, face-to-face standard encounter 19 Did not report attrition rate. |
Healthcare utilization: duration of appointment visit, wait time, number of patients seen per clinic day. Others: Measurement of clinical experience and patient’s overall satisfaction on quality of audio and visual presentation in the TE group |
Intervention: GI fellow at VA Palo Alto evaluated the patient and could have real-time or off-line consultation with local/remote radiologist, interventional endoscopist, surgeon, and/or pathologist. The GI fellow then returned to the patient-room for a real time Telemedicine Encounter session which included the fellow, patient and remotely located attending. Telemedicine encounter with IBD specialist remotely located. Control: standard encounter with fellow and attending in the clinic |
| 6 | Linn AJ, 2018 | Cluster RCT, Netherlands, adults, multicenter |
2 groups: 1. Intervention – patients received weekly text messages to improve medication adherence and counseling performed by IBD nurses 2. Control group – standard of care 201 patients were asked to participate → 29 patients refused, 12 patients did not meet inclusion criteria and for other reasons → 160 patients were included. Attrition rate: In part 2 of the study, only 28 of 52 patients in the experimental group completed the study, while 22 of 33 patients in the control group completed the study |
Treatment adherence: 5-item Medication Adherence Report Scale (MARS) Others: patient satisfaction, beliefs about medication, and self-efficacy |
Intervention: received a combination of weekly text messages and counseling by nurses specialized in IBD care who underwent a twelve weeks course on communication with IBD patients. Control: Received standard care – usual education. |
| 7 | Akobeng AK, 2015 | RCT, United Kingdom, children/ teenagers (age 8 – 16), single center |
2 groups: 1. Intervention – telephone consultation 2. Control – face-to-face consultation 246 patients were invited to participate → 86 randomized → 42 in control and 44 in intervention. IMPACT questionnaire returned at 12 months: 37 in control and 30 in intervention. IMPACT questionnaire returned at 24 months: 27 in control and 28 in intervention |
Disease activity: number of disease relapses using abbreviated Pediatric Crohn’s Disease Activity Index (aPCDAI) and Pediatric Ulcerative Colitis Activity Index (PUCAI) QoL: Pediatric IBD-specific IMPACT Healthcare utilization: number of hospital admissions, duration of consultations, and costs to the UK National Health Service Others: patient and parent satisfaction |
Intervention: Gastroenterology doctor would contact the patient and parents via telephone number that the parents and patient would provide. Control: routine face-to-face care |
| 8 | Carlsen K, 2017 | Open labeled randomized controlled trial, Denmark, children/adolescents (ages 10–17), single center *Non-biologic cohort |
2 groups:
1. eHealth – web-application young.constant-care.com (YCC). Web-based disease monitoring. 2. Control – standard visits every 3 months 99 invited → 53 were randomized (27 web, 26 control). 15 completed two years follow-up in web group while 18 completed two years follow-up in control group. |
Disease activity: aPCDAI or PUCAI Medication adherence: MARS QoL: IMPACT III and HRQoL Others: school absence |
Intervention: The pediatric adult eHealth program (www.young.constant-care.com) was based on the adult eHealth web program (www.constant-care.com). Individualized action plans based on a combination of fecal calprotectin, symptoms and web-based algorithm. On-demand outpatient visits in addition to 1 planned visit annually. Control: patients were managed according to the national pediatric IBD standard care in Denmark. |
| 9 | Carlsen K, 2017 | Open labeled intervention study, Denmark, children/adolescents (ages 10–17), single center *Biologic cohort |
2 groups: 1. eHealth – web-based program to help individualize infliximab (IFX) treatment based on disease activity assessment on a composite score. Using young.constant-care.com 2. Control – standard of care *Control group was followed for a shorter period than the eHealth group. eHealth – 29 patients; control – 21 patients. Ultimately, 13 patients dropped out of the study and only 16 completed the trial. |
Disease activity: aPCDAI or PUCAI QoL: HRQoL and IMPACT III Other: drug safety (blood samples were taken before IFX infusions to measure trough levels and levels of antibodies). |
Intervention: patients would enter weekly PUCAI/abbrPCDAI weekly until next IFX treatment. A fecal calprotectin stool sample was also collected. The TIBS score was reflected in a traffic light system where alarm symptoms would prompt the families to consult IBD provider for treatment decisions. Control: patients received IFX infusion every 8 weeks but intervals could be altered as needed to gain control of the disease. |
| 10 | Del Hoyo, 2019 | RCT, Spain, adults, single center |
3 groups: 1. Web-based telemedicine - remote monitoring (G_TECCU) 2. Nurse-assisted telephone care (G_NT) 3. Standard care with in-person visits (G_control) 68 patients were invited to participate and ultimately 63 patients were enrolled – 21 patients in each group. |
Disease activity: HBI and SCCAI. Partial Mayo score was used for face-to-face visits. Treatment adherence: Morisky-Green index QoL: IBDQ-9 Healthcare utilization: emergency room visits, hospitalizations, IBD-related surgeries, corticosteroid courses Others: work productivity, drug side effects, patient satisfaction |
Intervention: web-based telemanagement system (Telemonitoring of Crohn’s Disease and Ulcerative Colitis – TECCU) for remote monitoring of disease activity in patients on corticosteroids, immunosuppressants, and biological agents. Telephone calls or in-persons visits were available to train patients for administration of medications. Control: two groups. Nurse-assisted telephone care or standard face-to-face visits. |
| 11 | Heida A, 2017 | RCT, Netherlands, adolescents (ages 10 – 19), multicenter |
2 groups: 1. Intervention – telemonitoring (web-based) 2. Control – conventional follow-up. 170 teenagers were eligible and randomly assigned to home telemonitoring (n = 84) or standard follow-up (n = 86). |
Disease activity: PUCAI and shPCDAI QoL: IMPACT-III Healthcare utilization: cost-effectiveness analysis including direct and indirect medical and non-medical costs |
Intervention: Patients received automated email alerts to complete symptom score and send a stool sample for fecal calprotectin assessment. Symptom score and calprotectin stool test was uploaded to an IBD-live website and a color-coded system was used to advise patients the next best course of action. Control: Participants had regular checks and the interval of visits was based on the physician’s discretion. |
| 12 | McCombie A, 2020 | Noninferiority RCT, New Zealand, adults, multicenter |
2 groups: 1. Intervention – 2 smartphone apps for IBD monitoring and management (IBDsmart and IBDoc). 2. Control – usual IBD care from their physician. 107 randomized → 53 allocated to intervention group and 54 allocated to standard care group. 50 patients were ultimately included in each group. Attrition: At baseline, 50 (94%) and 50 (93%) patients completed the questionnaires in the intervention and control groups, respectively. At the end of 12 months of follow-up, 47 (89%) and 49 (91%) patients completed the questionnaire in the intervention and control group, respectively. |
Disease activity: HBI and SCCAI Other: usability and acceptability of IBDsmart and IBDoc. Adherence as measured by utilization of applications. |
Intervention: 2 smartphone applications for IBD monitoring symptoms and management with IBDsmart and IBDoc. The symptom scores are sent to health care providers. IBDoc calculates fecal calprotectin scores from stool samples provided by IBD patients at home. Patients self-completed HBI/SCCAI through the applications at least quarterly while in person visits were at baseline and 12 months. Control: usual IBD care from their physician. |
| 13 | Miloh T, 2017 | RCT, United States, children, single center |
2 groups: 1. Intervention – 2-way interactive text messaging to encourage medication adherence 2. Control – standard of care 51 children were randomized: 21 in the text-messaging group and 30 in the control group. Attrition rate: 8 (26.7%) and 6 (28.6%) dropped out in the intervention and control group, respectively |
Disease Activity: PCDAI and PUCAI Treatment adherence: Morisky questionnaire. |
Intervention: 2-way texting messaging (TM) using a centralized website/server. Text messages were sent to the patients and caregiver at set times based on patient preference. Control: standard of care All patients were followed in the pediatric gastroenterology clinic according to their condition and needs for 12 months. |
| 14 | Schliep M, 2020 | RCT, United States, adults, multicenter |
3 groups:
1. TELE-IBD weekly (W) 2. TELE IBD every other week (EOW) 3. Control 348 enrolled: 117 control, 115 EoW, and 116 W Attrition rates: 8.5% control, 13.9% EoW, and 19% W For this subgroup analysis, a total of 217 participants were included in the analysis. 90 in the control, 81 in TELE-IBD W, and 88 in TELE-IBD EOW. |
QoL: Short Form 12 Others: Depressive symptoms were assessed with the Mental Health Inventory 5 |
Intervention: web- and mobile-based (secure messaging to patients from web-portal) – TELE-IBD system. Bidirectional communication between patients and healthcare providers via text messages only. Patients would receive individualized alerts and action plans based on responses. Control: standard of care following evidence-based guidelines Both the intervention and control groups underwent study visits at baseline, 6 and 12 months in addition to their routine clinic visits. |
W: Weekly
EOW: Every other week
HBI: Harvey Bradshaw Index
SCCAI: Simple Clinical Colitis Activity Index
CD: Crohn’s disease
UC: Ulcerative colitis
QoL: Quality of Life
HRQoL: Health-related quality of life
IBD: Inflammatory bowel disease
IBDQ: IBD Questionnaire
IBDQ-9: Inflammatory Bowel Disease Questionnaire 9
SIBDQ: Short IBD Questionnaire
EMR: Electronic medical record
MARS: Medication Adherence Report Scale
PCDAI: Pediatric Crohn’s Disease Activity Index
aPCDAI: abbreviated Pediatric Crohn’s Disease Activity Index
shPCDAI: shortened Pediatric Crohn’s Disease Activity Index
PUCAI: Pediatric Ulcerative Colitis Activity Index
Characteristics of the patients included in the studies are described in detail in Supplementary Table 2 and include demographics data, disease phenotype, and concurrent medications at the time of enrolment. There was limited description of social determinants of health, including health literacy, affordability and access to care of study participants in the included trials, and whether the intervention was tailored to be culturally sensitive. In Tables 2–5, studies are organized by themes to allow for qualitative assessments of the impact of digital health technologies on specific domains of patient care. None of the RCTs blinded patients or investigators. All patients were recruited in the ambulatory setting. Median sample size was 98 patients (range, 34 to 909 patients), with relatively short follow-up period (most studies had follow-up between 6 and 12 months). High attrition was noted across all studies, ranging from 8% to 40%. Five studies were deemed to be at high-risk of bias due to significant methodologic flaws across multiple domains.(13–15, 21, 22) Figure 2 details the risk of bias assessment for each study.
Table 2.
Impact of digital health technologies on disease activity monitoring in patients with IBD
| Author, year | Type of Intervention | Outcome Measurements | Duration of Follow-up | Findings |
|---|---|---|---|---|
| Disease activity assessment | ||||
| Cross R, 2019 | 1. TELE-IBD weekly (W) 2. TELE-IBD every other week (EOW) 3. Control – standard of care |
Active disease defined as Harvey Bradshaw Index (HBI) 5+ (for CD) or Simple Clinical Colitis Activity Index (SCCAI) 3+ (for UC/indeterminate colitis) | 12 months |
Disease activity in CD
|
| Cross R, 2012 | 1. UCHAT – comprised of a home unit, decision support server, and a web-based clinician portal 2. Best available care (BAC) |
Seo index with activity index <120 = clinical remission. Scores of 120 to 150, 151 to 220, and > 222 correlate with mild, moderate, and severe disease respectively. A decrease in the index of 35 correlates with clinical response | 12 months |
|
| Elkjaer M, 2010 | 1. Web-based (Constant-Care) 2. Control |
SCCAI: disease activity; >5 = relapse Combination of systemic and topical 5-ASA use for disease relapse |
12 months |
Denmark
|
| Akobeng AK, 2015 | 1. Mobile-based (telephone consultation) 2. Control |
Number of disease relapses defined by abbreviated Pediatric Crohn’s Disease Activity Index (aPCDAI; clinical remission defined as ≤10) and Pediatric Ulcerative Colitis Activity Index (PUCAI; clinical remission defined as <10) | 12 months |
|
| Carlsen K, 2017 (Non-biologic cohort) | 1. Web-based (yong.constant-care.com) 2. Control |
Patients were required to record data on their disease activity – Pediatric Ulcerative Colitis Activity Index (PUCAI) and the abbreviated Pediatric Crohn’s Disease Activity Index (aPCDAI) | 24 months |
|
| Carlsen K, 2017 (Biologic cohort; IFX) | 1. Web-based (yong.constant-care.com) 2. Control |
Patients were required to record data on their disease activity – Pediatric Ulcerative Colitis Activity Index (PUCAI) and the abbreviated Pediatric Crohn’s Disease Activity Index (abbrPCDAI) | 24 months |
|
| Del Hoyo, 2019 | 1. Web-based telemedicine - remote monitoring (G_TECCU) 2. Nurse-assisted telephone care (G_NT) 3. Standard care with in-person visits (G_control) |
Clinical remission at 24 weeks (Harvey-Bradshaw index [HBI] for Crohn’s patients; Simple Clinical Colitis Activity Index [SCCAI] for UC patients – for remote check-ups. Partial Mayo score was used for face-to-face visits. Patients with CD and HBI <5 were deemed to be in remission while SCCAI ≤2 was remission for UC patients and a partial Mayo Score ≤2 and no individual Mayo subscore >1 was defined as remission. |
6 months |
|
| Heida, 2017 | 1. Telemonitoring (web-based) 2. Control |
Disease activity was assessed by Pediatric Ulcerative Colitis Activity Index (PUCAI) and the shortened Pediatric Crohn’s Disease Activity Index (shPCDAI). Cumulative incidence of disease flares, which was defined as disease activity requiring treatment intensification (e.g., steroid therapy, exclusive enteral nutrition, aminosalicylate dose escalation or introduction of anti-TNF). |
12 months |
|
| McCombie, 2020 | 1. Mobile application (IBDsmart and IBDoc) 2. Control |
HBI or SCCAI for disease activity assessment Remission was defined as SCCAI ≤2 or HBI ≤4 |
12 months |
|
| Miloh, 2017 | 1. Telemonitoring (2-way interactive text messaging [TM]) 2. Control |
Clinical score using Pediatric Crohn Disease Activity Index for patients with Crohn’s and Pediatric Ulcerative Colitis Activity Index for patients with ulcerative colitis. | 12 months |
|
HBI: Harvey Bradshaw Index
SCCAI: Simple Clinical Colitis Activity Index
CD: Crohn’s disease
UC: Ulcerative colitis
PCDAI: Pediatric Crohn’s Disease Activity Index
aPCDAI: abbreviated Pediatric Crohn’s Disease Activity Index
shPCDAI: shortened Pediatric Crohn’s Disease Activity Index
PUCAI: Pediatric Ulcerative Colitis Activity Index
Table 5.
Impact of digital health technologies on treatment adherence.
| Author, year | Type of Intervention | Outcome Measurements | Duration of Follow-up | Findings |
|---|---|---|---|---|
| Treatment adherence | ||||
| Cross R, 2012 | 1. UCHAT – comprised of a home unit, decision support server, and a web-based clinician portal 2. Best available care (BAC) |
Morisky Medication Adherence Score – Higher scores correlate with better medical adherence | 12 months |
|
| De Jong, 2017 | 1. Web-based (myIBDcoach) 2. Control |
8-item Morisky Medication Adherence Scale (<6 = low), | 12 months |
|
| Elkjaer M, 2010 | 1. Web (Constant-Care) 2. Control |
CQ: compliance questionnaire | 12 months |
Denmark
|
| Linn AJ, 2018 | 1. Mobile-based 2. Control |
5-item Medication Adherence Report Scale (MARS) | 6 months |
|
| Carlsen K, 2017 (Non-biologic cohort) | 1. Web-based (yong.constant-care.com) 2. Control |
MARS | 24 months |
|
| Del Hoyo, 2019 | 1. Web-based telemedicine - remote monitoring (G_TECCU) 2. Nurse-assisted telephone care (G_NT) 3. Standard care with in-person visits (G_control) |
Morisky-Green index | 6 months |
|
| Miloh, 2017 | 1. Telemonitoring (2-way interactive text messaging [TM]) 2. Control |
Morisky questionnaire | 12 months |
|
CQ: compliance questionnaire
MARS: Medication adherence report scale
Figure 2.
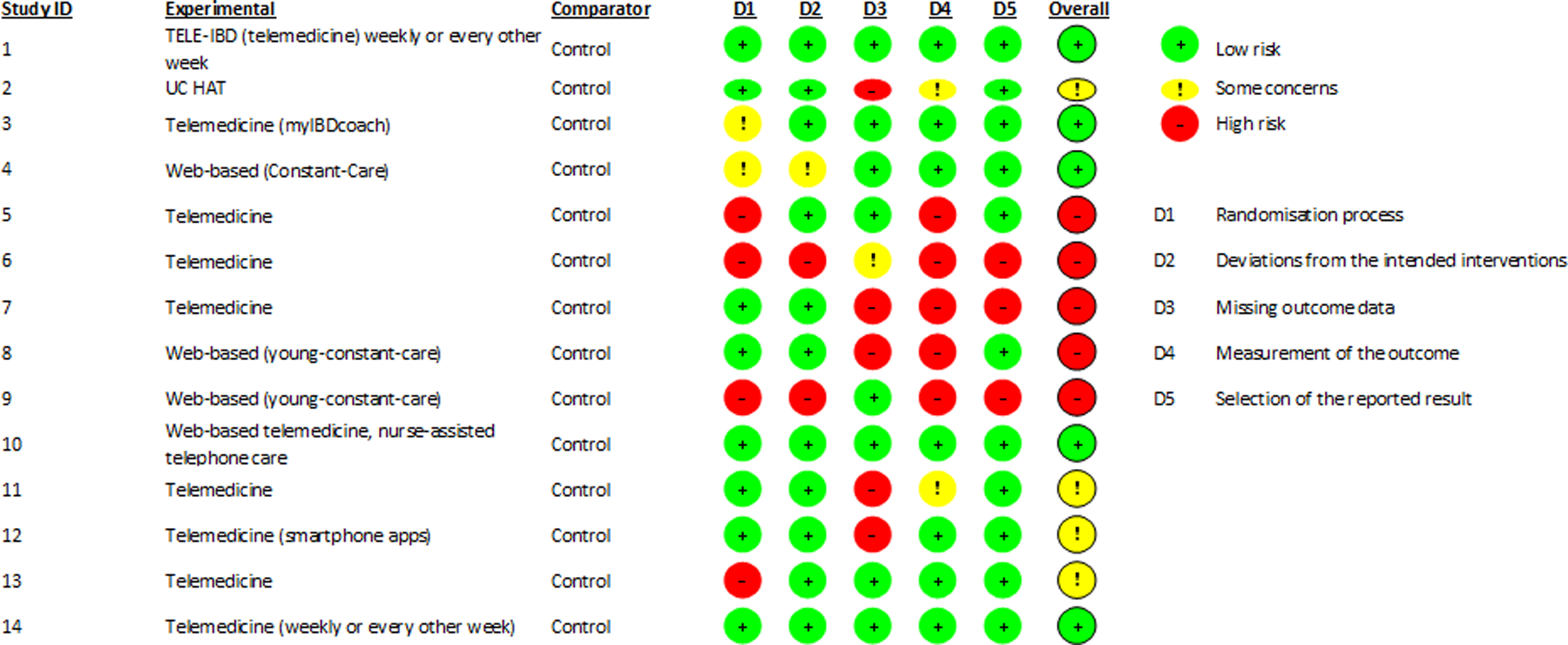
Risk of bias in included trials based on Cochrane risk-of-bias tool for randomized trials, version 2
Impact of digital health interventions on disease activity
Ten studies evaluated the impact of digital health interventions on disease activity and focused on web-based interventions, smartphone applications, and telemedicine to monitor patients’ disease activity level; five studies focused on children or adolescents only (Table 2).(13–20, 23, 24) All ten studies relied on symptom-based indices for evaluating disease activity. For adults with Crohn’s disease, the most commonly used disease activity index was the Harvey Bradshaw Index (HBI), and for children was the Pediatric Crohn’s Disease Activity Index (PCDAI). In patients with ulcerative colitis, studies with adults relied on Simple Clinical Colitis Activity Index (SCCAI), Seo Index, or Partial Mayo score, while studies in children relied on Pediatric Ulcerative Colitis Index (PUCAI).
In the largest RCT of web-based intervention, Elkjaer and colleagues randomized a total of 333 patients (233 patients in Denmark and 100 patients in Ireland) with mild-to-moderate UC, treated with aminosalicylates (5-ASAs), to ‘Constant-care’ (patients were asked to recognize signs of relapse and log onto the website and record daily disease activity score until they entered the green zone, considered as quiescent disease), or SoC.(19) Overall, the authors did not observe any significant differences in disease activity between the two groups. In a secondary analysis, the authors observed numerically more frequent, but significantly shorter duration of relapses in the intervention group compared to the SoC group [Denmark: median 18 days (95% CI, 10–21) vs 77 days (95% CI, 46–108), p<0.001; Ireland: median 30 days (95% CI, 2–37) vs 70 days (95% CI, 7–217), p<0.03]. Using the same platform as ‘Constant-care’, Carlsen and colleagues evaluated the effectiveness of web-based management vs. SoC in two cohorts: (1) monitoring disease activity in children/adolescents with IBD (young.constant-care.com) who were not on biologic therapy (non-biologic cohort), and (2) safety of personalizing infliximab therapy in patients receiving infliximab (biologic cohort).(14, 15) They observed no significant differences in C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) between the two groups in the non-biologic cohort. In the biologic cohort, patients in the intervention group could have significantly longer intervals between infliximab doses without an increase in the risk of developing clinically meaningful immunogenicity to infliximab, suggesting that web-based monitoring of infliximab was safe. Del Hoyo and colleagues developed a web-based system (Telemonitoring of Crohn’s Disease and Ulcerative Colitis – TECCU) to remotely monitor patient self-reported symptoms using a mobile- and web-based platform while Heida used a combination of email reminders, patient self-reported symptoms and a centralized website to monitor patients.(18, 20) Neither study observed a significant difference in the proportion of patients experiencing relapse between those randomized to digital health intervention vs. SoC.(18, 20) In the only RCT of web-based management in the United States, Cross and colleagues evaluated the impact of patients self-reporting symptoms to a web portal in 25 patients with UC (UC HAT) compared to “best available care” (BAC) for 12 months.(16) The authors did not observe a significant difference in disease activity and proportion of patients with clinical remission at the end of follow-up [60% of BAC and 68% of UC HAT patients were in remission at baseline (p=0.56); at 12 months, 77% of BAC and 76% of UC HAT patients were in remission (p=0.92)]. However, the intervention group had a 32% attrition rate, which could have affected these results.
In a separate large, multicenter RCT of telemedicine in the United States of 348 patients with IBD, Cross and colleagues randomized patients into two intervention groups (patients received disease-activity-based text messages weekly (W) or every other week (EOW) in addition to routine clinical care) or SoC and stratified by disease subtype.(17) While disease activity scores decreased and clinical remission rates increased from baseline to the end of follow-up for all groups, there were no significant differences among the three groups (CD patients: remission rates at baseline were 54%, 60% and 61% and increased to 64%, 61%, and 70% in the control, EOW, and W groups, respectively; UC patients: remission rates at baseline were 54%, 60%, and 61% and increased to 82%, 78%, and 67% in the control, EOW, and W groups, respectively). The intervention group that received weekly text messages had the highest attrition rate (19%) at the end of follow-up compared to the every-other-week (14%) and control (9%) groups. In a similar telemedicine study but in 51 children with IBD, Miloh and colleagues evaluated the effectiveness of interactive text messaging, in addition to SoC, to SoC alone and did not observe a significant difference in changes in disease activity between the two groups though both groups had high attrition rates (27% and 29% in the intervention and control, respectively).(24) Akobeng and colleagues attempted to evaluate the effectiveness of replacing clinic visits with telephone consultation in a group of 86 children/adolescents from the United Kingdom and assessed disease activity as a secondary outcome measure.(13) The authors did not find a significant difference in the frequency of relapses between the intervention group and SoC group though the number of respondents were very low (4 and 1 participants in the control and intervention group) to draw meaningful conclusions.
Using IBD-specific mobile applications (IBDsmart and IBDoc), McCombie and colleagues performed a non-inferiority RCT in 199 IBD patients and compared a mobile and web-based intervention to standard of care and evaluated disease activity as a secondary outcome.(23) At the end of 12 months of follow-up, the intervention group met its noninferiority outcome of no significant difference in symptom score compared to SoC.
Overall, there were no significant differences in different digital health technologies in impacting disease activity compared to SoC; however, the findings of these studies were limited by the small sample size, short duration of follow-up and evaluated disease activity as a secondary outcome. Additionally, many digital health interventions directed towards disease activity monitoring relied on patients self-reporting of their symptoms without accompanying objective markers of disease activity, such as biochemical and endoscopic data. Furthermore, the high attrition rates suggest difficulty with patient engagement, which may have affected the results of these studies. Overall, the benefit of using digital health interventions over standard of care for improving disease activity in patients with IBD is uncertain (very low certainty of evidence: evidence rated down for serious risk of bias, inconsistency and imprecision).
Impact of digital health interventions on quality of life
Eleven studies evaluated the impact of digital health technologies on IBD-related QoL (Table 3).(11, 13–20, 23, 25) QoL was measured using: IBD questionnaire, short IBDQ (SIBDQ), IBDQ-9, IBDQShort-Form 36/12 (SF-36 and SF-12), and Pediatric IBD-specific IMPACT-III.
Table 3.
Impact of digital health technologies on quality of life.
| Author, year | Type of Intervention | Outcome Measurements | Duration of Follow-up | Findings |
|---|---|---|---|---|
| Quality of life | ||||
| Cross R, 2019 | 1. TELE-IBD weekly (W) 2. TELE-IBD every other week (EOW) 3. Control |
IBDQ questionnaire. Higher scores associated with better quality of life. Score changes of 16 from baseline are significant. | 12 months |
|
| Cross R, 2012 | 1. UCHAT – comprised of a home unit, decision support server, and a web-based clinician portal 2. Best available care (BAC) |
IBDQ questionnaire. Higher scores associated with better quality of life. Score changes of 16 from baseline are significant. | 12 months |
|
| De Jong, 2017 | 1. Web-based (myIBDcoach) 2. Control |
SIBDQ (10-item questionnaire; 4 domains) | 12 months |
|
| Elkjaer M, 2010 | 1. Web (Constant-Care) 2. Control |
S-IBDQ: short IBD QoL questionnaire checking for bowel symptoms, systemic symptoms, emotional and social functions (10→70) Short Form-36 (SF-36) and Short Form-12 (SF-12) SF-36 for Denmark and SF-12 for Ireland: health survey |
12 months |
Denmark
|
| Akobeng AK, 2015 | 1. Mobile-based (telephone consultation) 2. Control |
Pediatric IBD-specific IMPACT quality of life (QOL) | 12 months |
|
| Carlsen K, 2017 (Non-biologic cohort) | 1. Web-based (yong.constant-care.com) 2. Control |
IMPACT III – self-reported HRQoL questionnaire developed by the Pediatric IBD working group | 24 months |
|
| Carlsen K, 2017 (Biologic cohort; IFX) | 1. Web-based (yong.constant-care.com) 2. Control |
IMPACT III – self-reported HRQoL questionnaire developed by the Pediatric IBD working group | 24 months |
|
| Del Hoyo, 2019 | 1. Web-based telemedicine - remote monitoring (G_TECCU) 2. Nurse-assisted telephone care (G_NT) 3. Standard care with in-person visits (G_control) |
Health-Related Quality of Life (HRQoL) as captured by Inflammatory Bowel Disease Questionnaire 9 (IBDQ-9). | 6 months |
|
| Heida, 2017 | 1. Telemonitoring (web-based) 2. Control |
QoL was measured with IBD-specific IMPACT-III | 12 months |
|
| McCombie, 2020 | 1. Mobile application (IBDsmart and IBDoc) 2. Control |
HRQOL was measured using IBDQ. | 12 months |
|
| Schljep M, 2020 | 1. TELE-IBD weekly (W) 2. TELE IBD every other week (EOW) 3. Control |
Quality of Life was measured by the Short Form 12 (SF-12) | 12 months |
|
W: Weekly
EOW: Every other week
QoL: Quality of life
HRQoL: Health related quality of life
BAC: Best available care
IMPACT-III: IBD Questionnaire
IBDQ: IBD Questionnaire
IBDQ-9: Inflammatory Bowel Disease Questionnaire 9
SIBDQ: Short IBD Questionnaire
In a large RCT of telemedicine in 348 adult patients with IBD in the United States, Cross and colleagues evaluated the impact of telemedicine on IBD-related QoL. They observed no significant difference in the QoL in patients receiving weekly vs. every other week text tailored messages vs. SoC; however, patients receiving text messages every other week had longitudinal improvement in QoL.(17) Using the same cohort but focusing on depressive symptoms and generic QoL, Schliep and colleagues did not observe a significant improvement in QoL in the intervention group compared to controls.(25) In two large multicenter RCTs of adult patients with IBD conducted in Europe, De Jong and colleagues observed a trend with improved QoL scores for patients enrolled in the intervention group (myIBDcoach) compared to SoC, while Elkjaer and colleagues only noted a significant improvement in QoL scores for the intervention group (Constant-care) in Denmark but not in Ireland compared to controls.(11, 19) In a smaller study of adult IBD patients in Spain, Del Hoyo and colleagues did not observe a significant difference in QoL between intervention group and control groups.(19) In four studies of web-based interventions in children/adolescents, there were no significant differences in improvement of QoL between the intervention and SoC groups.(14–16, 20)
In a non-inferiority RCT of mobile-based technology (mobile applications, IBDsmart and IBDoc) in adults with IBD in New Zealand by McCombie and colleagues, the intervention group met its primary endpoint of noninferiority to SoC with respect to QoL scores and symptoms after 12 months of follow-up suggesting that mobile-based technology could potentially replace face-to-face care.(23) In a separate study in children with IBD in the United Kingdom, Akobeng and colleagues enrolled patients into telephone encounter visits only compared to in-person clinic visits and did not notice worsening of QoL scores in the intervention group compared to the control.(13)
Overall, digital health interventions were not more effective than SoC in improving QoL and this was consistent across different countries and patient populations (children, adolescents, and adults). A potential limitation of this finding is the short follow-up time in these studies, which could have affected the adoption of these technologies. Overall, the benefit of using digital health interventions over standard of care for improving quality of life in patients with IBD is uncertain (very low certainty of evidence: evidence rated down for serious risk of bias, inconsistency and imprecision).
Impact of digital health interventions on healthcare utilization and cost-effectiveness
Ten studies evaluated the impact of digital health technologies on healthcare utilization and cost-effectiveness with many of the studies evaluating these outcomes as secondary aims (Table 4).(11, 13, 15, 17–21, 23, 24) Outcomes that were measured included proportion of hospitalizations, surgeries, emergency room visits, office visits, procedures, treatment with intravenous medications or corticosteroid use, and/or telephone/electronic encounters.
Table 4.
Impact of digital health technologies on healthcare utilization and cost-effectiveness.
| Author, year | Type of Intervention | Outcome Measurements | Duration of Follow-up | Findings |
|---|---|---|---|---|
| Healthcare utilization and cost-effectiveness | ||||
| Cross R, 2019 | 1. TELE-IBD weekly (W) 2. TELE-IBD every other week (EOW) 3. Control |
Electronic medical record (EMR) use 1 year before or after randomization, assessed hospitalizations, surgeries, ER visits, office visits, procedures, IV treatment, telephone/electronic encounters. | 12 months |
|
| De Jong, 2017 | 1. Web-based (myIBDcoach) 2. Control |
Number of flares; IBD-related hospitalizations, ER visits, surgeries, and/or corticosteroid use | 12 months |
|
| Elkjaer M, 2010 | 1. Web (Constant-Care) 2. Control |
Hospitalization, days lost through illness, and/or improvement of disease activity/extension | 12 months |
Denmark
|
| Akobeng AK, 2015 | 1. Mobile-based (telephone consultation) 2. Control |
Number of hospital admissions National Health Services (NHS) costs |
24 months |
|
| Carlsen K, 2017 (Non-biologic cohort) | 1. Web-based (yong.constant-care.com) 2. Control |
Contacts to the hospital and outpatient visits | 24 months |
|
| Del Hoyo, 2019 | 1. Web-based telemedicine - remote monitoring (G_TECCU) 2. Nurse-assisted telephone care (G_NT) 3. Standard care with in-person visits (G_control) |
Safety was a composite of ER visits, hospitalizations, IBD-related surgeries, corticosteroid courses, and adverse effects to medication. | 6 months |
|
| Heida, 2017 | 1. Telemonitoring (web-based) 2. Control |
The cost-effectiveness analysis included direct and indirect medical and non-medical costs. | 12 months |
|
| McCombie, 2020 | 1. Mobile application (IBDsmart and IBDoc) 2. Control |
IBD-related hospitalizations and outpatient appointments | 12 months |
|
| Miloh, 2017 | 1. Telemonitoring (2-way interactive text messaging [TM]) 2. Control |
Number of surgeries, admissions and emergency room visits in 1 year. Number of clinic no show. | 12 months | No significant differences in healthcare utilization between the two groups. |
| Krier M, 2011 | 1. Remote telemedicine encounter (TE) 2. Face-to-face standard encounter |
Duration of appointment visit, wait time, number of patients seen per clinic day, trainee and physician satisfaction using TE system, utilization of the network to gain insight into bandwidth requirements need for use | 9 months |
|
EMR: electronic medical record
ER: emergency room
W: weekly
EOW: every other week
IBD: Inflammatory bowel disease
SD: Standard deviation
NHS: National Health Services
HRQOL: Health-related quality of life
Two large studies, Cross and colleagues in the United States and De Jong and colleagues in Europe, demonstrated the positive impact of telemedicine and web-based interventions in reducing hospitalizations in adult patients with IBD compared to SoC.(11, 17) Four studies reported significantly lower number of outpatient visits/encounters in patients who received digital health interventions, without accompanying increase in disease-related complications (such as flares or hospitalizations) compared to SoC.(15, 18, 20, 23) In three studies that evaluated healthcare-related costs between digital health technologies and SoC, the authors found that there was significant cost savings with web-based interventions or replacing face-to-face clinic visits with telephone consultations.(13, 19, 20) One study demonstrated that the duration of appointment visit and wait time was not significantly different in patients who received remote telemedicine encounter with an IBD specialist compared to routine face-to-face standard encounter.(21)
No reduction in the proportion of surgeries, emergency room visits, procedures, or treatment with intravenous medications or corticosteroid use were associated with digital health technologies in the studies that reported these outcomes. However, a significant limitation of these studies is that the authors did not evaluate individual components of healthcare utilization such as IBD-related hospitalization, IBD-related surgeries, corticosteroid use, etc., so the studies were significantly underpowered to detect potential differences. Overall, digital health interventions may decrease overall healthcare resource utilization, primarily related to decreasing outpatient visits in patients with IBD (low certainty of evidence: evidence rated down for risk of bias, imprecision). The benefit of using digital health interventions over standard of care for decreasing unplanned healthcare utilization such as hospitalization or emergency department visits in patients with IBD is uncertain (very low certainty of evidence: evidence rated down for risk of bias, inconsistency and imprecision).
Impact of digital health interventions on treatment adherence
Seven studies evaluated the impact of web-based interventions and mobile technologies in evaluating treatment adherence (Table 5).(11, 15, 16, 18, 19, 22, 24) Studies used different measurement tools to evaluate treatment adherence: Morisky Medication Adherence Score (low adherence was defined as a score less than 6), Compliance Questionnaire, and Medical Adherence Report Scale (MARS).
There were conflicting data on the impact of web-based technologies on treatment adherence with smaller studies reporting no significant differences in treatment adherence in patients enrolled in web-based interventions compared to standard care while larger studies reported a significant difference. In a RCT in the United States, Cross and colleagues did not observe a significant difference in medication adherence in 25 adults with UC who were randomized to web-based intervention group (UCHAT) compared to SoC.(16) This finding was also observed in two small studies conducted in Europe by Carlsen and colleagues (young.constant-care.com) in 53 children and Del Hoyo and colleagues (TECCU) in 63 adults that evaluated the use of web-based technologies on medication adherence to SoC.(15, 18) Meanwhile, two large multicenter RCTs on web-based intervention in Europe conducted by De Jong (myIBDcoach; 905 adults) and Elkjaer (Constant-care; 485 adults) observed significantly higher rates of treatment adherence in patients enrolled in a web-based strategy compared to SoC.(11, 19). In two studies that evaluated mobile-based technology on the impact of treatment adherence, the authors did not observe any significant different in adherence at the end of follow-up. Linn and colleagues evaluated the effect of weekly personalized text messages compared to SoC in 160 adult patients and did not find a significant difference in treatment adherence at the end of six months, while Miloh and colleagues evaluated the effect of two-way interactive text messaging compared to SoC in 51 children and did not find a significant difference in treatment adherence at the end of 12 months.(22, 24) Overall, digital health technologies appeared to be most beneficial if they were web-based compared to mobile-based technologies though there were no trials directly comparing these different modalities. Overall, the benefit of using digital health interventions over standard of care for improving treatment adherecne in patients with IBD is uncertain (very low certainty of evidence: evidence rated down for serious risk of bias, inconsistency and imprecision).
Impact of digital health interventions on other outcomes
Eight studies evaluated outcomes on patient/parent satisfaction, work productivity and activity of daily living, acceptability and usability of digital health technologies, self-efficacy, school absence and knowledge base (Supplementary Table 3).(11, 13, 15, 18, 19, 21–23) Overall, patients, parents and providers were satisfied and willing to adopt digital health technologies. There were mixed results with improvement of self-efficacy and knowledge base though digital health interventions were not associated with worse scores in these domains.
DISCUSSION
Summary of evidence and key findings
In this systematic review of 14 RCTs comparing different digital health technologies vs. SoC in patients with IBD, we made some key observations. Digital health technologies did not result in lower rates of disease relapses compared to SoC. However, relapse rates were comparable to patients who received routine face-to-face encounters suggesting that digital health technologies may offer a safe and effective complimentary mechanism for chronic care management. Digital health interventions were associated with lower burden of healthcare utilization, primarily driven by lower rates of office visits, without any higher risk of unplanned hospitalizations, emergency room visits, surgeries, and/or corticosteroid use compared with SoC. Since IBD are chronic conditions, patients often require lifelong therapy, which may make treatment adherence difficult, especially in children/adolescents who may need supervision. Based on results from two large, multicenter RCTs in Europe (myIBDcoach and Constant-Care), web-based technologies were associated with significantly higher rates of treatment adherence compared to SoC.(11, 19) Furthermore, in studies that evaluated the impact of digital health technologies on healthcare costs, three studies demonstrated significant cost savings with web-based interventions or telephone consultations compared to face-to-face clinic visits.(13, 19, 20) Overall, QoL and satisfaction with the use of digital health technologies was comparable to SoC suggesting that patients, parents of children/adolescents, and providers were willing to adopt digital health technologies in routine clinical practice. Lastly, there were no negative outcomes associated with the application and adoption of digital health technologies reported in any of the studies included in this systematic review.
Our current findings suggest that digital health technologies may offer an opportunity to replace or at least supplement SoC traditional face-to-face office visits, with potentially a decline in healthcare utilization and costs without negative consequences on disease activity, QoL and treatment adherence. In a systematic review of digital health technologies in patients with viral hepatitis B or C, Haridy and colleagues evaluated 80 studies that evaluated electronic medical record interventions, telemedicine, mobile health, devices, clinical decision support, web-based, social media and electronic communication and found that digital health technologies significantly increased screening rates for HCV and HBV and not associated with worse virologic responses to treatment.(26)
Gaps in the literature and opportunities for future studies
An opportunity for research is the application of digital health technologies in community and rural settings, since many of the studies included in our systematic review recruited patients at tertiary referral centers which had expertise in managing patients with IBD. Application and adoption of digital health technologies may be beneficial in areas where there is limited IBD experience. There was intrinsic selection bias for participants who are savvy and have access of digital health tools in the included RCTs. Recruitment into these trials was challenging with a significant proportion of eligible patients declining to participate. Cross and colleagues reported several challenges in recruitment in their home telemanagement trial related to difficulty in randomization and blinding and defining a “control group” who receive variable degree of “best available care”.(27) Several other factors can contribute to low recruitment in clinical trials of IBD, with ongoing efforts to improve trial design, processes and methodology to improve recruitment.(28–31) Whether these interventions can be readily implemented and be similarly effective in a population enriched in negative social determinants of health such as racioethnic minorities, low literacy level, limited affordability, etc., is a major knowledge gap that merits future studies. Additionally, digital health interventions, either as supplement or as a replacement to SoC, may be beneficial in specific care settings in which patients are at high risk of unplanned healthcare utilization: 1) offering post-discharge nurse-led telemedicine visits in lieu of in-person clinic visit to improve patient engagement and identify patients at high-risk of readmission, 2) remote monitoring of high-risk patients starting biologic therapy and/or corticosteroids for severe disease, to identify early response to therapy, 3) preventative health to ensure timely administration of vaccinations, and 4) multi-disciplinary collaboration with other healthcare stakeholders (e.g., social worker, pharmacist, dietician, nurse case managers, primary care physicians, and surgeons).
Many studies in our systematic review recruited patients who were not on biologic therapies, immunosuppressants or corticosteroids which suggests that these patients had predominantly mild-moderate disease. Therefore, the findings of our systematic review may not be applicable to patients with moderate-severe disease. Furthermore, while some studies attempted to randomize patients by disease severity and IBD subtype, future studies on digital health interventions should adopt these randomization schemas to account for these factors. As the field has evolved from non-specific, symptom-driven treatment goals to more objective treat-to-targets such as endoscopic remission and/or normalization of biochemical results (such as CRP or fecal calprotectin), future studies on the impact of digital health interventions in disease monitoring should incorporate these newer targets, which are at less risk of bias compared to symptom-drive targets, which have not been shown to correlate well with disease activity.(32, 33)
While there were generally positive results associated with the use of digital health technologies, adherence to these technologies was a concern as studies reported high rates of attrition during follow-up in patients who received these interventions, which suggests fatigability with long term use. Future studies on understanding the reasons for this attrition would be crucial to the wide adoption of digital health interventions across various clinical settings and different populations. With the advancement in digital health technologies and the increased adoption of video visits during COVID-19 pandemic, studies should look at the impact of combining different digital health technologies on patient engagement, adherence as well as outcomes.
Limitations
Due to the heterogeneous clinical domains, patient populations, and different measurement metrics, we were unable to perform meta-analyses to quantitatively assess the impact of eHealth technologies in IBD patients compared to standard-of-care. In the absence of quantitative synthesis, statistical assessment of publication bias could not be assessed. While we conducted a thorough systematic review of published studies, the possibility of reporting bias or file drawer bias (negative studies not being reported) cannot be ruled out. Current RCTs of digital health interventions are at high risk of bias due to lack of blinding across all studies which could have affected the treatment effect sizes, patient-reported outcomes which are subject to recall bias, small sample sizes that were significantly affected by attrition rates, and short follow-up durations which could have affected the rate of adoption, and effectiveness, of digital health technologies. Additionally, social determinants of health factors (such as ethnicity, language, digital access, and literacy) that may influence implementation, adoption, and interpretability of digital health technologies were not considered in these studies.
In summary, digital health interventions may offer advantages for value-based care and population health management in patients with IBD by reducing healthcare utilization and costs, though the studies so far have failed to see benefits over SoC in improving disease activity, QoL and treatment adherence. Future studies addressing specific aspects of setting, design or delivery of digital health interventions that may improve patient engagement, efficiency of care and patient-centered outcomes are warranted.
Supplementary Material
STUDY HIGHLIGHTS.
WHAT IS KNOWN
Traditional clinical encounters in patients with IBD are often reactive and episodic, with significant variability in care.
Digital health technologies may be promising for chronic care models.
WHAT IS NEW HERE
Digital health technologies, including mobile applications, patient portals and telemedicine, may lower burden of unplanned healthcare utilization and costs, compared with traditional encounters in patients with IBD.
These interventions may not be more effective than traditional encounters in improving disease activity, quality of life or treatment adherence.
Digital health technologies may be effective population health management tools in patients with IBD.
Disclosures:
Dr. Nguyen is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number T32DK007202 and T15LM011271. Dr. Singh is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK117058 and R03DK129631, the American College of Gastroenterology Junior Faculty Development Award. Dr. Sandborn is supported in part by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Conflicts of Interest:
NHN – None to declare
IM – None to declare
AA – Research grants from Abbvie, Takeda, Janssen, AstraZeneca, Pfizer. Board member, Rx.Health
AMS – None to declare
WJS – Research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories; consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD, Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - employee, stock options; Escalier Biosciences - employee, stock options; Precision IBD - employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options
LOM – None to declare
SS – Research grants from AbbVie and Janssen, Personal fees from Pfizer (for ad hoc grant review)
REFERENCES
- 1.Olen O, Askling J, Sachs MC, Frumento P, Neovius M, Smedby KE, et al. Increased Mortality of Patients With Childhood-Onset Inflammatory Bowel Diseases, Compared With the General Population. Gastroenterology. 2019;156(3):614–22. [DOI] [PubMed] [Google Scholar]
- 2.Olen O, Askling J, Sachs MC, Neovius M, Smedby KE, Ekbom A, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Qian AS, Nguyen NH, Ho SKM, Luo J, Jairath V, et al. Trends in U.S. Health Care Spending on Inflammatory Bowel Diseases, 1996–2016. Inflamm Bowel Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen NH, Khera R, Ohno-Machado L, Sandborn WJ, Singh S. Annual Burden and Costs of Hospitalization for High-Need, High-Cost Patients With Chronic Gastrointestinal and Liver Diseases. Clin Gastroenterol Hepatol. 2018;16(8):1284–92 e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulai PS, Singh S, Ohno-Machado L, Sandborn WJ. Population Health Management for Inflammatory Bowel Disease. Gastroenterology. 2018;154(1):37–45. [DOI] [PubMed] [Google Scholar]
- 6.Siegel CA. Refocusing IBD Patient Management: Personalized, Proactive, and Patient-Centered Care. Am J Gastroenterol. 2018;113(10):1440–3. [DOI] [PubMed] [Google Scholar]
- 7.Siegel CA. Placing Value on Telemedicine for Inflammatory Bowel Disease. Am J Gastroenterol. 2019;114(3):382–3. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel B 2015 American Journal of Gastroenterology Lecture: How Digital Health Will Transform Gastroenterology. Am J Gastroenterol. 2016;111(5):624–30. [DOI] [PubMed] [Google Scholar]
- 9.Olivera P, Danese S, Jay N, Natoli G, Peyrin-Biroulet L. Big data in IBD: a look into the future. Nat Rev Gastroenterol Hepatol. 2019;16(5):312–21. [DOI] [PubMed] [Google Scholar]
- 10.George LA, Dominic MR, Cross RK. Integration of telemedicine into clinical practice for inflammatory bowel disease. Curr Opin Gastroenterol. 2020;36(4):304–9. [DOI] [PubMed] [Google Scholar]
- 11.de Jong MJ, Boonen A, van der Meulen-de Jong AE, Romberg-Camps MJ, van Bodegraven AA, Mahmmod N, et al. Cost-effectiveness of Telemedicine-directed Specialized vs Standard Care for Patients With Inflammatory Bowel Diseases in a Randomized Trial. Clin Gastroenterol Hepatol. 2020;18(8):1744–52. [DOI] [PubMed] [Google Scholar]
- 12.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 13.Akobeng AK, O’Leary N, Vail A, Brown N, Widiatmoko D, Fagbemi A, et al. Telephone Consultation as a Substitute for Routine Out-patient Face-to-face Consultation for Children With Inflammatory Bowel Disease: Randomised Controlled Trial and Economic Evaluation. EBioMedicine. 2015;2(9):1251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsen K, Houen G, Jakobsen C, Kallemose T, Paerregaard A, Riis LB, et al. Individualized Infliximab Treatment Guided by Patient-managed eHealth in Children and Adolescents with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23(9):1473–82. [DOI] [PubMed] [Google Scholar]
- 15.Carlsen K, Jakobsen C, Houen G, Kallemose T, Paerregaard A, Riis LB, et al. Self-managed eHealth Disease Monitoring in Children and Adolescents with Inflammatory Bowel Disease: A Randomized Controlled Trial. Inflamm Bowel Dis. 2017;23(3):357–65. [DOI] [PubMed] [Google Scholar]
- 16.Cross RK, Cheevers N, Rustgi A, Langenberg P, Finkelstein J. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis (UC HAT). Inflamm Bowel Dis. 2012;18(6):1018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross RK, Langenberg P, Regueiro M, Schwartz DA, Tracy JK, Collins JF, et al. A Randomized Controlled Trial of TELEmedicine for Patients with Inflammatory Bowel Disease (TELE-IBD). Am J Gastroenterol. 2019;114(3):472–82. [DOI] [PubMed] [Google Scholar]
- 18.Del Hoyo J, Nos P, Faubel R, Munoz D, Dominguez D, Bastida G, et al. A Web-Based Telemanagement System for Improving Disease Activity and Quality of Life in Patients With Complex Inflammatory Bowel Disease: Pilot Randomized Controlled Trial. J Med Internet Res. 2018;20(11):e11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkjaer M, Shuhaibar M, Burisch J, Bailey Y, Scherfig H, Laugesen B, et al. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59(12):1652–61. [DOI] [PubMed] [Google Scholar]
- 20.Heida A, Dijkstra A, Muller Kobold A, Rossen JW, Kindermann A, Kokke F, et al. Efficacy of Home Telemonitoring versus Conventional Follow-up: A Randomized Controlled Trial among Teenagers with Inflammatory Bowel Disease. J Crohns Colitis. 2018;12(4):432–41. [DOI] [PubMed] [Google Scholar]
- 21.Krier M, Kaltenbach T, McQuaid K, Soetikno R. Potential use of telemedicine to provide outpatient care for inflammatory bowel disease. Am J Gastroenterol. 2011;106(12):2063–7. [DOI] [PubMed] [Google Scholar]
- 22.Linn AJ, van Dijk L, van Weert JCM, Gebeyehu BG, van Bodegraven AA, Smit EG. Creating a synergy effect: A cluster randomized controlled trial testing the effect of a tailored multimedia intervention on patient outcomes. Patient Educ Couns. 2018;101(8):1419–26. [DOI] [PubMed] [Google Scholar]
- 23.McCombie A, Walmsley R, Barclay M, Ho C, Langlotz T, Regenbrecht H, et al. A Noninferiority Randomized Clinical Trial of the Use of the Smartphone-Based Health Applications IBDsmart and IBDoc in the Care of Inflammatory Bowel Disease Patients. Inflamm Bowel Dis. 2020;26(7):1098–109. [DOI] [PubMed] [Google Scholar]
- 24.Miloh T, Shub M, Montes R, Ingebo K, Silber G, Pasternak B. Text Messaging Effect on Adherence in Children With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2017;64(6):939–42. [DOI] [PubMed] [Google Scholar]
- 25.Schliep M, Chudy-Onwugaje K, Abutaleb A, Langenberg P, Regueiro M, Schwartz DA, et al. TELEmedicine for Patients With Inflammatory Bowel Disease (TELE-IBD) Does Not Improve Depressive Symptoms or General Quality of Life Compared With Standard Care at Tertiary Referral Centers. Crohns Colitis 360. 2020;2(1):otaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haridy J, Iyngkaran G, Nicoll A, Hebbard G, Tse E, Fazio T. eHealth Technologies for Screening, Diagnosis, and Management of Viral Hepatitis: A Systematic Review. Clin Gastroenterol Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 27.Cross RK, Finkelstein J. Challenges in the design of a Home Telemanagement trial for patients with ulcerative colitis. Clin Trials. 2009;6(6):649–57. [DOI] [PubMed] [Google Scholar]
- 28.Dubinsky MC, Collins R, Abreu MT, International Organization for the Study of Inflammatory Bowel D. Challenges and Opportunities in IBD Clinical Trial Design. Gastroenterology. 2021;161(2):400–4. [DOI] [PubMed] [Google Scholar]
- 29.Khanna R, Zou G, Feagan BG. Evolution of the Randomized Controlled Trial in Inflammatory Bowel Disease: Current Challenges and Future Solutions. Inflamm Bowel Dis. 2018;24(10):2155–64. [DOI] [PubMed] [Google Scholar]
- 30.Noor NM, Parkes M, Raine T. Moving towards more patient-centred clinical trials in IBD. Nat Rev Gastroenterol Hepatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inan OT, Tenaerts P, Prindiville SA, Reynolds HR, Dizon DS, Cooper-Arnold K, et al. Digitizing clinical trials. NPJ Digit Med. 2020;3:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombel JF, D’Haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis. 2020;14(2):254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vanasek T, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


