Abstract
Oncolytic viruses (OVs) are emerging as potentially useful platforms in treatment methods for patients with tumors. They preferentially target and kill tumor cells, leaving healthy cells unharmed. In addition to direct oncolysis, the essential and attractive aspect of oncolytic virotherapy is based on the intrinsic induction of both innate and adaptive immune responses. To further augment this efficacious response, OVs have been genetically engineered to express immune regulators that enhance or restore antitumor immunity. Recently, combinations of OVs with other immunotherapies, such as immune checkpoint inhibitors (ICIs), chimeric antigen receptors (CARs), antigen-specific T-cell receptors (TCRs) and autologous tumor-infiltrating lymphocytes (TILs), have led to promising progress in cancer treatment. This review summarizes the intrinsic mechanisms of OVs, describes the optimization strategies for using armed OVs to enhance the effects of antitumor immunity and highlights rational combinations of OVs with other immunotherapies in recent preclinical and clinical studies.
Subject terms: Cancer therapy, Tumour immunology
Introduction
Naturally, carcinogenesis proceeds through a multistep process involving the accumulation of genetic and epigenetic aberrations leading to the production of antigens that differ quantitatively or qualitatively from those produced by healthy cells.1 These cancer-specific antigens are processed by antigen-presenting cells (APCs), such as dendritic cells (DCs). They first bind to major histocompatibility complex (MHC) molecules and then are presented on the cell APC surface in antigen–MHC complexes. T lymphocytes interact with their cognate T cell receptors (TCRs) to recognize antigen-MHC complexes in lymph nodes. Although antigen stimulation of a TCR is necessary for T-cell activation and proliferation, an additional costimulation signal is needed. CD28, the primary costimulatory molecule on T cells, stimulates the activation of naive T-cells and promotes cytokine secretion. Upon antigen stimulation and costimulation signaling, cytotoxic lymphocytes (CTLs) are primed and trafficked via the circulatory system to the tumor, ultimately eliminating cancer cells. Killing tumor cells requires not only the generation of CTLs but also physical contact between these T cells and cancer cells.2
However, the tumor microenvironment (TME) exhibits highly complex heterogeneity and is characterized by acidic conditions, hypoxia, low immunogenicity and suppressed immune cell function.3,4 In addition to a dense extracellular matrix (ECM), the cellular components of the TME consist mostly of tumor cells, stem cells (CSCs), endothelial cells (ECs), cancer-associated fibroblasts (CAFs), and tumor-infiltrating immune cells.5 Tumor-infiltrating immune cells include macrophages, neutrophils, DCs, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, T cells, and B cells.4 The immunosuppressive TME is extensively populated with suppressive immune cells such as MDSCs, regulatory T cells (Tregs) and tumor-associated macrophages (TAMs), but CTLs are lacking in the tumor core.6 Despite the high infiltration of CTLs in certain types of tumor tissues, immune checkpoint axes (programmed death ligand-1 (PD-1)/PD-L1, etc.) populate the surface of CTLs or tumor cells.6 Hence, the immunosuppressive TME poses great challenges to cancer immunotherapy.
Multiple strategies are used to enhance the role of T cells in cancer immunotherapy. Cancer vaccines aim to elicit antigen-specific T-cell cytotoxicity. Adoptive cell therapies are based on autologous tumor-infiltrating lymphocyte (TIL) therapies, chimeric antigen receptor (CAR) T-cell therapies and antigen-specific TCR therapies, which are all aimed at increasing the infusion of tumor-fighting immune cells. Immune checkpoint inhibitor (ICI) therapies unleash powerful antitumor T cell responses.7 These immunotherapies have revolutionized the field of cancer immunotherapy. However, these immunotherapies benefit only a minority of patients for multiple reasons, such as immune system suppression, lack of cytokine variety, poor APC function, few TILs and weak activity of effector T cells.8
Viruses have been used as possible agents to treat cancer for more than a century.9 With the development of cloning technology, a variety of viruses could be genetically engineered to selectively infect and lyse tumor cells. The increased understanding of viral mechanisms of action, including activating innate and adaptive antitumor immunity and modulating the TME, prospered virotherapy.10 Four OVs have been approved for the treatment of various cancers. Despite the approved OVs, a number of OVs that were used as transgene carriers or combined with other immunotherapies were investigated for their antitumor effects in preclinical or clinical studies. In this review, on the basis of the intrinsic mechanism of OVs, we provide a brief overview of each OV and emphasize the role of unarmed or armed OVs in effectively enhancing antitumor immunity in four ways: abrogating immune suppression, producing cytokine variety, enhancing APC function, and proving effector T-cell function.8,11 Furthermore, we discuss the rational combinations of OVs with other immunotherapies that have been tested in recent preclinical and clinical studies.
OVs
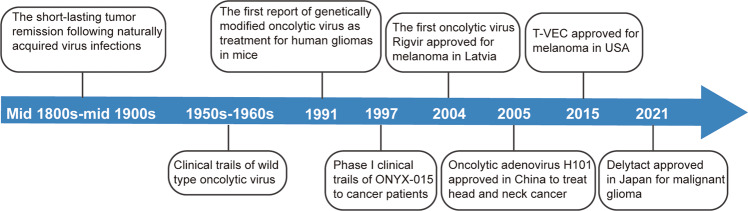
Oncolytic virus therapy (OVT) is a novel immunotherapy that uses natural or genetically modified viruses to specifically infect and lyse cancer cells but does not harm normal cells.12 Some milestones in the development of OVT are shown in Fig. 1. Historically, the possible use of natural viruses occurred in the early 1900s.9 From the early and mid-1900s, patients appeared to have short-lasting tumor remission following naturally acquired virus infections. For example, a 42-year-old woman with acute leukemia presented temporary remission after a presumed influenza infection in 1896.13 In the 1950s and 1960s, tests of the in vivo antitumor activity of OVs in patients were capable of being conducted, which benefited from the development of cell and tissue culture systems and the establishment of xenograft murine cancer models.14 In 1950, 30 patients with epidermoid cervical carcinomas were treated with 10 different adenovirus serotypes.15 Sixty-five percent of the patients formed necrosis and cavitiy in the central portion of cancer tissue. Subsequently, the application of biotechnology technology to genetically engineered viruses accelerated the field of virotherapy.15 In 1991, Martuza et al. first reported a thymidine kinase-negative mutant of herpes simplex virus-1 (dlsptk) with attenuated neurovirulence, which prolonged survival in glioma-bearing nude mice.16
Fig. 1.
A timeline of important milestones in the development of oncolytic virus as a cancer therapy
In this period, the modification strategy focused on obtaining tumor selectivity and improving safety. ONYX-015, which was described in 1997, is an attenuated adenovirus with the deletion of E1B55K gene, showing tumor-specific cytolysis and antitumoral efficacy.17 The first oncolytic virus, Rigvir, was approved in Latvia in 2004. Rigvir is an unmodified ECHO-7 virus but has been selected for melanoma.18 Oncolytic adenovirus H101, with E1B-55KD and partial E3 deleted, became the first approved OV in China in 2005 to treat head and neck cancer.19 Engineering OVs with transgenes potentially enhances OV oncolytic activity. T-VEC (IMLYGIC) is a modified form of herpes simplex type-1 virus (HSV-1) that encodes a human granulocyte macrophage colony-stimulating factor (GM-CSF) gene.20. T-VEC was approved by the US Food and Drug Administration (FDA) for the treatment of melanoma in October 2015.21 The approval of T-VEC has attracted increasing attention to OVT. In 2021, a modified HSV, named Delytact, was approved in Japan for malignant glioma. A multitude of different viruses have been presently exploited as OVs, including adenovirus,22 herpes simplex virus,23 measles virus,24 newcastle disease virus,25 reovirus,26 vesicular stomatitis virus27 and coxsackievirus.28
Adenoviruses
Adenoviruses (AdV) belong to the family of Adenoviridae, genus Mastadenovirus. They are nonenveloped viruses with double-stranded linear DNA genomes (~30–40 kb) and an icosahedral capsid.29 AdV are characterized by hexon, penton-base and fiber proteins, which are responsible for their tropism. Human AdVs are divided into seven different species (A–G) that contain 104 candidate serotypes by April 2021. Serotype 5 adenovirus (Ad5) is the most commonly used viral vector in clinical studies. Ad5 enters the targeted cells via the interaction of fiber knob with coxsackievirus and adenovirus receptors.30 Three general strategies have been employed to modify AdV to obtain cancer selectivity. The deletion of the E1A and E1B 55K genes make the AdV selectively replicate in retinoblastoma (pRb)- and p53-mutated tumor cells.31 The partial deletion in the E3 region allows AdV to encode immunostimulatory transgenes, which can enhance antitumor immunity.32 The Arg-Gly-Asp (RGD) motif was inserted into the HI loop of the AdV fiber protein to improve the infectivity of AdV.33
Herpes simplex virus
HSV, especially HSV type 1 (HSV-1), as an OV, has been tested widely in patients. HSV-1 belongs to the Alphaherpesvirinae subfamily of the Herpesviridae family. It is ~200 nm in diameter and is a double-stranded DNA virus with a 152 kb genome encoding over 74 distinct genes.34,35 The nonessential genes for replication in the large genome could be deleted and replaced with the engineered transgenes, which has no effect on the packaging efficiency of the virus. T-VEC is genetically created through deletion of ICP34.5 and ICP47 and insertion of GM-CSF.36 The deletion of ICP34.5, encoding the neurovirulence factor, stops virus replication in neurons but supports virus replication in tumor cells.37 Furthermore, in the placement of ICP34.5 T-VEC contains two copies of GM-CSF, which promotes dendritic cell maturation. ICP47 encodes an inhibitor of antigen presentation that blocks MHC class I antigen presentation to CD8+ T cells.38 Deletion of ICP47 can promote immune responses against tumor cells.39
Vaccinia virus
Vaccinia virus (VV) is an enveloped virus and comprises double-stranded DNA belonging to the genus Orthopoxvirus of the Poxviridae family.40 The genome of VV (70–100 nm in diameter) is approximately 190 kb in length, which allows the insertion and high-level expression of large foreign genes.41,42 The deletion of viral thymidine kinase (TK), vaccinia type I IFN-binding protein (B18R) or vaccinia growth factor (VGF) is one of the most common policies to increase the selective replication and lytic capability of VV.43 As an oncolytic agent, VV showed a natural selectivity to tumors and a possibility for use with systemic administration.44 JX-594 is a Wyeth strain VV-derived OV that lacks the TK gene and is armed with GM-CSF and β-galactosidase.45 The deletion of the viral TK gene significantly increased vaccinia specificity to tumors.46 The clinical trial JX-594 is discussed below.
Reovirus
Reovirus (RV) is a nonenveloped, double-stranded RNA (~23.5 kb) virus that belongs to the family Reoviridae and has found various hosts in fungi, plants, fish, reptiles, birds and mammals.47,48 The double-stranded RNA is structured into 10 segments according to size: large (L1–3), medium (M1–3) and small (S1–4).49 Evidence has demonstrated that the Ras signaling pathway is essential for RV replication and the release of virus progeny.50 Moreover, RV can induce cell apoptosis through the Ras/RalGEF/p38 pathway.51 This makes RV specifically target tumor cells overexpressing Ras. Three different RV serotypes have been identified: type one Lang, type two Jones, and type three Abney and Dearing.52 Reolysin (also known as Pelareorep), serotype 3 RV, is the most advanced oncolytic RNA virus in the clinic for cancer therapy and has completed numerous clinical trials as monotherapy or in combination with other therapies.53
Newcastle disease virus
Newcastle disease virus (NDV) is an enveloped virus with negative sense single-stranded RNA from the genus Avulavirus of the Paramyxoviridae family.54 Its diameter is 100–500 nm, and its genome is ~15 kb in length and encodes at least eight proteins (3′-N-P/V/W-M-F-HN-L-5′): nucleocapsid (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin-neuraminidase protein (HN) and large polymerase protein (L)- and two other proteins, V and W.55 NDV binds tumor cells through the HN protein, which interacts with sialic acid receptors on the surface of host cells, and then with the activated F protein, the virus and membrane of the host cells fuse with the HN protein. Therefore, the genome of the virus enters the host cytoplasm.56,57 The genomes have a large capacity (>5 kb) for the insertion of transgenes, and the insertion site of foreign genes between P/M is recommended. As an oncolytic virus, several clinical studies have demonstrated that NDV has a very high safety profile for cancer patients and shows notable antitumor capacity.58
Measles virus
Measles virus (MeV) is an enveloped virus with negative sense single-stranded RNA from the genus Morbillivirus of the Paramyxoviridae family. Its diameter is 100–200 nm, and its genome is ~16 kb in length, which includes six genes encoding for eight proteins: six anti-genome arrangements (5′-N-P-M-F-H-L-3′) and two accessory proteins (V and C).59 MeV interacts with host cells through three receptors: CD46, signaling lymphocyte-activation molecule (SLAM/CD150) and poliovirus-receptor-like-4 (PVRL4).60 SLAM/CD150 is often overexpressed on many hematological malignancies, while CD46 is constitutively overexpressed on many tumor cells, which makes MeV naturally selective for infecting tumor cells.61 However, CD46 is also expressed at the basal level in normal cells, so it is not a tumor-selective receptor.60 Its favorable safety profile with no dose-limiting toxicities and natural oncotropism makes MeV a promising OV candidate.62
Other oncolytic viruses
Apart from the previously mentioned viruses, several other viruses, such as seneca valley virus,63 poliovirus,64 vesicular stomatitis virus65 and parvovirus66 have been developed into oncolytic viruses.
Mechanism of OV action
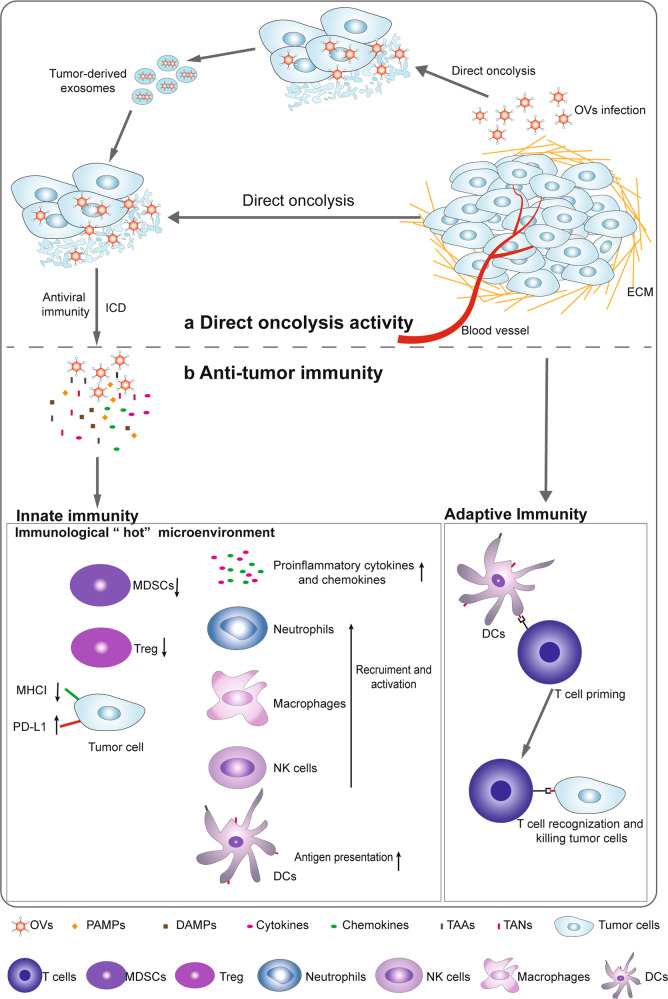
The direct oncolytic activity of OVs is considered the initial mechanism by which OVs kill cancers.67 OVs induce antiviral immunity and antitumor immunity. Antitumor immunity is obviously beneficial for tumor treatment. Based on the premise that the amplification and spread of OVs are limited by the antiviral immune response, host immune responses have been largely assumed to be detrimental to the success of OVs.68–70 However, the antiviral immune response has recently been viewed as beneficial in the treatment of tumors for the initial priming of antitumor immunity by OVs.71 Here, the direct killing activity and immune response of OVs are described. OVs preferentially target and kill tumor cells without affecting healthy cells. OVs induce innate immunity and turn “cold” tumors into “hot” tumors by facilitating the recruitment of immune cells and activating systemic anticancer adaptive immunity to suppress tumor growth (Fig. 2).
Fig. 2.
Mechanisms of oncolytic virus (OV) action. a Direct oncolysis: new viral particles are released from OV-lysed tumor cells to infect unaffected tumor cells. Moreover, exosomes derived from OV-infected tumors contain OVs and can exhibit high tumor tropism. b Antitumor immunity: immunogenic cell death (ICD) induced by OV exposure leads to the release of multiple molecules, including pathogen-associated molecular pattern molecules (PAMPs), damage-associated molecular pattern molecules (DAMPs), tumor-associated antigens (TAAs) and tumor-associated neoantigens (TANs). The identification of PAMPs/DAMPs through pattern recognition receptors (PRRs) in cancer or immune cells triggers the expression of proinflammatory cytokines such as type I interferons (IFNs), interleukin (IL)-1β, IL-6, IL-12, TNF-α, granulocyte macrophage colony-stimulating factor (GM-CSF), and chemokines such as CCL2, CCL3, CCL5 and CXCL10. Chemokines recruit neutrophils and macrophages to infection sites, and these cytokines stimulate the activity of innate immune cells such as NK cells and DCs, which further stimulate the production of IFNs, TNF-α, IL-12, IL-6, and chemokines, resulting in the amplification of the initial innate response and turning immunologically “cold” tumors into “hot” tumors. Type I IFNs increased the levels of MHC class I and II molecules and costimulatory molecules such as CD40, CD80, and CD86 on the surface of DCs. The released TAAs and TANs are processed and ultimately presented on the APC surface in complex with MHC molecules. Multiple cytokines and chemokines contribute to the recruitment and activation of antitumor CD8+ T cells and B cells
OVs directly lyse tumor cells
Normal host cells sense viral components and clear viruses by activating signaling pathways. However, abnormalities in the antiviral machinery in tumor cells allow the survival and replication of viruses.72,73 OVs are classified as naturally occurring or genetically modified, with the latter targeted to defective antiviral pathways within tumor cells for selectively infecting, replicating and lysing cancer cells, leaving normal cells unharmed.74,75 The release of infectious OVs from lysed tumor cells spread to surrounding uninfected tumor cells, resulting in the amplification of their oncolytic activity.14
Recently, tumor-derived exosomes secreted after OV infection have been shown to contribute to activated antitumor efficacy. Tumor-derived extracellular vesicles which were obtained from HCT116 tumor-bearing mice infected with oncolytic adenovirus (OAd) OBP-301 contained OBP-301 and exhibited high tumor tropism in orthotopic HCT116 rectal tumors.76
OVs activate innate immunity
Following administration, viral elements known as pathogen-associated molecular patterns (PAMPs), including viral capsids, DNAs, RNAs and proteins, are exposed to the host immune system.12 Moreover, OVs can activate various forms of immunogenic cell death (ICD), including immunogenic apoptosis, necroptosis and pyroptosis, by inducing endoplasmic reticulum (ER) stress,77,78 leading to the release of hallmark immunostimulatory damage-associated molecular patterns (DAMPs), such as ATP, high mobility group box 1 protein (HMGB1), heat shock protein, ecto-calreticulin and proinflammatory cytokines.79,80 These PAMPs and DAMPs are sensed by pattern recognition receptors (PRRs), such as stimulator of IFN genes (STING), Toll-like receptor (TLR) adaptor molecule 1 and TLR3 on immune cells,81–83 establishing a proinflammatory microenvironment by stimulating the production of proinflammatory cytokines, such as type I IFNs, interleukin (IL)-1β, IL-6, IL-12, TNF-α, GM-CSF, and chemokines, such as CCL2, CCL3, CCL5, and CXCL10, leading to the transformation of immunologically “cold” tumors into “hot” tumors.71 First, locally secreted chemokines, such as CCL3 and CXCL10, recruit the first cell responders, such as neutrophils and macrophages, to the site of infection,84 and these cytokines are involved in the induction of effective antitumor responses.85 The aggregation of PAMPs with virus-recognizing receptors on NK cells results in the early influx of NK cells. Activated cytotoxic NK cells might kill virus-infected cells by releasing cytolytic components and triggering FAS-FASL signaling.86 In addition, activated NK cells express IFN-γ and TNF-α to further contribute to the activation of macrophages, DCs, and T cells.71 This NK cell and DC activation further stimulates the production of IFNs, TNF-α, IL-12, IL-6, and chemokines that act in an autocrine and paracrine fashion to amplify the initial innate response.71,87,88
OVs prime antitumor adaptive immunity
The mainstay of adaptive immunity against tumor cells during OV infection is the tumor-specific T-cell response. Successful activation of antigen-specific T-cell responses requires three signals from APCs: antigens presented in the context of an MHC molecule, costimulation, and cytokines. OV-mediated oncolysis of tumor cells initiates the release of tumor-associated antigens and neoantigens (TAAs and TANs, respectively), which are processed by APCs to produce antigen epitopes ultimately presented on the APC surface in complex with MHC molecules. In the cytokine milieu produced after immune and tumor cell exposure to OVs, type I IFNs enhance the expression of MHC class I and II molecules and costimulatory molecules, such as CD40, CD80, and CD86 on the surface of DCs.89 Many reports have documented the ability of OVs to induce the activation of MHC class I pathway-related molecules90,91 and costimulatory molecules.92,93 Notably, multiple cytokines and chemokines produced by OV-infected cells or mature APCs contribute to the recruitment and reactivation of T cells. Once activated, these antitumor CD8+ T cells and B cells cause tumor regression and can clear either newly grafted tumors or distant tumors in an OV-independent manner.94,95 Therefore, it is being increasingly acknowledged that OVs, including HSV-1,96 oncolytic VV, (OVV)97 vesicular stomatitis virus (VSV),98 MeV,99 and OAd,100 mainly generate specific and efficacious T-cell immunity to protect against tumors in an antigen-specific manner.
Effects of OVs on the tumor ECM and vasculature
The ECM, a noncellular compartment, is generated by activated CAFs and comprises up to 60% of a solid tumor mass.101 The excessive accumulation of collagenous matrix, proteoglycans, and hyaluronan leads to an impermeable and rigid ECM, forming a shield surrounding tumor cells.102 These physical barriers make it difficult for OVs to effectively reach the whole tumor mass.
Ilkow et al. demonstrated that VSV-based therapeutics were enhanced via crosstalk between CAFs and cancer cells.103 In contrast, transforming growth factor-beta 1 (TGF-β1) secreted by tumor cells was involved in promoting OV infection of CAFs. In addition, high levels of fibroblast growth factor 2 (FGF2) produced by tumor cells rendered the cells sensitive to viral infection.103 Moreover, in addition to killing tumor cells, OAd targeted both glioblastoma cells and glioblastoma‑associated stromal FAP+ cells.104
OVs have been reported to affect tumor vasculature by infecting and lysing vascular endothelial cells (VECs). Vascular endothelial growth factor (VEGF) suppresses the intrinsic antiviral response and sensitizes tumor vasculature to VV infection by signaling mediated through Erk1/2 and Stat3 and upregulating PRD1-BF1/Blimp1 expression in the tumor vasculature.105 Three-dimensional imaging of infected tumors in a murine colon cancer model revealed that VSV replicated in the tumor neovasculature and spread within the tumor mass.106 Engineered OVVs were shown to selectively target and disrupt established tumor vasculature, resulting in the destruction of systemic tumors in humans.107
Strategies to enhance OVs’ selective activity
Highly lytic viruses efficiently lyse tumor cells.108 There are numerous ways to improve the selective activity of OVs. Early OVs showed a degree of intrinsic oncolytic selectivity that was associated with different gene and protein expression profiles of tumor cells. However, based on the lack of higher specificity, many methods have been used largely to further improve the direct tumor specificity of OVs.109 Virulence gene deletion or viral factor modulation is used mainly to maintain OV proliferation and downregulate proapoptotic pathways. T-VEC, a modified HSV-1, was genetically altered through deletion of two nonessential viral genes. Functional deletion of ICP34.5 and the ICP47 gene attenuated viral pathogenicity, enhanced tumor-specific cell lysis in a broad range of human tumors and blocked antigen presentation in HSV-infected cells.110,111 The approved OAd oncorine was generated by deletion of E1B-55 kDa which binds to the tumor suppressor p53 in normal cells and causes cell cycle progression and viral replication.112 Therefore, oncorine does not generally replicate in normal cells but selectively replicates in p53-deficient tumors.113 Similarly, to generate oncolytic poxviruses, the viral TK gene is deleted, which increases the selectivity of the virus for rapidly dividing cancerous cells.114
Tumor-specific promoters have also been used for the specific delivery of essential genes that induce virus proliferation, particularly OAd proliferation. E1A is an essential gene in adenoviral replication and the first gene expressed upon oncolytic adenoviral infection.115 Many tumor-specific promoters that have been utilized to drive E1A expression are strategically applied to improve the specific antitumor activity of OAd, including the human telomerase reverse transcriptase promoter (hTERT), hypoxia-responsive promoter (HRE), prostate-specific antigen promoter (PSA), alpha-fetoprotein promoter (AFP), alpha-lactalbumin promoter (ALA) and mucin1 promoter (DF3/MUC1).116,117
Based on mammalian synthetic biology, gene circuits have been creatively engineered to integrate tumor-specific promoters and microRNA (miRNA) inputs for the identification of specific cancer cells.118 Huang et al. engineered an innovative sensory switch circuit consisting of a Gal4VP16 activator gene driven by the AFP promoter and two mutually inhibiting repressor genes controlled by miR-142, miR-199a-3p, and miR-142.119 In this circuit setup, a high E1A level can be specifically achieved to trigger adenoviral replication in tumor cells.119
miRNAs are short small endogenous noncoding RNAs that serve as posttranscriptional regulators of gene expression by interfering with the translation of target mRNAs.120 It is now generally accepted that miRNAs are involved in multiple physiological and pathological processes. Dysregulation of miRNAs contributes to tumor progression, invasion, angiogenesis and metastasis in many types of cancers.121 miRNAs have been categorized into two classes according to their altered expression in tumor cells: oncogenic miRNA upregulation promotes tumorigenesis by blocking the translation of tumor suppressor protein mRNAs, and tumor suppressor miRNA downregulation generally suppresses the translation of oncoprotein mRNAs.122 Hence, elevating tumor suppressor miRNA levels or inhibiting oncogenic miRNA expression is a promising potential therapeutic approach. OV vectors effectively deliver tumor suppressive interfering pre-miRNAs into tumor cells. Specifically, interfering pre-miRNAs are free in the cytoplasm and are cleaved to form mature miRNAs, leading to the inactivation of target mRNAs. OAd carrying the tumor suppressor miRNA-143 (miR-143) induced apoptosis, decreased the expression level of KRAS and reduced tumor growth in HCT116 xenograft cells.123 The same antitumor effect of miR-143 was observed in osteosarcoma cells when oncolytic VSV was the carrier.124 To enhance oncolytic specificity, Jia et al. inserted miR-34a targets in both the 5′ untranslated region (UTR) and 3′UTR of the virus to obtain double-miR-34a targeting oncolytic coxsackievirus B3, and this engineered virus maintained nearly full oncolytic activity but showed reduced toxicity.125 Similarly, oncogenic miRNAs can be used to improve oncolytic safety and specificity. The UL9 protein is required for HSV replication, but a dominant-negative mutant inhibits HSV replication by blocking the Ori-binding sites in UL9.126 miR-21, an oncogenic miRNA, is nearly universally upregulated in cancer cells. Marzulli et al. engineered miR-21-binding sites in the 3′UTR of the dominant-negative UL9 gene to enable pre-existing oncogenic miR-21 contact with miR-21-binding sites to restart HSV replication.126 In addition, it is thought that cellular miRNAs play important roles via proviral or antiviral effects exerted during the viral life cycle in mammals.127 Therefore, the delivery of antiviral miRNAs or the inhibition of proviral miRNA function by OVs is a promising strategy for enhancing oncolytic specificity in tumor cells. miR-222 is a limiting factor for viral propagation, and OAd was engineered with miR-222-binding sites to inhibit high miR-222 expression, leading to cancer cell sensitization to oncolysis.128
Although the antiviral immune response has recently been viewed as beneficial in priming antitumor immunity by OVs, antiviral immunity is still considered a hurdle to OV proliferation. Targeting the central mediator of antiviral responses was used to overcome the antiviral response to allow OV proliferation and enhance transgene persistence. Low expression of STAT1, a target gene of IFN signaling of antiviral responses, and its target genes sensitizes melanoma cells to the oncolytic virus EHDV-TAU.129 Mutations in the IFNγ–JAK–STAT pathway simultaneously render melanomas susceptible to OV therapy.130 CCDC6 has an antiviral influence against the oncolytic alphavirus M1 by regulating IFN-stimulated genes; the epigenetic silencing of CCDC6 sensitizes orthotopic bladder tumors to M1 virus.131 Similarly, T-VEC induces ICD in vitro and promotes tumor immunity in low STING-expressing melanoma.132 However, Froechlich et al. observed that oncolytic viral replication and cytotoxicity were improved in STING-deficient tumor cells, where oncolytic viruses showed impaired immunogenicity.133 Therefore, there is a need to demonstrate the role of antiviral immunity in OV proliferation and the priming of antitumor immunity and propose more strategies to achieve balance, obtaining the maximum effect of OVs.
Strategies for using armed OVs
OVs armed with costimulatory molecules enhance APC function
Costimulatory molecules are necessary for the full activation of T cells. In the TME, immunity is suppressed by the lack of costimulatory molecules on the surface of cancer cells. Thus, targeting costimulatory pathways to enhance antitumor immunity seems to be an attractive approach.134 Scientists have encoded OVs to express T-cell costimulatory molecules (such as OX40, CD40, intercellular adhesion molecule-1 (ICAM-1), B7-1, lymphocyte function-associated antigen 3 (LFA3), glucocorticoid-induced tumor necrosis family receptor family-related gene (GITR) or 4-1BB) to enhance the antitumor effects of OVs.135–144 The latest evidence showed that VALO-D102, a novel AdV encoding CD40L and OX40L, improved tumor growth control and induced robust infiltration of tumor-specific CD8+ effector T cells in two mouse models of melanoma. When combined with an anti-PD-1 antibody, VALO-D102 significantly improved tumor suppression compared with either monotherapy alone.144 Another OAd, LOAd 703, armed with CD40L and 4-1BBL, promoted the activation of cytotoxic T cells and limited tumor growth in a multiple myeloma xenograft model.143 Recent evidence has demonstrated that LOAd 703 can enhance the immunogenic profile by upregulating the costimulatory molecules CD80, CD86, and CD70, MHC molecules, the death receptor Fas and the adhesion molecule ICAM-1.145 Currently, two AdVs engineered to express anti-CD40 antibodies or OX40 ligands are being investigated in the clinic: NG-350A and DNX-2440. NG-350A is an OAd vector that expresses a full-length agonist anti-CD40 antibody at the site of viral replication, and DNX-2440 is a replication-competent OAd expressing human OX40 ligand (Table 1).
Table 1.
Ongoing or completed clinical trials with OVs encoding immunostimulatory transgenes
| Virus | Name (Institution) | Transgenes | Tumor type | Reference/identifier | Phase/status |
|---|---|---|---|---|---|
| CG0070 (CG Oncology) | GM-CSF | Bladder cancer | NCT02365818 | Phase II Completed | |
| Nonmuscular invasive bladder cancer | NCT04452591 | Phase III Recruiting | |||
| TILT-123 (TILT Biotherapeutics) | TNF-α and IL-2 | Solid tumor | NCT04695327 | Phase I Recruiting | |
| Metastatic melanoma | NCT04217473 | Phase I Recruiting | |||
| Adenovirus | NG-641 (PsiOxus Therapeutics) | Anti-FAP-TAc antibody CXCL9/CXCL10/IFNα |
Metastatic cancer Epithelial tumor |
NCT04053283 | Phase I Recruiting |
| ONCOS-102 (Targovax) | GM-CSF | Malignant solid tumor | NCT01598129 | Phase I Completed | |
| NG-350A (PsiOxus Therapeutics) | Anti-CD40 antibody |
Metastatic cancer Epithelial tumor |
NCT03852511 | Phase I Recruiting | |
| DNX-2440 (DNAtrix) | OX40 ligand |
Liver metastases Liver metastasis of Colon cancer Colorectal cancer Breast cancer Gastric cancer Periampullary cancer Melanoma Renal cell cancer Sarcoma Squamous cell carcinoma Gastrointestinal stromal tumors |
NCT04714983 | Phase I Recruiting | |
| Glioblastoma | NCT03714334 | Phase I Recruiting | |||
| OH2 (Wuhan Binhui Biotechnology) | GM-CSF |
Solid tumor Gastrointestinal cancer |
NCT03866525 | Phase I/II Recruiting | |
| Pancreatic cancer | NCT04637698 | Phase I/II Recruiting | |||
| Talimogene laherparepvec (Amgen) | GM-CSF | Peritoneal surface malignancies | NCT03663712 | Phase I Recruiting | |
| Kaposi sarcoma | NCT04065152 | Phase II Recruiting | |||
| Melanoma | NCT04427306 | Phase II Recruiting | |||
| HSV | VG161 (CNBG-Virogin Biotech) | IL12/15/PDL1B | Advanced malignant solid tumor | NCT04758897 | Phase I Recruiting |
| Primary liver cancer | NCT04806464 | Phase I Recruiting | |||
| M032 (University of Alabama at Birmingham) | IL12 |
Recurrent glioblastoma Multiforme progressive glioblastoma Multiforme anaplastic astrocytoma or gliosarcoma |
NCT02062827 | Phase I Recruiting | |
| VV | JX-594 (Jennerex Biotherapeutics Green Cross Corporation) | GM-CSF | Liver cancer | NCT00629759 | Phase I Completed |
|
Melanoma Lung cancer Renal cell carcinoma Squamous cell Carcinoma of the head and neck |
NCT00625456 | Phase I Completed | |||
|
Hepatocellular carcinoma liver cancer (HCC) |
NCT01387555 | Phase II Completed | |||
|
Neuroblastoma Rhabdomyosarcoma Lymphoma Wilm’s tumor Ewing’s sarcoma |
NCT01169584 | Phase I Completed | |||
| Melanoma | NCT00429312 | Phase I/II Completed | |||
| ASP9801 (Astellas Pharma) | IL-7 IL-12 |
Metastatic cancer Solid tumors Advanced cancer |
NCT03954067 | Phase I Recruiting | |
| RGV004 (Second Affiliated Hospital, School of Medicine, Zhejiang University) | Anti-CD19/anti-CD3 bispecific antibody | Relapsed or refractory B-cell lymphoma | NCT04887025 | Phase I Not, yet recruiting | |
| VSV | Given IV (Mayo Clinic) | IFN-β NIS |
Relapsed or refractory multiple myeloma Acute myeloid leukemia T-cell lymphoma |
NCT03017820 | Phase I Recruiting |
OVs armed with chemokines recruit antitumor lymphocytes
Chemokines are small secreted proteins that can mediate the migration and positioning of immune cells within various tissues and are involved in the induction and effector phases of immune responses against infections and tumors.146,147 Increasing evidence suggests that chemokines play important roles in the TME because of their ability to attract immune cells to tumor lesion sites. Because of this ability, OVs have been armed with chemokines to enhance their antitumor efficacy, especially for turning “cold” tumors into “hot” tumors.68 The chemotactic cytokine CCL5 (also known as RANTES), which binds to the receptors CCR1, CCR3, and CCR5 residing on several types of immune cells, including CTLs148 and NK cells,149 can direct the infiltration of T cells and recruit NK cells via CCR5.149 CCL5-armed OVV (vvCCL5)-induced chemotaxis of lymphocyte populations, exerted a great tumor suppressive effect and showed increased levels of TILs when used to simultaneously vaccinate its receptor type-1-polarized dendritic cells.148 CCL5-expressing OVV (OV-ffLuc-CCL5) enhanced NK cell accumulation within tumors in vivo.150 Additionally, another OAd, Ad-RANTES-E1A, expressed CCL5 in tumors and induced tumor-specific cellular immunity by recruiting myeloid DCs and macrophages to tumor sites.151–153 Liu et al. armed an OVV (vvDD) with CXCL11 and found that vvDD-CXCL11 significantly increased CXCL11 protein levels within tumors and recruited CD8+ T cells and, to a lesser extent, NK cells to the TME to trigger a systemic antitumor immune response.154 Moon et al. also proved that vvDD-CXCL11 was successful in recruiting T cells and augmenting antitumor efficacy.155
OVs armed with cytokines improve antitumor lymphocyte function
Cytokines are soluble proteins that mediate cell-to-cell communication and regulate homeostasis of the immune system.156,157 In the TME, cytokines can suppress tumor cell growth through anti-proliferative and proapoptotic activity or recognition by cytotoxic effector cells.158 They play very important roles in cancer treatment. Numerous cytokines, including GM-CSF, IL-2, IL-7, IL-12, IL-15, IL-18, IL-21, IL-24, IFN-α, IFN-β, and IFN-γ, can modulate the antitumoral response and have shown antitumor properties in clinical trials and preclinical studies. There are thousands of clinical trials registered with ClinicalTrials.gov that completed recruitment through January 2021 with patients to be treated with cytokines. Among these cytokines, G-CSF, GM-CSF, VEGF, IL-2 and IFN-γ have been the most extensively studied.159 However, cytokines generally have short half-lives and act over short distances, limiting their widespread adoption in treatment regimens. Therefore, many OVs have been engineered to express immunostimulatory cytokines in an effort to enhance the antineoplastic immune response.160
GM-SCF
GM-CSF is produced by a variety of cell types, including activated T cells, macrophages, ECs, fibroblasts and cancer cells. It is a potent cytokine that promotes the development and maturation of DCs and the proliferation and activation of T cells, which enhance antitumor immune responses in cancer therapy.161,162 GM-CSF is one of the most frequently adopted cytokines for arming OVs. T-VEC encoding GM-CSF was the first OV approved by the US FDA for the treatment of melanoma in October 2015.20 Injection with T-VEC induces local and systemic antigen-specific T-cell responses and decreases the number of Tregs, suppressor T cells (Ts), and MDSCs in injected lesions, ultimately leading to an improved durable response rate (DRR) and a long-lasting complete response (CR).111,163 Despite T-VEC, GM-CSF is widely used for other types of OVs (HSV,110 VV,164 VSV,165 MV,166 AdV,167,168 and RV169) to enhance its antitumor efficacy. OH2 is derived from wild-type HSV-2 strain HG52, created with the deletion of the ICP34.5 neurovirulence gene and ICP47 gene and expressing the gene encoding human GM-CSF to enhance antitumor immunity.170 A single OH2 injection altered the TME with an increase in CD3+ and CD8+ T cell density and PD-1 expression in patients with metastatic esophageal and rectal cancer.171 On August 20, 2021, the US FDA approved OH2 for use in US clinical trials enrolling people with a variety of solid tumors. Studies have proven that JX-594 administered through intravenous infusion continuously spreads infection within tumors but does not harm normal tissues.172–174 In phase I/II clinical trials, JX-594 was shown to be well tolerated after intravenous infusion and to induce no dose-limiting toxicities; the maximum tolerated dose was not reached.172,173,175 However, JX-594 in combination with sorafenib failed to show a survival benefit in a phase III trial in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy (NCT02562755). There are still many issues for JX-594 application to be solved, such as in combination with other immunotherapies.
Interleukin
Interleukins constitute a class of small-molecule proteins that mediate communication between immune cells and tissue cells, playing important roles in the development and progression of cancers.176 Some interleukins can promote tumor growth and metastatic spread (e.g., IL-4, IL-6, and IL-10),177–179 while others regulate immunosurveillance and thus tumor control (e.g., IL-2, IL-7 IL-12, IL-15, IL-18, IL-21, IL-23, and IL-24).180–183 Therefore, many OVs have been engineered to carry antitumoral ILs.
IL-2 is mainly produced by CD4+ T cells and is secreted to a lesser degree by CD8+ T cells, B cells, DCs and other innate immune cells.184,185 IL-2 can activate both innate and adaptive immunity mainly through effector and regulatory T lymphocytes. IL-2 has been shown to be effective in cancer therapy.186 However, the half-life of IL-2 is short (10–85 min in serum),187 and therefore, it must be repeatedly administered in short intervals to maintain efficient bioavailability, which limits its clinical use. Recently, scientists have constructed OVs coding IL-2 to ensure that IL-2 can be locally expressed in tumors and to thus enhance OV antitumor activity. Liu et al. demonstrated that IL-2 expressed by OVV was used to treat a variety of murine tumor models and showed no systemic toxicity, and this treatment created an optimal immune microenvironment. Moreover, when combined with an anti-PD-1/PD-L1 antibody, this viral therapy cured most late-stage tumors in mice.188 Despite this outcome, OVVs armed with both IL-2 and TNF-α showed even greater effective antitumor efficacy without treatment-related signs of systemic toxicity. This combination treatment enhanced adoptive cell therapy by diminishing the immunosuppressive characteristics of the TME.189 IL-2 can be encoded by NDV190–192 and HSV,193 and it has shown antitumor efficacy against the TME and in the spleen of a late-stage tumor model, as determined by the percentages of activated CD4+Foxp3− and CD8+IFN-γ+ T cells.
IL-12 is known to promote the development of T cells and NK cells and the production of IFN-γ and the TH1 response.194 All these responses benefit cancer therapy, but in clinical trials, the antitumor efficacy was unsatisfactory.195,196 This outcome may have been a result of insufficient IL-12 delivery to the TME or exhaustion of lymphocytes (including T cells, NK cells, TAMs, and/or MDSCs) in the TME. Scientists have used several kinds of OVs armed with IL-12 to solve this problem in many preclinical studies and clinical studies (reviewed by Nguyen et al. 197), among which the most commonly used OVs are Ad and HSV. For example, AdV encoding IL-12 (Ad-IL-12) has shown promise as a treatment for cancers (including prostate adenocarcinoma,198,199 breast carcinomas,200, pancreatic cancer,201,202 melanoma,203 gliomas204 and colorectal carcinomas205). In animal tumor models, Ad-IL-12 showed significant antitumor efficacy and prolonged the survival of the animals. The antitumor immune response was mainly mediated by CD8+ T cells. Some treated model animals rejected a subsequent rechallenge with the same tumor cells, demonstrating the induction of antitumor immune memory.195 In clinical studies, Ad-IL-12 was well tolerated by 21 patients with advanced digestive tumors, and it did not show dose-limiting toxicity.
HSVs armed with IL-12 have also been widely used.206 Oncolytic HSV encoding IL-12, oHSV-IL-12, exhibited significant antitumor activity against hepatic tumors and was more effective in rejecting tumor rechallenge. This antitumor efficacy was associated with marked IL-12 and IFN-γ expression, which induced an increase in the number of CD4+ and CD8+ lymphocytes in the TME.207 oHSV-IL-12 elicited local and systemic immune responses, completely preventing the growth of distant untreated lung tumors in mice.208 Scientists have also tested the antitumor efficacy of oHSV-IL-12 in ovarian carcinomas,209 glioblastoma,210–212 neuroblastoma,213,214 colorectal cancer215 and prostate cancer.216 oHSV-IL-12 showed enhanced antitumor efficacy that is mainly mediated by T-cell immune responses. IL-12 was also engineered to be expressed by oncolytic MeV (MeVac FmIL-12), which led to complete remission in 90% of MC38 tumor models.216 Enhanced therapeutic efficacy was realized by activation of the systemic antitumor immune response through increased expression of inflammatory cytokines (IFN-γ, TNF-α, and IL-6).217
Other viruses, such as VSV,218 VV,219,220 NDV,221,222 and Maraba virus223 armed with IL-12, have been used for the rapid improvement in OV antitumor efficacy. To avoid potential systemic toxicity, a number of IL-12 modifications have been explored.224 Recently, a double-deleted mutant oncolytic vaccinia virus (vvDD) genetically engineered a membrane-bound IL-12 (vvDD-IL-12-FG) that delivered IL-12 to the tumor bed and tethered IL-12 to cell membranes. vvDD-IL-12-FG inhibited tumor growth and promoted survival without inducing toxic side effects.225 The same team also engineered secreted or membrane-bound IL-23, a cytokine in the IL-12 cytokine family, into vvDD to elicit potent antitumor effects by modulating the TME.226
IL-15, mainly produced by activated monocytes and macrophages,227 primarily promotes the proliferation, activation and cytotoxic functions of CD8+ T cells and NK cells.228 Studies have reported that IL-15 expressed in the TME may lead to rejection of large tumors by enabling T cells.229 Multiple OVs were genetically engineered with IL-15 and have shown promising immunostimulatory and antitumor efficacy.115,230–232 IL-15Rα is the IL-15-specific receptor with high affinity. To further enhance IL-15 activity, Kowalsky et al. recently engineered oncolytic VV to express a superagoinst IL-15 (a fusion protein of IL-15 and IL-15Rα) and named it vvDD-IL15-Rα.232 As a result, vvDD-IL15-Rα induced strong antitumor activity and prolonged the survival time of tumor-bearing mice. More interestingly, IL-15 promoted the expression of the PD1/PD-L1 axis, which further resulted in a great improvement in the therapeutic outcome via the combination of vvDD-IL15-Rα with PD-1 blockade.
Despite the above common interleukin, a number of other interleukins are armed into OVs to improve antitumor activity, such as IL-7,220 IL-36γ, 233 IL-21,234 IL-24,235 and IL-18.236 The promising results will advance the clinical applications of IL-armed OVs for tumor treatment as research proceeds.
Interferons
IFNs, including type I IFNs (IFNα and IFNβ) and type II IFN (IFNγ), comprise a family of cytokines that are recognized as crucial molecules that interfere with viral replication. However, numerous studies have demonstrated that IFNs also play important roles in protecting a host against tumor development through their direct effects on target cells and by activating immune responses.237 IFNγ exerts indirect effects on tumor cells via the TME and modulation of the immune response,238 and type I IFNs exert direct effects (on cancer cells) and indirect effects (through immune effector cells and vasculature) on tumors.239 However, their systemic toxicities and short half-life following administration limit their overall bioavailability.240
An engineered OV generated from VSV encoding IFNγ demonstrated greater activation of DCs and induced greater secretion of proinflammatory cytokines than the parental virus and showed pronounced antitumor effects in several murine tumor models.241 OAd armed with IFNγ (CNHK300-hIFN-γ) showed antitumor effects through triplex mechanisms, including selective oncolysis, antiangiogenic effects, and immune responses.242
Type I IFNs have been frequently inserted into OVs to improve their antitumor efficiency. An IFNα-expressing OAd (RGD-ΔE3-ADP-ham-IFN) showed great therapeutic potential for the treatment of pancreatic cancer in a syngeneic Syrian hamster model.243 More recently, an IFNα-expressing OAd (5/3 Cox2 ΔE3 ADP IFN) showed significant tumor growth suppression in an esophageal adenocarcinoma (EAC) xenograft model.244 Similarly, multiple types of OVs can be engineered to overexpress IFNβ to improve anticancer efficacy, including VSV,245–248 AdV,249,250 MeV,251 VV,252 Sendai virus (SeV),253 and NDV.254 Despite the effective therapeutic effect of OV-encoding IFN, the potential toxicity should attract attention. Recently, the safety and efficacy of VSV-IFN-NIS, an oncolytic VSV incorporating IFN beta and sodium iodine symporter transgenes, was tested in a phase I clinical trial.255 Although a single high-dose intravenous VSV-IFNβ-NIS treatment is safe in heavily pretreated patients with hematologic malignancies, patients still experienced drug adverse events (AEs). A total of 73% (11/15) of patients experienced hematological AEs, particularly lymphopenia (grade 3–4). Nonhematologic AEs of interest were grade 1 (6.7%) and 2 (46.6%) cytokine release syndromes, of which 1 patient required transient norepinephrine support. More strategies and concerns should be provided to achieve the optimal therapeutic effect in patients with OVs or in combination with other immunotherapies but not trigger toxicity (e.g., cytokine storm).
OVs armed with antigens as cancer vaccines
OV-infected tumor cells resulting from various forms of ICD have been described previously. Their released PAMPs and DAMPs activate innate immunity in the TME, serving as important drivers of tumor cell adjuvanticity.256,257 Available TAA and TAN targets derived from OV-infected tumor cells prime antitumor adaptive immunity, which makes antigenicity the other critical advantage of OVs.257 Consequently, an oncolytic virus could act as an effective tumor in situ vaccine. An engineered oncolytic herpes virus (OVH) initiates TAA-specific immune responses induced by ICD, which leads to systemic tumor regression in an antigen-targeting therapeutic antibody-dependent manner.94 In situ therapeutic cancer vaccination with membrane-tethered IL-2-armed OV (vvDD-mIL2) plus a TLR 9 ligand (CpG) yielded systemic immunization.258 Moreover, OVs can also be further armed with tumor antigens to enhance the antitumor immune response. Indeed, in early explorations of this strategy, OV-expressing TAAs (e.g., HPV-16 E7 antigen) were directly used as a vaccine vector to generate an antitumor immune response against TAAs.259 Further advancement of this strategy was made through arming OVs to coexpress TAAs and immunomodulatory molecules (e.g., OAd encoding SA-4-1BBL and HPV-16 E7 Antigen260), enhancing systemic antitumor immunity. More promising of this approach, investigators have created the heterologous prime-boost cancer vaccination to further expand tumor antigen-specific T cells.261 PROSTVAC is a viral but non-OV vector–base cancer vaccine using a prime with vaccinia (PROSTVAC-V) followed by a boost with fowlpox (PROSTVAC-F), each with insertions of four human genes: PSA and three costimulatory molecules LFA-3, B7.1 and ICAM-1.139 In a phase II study, PROSTVAC prolonged median overall survival versus placebo in metastatic castration-resistant prostate cancer.262 However, PROSTVAC had no effect on median overall survival in metastatic castration-resistant prostate cancer in the phase III study (NCT01322490). The sponsor considered that a few possibilities may account for the findings, including a false-positive signal and/or an imbalance in prognostic factors in phase II and sufficient immune responses or other negative regulatory influences in the TME in phase III.263 Then, the sponsor tried the combination therapy in a clinical trial. Bridle et al. applied a replication-incompetent adenovirus vector expressing TAAs (e.g., human dopachrome tautomerase (DCT)) to prime and an oncolytic replication-competent rhabdovirus encoding the same TAA as the boosting vaccine. The prime-boost regimens provided outstanding DCT-specific systemic CD4+ and CD8+ T cell responses,261 which were further enhanced by using cyclophosphamide preconditioning.264 A recent preclinical study also applied oncolytic Maraba MG1 rhabdovirus encoding MAGE-A3 as a boosting vaccine in primates, and the prime-boost regimen induced an expanded and persistent MAGE-A3-specific CD4+ and CD8+ T cells. These promising results in preclinical experiments resulted in multiple clinical studies for the treatment of HPV-associated cancers (NCT03618953) and MAGE-A3-positive solid malignancies (NCT02285816, NCT02879760).
OVs armed with ICIs eliminate immune suppression
Tumor-specific T-cell priming and activation are involved in antigen-specific signaling through TCRs and coactivating signals mediated by cosignaling receptors and costimulatory ligands, but these signaling pathways are disrupted by coinhibitory signaling induced by T cells. Checkpoint receptors such as CTLA4, PD-1, TIGIT, TIM-3, BTLA and CD160.265 reside on the T-cell surface. The physical interaction between these checkpoint receptors and their ligands expressed on tumors, APCs and stromal cells leads to coinhibitory signaling, which causes cytotoxic T-cell exhaustion in the tumor environment.266 ICI blockade of coinhibitory signaling reverses the exhaustion of CTLs, resulting in the death of tumor cells via restored T-cell functions. Multiple ICIs targeting CTLA4 and PD-1 or PD-L1 have been approved for use in cancer therapy due to their promising long-lasting therapeutic efficacies in many types of cancer.267 In addition, ICIs targeting TIGIT,268,269 TIM-3270 and BTLA271 have also demonstrated unprecedented preclinical results and are in clinical development. Scientists have reported that these ICIs cause many immune-related AEs, such as pneumonitis, colitis, and autoimmune diseases.272 ICIs can also mediate cardiotoxic effects, which are serious complications that can lead to high mortality.273,274 The price of these drugs is very high for patients and health-care systems. Engineering OVs that encode ICIs may be a potential solution to these problems.
A novel recombinant myxoma virus (MYXV) can induce the secretion of the soluble form of PD1 from infected cells. It has been shown to induce and maintain CD8+ T cell responses intratumorally. Compared with combination therapy with unmodified myxoma and systemic αPD1 antibodies, MYXV was safer and more effective in a melanoma model.275 OAd overexpressing the soluble fusion protein PD-1/CD137L, containing the extracellular domains of PD-1 and CD137L at each terminus, induced tumor-specific and systemic protection against tumors.276 MeV-encoding antibodies against CTLA-4 and PD-L1 (MV-αCTLA-4 and MV-αPD-L1) showed high rates of complete tumor remission (>80%) in melanoma xenografts compared with parental MeV.277 Kleinpeter et al. demonstrated that OVV-encoded anti-PD1 antibodies (including whole antibodies (mAbs), antigen-binding fragments (Fabs) or single-chain variable fragments (ScFvs)) induced better therapeutic control of tumor growth than either OV or anti-PD1 therapy alone.278 In murine models, anti-PD-1 mAb-armed oncolytic HSV showed an enhanced antitumor response, similar to that of unloaded virus combined with anti-PD-1 antibodies, which was superior to that of unloaded virus or anti-PD-1 therapy alone.279 T3011 is a genetically modified oncolytic HSV-1 encoding IL-12 and an anti-PD-1 antibody. Locally produced IL-12 induced the synthesis of IFN-γ, enhancing the cytolytic activity of NK cells and CTLs. The anti-PD-1 antibody blocked checkpoint inhibition of T effector cells. The most recent phase I clinical trial reported that T3011 was well tolerated in patients with advanced cutaneous or subcutaneous malignancies.280 In another study, a novel oncolytic VV encoding a full mAb against TIGIT showed improved antitumor efficacy and induced long-term tumor-specific immunological memory.281 Recently, Lei et al. engineered influenza A virus to express CTLA4-specific scFv to suppress the growth of treated tumors and increase the overall survival of mice.282
OVs combined with immunotherapies
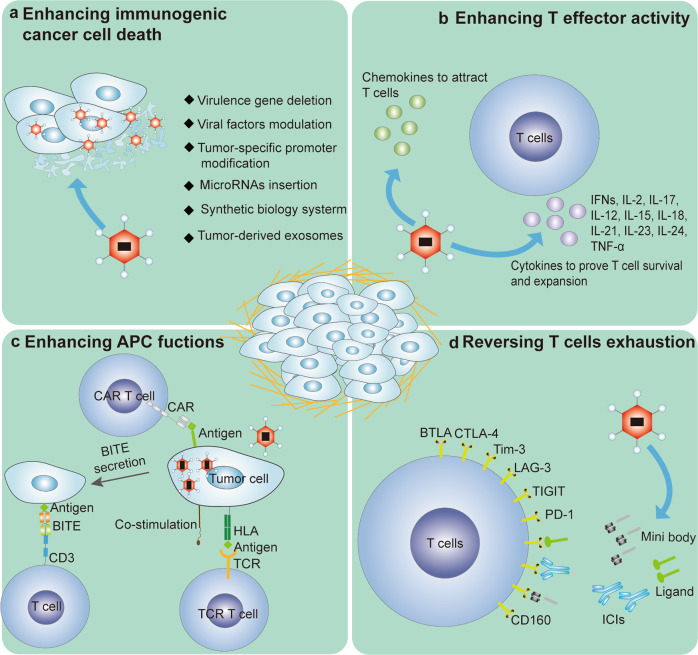
Unarmed or armed OVs as single agents have demonstrated excellent safety and promising therapeutic effects in tumor treatment. However, monotherapies are unlikely to completely overcome the loss of T-cell function caused by tumor heterogeneity and an immunosuppressive microenvironment. Promising OVs genetically modified with other antitumor agents have achieved tumor eradication in several clinical studies. Recently, the combination of armed OVs with ICIs and adoptive T-cell therapy (ACT) achieved extremely high efficacy by activating multiple antitumor steps, including increasing T-cell trafficking to tumors, supporting T-cell survival and expansion, enhancing APC function and reversing T-cell exhaustion (Fig. 3 and Table 2).
Fig. 3.
Armed oncolytic virus (OV) enhances antitumor activity. a There are numerous means to prove the lytic activity of OVs, some of which might be more immunogenic and prime antiviral adaptive immune responses. b The administration of OV-expressing chemokines promotes the secretion of chemokines into the tumor microenvironment (TME), which increases T-cell trafficking to tumors. The secretion of cytokines induced by OVs maintains T-cell survival and expansion. c Armed OVs can provide local antigen targets for chimeric antigen receptor T-cell therapy (CAR T) cells or human leukocyte antigen (HLA)/costimulation molecules directed to T-cell receptor (TCR)-T cells. Furthermore, OVs expressing bispecific T-cell engagers (BiTEs) are capable of overcoming antigen heterogeneity and inducing tumor cell death. d Immune checkpoint inhibitors (ICIs) or mini bodies and immunosuppressive ligands locally delivered by armed OVs reverse T-cell exhaustion
Table 2.
Ongoing or completed preclinical/clinical studies with OVs and ICIs or ACT therapies
| Immunotherapy type | Oncolytic virus | Transgenes | Combination agent/target | Tumor type | Reference/identifier | Phase/status |
|---|---|---|---|---|---|---|
| Adenovirus (CG0070) | GM-CSF | Pembrolizumab | Nonmuscle invasive bladder cancer | NCT04387461 | Phase II Recruiting | |
| Adenovirus (DNX2401) | None | Pembrolizumab | Glioblastoma and gliosarcoma | NCT02798406 | Phase II Completed | |
| Adenovirus (ONCOS-102) | GM-CSF | Pembrolizumab | Unresectable melanoma | NCT03003676 | Phase I Pilot study Completed | |
| Adenovirus (Telomelysin) | None | Pembrolizumab | Head and beck squamous cell carcinoma (HNSCC) | NCT04685499 | Phase II Recruiting | |
| Vaccinia Virus (BT-001) | CTLA4 Antibody and GM-CSF | Pembrolizumab | Metastatic/advanced solid tumors | NCT04725331 | Phase I/II Recruiting | |
| Vaccinia Virus (TBio-6517) | None | Pembrolizumab | Triple negative breast cancer; Microsatellite instability in colorectal cancer | NCT04301011 | Phase I/IIa Recruiting | |
| Herpes Simplex Virus Type 1 (IMLYGIC) | GM-CSF | Pembrolizumab | Stage IIIB-IVM1d melanoma | NCT04068181 | Phase II Active, Not Recruiting | |
| Herpes Simplex Virus Type 1 (IMLYGIC) | GM-CSF | Pembrolizumab + placebo | Unresectable stage IIIB–IVM1c melanoma (MEL) | NCT02263508 | Phase Ib/III Terminated | |
| Herpes Simplex Virus (ONCR-177) | None | Pembrolizumab | Melanoma; HNSCC; Breast cancer; Triple-negative breast cancer; Colorectal carcinoma; Nonmelanoma skin cancer | NCT04348916 | Phase I Recruiting | |
| Herpes Simplex Virus Type 2 (OH2) | None | Pembrolizumab | Melanoma | NCT04386967 | Phase I Recruiting | |
| Maraba Virus (MG1-MAGEA3) + Ad MAGEA3) | MAGE-A3 | Pembrolizumab | Non-small-cell lung carcinoma (NSCLC) | NCT02879760 | Phase I/II Completed | |
| Immune checkpoint inhibitors | OVV-01 | None | Pembrolizumab or atezolizumab | Advanced solid tumors | NCT04787003 | Phase I Recruiting |
| Reovirus (REOLYSIN) | None | Pembrolizumab | Pancreatic adenocarcinoma | NCT02620423 | Phase Ib Completed | |
| Coxsackie Virus (CAVATAK) | None | Pembrolizumab | NSCLC | NCT02824965 | Phase I/II Active, not recruiting | |
| Vesicular Stomatitis Virus (VSV-IFNβ-NIS) | IFNβ and the Sodium iodide symporter (NIS) | Pembrolizumab | Refractory NSCLC and HNSCC | NCT03647163 | Phase I/II Recruiting | |
| Vaccinia Viruses (Pexa-Vec) | GM-CSF | Ipilimumab | Metastatic/advanced solid tumors | NCT02977156 | Phase I Recruiting | |
| Herpes Simplex Virus Type 1 (IMLYGIC) | GM-CSF | Ipilimumab + Nivolumab | Triple-negative or estrogen receptor-positive, HER2-negative localized breast cancer | NCT04185311 Phase I | Active, Not Recruiting | |
| Herpes Simplex Virus Type 1 (HF10) | None | Ipilimumab | Unresectable or metastatic melanoma | NCT03153085 | Phase II Completed | |
| Herpes Simplex Virus Type 1 (HF10) | None | Ipilimumab | Unresectable or metastatic melanoma | NCT02272855 | Phase II Completed | |
| Coxsackievirus (CVA21) | None | Ipilimumab | Uveal melanoma metastases to liver | NCT03408587 | Phase I b Completed | |
| Herpes Simplex Virus Type 1 (RP1) | None | Nivolumab | Advanced and/or refractory solid tumors | NCT03767348 | Phase I/II Recruiting | |
| Herpes Simplex Virus Type 1 (HF10) | None | Nivolumab | Resectable stage IIIB, IIIC, IVM1a melanoma | NCT03259425 | Phase II Terminated with Results | |
| Reovirus (REOLYSIN) | None | Nivolumab + carfilzomib+ dexamethasone | Recurrent plasma cell myeloma | NCT03605719 | Phase I Recruiting | |
| Reovirus (REOLYSIN) | None | Avelumab + paclitaxel | Breast cancer metastatic | NCT04215146 | Phase II/III Recruiting | |
| Poxvirus (JX-594) | GM-CSF and beta-galactosidase | Avelumab + metronomic cyclophosphamide | Sarcoma; Advanced breast cancer | NCT02630368 | Phase II Recruiting | |
| Adenovirus (LOAd703) | 4-1BBL+CD40L | Atezolizumab | Pancreatic cancer | NCT02705196 | Phase I/IIa Recruiting | |
| Adenovirus (LOAd703) | 4-1BBL+CD40L | Atezolizumab | Malignant melanoma | NCT04123470 | Phase I/II Recruiting | |
| Reovirus (REOLYSIN) | None | Atezolizumab | Early breast cancer | NCT04102618 | Early Phase I Recruiting | |
| Maraba Virus (MG1-E6E7) | Mutant HPV E6 and E7 | Atezolizumab | HPV-associated cancers | NCT03618953 | Phase I/Ib Active, Not Recruiting | |
| OVV-01 | None | Pembrolizumab or atezolizumab | Advanced solid tumors | NCT04787003 | Phase I Recruiting | |
| Adenovirus (VCN-01) | PH20 | Durvalumab | R/M head and neck squamous cell carcinoma | Phase I Recruiting | ||
| Poxvirus (JX-594) | GM-CSF and beta-galactosidase | Durvalumab + tremelimumab | Refractory colorectal cancer | NCT03206073 | Phase I/II Active, Not Recruiting | |
| CAR T Cells | Adenovirus (CAdVEC) | None | HER2-CAR T | HER2 positive cancer | NCT03740256 | Phase I Recruiting |
| Vaccinia Virus (VV.CXCL11) | CXCL11 | Mesothelin-CAR T | Lung cancer | 155 | Preclinical Study | |
| Adenovirus (OAd-TNFa-IL2) | TNF-α and IL-2 | Mesothelin-CAR T | Pancreatic ductal adenocarcinoma | 68 | ||
| Vaccinia Virus (rTTVΔTK-IL21) | IL-21 | CD19-CAR T | Lung cancer | 68 | ||
| Adenovirus (oAD-IL7) | IL-7 | B7H3-CAR T | Glioblastoma | 20 | ||
| Adenovirus (Ad.sTbRFc) | sTGFβRIIFc (targeting TGFβ) | Mesothelin-CAR T | Breast cancer | 20 | ||
| Adenovirus (OAd-BiTE) | BiTE (targeting EGFR and CD3) | Folate receptor alpha (FR-α)-CAR T | Pancreatic ductal adenocarcinoma | 20 | ||
| Adenovirus (CAdTrio) | BiTE (targeting CD44v6 and CD3), PD-L1Ab, IL-12p70 | HER2-CAR T | Pancreatic ductal adenocarcinoma; Squamous cell carcinoma | 68 | ||
| Chimeric Orthopoxvirus (OV19t) | CD19 | CD19-CAR T | Breast cancer | 68 | ||
| Vaccinia Virus (mCD19 VV) | mCD19 | mCD19-CAR T | Melanoma | 68 | ||
| Adenovirus (CAd-VECPDL1) | PD-L1 mini-body | HER2-CAR T | Prostate; squamous cell carcinoma | 68 | ||
| CAR NK | Vaccinia Virus (OV-ffLuc-CCL5) | CCL5 | CCR5- NK | Colon cancer | 68 | |
| Herpes Simplex Virus 1 (OV-IL15C) | IL15/IL15Rα Sushi domain | EGFR-CAR NK | Glioblastoma | 68 | ||
| Adoptive TILs | Poxvirus (vvDD-IL2) | IL-2 | TILs | Colon cancer | 68 |
Combining OVs with ICIs
OVs engineered to encode ICIs are promising beneficial therapies. However, the most commonly used method for treating tumors with ICIs is based on the use of ICI antibodies, such as the approved drugs ipilimumab (anti-CTLA-4), pembrolizumab (anti-PD-1), nivolumab (anti-PD-1), cemiplimab (anti-PD-1), avelumab (anti-PD-L1), and atezolizumab (anti-PD-L1).283 Despite the success of these ICIs, only an estimated 12.5% of patients who receive ICI therapy have benefitted.284 One of the most commonly recognized reasons for primary resistance to ICI therapy is the absence or low level of PD-L1 on tumor cells.285 Initial studies have revealed that primary resistance to ICI therapies was observed when antigen presentation and CD8+ T cells were absent in nonimmunogenic tumors.286 OVs have been shown to induce a significant increase in PD-L1 levels,287 which are beneficial to ICI therapy. Furthermore, OV treatments can turn immunologically “cold” tumors into “hot” tumors, especially through the accumulation of TILs in the tumor tissue.288 OVs armed with cytokines broaden the reshaped form of the TME into a proinflammatory microenvironment, rendering tumors more susceptible to ICI therapy.288 In a phase Ib study, patients with advanced melanoma exhibited increased CD8+ T cells, elevated PD-L1 protein expression, and IFN-γ gene expression in several cell subsets in tumors after IMLYGIC treatment, which benefited from pembrolizumab treatment, resulting in a 62% objective response rate with a 33% CR rate.289 Similar results were obtained in a phase II trial evaluating the efficacy and safety of the combination IMLYGIC and ipilimumab in patients with advanced, unresectable melanoma; a higher objective response was observed upon combinatorial treatment compared to ipilimumab alone.290 The studies provide a clinical demonstration that the combination of OVs and ICIs could improve therapeutic effects in cancer patients who are resistant to ICIs alone. Based on the evidence, there has been an immense number of preclinical and clinical studies in which both unarmed and armed OVs are combined with ICIs to achieve effective tumor eradication (Table 2).
However, recently, a phase III, randomized, placebo-controlled study of IMLYGIC plus pembrolizumab for unresectable stage IIIB–IVM1c melanoma (MEL) demonstrated that IMLYGIC plus pembrolizumab did not significantly improve progression-free survival or overall survival compared with placebo plus pembrolizumab (NCT02263508). The negative results indicated that the sponsor should consider crucial concerns when selecting the combination of OVs and ICIs, such as tumor subtype and progression, the framework for evaluating changes in tumor size,291 the optimal timing of OVs, ICI administration,292 etc.
Combining OVs with CAR T-cell and TCR T-cell therapies
Genetically engineered T-cell immunotherapies have recently achieved inspiring clinical success in the treatment of hematologic malignancies.293 The two main approaches to T-cell engineering are the expression of CAR or antigen-specific TCR on T cells, which allows T cells to recognize tumor antigens and ultimately results in the induction of antigen-specific T cell responses.294 The most basic framework of CAR involves a genetically incorporated extracellular antigen-specific scFv (the antigen-binding domain), an extracellular hinge region, a transmembrane domain, and an intracellular signaling domain (including CD3ζ and two or more costimulatory domains).295 The intracellular signaling domain is designed and enhanced to promote robust cell proliferation, longevity and tumor cytotoxicity in the TME.293 TCR-engineered T cells express a recombinant TCR with α and β chains recognizing a TAA to promote antigen-specific immunotherapy.296 Recently, CAR T-cell therapy has become a potentially promising treatment for cancer, especially blood cancers. The US FDA has approved CAR T-cell products, Kymriah for treating acute lymphoblastic leukemia,297 Yescarta298 and Breyanzi299 for treating B-cell lymphoma, and Tecartus for treating mantle cell lymphoma.300 Clinical trials assessing the effectiveness of TCR T cells showed good outcomes against solid tumors.301 However, CAR T cells and TCR T cells have shown suboptimal efficacy against solid tumors.
The efficacy of CAR T and TCR T cells in solid tumors is reduced because of several problems, such as suboptimal trafficking of engineered T cells to tumors, antigen loss or heterogeneity, and poor fit with the tumor immune microenvironment (TIM).302 Based on the mechanism, unarmed or armed OVs can overcome barriers to T-cell trafficking to tumors, provide antigens and reverse the immunosuppressive TIM.
OVs overcome barriers to T-cell trafficking to tumors
The prerequisite for ACT is that CAR T or TCR T cells injected into the bloodstream localize to and infiltrate the tumor core to induce killing of cancer cells. T-cell trafficking to tumors is a multistep process involving adhesion of engineered T cells and local blood vessels, sequentially attaching, rolling, extravasating the vessel and migrating into the tumor core.303 However, aberrant chemotactic signaling of chemokine receptors on T cells and chemokines released in the TIM results in inefficient extravasation and recruitment of engineered T cells to the tumor.2 Even with proper chemotactic signaling, the tumor vasculature is detrimental to engineered T-cell recruitment because of its high level of disorganization, anergy toward inflammatory stimuli,303 and induced endothelial FasL expression that mediates CD8+ T cell killing.2 Furthermore, Ly6Clo F4/80hi TAMs along the epithelial tumor margins block engineered T-cell infiltration into the tumor.304
Positive chemokine–chemokine receptor signaling benefits T-cell trafficking into tumors, including that of the signaling pairs CXCL9,10,11/CXCR3, CXCL16/CXCR6, CCL2/CCR2, CCL3, 4, 5/CCR5, CCL21/CCR7 and CCL27/CCR10.305 CAR T cells have been engineered to coexpress CCR2,306 CXCR1 or CXCR2,307,308 and CCR4309 to enhance the ability of T cells to kill tumor cells. TCR T cells armed with CXCR2 markedly improved T-cell homing to a tumor site.310 CAR NK cells overexpressing CXCR4 exhibited enhanced migratory capacity compared to conventional CAR NK cells. However, tumor cell- and stromal cell-secreted chemokines that interact with chemokine receptors are essential prerequisites for regulating T-cell infiltration into tumors and influencing therapeutic outcomes in patients. Tumors mostly produce a minute number of chemokines, resulting in inefficient targeting of effectors to tumors.155 Despite their intrinsic enhancement of T-cell infiltration, OVs have been genetically engineered to express chemokines such as CCL2,214 CCL5,148 CCL19,311 CXCL11154 or CXCL9312 to recruit DCs, memory T lymphocytes, CD8+ cytotoxic T cells, and CD4+ T helper cells into the tumor core, resulting in the expansion of antitumor activity. Based on this idea, chemokine-armed OVs potentially act as powerful enhancers for engineered T-cell immunotherapy. Moon et al. modified CAR T cells to express CXCL11 (CAR/CXCL11) and engineered OVV with CXCL11 (VV.CXCL11).155 Although both CAR/CXCL11 and VV. CXCL11 significantly elevated CXCL11 protein levels within tumors; only VV. CXCL11 treatment effectively recruited T cells and augmented antitumor efficacy, which demonstrated the possibility and superiority of OVs as efficient partners in CAR T-cell therapy. OAd engineered to express CCL5 improved the migration of CAR T cells in solid tumors, resulting in increased antitumor effects.313 Then, an artificial CCL5–CCR5 axis was activated by inducing CCR5, promoting the differentiation of NK cells in ACT, and OVV was modified with CCL5, inducing the accumulation of NK cells in solid tumors and improving the therapeutic efficacy of NK cells.150
OVs support T-cell survival and expansion
When engineered T cells enter a tumor and confront a hostile TME, the resulting functional exhaustion and insufficient expansion and persistence of the T cells have been identified as major obstacles in ACT.314 Cytokines are key contributors to the survival and expansion of T-cell therapies. Therefore, CAR T cells have been genetically engineered to be carriers that deliver cytokines, such as IL-12,315,316 IL-15,317,318, IL-18,319 IL-7,320 and IL-23,321 into tumors. Additionally, intratumoral production of IL18322 or inducible expression of IL-12323 with TCR T cells improved the performance of engineered T cells. Compared to CAR T cells and TCR T cells serving as carriers, OVs show superior capacity for delivering cytokines into tumors in ACTs. To date, multiple cytokines, including IFNs, IL-2, IL-17, IL-12, IL-15, IL-18, IL-23, IL-24, and TNF-α, have been introduced into OVs to enhance antitumor immunity.324
To date, few preclinical studies using cytokine-armed OVs to improve CAR T-cell therapies have been reported. TNF-α and IL-2 expressed by genetically engineered OAd enhanced the efficacy of mesothelin-CAR T cells in “cold” pancreatic ductal adenocarcinoma. This combination therapy shaped the immunosuppressive TME into a “hot” TME by increasing T-cell recruitment, enhancing T-cell function, driving macrophage polarization into the M1 phenotype and promoting DC maturation.325 Treatment with IL21-armed OVV was shown to enhance TIL activity and showed notable synergy with CAR T-cell therapy in tumor treatment.234 In another study, an engineered OAd loaded with IL-7 was used in combination with B7H3-targeted CAR T cells, and this combination treatment enhanced T-cell proliferation, reduced the T-cell apoptosis rate and improved the therapeutic efficacy of B7H3-CAR T cells in glioblastoma.326 Similarly, a combination of armed OVs with CAR NK cells achieved a profound therapeutic effect. Ma et al. constructed an oncolytic HSV-1 to express the human IL15/IL15Rα complex (named OV-IL15C) to investigate its efficacy when administered with EGFR-CAR NK cells in multiple glioblastoma mouse models.327 Compared with monotherapy, the combination therapy increased intracranial infiltration and activation of NK and CD8+ T cells and prolonged the persistence of CAR NK cells, leading to tumor growth inhibition and prolonged survival of tumor-bearing mice.327
TGFβ plays a critical role in T-cell exclusion and immunosuppressive microenvironment formation.328 Targeting TGFβ activity has demonstrated promise and efficacy in tumor therapy.329 Soluble TGFβ receptor II fusion protein (sTGFβRIIFc), a TGFβ antagonist, has been demonstrated to suppress metastasis in mice.330 Combining the effect of OV oncolytic activity on tumor cells and the function of sTGFβRIIFc to block TGFβ signaling, OAd expressing sTGFbRIIFc (Ad.sTbRFc) significantly inhibited breast cancer metastasis in mice.331,332 Furthermore, Li et al. combined Ad.sTbRFc with mesothelin-targeted CAR T cells to develop a better therapeutic strategy.333 According to the results of their study, Ad.sTbRFc obviously inhibited tumor growth at the early stage of treatment. In contrast, mesothelin CAR T cells showed greater antitumor responses at a later stage. The combined therapy mediated a stronger long-term antitumor response than monotherapy.333
OVs overcome antigen loss or diversity
Identifying and clearing tumor cells by CAR T and TCR T cells require that target antigens are presented on cells. CAR T cells recognize tumor antigens on the cell surface; in contrast, TCR T cells target intracellular antigens or cell surface antigens. Antigens exclusively presented on tumor cells but not healthy cells are prerequisites for safe and effective CAR T and TCR T-cell therapy for solid cancers.334
However, solid tumors are in an immunosuppressive TME characterized by heterogeneous antigens and lack of targetable tumor antigens, creating a challenge to the effective clinical use of CAR T and TCR T-cell therapeutics.335 Considerable effort has been devoted to developing ACT strategies for overcoming antigen heterogeneity in solid tumors.336
OVs combined with bispecific T-cell engagers (BiTEs) target various antigens and overcome antigen escape during ACT. For example, OAd delivering an EGFR-targeting BiTE (OAd-BiTE) was used to improve the efficacy of folate receptor alpha (FR-α)-specific CAR T-cell therapy by overcoming the problem of tumor heterogeneity in solid tumors.337,338 The cytotoxicity of FR-α-targeted CAR T cells is closely associated with FR-α density. FR-α-negative cancer cells can escape recognition and killing by CAR T cells. However, BiTEs expressed by OAd-infected cells effectively redirected CAR T cells toward EGFR-positive and FR-α-negative cancer cells, resulting in a reduction in tumor heterogeneity, improved antitumor efficacy and prolonged survival in mouse models of cancer.337 Furthermore, Suzuki and collaborators constructed an OV that produced IL-12, an anti-PD-L1 antibody, and a CD44v6-targeted BiTE molecule (forming CAdTrio), enhancing the breadth, potency, and duration of the antitumor activity of HER2-specific CAR T cells.339 CD44v6 BiTEs secreted from CAdTrio redirected HER2-specific CAR T cells to kill CD44v6-positive cancer cells and induce dual targeting of orthotopic HER2+ and HER2−/− CD44v6+ tumors.339 Based on the confirmed capability of BiTEs, bi and tri specific T-cell engager-armed OVs might be promising in tumor treatment.340
Recently, local intratumoral delivery of antigens by OVs improved CAR T-cell immunotherapy and demonstrated remarkable efficacy with nonimmunogenic solid tumors. Park et al. engineered an OVV to generate a nonsignaling truncated CD19 protein (CD19t) that was a B-cell-lineage-restricted molecule.341 Infected with this armed OV, CD19-negative triple-negative breast and glioma tumor cells specifically expressed CD19t residing on the cell surface. When these cells were cocultured with CD19-targeted CAR T cells in vitro, the cytotoxicity of the T cells was significantly increased, as indicated by the upregulated expression of activation markers (CD25 and CD137), increased levels of secreted cytokines IFN-γ and IL-2, and lysis of CD19+ tumor cells. This combination therapy resulted in remarkable tumor regression compared to monotherapy in immunodeficient NSG mice. Additionally, the authors demonstrated that OV19t promoted endogenous T-cell and CAR T-cell infiltration into tumors and induced immunological memory in immunocompetent mouse tumor models.341 Furthermore, Aalipour et al. confirmed the efficacy of OVs as carriers to induce targets of CAR T cells.342 However, some strategies are focused on delivering CAR T targets into tumors. For example, CAR T cells engineered to coexpress antigen peptides can transfer antigen peptides to tumor cells via extracellular vesicles, improving the presentation and targeting by antigen-specific CTLs for the treatment of nonimmunogenic tumors.343 In another study, recombinant AdV was used to deliver truncated CD19 tags into a number of cancer cell lines to improve CD19 CAR T-cell therapeutic efficacy, overcoming the problem of endogenous antigen dependence.344 In summary, the multiple advantages of a tumor-tagging strategy combining OVs with CAR T cells make this combination a novel and promising solution for the heterogeneity and antigen loss in solid tumors.345
OVs attenuate exhaustion of CAR T and TCR T cells
Exhaustion and senescence, two crucial dysfunctional states of T cells in the TME, limit the efficacy and application of ACT.346 Exhausted CAR T cells are dysfunctional, and this state is acquired mainly through the upregulation of multiple inhibitory receptors, such as PD-1, CTLA-4, and TIM-3.347 High expression of PD-1 has been observed in TCR T cells following infusion, and this expression was associated with reduced production of IFN-γ and a decreased immune response.348
Hence, disrupting the PD-1/PD-L1 axis is an effective way to relieve adoptive T-cell exhaustion and improve persistence. An anti-PD-1 blockade antibody was used to enhance the function of CAR T or TCR T cells and thereby promote tumor eradication,349,350 but this approach might lead to the development of systemic toxicity.351 Self-delivery of PD-1 blocking ScFv via engineered CAR T cells is a safe strategy to augment these cell functions and persistence in the TME.352 Local secretion of functional checkpoint blockade factors by armed OVs may be a simple, safe and efficacious approach to boost the efficacy of CAR T cells. For example, Suzuki and colleagues engineered OAV to express an anti-PD-L1-blocking mini-antibody (CAd-VECPDL1) to enhance CAR T-cell killing action.353 This anti-PD-L1 mini antibody was detected at the tumor site after CAd-VECPDL1 administration. The combination of CAd-VECPDL1 with HER2-targeted CAR T cells showed enhanced antitumor activity compared to treatment with HER2-targeted CAR T cells alone, HER2-targeted CAR T cells plus unarmed OAd and even anti-PD-L1 blocking antibody plus HER2-targeted CAR T cells in a HER2 prostate cancer xenograft model. These data demonstrated the superiority of the local production of anti-PD-L1 mini antibodies by OVs in combination with ACT.353
OVs enhance the adoptive transfer of TILs
Immunotherapy using autologous TILs is an adoptive cell transfer therapy and has emerged as a powerful treatment option for patients with advanced solid tumors, especially metastatic melanoma.354 TIL therapy refers to the surgical excision of tumors from patients, isolation and expansion of TILs ex vivo and then the transfer of TILs back into the same patient.355 Adoptive transfer of TILs for the treatment of metastatic melanoma has shown high efficacy, with objective responses ranging from 40% to 70%,356 which were largely associated with the high mutational load and abundant tumor-reactive lymphocytes in the tumors. However, most solid tumors are poorly immunogenic, and tumor tissues lack TILs, which become major hurdles in sourcing TILs.357 Unarmed or armed OV-mediated ICD causes the release of TAAs/TANs and can turn immunologically “cold” tumors into “hot” tumors accompanied by TIL accumulation, which makes OVs great partners for TIL therapy. Recently, one study reported a combination of OV and TIL therapy. Feist et al. intratumorally injected IL2-armed oncolytic poxvirus into MC38 tumors with low immunogenicity, and the results showed the accumulation of tumor-specific TILs that contained a lower percentage of exhausted PD-1hiTim-3+CD8+ T cells and Tregs. TILs, undergoing isolation, expansion and transfer, significantly delayed the growth of tumors and improved the survival of mice with established MC38 tumors.288
Outlook
OVs can selectively kill tumor cells, but first-generation OVs (wild-type and natural variant strains of weak viruses) have low clinical activity. Between first-generation OVs and third-generation OVs (exogenous therapeutic gene-“armed” OVs), a great deal of effort has been directed to understanding the activating effect of OVs on antitumor immunity. As OVs can turn “cold” tumors into “hot” tumors and can be readily genetically engineered with immunomodulatory therapeutic genes, it is possible and promising to use these OVs as platforms to enhance T-cell function against tumors. For currently used cytokines and ICIs expressed by OVs, more effective drug targets will certainly be found in the near future. To date, the combination of OVs with ICIs or ACT to promote a sustained antitumoral immune response has been successfully tested in preclinical studies and in clinical trials. Further efforts should be directed to realize oncolytic monotherapy or combinations of OVs with other immunotherapies in cancer treatment to improve T-cell responses.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) under grant No. 82073366; by the National Major Scientific and Technological Special Project for “Significant New Drugs Development” under grant No. 2018ZX09201018-013; and by the Talents project of Sichuan University of Science & Engineering under grant number E10402637.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yaomei Tian, Daoyuan Xie.
References
- 1.Leko V, Rosenberg SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 2020;38:454–472. doi: 10.1016/j.ccell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 3.Ferro S, Huber V, Rivoltini L. Mechanisms of tumor immunotherapy, with a focus on thoracic cancers. J. Thorac. Dis. 2018;10:4619–4631. doi: 10.21037/jtd.2018.07.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019;20:840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binnewies M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 9.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 10.Malfitano AM, et al. Virotherapy: from single agents to combinatorial treatments. Biochem. Pharmacol. 2020;177:113986. doi: 10.1016/j.bcp.2020.113986. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson C, et al. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget. 2015;6:19976–19989. doi: 10.18632/oncotarget.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davola ME, Mossman KL. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy? Oncoimmunology. 2019;8:e1581528. doi: 10.1080/2162402X.2019.1596006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 16.Martuza RL, et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 17.Heise C, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 18.Alberts P, et al. The advent of oncolytic virotherapy in oncology: the Rigvir(R) story. Eur. J. Pharmacol. 2018;837:117–126. doi: 10.1016/j.ejphar.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 20.Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5:e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez MC, et al. Talimogene Laherparepvec (TVEC) for the treatment of advanced melanoma: a single-institution experience. Ann. Surg. Oncol. 2018;25:3960–3965. doi: 10.1245/s10434-018-6803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin. Cancer Biol. 2000;10:453–459. doi: 10.1006/scbi.2000.0336. [DOI] [PubMed] [Google Scholar]
- 23.Kemeny N, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum. Gene Ther. 2006;17:1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- 24.Peng K-W, et al. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 25.Sinkovics JG, Horvath JC. Newcastle disease virus (NDV): brief history of its oncolytic strains. J. Clin. Virol. 2000;16:1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 26.Hashiro G, Loh PC, Yau JT. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch. Virol. 1977;54:307–315. doi: 10.1007/BF01314776. [DOI] [PubMed] [Google Scholar]
- 27.Balachandran S, Barber G. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto S, et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 2012;72:2609–2621. doi: 10.1158/0008-5472.CAN-11-3185. [DOI] [PubMed] [Google Scholar]
- 29.Vellinga J, Van der Heijdt S, Hoeben RC. The adenovirus capsid: major progress in minor proteins. J. Gen. Virol. 2005;86:1581–1588. doi: 10.1099/vir.0.80877-0. [DOI] [PubMed] [Google Scholar]
- 30.Arnberg N. Adenovirus receptors: implications for targeting of viral vectors. Trends Pharm. Sci. 2012;33:442–448. doi: 10.1016/j.tips.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Moure M, Martinez-Velez N, Patino-Garcia A, Alonso MM. Oncolytic adenoviruses as a therapeutic approach for osteosarcoma: a new hope. J. Bone Oncol. 2017;9:41–47. doi: 10.1016/j.jbo.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koski A, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds P, Dmitriev I, Curiel D. Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther. 1999;6:1336–1339. doi: 10.1038/sj.gt.3300941. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe D, Goshima F. Oncolytic Virotherapy by HSV. Adv. Exp. Med. Biol. 2018;1045:63–84. doi: 10.1007/978-981-10-7230-7_4. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald SJ, Mostafa HH, Morrison LA, Davido DJ. Genome sequence of herpes simplex virus 1 strain KOS. J. Virol. 2012;86:6371–6372. doi: 10.1128/JVI.00646-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bommareddy PK, Patel A, Hossain S, Kaufman HL. Talimogene Laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am. J. Clin. Dermatol. 2017;18:1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanai R, et al. Effect of gamma34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J. Virol. 2012;86:4420–4431. doi: 10.1128/JVI.00017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jugovic P, et al. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J. Virol. 1998;72:5076–5084. doi: 10.1128/jvi.72.6.5076-5084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat. Cell Biol. 2001;3:745–750. doi: 10.1038/35087061. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira GP, et al. Poxvirus host range genes and virus–host spectrum: a critical review. Viruses. 2017;9:331. doi: 10.3390/v9110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs BL, et al. Vaccinia virus vaccines: past, present and future. Antivir. Res. 2009;84:1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parato KA, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 2012;20:749–758. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholl SM, et al. Recombinant vaccinia virus encoding human MUC1 and IL2 as immunotherapy in patients with breast cancer. J. Immunother. 2000;23:570–580. doi: 10.1097/00002371-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Haddad D. Geneticallyengineered vaccinia viruses as agents for cancer treatment, imaging, and transgene delivery. Front. Oncol. 2017;7:96. doi: 10.3389/fonc.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Stanford MM, McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin. Biol. Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- 47.Millward S, Graham AF. Structural studies on reovirus: discontinuities in the genome. Proc. Natl Acad. Sci. USA. 1970;65:422–429. doi: 10.1073/pnas.65.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steyer A, et al. High similarity of novel orthoreovirus detected in a child hospitalized with acute gastroenteritis to mammalian orthoreoviruses found in bats in Europe. J. Clin. Microbiol. 2013;51:3818–3825. doi: 10.1128/JCM.01531-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shatkin AJ, Sipe JD, Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J. Virol. 1968;2:986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcato P, et al. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol. Ther. 2007;15:1522–1530. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 51.Errington F, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15:1257–1270. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosen L, et al. Observations on a newly recognized virus (Abney) of the reovirus family. Am. J. Hyg. 1960;71:258–265. doi: 10.1093/oxfordjournals.aje.a120109. [DOI] [PubMed] [Google Scholar]
- 53.Chaurasiya S, Fong Y, Warner SG. Oncolytic virotherapy for cancer: clinical experience. Biomedicines. 2021;9:419. doi: 10.3390/biomedicines9040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schirrmacher V, van Gool S, Stuecker W. Breaking therapy resistance: an update on oncolytic Newcastle disease virus for improvements of cancer therapy. Biomedicines. 2019;7:66. doi: 10.3390/biomedicines7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng Q, He J, Zhong L, Zhao Y. Advances in the study of antitumour immunotherapy for Newcastle disease virus. Int. J. Med. Sci. 2021;18:2294–2302. doi: 10.7150/ijms.59185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schirrmacher V. Immunobiology of Newcastle disease virus and its use for prophylactic vaccination in poultry and as adjuvant for therapeutic vaccination in cancer patients. Int. J. Mol. Sci. 2017;18:1103. doi: 10.3390/ijms18051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schirrmacher V. Fifty years of clinical application of Newcastle disease virus: time to celebrate! Biomedicines. 2016;4:16. doi: 10.3390/biomedicines4030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horikami SM, Moyer SA. Structure, transcription, and replication of measles virus. Curr. Top. Microbiol. Immunol. 1995;191:35–50. doi: 10.1007/978-3-642-78621-1_3. [DOI] [PubMed] [Google Scholar]
- 60.Bhattacharjee S, Yadava PK. Measles virus: background and oncolytic virotherapy. Biochem. Biophys. Rep. 2018;13:58–62. doi: 10.1016/j.bbrep.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leber MF, et al. Engineering and combining oncolytic measles virus for cancer therapy. Cytokine Growth Factor Rev. 2020;56:39–48. doi: 10.1016/j.cytogfr.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muhlebach MD. Measles virus in cancer therapy. Curr. Opin. Virol. 2020;41:85–97. doi: 10.1016/j.coviro.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 63.Schenk EL, et al. A randomized double-blind phase II study of the Seneca Valley Virus (NTX-010) versus placebo for patients with extensive-stage SCLC (ES SCLC) who were stable or responding after at least four cycles of platinum-based chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 study. J. Thorac. Oncol. 2020;15:110–119. doi: 10.1016/j.jtho.2019.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beasley GM, et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer. 2021;9:e002203. doi: 10.1136/jitc-2020-002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gebremeskel S, et al. Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. J. Immunother. Cancer. 2021;9:e002096. doi: 10.1136/jitc-2020-002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hajda J, et al. Phase 2 trial of oncolytic H-1 parvovirus therapy shows safety and signs of immune system activation in patients with metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2021;27:5546–5556. doi: 10.1158/1078-0432.CCR-21-1020. [DOI] [PubMed] [Google Scholar]
- 67.Chiocca EA. Oncolytic viruses. Nat. Rev. Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 68.Chaurasiya S, Fong Y, Warner SG. Optimizing oncolytic viral design to enhance antitumor efficacy: progress and challenges. Cancers. 2020;12:1699. doi: 10.3390/cancers12061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filley AC, Dey M. Immune system, friend or foe of oncolytic virotherapy? Front. Oncol. 2017;7:106. doi: 10.3389/fonc.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaurasiya S, Chen NG, Fong Y. Oncolytic viruses and immunity. Curr. Opin. Immunol. 2018;51:83–90. doi: 10.1016/j.coi.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gujar S, et al. Antitumor benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. Trends Immunol. 2018;39:209–221. doi: 10.1016/j.it.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Hindupur SV, et al. STAT3/5 inhibitors suppress proliferation in bladder cancer and enhance oncolytic adenovirus therapy. Int. J. Mol. Sci. 2020;21:1106. doi: 10.3390/ijms21031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLaughlin M, et al. The PERK inhibitor GSK2606414 enhances reovirus infection in head and neck squamous cell carcinoma via an ATF4-dependent mechanism. Mol. Ther. Oncolytics. 2020;16:238–249. doi: 10.1016/j.omto.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 75.Lin Y, et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc. Natl Acad. Sci. USA. 2014;111:E4504–E4512. doi: 10.1073/pnas.1408759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kakiuchi Y, et al. Local oncolytic adenovirotherapy produces an abscopal effect via tumor-derived extracellular vesicles. Mol. Ther. 2021;29:2920–2930. doi: 10.1016/j.ymthe.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020;14:2994–3006. doi: 10.1002/1878-0261.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma J, et al. Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death Dis. 2020;11:48. doi: 10.1038/s41419-020-2236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Somma S, et al. The oncolytic virus dl922-947 triggers immunogenic cell death in mesothelioma and reduces xenograft growth. Front. Oncol. 2019;9:564. doi: 10.3389/fonc.2019.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao X, et al. STAT3 contributes to oncolytic newcastle disease virus-induced immunogenic cell death in melanoma cells. Front. Oncol. 2019;9:436. doi: 10.3389/fonc.2019.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melchjorsen J. Learning from the messengers: innate sensing of viruses and cytokine regulation of immunity—clues for treatments and vaccines. Viruses. 2013;5:470–527. doi: 10.3390/v5020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front. Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.An Y, et al. Oncolytic reovirus induces ovarian cancer cell apoptosis in a TLR3-dependent manner. Virus Res. 2021;301:198440. doi: 10.1016/j.virusres.2021.198440. [DOI] [PubMed] [Google Scholar]
- 84.Kleijn A, et al. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS ONE. 2014;9:e97495. doi: 10.1371/journal.pone.0097495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang H, et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE. 2014;9:e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramelyte E, et al. Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell. 2021;39:394–406.e394. doi: 10.1016/j.ccell.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prestwich RJ, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin. Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gujar SA, Marcato P, Pan D, Lee PW. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol. Cancer Ther. 2010;9:2924–2933. doi: 10.1158/1535-7163.MCT-10-0590. [DOI] [PubMed] [Google Scholar]
- 90.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl Acad. Sci. USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao L, Liu H. Newcastle disease virus: a promising agent for tumour immunotherapy. Clin. Exp. Pharm. Physiol. 2012;39:725–730. doi: 10.1111/j.1440-1681.2011.05662.x. [DOI] [PubMed] [Google Scholar]
- 92.Guillerme JB, et al. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin. Cancer Res. 2013;19:1147–1158. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 93.Fonteneau JF, Guillerme JB, Tangy F, Gregoire M. Attenuated measles virus used as an oncolytic virus activates myeloid and plasmacytoid dendritic cells. Oncoimmunology. 2013;2:e24212. doi: 10.4161/onci.24212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo Y, et al. Tumor-targeting oncolytic virus elicits potent immunotherapeutic vaccine responses to tumor antigens. Oncoimmunology. 2020;9:1726168. doi: 10.1080/2162402X.2020.1726168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gujar SA, et al. Oncolytic virus-initiated protective immunity against prostate cancer. Mol. Ther. 2011;19:797–804. doi: 10.1038/mt.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bommareddy PK, et al. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci. Transl. Med. 2018;10:eaau0417. doi: 10.1126/scitranslmed.aau0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang G, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 2020;11:1395. doi: 10.1038/s41467-020-15229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim DS, et al. Smac mimetics and oncolytic viruses synergize in driving anticancer T-cell responses through complementary mechanisms. Nat. Commun. 2017;8:344. doi: 10.1038/s41467-017-00324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Packiriswamy N, et al. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia. 2020;34:3310–3322. doi: 10.1038/s41375-020-0828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qiao J, et al. Intratumoral oncolytic adenoviral treatment modulates the glioma microenvironment and facilitates systemic tumor-antigen-specific T cell therapy. Oncoimmunology. 2015;4:e1022302. doi: 10.1080/2162402X.2015.1022302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henke E, Nandigama R, Ergun S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front. Mol. Biosci. 2019;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Everts A, Bergeman M, McFadden G, Kemp V. Simultaneous tumor and stroma targeting by oncolytic viruses. Biomedicines. 2020;8:474. doi: 10.3390/biomedicines8110474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ilkow CS, et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015;21:530–536. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- 104.Li M, et al. Characterization and oncolytic virus targeting of FAP-expressing tumor-associated pericytes in glioblastoma. Acta Neuropathol. Commun. 2020;8:221. doi: 10.1186/s40478-020-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arulanandam R, et al. VEGF-mediated induction of PRD1-BF1/Blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell. 2015;28:210–224. doi: 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 106.Breitbach CJ, et al. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 2011;19:886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Breitbach CJ, et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 108.Harrington K, et al. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019;18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 109.Maroun J, et al. Designing and building oncolytic viruses. Future Virol. 2017;12:193–213. doi: 10.2217/fvl-2016-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu BL, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 111.Andtbacka RH, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 112.Ady JW, Heffner J, Klein E, Fong Y. Oncolytic viral therapy for pancreatic cancer: current research and future directions. Oncolytic Virother. 2014;3:35–46. doi: 10.2147/OV.S53858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liang M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr. Cancer Drug Targets. 2018;18:171–176. doi: 10.2174/1568009618666171129221503. [DOI] [PubMed] [Google Scholar]
- 114.Chan WM, McFadden G. Oncolytic poxviruses. Annu. Rev. Virol. 2014;1:119–141. doi: 10.1146/annurev-virology-031413-085442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Q, et al. Efficacy of a novel double-controlled oncolytic adenovirus driven by the Ki67 core promoter and armed with IL-15 against glioblastoma cells. Cell Biosci. 2020;10:124. doi: 10.1186/s13578-020-00485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hardcastle J, Kurozumi K, Chiocca EA, Kaur B. Oncolytic viruses driven by tumor-specific promoters. Curr. Cancer Drug Targets. 2007;7:181–189. doi: 10.2174/156800907780058880. [DOI] [PubMed] [Google Scholar]
- 117.Montano-Samaniego M, et al. Strategies for targeting gene therapy in cancer cells with tumor-specific promoters. Front. Oncol. 2020;10:605380. doi: 10.3389/fonc.2020.605380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie Z, et al. Multi-input RNAi-based logic circuit for identification of specific cancer cells. Science. 2011;333:1307–1311. doi: 10.1126/science.1205527. [DOI] [PubMed] [Google Scholar]
- 119.Huang H, et al. Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy. Nat. Commun. 2019;10:4801. doi: 10.1038/s41467-019-12794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stark A, et al. Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3’UTR evolution. Cell. 2005;123:1133–1146. doi: 10.1016/j.cell.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 121.Forterre A, Komuro H, Aminova S, Harada M. A comprehensive review of cancer MicroRNA therapeutic delivery strategies. Cancers. 2020;12:1852. doi: 10.3390/cancers12071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hosseinahli N, Aghapour M, Duijf PHG, Baradaran B. Treating cancer with microRNA replacement therapy: a literature review. J. Cell Physiol. 2018;233:5574–5588. doi: 10.1002/jcp.26514. [DOI] [PubMed] [Google Scholar]
- 123.Luo Q, et al. A triple-regulated oncolytic adenovirus carrying microRNA-143 exhibits potent antitumor efficacy in colorectal cancer. Mol. Ther. Oncolytics. 2020;16:219–229. doi: 10.1016/j.omto.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakuda T, et al. Development of an oncolytic recombinant vesicular stomatitis virus encoding a tumor-suppressor microRNA. Anticancer Res. 2020;40:6319–6325. doi: 10.21873/anticanres.14652. [DOI] [PubMed] [Google Scholar]
- 125.Jia Y, et al. Extremely low organ toxicity and strong antitumor activity of miR-34-regulated oncolytic coxsackievirus B3. Mol. Ther. Oncolytics. 2019;12:246–258. doi: 10.1016/j.omto.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marzulli M, et al. A novel oncolytic herpes simplex virus design based on the common overexpression of microRNA-21 in tumors. J. Gene Ther. 2018;3:2381–3326. doi: 10.13188/2381-3326.1000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Girardi E, Lopez P, Pfeffer S. On the importance of host MicroRNAs during viral infection. Front. Genet. 2018;9:439. doi: 10.3389/fgene.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raimondi G, Gea-Sorli S, Otero-Mateo M, Fillat C. Inhibition of miR-222 by oncolytic adenovirus-encoded miRNA sponges promotes viral oncolysis and elicits antitumor effects in pancreatic cancer models. Cancers. 2021;13:3233. doi: 10.3390/cancers13133233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dellac S, et al. Constitutive low expression of antiviral effectors sensitizes melanoma cells to a novel oncolytic virus. Int. J. Cancer. 2021;148:2321–2334. doi: 10.1002/ijc.33401. [DOI] [PubMed] [Google Scholar]
- 130.Nguyen TT, et al. Mutations in the IFNgamma–JAK–STAT pathway causing resistance to immune checkpoint inhibitors in melanoma increase sensitivity to oncolytic virus treatment. Clin. Cancer Res. 2021;27:3432–3442. doi: 10.1158/1078-0432.CCR-20-3365. [DOI] [PubMed] [Google Scholar]
- 131.Liu Y, et al. Suppression of CCDC6 sensitizes tumor to oncolytic virus M1. Neoplasia. 2021;23:158–168. doi: 10.1016/j.neo.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bommareddy PK, Zloza A, Rabkin SD, Kaufman HL. Oncolytic virus immunotherapy induces immunogenic cell death and overcomes STING deficiency in melanoma. Oncoimmunology. 2019;8:1591875. doi: 10.1080/2162402X.2019.1591875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Froechlich G, et al. Integrity of the antiviral STING-mediated DNA sensing in tumor cells is required to sustain the immunotherapeutic efficacy of herpes simplex oncolytic virus. Cancers. 2020;12:3407. doi: 10.3390/cancers12113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Capece D, et al. Targeting costimulatory molecules to improve antitumor immunity. J. Biomed. Biotechnol. 2012;2012:926321. doi: 10.1155/2012/926321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eriksson E, et al. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin. Cancer Res. 2017;23:5846–5857. doi: 10.1158/1078-0432.CCR-17-0285. [DOI] [PubMed] [Google Scholar]
- 136.Kaufman HL, et al. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J. Clin. Investig. 2005;115:1903–1912. doi: 10.1172/JCI24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Autio K, et al. Safety and biodistribution of a double-deleted oncolytic vaccinia virus encoding CD40 ligand in laboratory Beagles. Mol. Ther. Oncolytics. 2014;1:14002. doi: 10.1038/mto.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang JH, et al. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol. Ther. 2010;18:264–274. doi: 10.1038/mt.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DiPaola RS, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J. Transl. Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andarini S, et al. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res. 2004;64:3281–3287. doi: 10.1158/0008-5472.can-03-3911. [DOI] [PubMed] [Google Scholar]
- 141.Calmels B, et al. Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther. 2005;12:198–205. doi: 10.1038/sj.cgt.7700781. [DOI] [PubMed] [Google Scholar]
- 142.Eriksson E, et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 2017;24:92–103. doi: 10.1038/gt.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wenthe J, et al. Immunostimulatory oncolytic virotherapy for multiple myeloma targeting 4-1BB and/or CD40. Cancer Gene Ther. 2020;27:948–959. doi: 10.1038/s41417-020-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ylosmaki E, et al. Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform. Mol. Ther. Oncolytics. 2021;20:459–469. doi: 10.1016/j.omto.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wenthe J, et al. Boosting CAR T-cell responses in lymphoma by simultaneous targeting of CD40/4-1BB using oncolytic viral gene therapy. Cancer Immunol. Immunother. 2021;70:2851–2865. doi: 10.1007/s00262-021-02895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vilgelm AE, Richmond A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front. Immunol. 2019;10:333. doi: 10.3389/fimmu.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 148.Li J, et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol. Ther. 2011;19:650–657. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mgrditchian T, et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl Acad. Sci. USA. 2017;114:E9271–E9279. doi: 10.1073/pnas.1703921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li F, et al. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J. Immunother. Cancer. 2019;8:e000131. doi: 10.1136/jitc-2019-000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lapteva N, et al. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J. Immunother. 2009;32:145–156. doi: 10.1097/CJI.0b013e318193d31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hensbergen PJ, et al. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J. Immunother. 2005;28:343–351. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 153.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68:8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 154.Liu Z, et al. CXCL11-Armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. OncoImmunology. 2015;5:e1091554. doi: 10.1080/2162402X.2015.1091554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moon EK, et al. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. OncoImmunology. 2017;7:e1395997. doi: 10.1080/2162402X.2017.1395997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Amedei A, Prisco D, D’ Elios MM. The use of cytokines and chemokines in the cancer immunotherapy. Recent Pat. Anticancer Drug Discov. 2013;8:126. [PubMed] [Google Scholar]
- 157.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3:3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 159.Qiu Y, et al. Clinical application of cytokines in cancer immunotherapy. Drug Des. Devel Ther. 2021;15:2269–2287. doi: 10.2147/DDDT.S308578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pearl TM, Markert JM, Cassady KA, Ghonime MG. Oncolytic virus-based cytokine expression to improve immune activity in brain and solid tumors. Mol. Ther. Oncolytics. 2019;13:14–21. doi: 10.1016/j.omto.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shiomi A, Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015:568543. doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp. Mol. Med. 2016;48:e242. doi: 10.1038/emm.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kaufman HL, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 164.Lee JH, et al. Oncolytic and immunostimulatory efficacy of a targeted oncolytic poxvirus expressing human GM-CSF following intravenous administration in a rabbit tumor model. Cancer Gene Ther. 2010;17:73–79. doi: 10.1038/cgt.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lemay CG, et al. Harnessing oncolytic virus-mediated antitumor immunity in an infected cell vaccine. Mol. Ther. 2012;20:1791–1799. doi: 10.1038/mt.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grossardt C, et al. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum. Gene Ther. 2013;24:644–654. doi: 10.1089/hum.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Robinson M, et al. Novel immunocompetent murine tumor model for evaluation of conditionally replication-competent (oncolytic) murine adenoviral vectors. J. Virol. 2009;83:3450–3462. doi: 10.1128/JVI.02561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lei N, et al. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- 169.Kemp V, et al. Arming oncolytic reovirus with GM-CSF gene to enhance immunity. Cancer Gene Ther. 2019;26:268–281. doi: 10.1038/s41417-018-0063-9. [DOI] [PubMed] [Google Scholar]
- 170.Zhao Q, et al. A novel oncolytic herpes simplex virus type 2 has potent anti-tumor activity. PLoS ONE. 2014;9:e93103. doi: 10.1371/journal.pone.0093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang B, et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial. J. Immunother. Cancer. 2021;9:e002224. doi: 10.1136/jitc-2020-002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Breitbach CJ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 173.Park SH, et al. Phase 1b trial of biweekly intravenous Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol. Ther. 2015;23:1532–1540. doi: 10.1038/mt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cripe TP, et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015;23:602–608. doi: 10.1038/mt.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Breitbach CJ, et al. A Phase 2, open-label, randomized study of Pexa-Vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol. Biol. 2015;1317:343–357. doi: 10.1007/978-1-4939-2727-2_19. [DOI] [PubMed] [Google Scholar]
- 176.Briukhovetska D, et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer. 2021;21:481–499. doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Suzuki A, Leland P, Joshi BH, Puri RK. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine. 2015;75:79–88. doi: 10.1016/j.cyto.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 178.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 179.Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 180.Lin J, et al. The role of IL-7 in immunity and cancer. Anticancer Res. 2017;37:963–967. doi: 10.21873/anticanres.11405. [DOI] [PubMed] [Google Scholar]
- 181.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3:3856–3893. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb. Perspect. Biol. 2018;10:a028530. doi: 10.1101/cshperspect.a028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 2018;10:a028472. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pol JG, et al. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 2020;217:e20191247. doi: 10.1084/jem.20191247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zelante T, Fric J, Wong AY, Ricciardi-Castagnoli P. Interleukin-2 production by dendritic cells and its immuno-regulatory functions. Front. Immunol. 2012;3:161. doi: 10.3389/fimmu.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alva A, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2016;65:1533–1544. doi: 10.1007/s00262-016-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Konrad MW, et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50:2009–2017. [PubMed] [Google Scholar]
- 188.Liu Z, et al. Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2. Nat. Commun. 2018;9:4682. doi: 10.1038/s41467-018-06954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Havunen R, et al. Oncolytic adenoviruses armed with tumor necrosis factor alpha and interleukin-2 enable successful adoptive cell therapy. Mol. Ther. Oncolytics. 2017;4:77–86. doi: 10.1016/j.omto.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vigil A, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 191.Zamarin D, et al. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 2009;16:796–804. doi: 10.1038/gt.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhao H, Janke M, Fournier P, Schirrmacher V. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res. 2008;136:75–80. doi: 10.1016/j.virusres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 193.Carew JF, et al. A novel approach to cancer therapy using an oncolytic herpes virus to package amplicons containing cytokine genes. Mol. Ther. 2001;4:250–256. doi: 10.1006/mthe.2001.0448. [DOI] [PubMed] [Google Scholar]
- 194.Hsieh C, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 195.Sangro B, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J. Clin. Oncol. 2004;22:1389–1397. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 196.Leonard JP, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 197.Nguyen KG, et al. Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 2020;11:575597. doi: 10.3389/fimmu.2020.575597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Raja Gabaglia C, et al. Attenuation of the glucocorticoid response during Ad5IL-12 adenovirus vector treatment enhances natural killer cell-mediated killing of MHC class I–negative LNCaP prostate tumors. Cancer Res. 2007;67:2290–2297. doi: 10.1158/0008-5472.CAN-06-3399. [DOI] [PubMed] [Google Scholar]
- 199.Raja Gabaglia C, et al. Attenuation of the glucocorticoid response during Ad5IL-12 adenovirus vector treatment enhances natural killer cell-mediated killing of MHC class I-negative LNCaP prostate tumors. Cancer Res. 2007;67:2290–2297. doi: 10.1158/0008-5472.CAN-06-3399. [DOI] [PubMed] [Google Scholar]
- 200.Bramson JL, et al. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum. Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- 201.Poutou J, et al. Safety and antitumor effect of oncolytic and helper-dependent adenoviruses expressing interleukin-12 variants in a hamster pancreatic cancer model. Gene Ther. 2015;22:696–706. doi: 10.1038/gt.2015.45. [DOI] [PubMed] [Google Scholar]
- 202.Wang P, et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017;8:1395. doi: 10.1038/s41467-017-01385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee YS, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin. Cancer Res. 2006;12:5859–5868. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- 204.Thaci B, et al. Depletion of myeloid-derived suppressor cells during interleukin-12 immunogene therapy does not confer a survival advantage in experimental malignant glioma. Cancer Gene Ther. 2014;21:38–44. doi: 10.1038/cgt.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gambotto A, et al. Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12. Cancer Gene Ther. 1999;6:45–53. doi: 10.1038/sj.cgt.7700013. [DOI] [PubMed] [Google Scholar]
- 206.Nguyen H-M, Guz-Montgomery K, Saha D. Oncolytic virus encoding a master pro-inflammatory cytokine interleukin 12 in cancer immunotherapy. Cells. 2020;9:400. doi: 10.3390/cells9020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leoni V, et al. A fully-virulent retargeted oncolytic HSV armed with IL-12 elicits local immunity and vaccine therapy towards distant tumors. PLoS Pathog. 2018;14:e1007209. doi: 10.1371/journal.ppat.1007209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Thomas ED, et al. IL-12 Expressing oncolytic herpes simplex virus promotes anti-tumor activity and immunologic control of metastatic ovarian cancer in mice. J. Ovarian Res. 2016;9:70. doi: 10.1186/s13048-016-0282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cheema TA, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc. Natl Acad. Sci. USA. 2013;110:12006–12011. doi: 10.1073/pnas.1307935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Alessandrini F, et al. Eradication of glioblastoma by immuno-virotherapy with a retargeted oncolytic HSV in a preclinical model. Oncogene. 2019;38:4467–4479. doi: 10.1038/s41388-019-0737-2. [DOI] [PubMed] [Google Scholar]
- 211.Parker JN, et al. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc. Natl Acad. Sci. USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32:253–267.e255. doi: 10.1016/j.ccell.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Derubertis BG, et al. Cytokine-secreting herpes viral mutants effectively treat tumor in a murine metastatic colorectal liver model by oncolytic and T-cell-dependent mechanisms. Cancer Gene Ther. 2007;14:590–597. doi: 10.1038/sj.cgt.7701053. [DOI] [PubMed] [Google Scholar]
- 214.Parker JN, et al. Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther. 2005;12:359–368. doi: 10.1038/sj.cgt.7700784. [DOI] [PubMed] [Google Scholar]
- 215.Varghese S, et al. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–265. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- 216.Veinalde R, et al. Oncolytic measles virus encoding interleukin-12 mediates potent antitumor effects through T cell activation. Oncoimmunology. 2017;6:e1285992. doi: 10.1080/2162402X.2017.1285992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang J, Liu T, Chen J. Oncolytic measles virus encoding interleukin-12 mediated antitumor activity and immunologic control of colon cancer in vivo and ex vivo. Cancer Biother. Radiopharm. 2021;36:774–782. doi: 10.1089/cbr.2019.3084. [DOI] [PubMed] [Google Scholar]
- 218.Shin EJ, et al. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope. 2007;117:210–214. doi: 10.1097/01.mlg.0000246194.66295.d8. [DOI] [PubMed] [Google Scholar]
- 219.Chen B, et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J. Immunother. 2001;24:46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 220.Nakao S, et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020;12:eaax7992. doi: 10.1126/scitranslmed.aax7992. [DOI] [PubMed] [Google Scholar]
- 221.Vijayakumar G, McCroskery S, Palese P. Engineering Newcastle disease virus as an oncolytic vector for intratumoral delivery of immune checkpoint inhibitors and immunocytokines. J. Virol. 2020;94:e01677–01619. doi: 10.1128/JVI.01677-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ren G, et al. Recombinant Newcastle disease virus encoding IL-12 and/or IL-2 as potential candidate for hepatoma carcinoma therapy. Technol. Cancer Res Treat. 2016;15:NP83–NP94. doi: 10.1177/1533034615601521. [DOI] [PubMed] [Google Scholar]
- 223.Alkayyal AA, et al. NK-cell recruitment is necessary for eradication of peritoneal carcinomatosis with an IL12-expressing Maraba virus cellular vaccine. Cancer Immunol. Res. 2017;5:211–221. doi: 10.1158/2326-6066.CIR-16-0162. [DOI] [PubMed] [Google Scholar]
- 224.Ge Y, et al. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J. Immunother. Cancer. 2020;8:e000710. doi: 10.1136/jitc-2020-000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chen L, et al. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics. 2021;11:6668–6681. doi: 10.7150/thno.56494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Skov S, Bonyhadi M, Odum N, Ledbetter JA. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J. Immunol. 2000;164:3500–3505. doi: 10.4049/jimmunol.164.7.3500. [DOI] [PubMed] [Google Scholar]
- 227.Robinson TO, Schluns KS. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017;190:159–168. doi: 10.1016/j.imlet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu RB, et al. IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proc. Natl Acad. Sci. USA. 2013;110:8158–8163. doi: 10.1073/pnas.1301022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hock K, et al. Oncolytic influenza A virus expressing interleukin-15 decreases tumor growth in vivo. Surgery. 2017;161:735–746. doi: 10.1016/j.surg.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 230.Backhaus PS, et al. Immunological effects and viral gene expression determine the efficacy of oncolytic measles vaccines encoding IL-12 or IL-15 agonists. Viruses. 2019;11:914. doi: 10.3390/v11100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cai, L. et al. The construction of a new oncolytic herpes simplex virus expressing murine interleukin-15 with gene-editing technology. J. Med. Virol. 92, 3617–3627 (2020). [DOI] [PubMed]
- 232.Kowalsky SJ, et al. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 2018;26:2476–2486. doi: 10.1016/j.ymthe.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang M, et al. IL-36gamma-armed oncolytic virus exerts superior efficacy through induction of potent adaptive antitumor immunity. Cancer Immunol. Immunother. 2021;70:2467–2481. doi: 10.1007/s00262-021-02860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen T, et al. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J. Immunother. Cancer. 2021;9:e001647. doi: 10.1136/jitc-2020-001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hu HJ, et al. The armed oncolytic adenovirus ZD55-IL-24 eradicates melanoma by turning the tumor cells from the self-state into the nonself-state besides direct killing. Cell Death Dis. 2020;11:1022. doi: 10.1038/s41419-020-03223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang C, et al. Oncolytic adenovirus expressing interleukin-18 improves antitumor activity of dacarbazine for malignant melanoma. Drug Des. Devel Ther. 2016;10:3755–3761. doi: 10.2147/DDDT.S115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 238.Bui JD, et al. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J. Immunol. 2006;176:905–913. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 239.Borden EC. Interferons alpha and beta in cancer: therapeutic opportunities from new insights. Nat. Rev. Drug Discov. 2019;18:219–234. doi: 10.1038/s41573-018-0011-2. [DOI] [PubMed] [Google Scholar]
- 240.Koeller JM. Biologic response modifiers: the interferon alfa experience. Am. J. Hosp. Pharm. 1989;46:S11–S15. [PubMed] [Google Scholar]
- 241.Bourgeois-Daigneault M-C, et al. Oncolytic vesicular stomatitis virus expressing interferon-σ has enhanced therapeutic activity. Mol. Ther. Oncolytics. 2016;3:16001. doi: 10.1038/mto.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Su C, et al. Immune gene–viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-γ gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol. Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 243.LaRocca CJ, et al. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery. 2015;157:888–898. doi: 10.1016/j.surg.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.LaRocca CJ, et al. Interferon alpha-expressing oncolytic adenovirus for treatment of esophageal adenocarcinoma. Ann. Surg. Oncol. 2021;28:8556–8564. doi: 10.1245/s10434-021-10382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Naik S, Nace R, Barber GN, Russell SJ. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β. Cancer Gene Ther. 2012;19:443–450. doi: 10.1038/cgt.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jenks N, et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-β in rodents and nonhuman primates. Hum. Gene Ther. 2010;21:451–462. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Saloura V, et al. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-β for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy. Hum. Gene Ther. 2010;21:51–64. doi: 10.1089/hum.2009.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Willmon CL, et al. Expression of IFN-β enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Huang H, et al. Interferon-β-armed oncolytic adenovirus induces both apoptosis and necroptosis in cancer cells. Acta Biochim. Biophys. Sin. 2012;44:737–745. doi: 10.1093/abbs/gms060. [DOI] [PubMed] [Google Scholar]
- 250.He LF, et al. Significant antitumor activity of oncolytic adenovirus expressing human interferon-β for hepatocellular carcinoma. J. Gene Med. 2008;10:983–992. doi: 10.1002/jgm.1231. [DOI] [PubMed] [Google Scholar]
- 251.Li H, et al. Oncolytic measles viruses encoding interferon β and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17:550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kirn DH, et al. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hasegawa Y, et al. Urokinase-targeted fusion by oncolytic sendai virus eradicates orthotopic glioblastomas by pronounced synergy with interferon-β gene. Mol. Ther. 2010;18:1778–1786. doi: 10.1038/mt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Buijs P, et al. Recombinant immunomodulating lentogenic or mesogenic oncolytic Newcastle disease virus for treatment of pancreatic adenocarcinoma. Viruses. 2015;7:2980–2998. doi: 10.3390/v7062756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cook, J. et al. TCL-461: clinical activity of systemic VSV-IFNβ-NIS oncolytic virotherapy in patients with relapsed refractory hematologic malignancies. Best Pract. Res. Clin. Rheumatol.21, S416–S417 (2021).
- 256.Schock SN, et al. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway. Cell Death Differ. 2017;24:615–625. doi: 10.1038/cdd.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Russell SJ, Barber GN. Oncolytic viruses as antigen-agnostic cancer vaccines. Cancer Cell. 2018;33:599–605. doi: 10.1016/j.ccell.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu W, et al. In situ therapeutic cancer vaccination with an oncolytic virus expressing membrane-tethered IL-2. Mol. Ther. Oncolytics. 2020;17:350–360. doi: 10.1016/j.omto.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liao JB, Publicover J, Rose JK, DiMaio D. Single-dose, therapeutic vaccination of mice with vesicular stomatitis virus expressing human papillomavirus type 16 E7 protein. Clin. Vaccin. Immunol. 2008;15:817–824. doi: 10.1128/CVI.00343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Martinez-Perez AG, et al. An oncolytic adenovirus encoding SA-4-1BBL adjuvant fused to HPV-16 E7 antigen produces a specific antitumor effect in a cancer mouse model. Vaccines. 2021;9:149. doi: 10.3390/vaccines9020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bridle BW, et al. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol. Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kantoff PW, Gulley JL, Pico-Navarro C. Revised overall survival analysis of a phase II, randomized, double-blind, controlled study of PROSTVAC in men with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2017;35:124–125. doi: 10.1200/JCO.2016.69.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gulley JL, et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019;37:1051–1061. doi: 10.1200/JCO.18.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pol JG, et al. Enhanced immunotherapeutic profile of oncolytic virus-based cancer vaccination using cyclophosphamide preconditioning. J. Immunother. Cancer. 2020;8:e000981. doi: 10.1136/jitc-2020-000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jeong S, Park SH. Co-stimulatory receptors in cancers and their implications for cancer immunotherapy. Immune Netw. 2020;20:e3. doi: 10.4110/in.2020.20.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Crawford A, et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vaddepally RK, et al. Review of indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hansen K, et al. COM902, a novel therapeutic antibody targeting TIGIT augments anti-tumor T cell function in combination with PVRIG or PD-1 pathway blockade. Cancer Immunol. Immunother. 2021;70:3525–3540. doi: 10.1007/s00262-021-02921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kong Y, et al. T-Cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-cell exhaustion and poor clinical outcome in aml patients. Clin. Cancer Res. 2016;22:3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 270.Shayan G, et al. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K–Akt pathway in head and neck cancer. Oncoimmunology. 2017;6:e1261779. doi: 10.1080/2162402X.2016.1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sekar D, et al. Downregulation of BTLA on NKT cells promotes tumor immune control in a mouse model of mammary carcinoma. Int. J. Mol. Sci. 2018;19:752. doi: 10.3390/ijms19030752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 273.Lyon AR, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 274.Varricchi G, et al. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Curr. Med. Chem. 2018;25:1327. doi: 10.2174/0929867324666170407125017. [DOI] [PubMed] [Google Scholar]
- 275.Bartee MY, Dunlap KM, Bartee E. Tumor-localized secretion of soluble pd1 enhances oncolytic virotherapy. Cancer Res. 2017;77:2952–2963. doi: 10.1158/0008-5472.CAN-16-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhang H, et al. Recombinant oncolytic adenovirus expressing a soluble PVR elicits long-term antitumor immune surveillance. Mol. Ther. Oncolytics. 2021;20:12–22. doi: 10.1016/j.omto.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Engeland CE, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014;22:1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kleinpeter P, et al. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death-1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. OncoImmunology. 2016;5:e1220467. doi: 10.1080/2162402X.2016.1220467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tian C, et al. Enhanced anti-tumor response elicited by a novel oncolytic HSV-1 engineered with an anti-PD-1 antibody. Cancer Lett. 2021;518:49–58. doi: 10.1016/j.canlet.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 280.Andrew Mark Haydon GK, et al. A phase 1, open-label, dose escalation study of the safety and tolerability of T3011 in advanced cutaneous or subcutaneous malignancies. J. Clin. Oncol. 2021;39:2526. [Google Scholar]
- 281.Zuo S, et al. Enhanced antitumor efficacy of a novel oncolytic vaccinia virus encoding a fully monoclonal antibody against T-cell immunoglobulin and ITIM domain (TIGIT) EBioMedicine. 2021;64:103240. doi: 10.1016/j.ebiom.2021.103240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lei GL, et al. A recombinant influenza virus with a CTLA4-specific scFv inhibits tumor growth in a mouse model. Cell Biol. Int. 2021;45:1202–1210. doi: 10.1002/cbin.11559. [DOI] [PubMed] [Google Scholar]
- 283.Vaddepally RK, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw. Open. 2020;3:e200423. doi: 10.1001/jamanetworkopen.2020.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Daud AI, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J. Clin. Oncol. 2016;34:4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kelly KR, et al. Oncolytic reovirus sensitizes multiple myeloma cells to anti-PD-L1 therapy. Leukemia. 2018;32:230–233. doi: 10.1038/leu.2017.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Feist M, et al. Oncolytic virus promotes tumor-reactive infiltrating lymphocytes for adoptive cell therapy. Cancer Gene Ther. 2021;28:98–111. doi: 10.1038/s41417-020-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ribas A, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031–1032. doi: 10.1016/j.cell.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 290.Chesney J, et al. Randomized, open-Label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Goldmacher GV, et al. Response criteria for intratumoral immunotherapy in solid tumors: itRECIST. J. Clin. Oncol. 2020;38:2667–2676. doi: 10.1200/JCO.19.02985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nguyen HM, Bommareddy PK, Silk AW, Saha D. Optimal timing of PD-1 blockade in combination with oncolytic virus therapy. Semin. Cancer Biol. 2021;S1044-579X:00149–00148. doi: 10.1016/j.semcancer.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 293.Han D, et al. Current progress in CAR-T cell therapy for hematological malignancies. J. Cancer. 2021;12:326–334. doi: 10.7150/jca.48976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. 2020;181:46–62. doi: 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Boucher JC, Davila ML. Chimeric antigen receptor design today and tomorrow. Cancer J. 2021;27:92–97. doi: 10.1097/PPO.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 296.Xu Y, et al. Preclinical development of T-cell receptor-engineered T-cell therapy targeting the 5T4 tumor antigen on renal cell carcinoma. Cancer Immunol. Immunother. 2019;68:1979–1993. doi: 10.1007/s00262-019-02419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ali S, et al. The European Medicines Agency review of Kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist. 2020;25:e321–e327. doi: 10.1634/theoncologist.2019-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Papadouli I, et al. EMA review of Axicabtagene Ciloleucel (Yescarta) for the treatment of diffuse large B-cell lymphoma. Oncologist. 2020;25:894–902. doi: 10.1634/theoncologist.2019-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Aschenbrenner DS. New treatment for relapsed or refractory large B-cell lymphoma. Am. J. Nurs. 2021;121:21–22. doi: 10.1097/01.NAJ.0000753644.07326.f6. [DOI] [PubMed] [Google Scholar]
- 300.Silkenstedt E, Dreyling M. Mantle cell lymphoma—advances in molecular biology, prognostication and treatment approaches. Hematol. Oncol. 2021;39:31–38. doi: 10.1002/hon.2860. [DOI] [PubMed] [Google Scholar]
- 301.Lo Presti V, Buitenwerf F, van Til NP, Nierkens S. Gene augmentation and editing to improve TCR engineered T cell therapy against solid tumors. Vaccines (Basel). 2020;8:733. doi: 10.3390/vaccines8040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ellis GI, Sheppard NC, Riley JL. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 2021;22:427–447. doi: 10.1038/s41576-021-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ager A, Watson HA, Wehenkel SC, Mohammed RN. Homing to solid cancers: a vascular checkpoint in adoptive cell therapy using CAR T-cells. Biochem. Soc. Trans. 2016;44:377–385. doi: 10.1042/BST20150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Beatty GL, et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhang Y, Guan XY, Jiang P. Cytokine and chemokine signals of T-cell exclusion in tumors. Front. Immunol. 2020;11:594609. doi: 10.3389/fimmu.2020.594609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Moon EK, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jin L, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 2019;10:4016. doi: 10.1038/s41467-019-11869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Liu G, et al. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur. J. Immunol. 2020;50:712–724. doi: 10.1002/eji.201948457. [DOI] [PubMed] [Google Scholar]
- 309.Di Stasi A, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Idorn M, et al. Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model. Oncoimmunology. 2018;7:e1450715. doi: 10.1080/2162402X.2018.1450715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chen Z, Liu J, Xu D. [Oncolytic adenovirus expressing CCL19 enhances immunity against gastric cancer in mice] Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi. 2021;37:119–124. [PubMed] [Google Scholar]
- 312.Eckert EC, et al. Generation of a tumor-specific chemokine gradient using oncolytic vesicular stomatitis virus encoding CXCL9. Mol. Ther. Oncolytics. 2020;16:63–74. doi: 10.1016/j.omto.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nishio N, Dotti G. Oncolytic virus expressing RANTES and IL-15 enhances function of CAR-modified T cells in solid tumors. Oncoimmunology. 2015;4:e988098. doi: 10.4161/21505594.2014.988098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Schmidts A, Maus MV. Making CAR T cells a solid option for solid tumors. Front. Immunol. 2018;9:2593. doi: 10.3389/fimmu.2018.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kueberuwa G, et al. CD19 CAR T cells expressing IL-12 eradicate lymphoma in fully lymphoreplete mice through induction of host immunity. Mol. Ther. Oncolytics. 2018;8:41–51. doi: 10.1016/j.omto.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Koneru M, et al. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lanitis E, et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression. J. Exp. Med. 2021;218:e20192203. doi: 10.1084/jem.20192203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Hurton LV, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hu B, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.He C, et al. Co-expression of IL-7 improves NKG2D-based CAR T cell therapy on prostate cancer by enhancing the expansion and inhibiting the apoptosis and exhaustion. Cancers (Basel) 2020;12:1969. doi: 10.3390/cancers12071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ma X, et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kunert A, et al. Intra-tumoral production of IL18, but not IL12, by TCR-engineered T cells is non-toxic and counteracts immune evasion of solid tumors. Oncoimmunology. 2017;7:e1378842. doi: 10.1080/2162402X.2017.1378842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Alsaieedi A, et al. Safety and efficacy of Tet-regulated IL-12 expression in cancer-specific T cells. Oncoimmunology. 2019;8:1542917. doi: 10.1080/2162402X.2018.1542917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Pol JG, et al. Cytokines in oncolytic virotherapy. Cytokine Growth Fact. Rev. 2020;56:4–27. doi: 10.1016/j.cytogfr.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 325.Watanabe K, et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3:e99573. doi: 10.1172/jci.insight.99573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Huang J, et al. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol. Immunother. 2021;70:2453–2465. doi: 10.1007/s00262-021-02856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ma R, et al. An oncolytic virus expressing IL15/IL15Ralpha combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 2021;81:3635–3648. doi: 10.1158/0008-5472.CAN-21-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mariathasan S, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Neuzillet C, et al. Targeting the TGFbeta pathway for cancer therapy. Pharm. Ther. 2015;147:22–31. doi: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 330.Yang YA, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J. Clin. Investig. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hu Z, Zhang Z, Guise T, Seth P. Systemic delivery of an oncolytic adenovirus expressing soluble transforming growth factor-beta receptor II-Fc fusion protein can inhibit breast cancer bone metastasis in a mouse model. Hum. Gene Ther. 2010;21:1623–1629. doi: 10.1089/hum.2010.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang Z, et al. Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther. 2012;19:630–636. doi: 10.1038/cgt.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Li Y, et al. Oncolytic adenovirus targeting TGF-beta enhances anti-tumor responses of mesothelin-targeted chimeric antigen receptor T cell therapy against breast cancer. Cell. Immunol. 2020;348:104041. doi: 10.1016/j.cellimm.2020.104041. [DOI] [PubMed] [Google Scholar]
- 334.Kailayangiri S, et al. Overcoming heterogeneity of antigen expression for effective CAR T cell targeting of cancers. Cancers (Basel) 2020;12:1075. doi: 10.3390/cancers12051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Dagher O, King TR, Wellhausen N, Posey AD., Jr. Combination therapy for solid tumors: taking a classic car on new adventures. Cancer Cell. 2020;38:621–623. doi: 10.1016/j.ccell.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 336.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wing A, et al. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol. Res. 2018;6:605–616. doi: 10.1158/2326-6066.CIR-17-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Fajardo CA, et al. Oncolytic Adenoviral Delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017;77:2052–2063. doi: 10.1158/0008-5472.CAN-16-1708. [DOI] [PubMed] [Google Scholar]
- 339.Porter CE, et al. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol. Ther. 2020;28:1251–1262. doi: 10.1016/j.ymthe.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Guo ZS, et al. Bi- and tri-specific T cell engager-armed oncolytic viruses: next-generation cancer immunotherapy. Biomedicines. 2020;8:204. doi: 10.3390/biomedicines8070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Park AK, et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020;12:eaaz1863. doi: 10.1126/scitranslmed.aaz1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Aalipour A, et al. Viral delivery of CAR targets to solid tumors enables effective cell therapy. Mol. Ther. Oncolytics. 2020;17:232–240. doi: 10.1016/j.omto.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lee, D., Minn, A. J., Johnson L. R. & More, S. CAR-T cells to deliver engineered peptide antigens and reprogram antigen specific T cell responses against solid tumors. J. Clin. Oncol. 39, 2530–2530 (2021).
- 344.Tang X, et al. Adenovirus-mediated specific tumor tagging facilitates CAR-T therapy against antigen-mismatched solid tumors. Cancer Lett. 2020;487:1–9. doi: 10.1016/j.canlet.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 345.Tang XY, et al. Tumor-tagging by oncolytic viruses: a novel strategy for CAR-T therapy against solid tumors. Cancer Lett. 2021;503:69–74. doi: 10.1016/j.canlet.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 346.Zhao Y, Shao Q, Peng G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell. Mol. Immunol. 2020;17:27–35. doi: 10.1038/s41423-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Poorebrahim M, et al. Counteracting CAR T cell dysfunction. Oncogene. 2021;40:421–435. doi: 10.1038/s41388-020-01501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Abate-Daga D, et al. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood. 2013;122:1399–1410. doi: 10.1182/blood-2013-04-495531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2:e26286. doi: 10.4161/onci.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Moon EK, et al. Blockade of programmed death 1 augments the ability of human T cells engineered to target NY-ESO-1 to control tumor growth after adoptive transfer. Clin. Cancer Res. 2016;22:436–447. doi: 10.1158/1078-0432.CCR-15-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wang DY, Johnson DB, Davis EJ. Toxicities associated with PD-1/PD-L1 blockade. Cancer J. 2018;24:36–40. doi: 10.1097/PPO.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ping Y, et al. Augmenting the effectiveness of CAR-T cells by enhanced self-delivery of PD-1-neutralizing scFv. Front. Cell Dev. Biol. 2020;8:803. doi: 10.3389/fcell.2020.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Tanoue K, et al. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Res. 2017;77:2040–2051. doi: 10.1158/0008-5472.CAN-16-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Mullinax JE, et al. Expanded tumor-infiltrating lymphocytes from soft tissue sarcoma have tumor-specific function. J. Immunother. 2021;44:63–70. doi: 10.1097/CJI.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci. Transl. Med. 2015;7:280ps287. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 356.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]