Abstract
Oral cancer is the fourth most common cancer among men in Taiwan. The age-standardized incidence rate of oral cancer among men in Taiwan has increased since 1980 and became six times greater in 2014. To enable effective public health planning for oral cancer, research on the projection of oral cancer burden is essential. We conducted an age-period-cohort analysis on the incidence of oral cancer among men in Taiwan from 1997 to 2017 and extrapolated the trend to 2025. We found that the period trends for young adults aged between 25 and 44 have already peaked before 2017; the younger, the earlier, and then the trends declined. The cohort trends have peaked roughly at the 1972 birth cohort and then declined for all ages. Despite the increasing trend in the age-standardized incidence rate for oral cancer among men in Taiwan from 1997 to 2017, we forecast a peak attained, an imminent decline after 2017, and a decrease of 8.4% in age-standardized incidence rate from 2017 to 2025. The findings of this study contribute to developing efficient and comprehensive strategies for oral cancer prevention and control.
Subject terms: Diseases, Oncology, Mathematics and computing
Introduction
Worldwide increases in the incidence of various cancers impose a significant burden on health and economics1. A total of 14.1 and 18.1 million new cancer cases were diagnosed in 2012 and 2018, respectively, an increase of approximately 28.4%2,3. Among all cancers, oral cancer (including lip, oral cavity, oropharynx, and hypopharynx cancers) is a major disease category because of its substantial impact on patients’ quality of life4–8. From 1990 to 2017, the global incidence of oral cancer exhibited an upward trend, and increases were primarily for people aged 15–49 years and with low-middle socioeconomic status9. Men are more than twice as likely as women to develop oral cancer. In Taiwan, male predominance is even more notable; oral cancer is the fourth most common cancer among men with an incidence of 7,400 cases (42.15 per 100,000 people), but only the fifteenth common cancer among women with an incidence of 770 cases (3.92 per 100,000 people) in 201810.
In recent years, the escalating global cancer burden motivates cancer incidence projections for cancer prevention and control11–27. Two studies regarding the prediction of oral cancer incidence were successively conducted in the U.S.26,27. The first one made projections with the joinpoint regression28, and their forecasts supported the argument that strengthened vaccination efforts of human papillomavirus virus (HPV) to reduce the future burden. The second one used the age-period-cohort (APC) model29 instead, given the strong cohort effect of oral cancer incidence in the U.S. They forecast a continued shift in the incidence to an older population because of the low HPV vaccination rates among older individuals. In Taiwan, the age-standardized incidence rate of oral cancer among men has increased since 1980, and in 2014 the incidence rate was sixfold that of 198030. Such a rapid increase may be associated with cigarette smoking and betel quid chewing in the population30–36. To enable effective public health planning for oral cancer, research projecting the oral cancer burden is essential. Therefore, we studied the incidence trend of oral cancer among men in Taiwan from 1997 to 2017 and extrapolated the trend to 2025 by the APC model. We discussed possible factors driving these trends and suggested improving oral cancer prevention and control.
Materials and methods
Ethics
This study protocol was approved by the National Taiwan University Research Ethics Committee (202101HM030) and the Data Release Review Board of the Health Promotion Administration, Ministry of Health and Welfare in Taiwan. All methods were performed following the relevant guidelines and regulations. In addition, the Research Ethics Committee waived the requirement for informed consent due to the lack of personal information and secondary data in the study.
Data source, case definition, and study population
All information on newly diagnosed malignant neoplasms from hospitals with capacities of > 50 beds has been recorded in the Taiwan Cancer Registry dataset, a nationwide, population-based registry, since 1979. Every patient in the dataset comprises demographics (sex, date of birth, and residential area code) and diagnostic data (date of diagnosis, tumor site, histopathological information, and tumor grade). To strengthen the validity, completeness, and timeliness of the Taiwan Cancer Registry Dataset, multiple verification processes were conducted, containing logical and consistency assessments, duplicate checks, and trace-back of death certificate-only cases. The registry has been in its maturity stage since 2003 and with stable high quality (timeliness < 14 months, completeness > 98%, a morphological verified rate ≈ 93%, and a percentage of cases registered only in death certificate < 1%)37–39. Cases with a definitive diagnosis before 2002 were encoded according to the Field Trial Edition of the International Classification of Disease for Oncology (ICD-O-FT). The newest version, ICD-O-3, has been used as the standard code source since 200340. Yearly cancer incidence data (ICO-O-FT: 140, 141, 143–146, 148, 149; ICD-O-3: C00–C06, C09, C10, C12–C14) for men from 1997 to 2017 were included in the study.
Patients were categorized into twelve 5-year age groups (25–29, 30–34, 35–39, 40–44, 45–49, …, 80–84). We excluded patients aged < 25 years or > 84 years because of the scarcity of cases. The period between 1997 and 2017 was divided into twenty 1-year groups. The corresponding periods and age group population were obtained from the online database maintained by the Department of Statistics of Taiwan’s Ministry of the Interior. The World Health Organization 2000 World Standard Population with the truncated age interval of 25–84 was used for age standardization (Table S6).
Age‑period‑cohort model
The APC model was used to analyze the oral cancer incidence rate and project the trend to 2025, given the strong cohort effect in Taiwan36. However, because of the perfect linearity of the temporal variables (cohort + age = period), an infinite set of parameter estimates with equal goodness of fit exists, causing the non-identifiability problem. In addition, we used data provided in 5-year age groups and 1-year periods in the study, which might cause additional identifiability issues with the unequal intervals in their definition of cohort indices41. To circumvent the non-identifiability problem inherent in the APC model, the linear cohort effect was not estimated in the APC model, presented as follows:
where g(.) was the link function, was the expected incidence cases, m was the person-years, a, p, and c were the age variable, the period variable, and the cohort variable, respectively, was the intercept, and were the linear age effect and the linear period effect, respectively, and , , and were functions of the age variable, the period variable, and the cohort variable, respectively, denoting the nonlinear effects. Maximum likelihood estimation was used for estimating the parameters in the APC model.
Ensemble learning and model selection
We applied an ensemble technique to obtain an APC model with the best predictive performance. In this technique, various APC models in the ensemble are trained on the given training dataset. Finally, the model that performs best on the validation set is chosen for future use. We considered a threefold validation, splitting the complete data (21 calendar years from 1997 to 2017) into a training set (14 calendar years from 1997 to 2010) and a validation set (7 calendar years from 2011 to 2017). A total of 52 types of APC models (with the formula presented in Table S4) were considered for training. The model types referred to in previous research11–13,42–45, included polynomial APC prediction models (Type 1 to Type 17)42, Tzeng and Lee’s APC prediction model (Type 18 to Type 22)43,44, and cubic splines APC prediction models (Type 23 to Type 52)11–13,45. For the cubic splines APC models, the knot locations were placed at:
where , , and were the number of age groups, period groups, and cohort groups, respectively, and , , and were the number of knots for age, period, and cohort, respectively. We considered five types of link functions (log, power 2, power 3, power 4, and power 5) for the 52 model types. Also, with the assumption that historical trends will not continue indefinitely11–13,42, each APC model projection was applied to 21 levels (0%, 5%, 10%, 15%, …, or 100%) of year-on-year attenuation (, where was the attenuated predicted value, was the predicted value, was the last observed value, and A was the attenuation level). A total of 5460 models (52 5 21) were constructed and formed the ensemble APC model.
The ensemble APC model was used to predict the incidence rate of the validation set (calendar years from 2011 to 2017). Model selection was based on the index of the symmetric mean absolute percentage error (, where was the predicted value and was the observed value) and the logarithmic score (, where was the probability density function and was the observed value). We selected a final model with the lowest SMAPE or the maximal LOGS. Finally, we re-estimated the model parameters based on oral cancer incidence data from 1997 to 2017 (all available data) and made projections for 2025. We presented a diagram of the method for building APC prediction models in Fig. S1.
We also conducted sensitivity analyses and presented results in the supplementary materials. The sensitivity analyses included working the model selection with a fivefold validation (16 calendar years from 1997 to 2012 for training and the remaining five calendar years for validating), applying log-transformation on the SMAPE (), considering another attenuation strategy with and , where , and performing the same analysis for women. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). The SAS code for analysis was presented in the supplementary materials.
Results
We selected the optimal model as a cubic splines APC model (type 26 in Table S4) with 80% attenuation, presented as follows: where . The validation statistics of the selected model were SMAPE = 8.59 and LOGS = − 469.75. The age-standardized oral cancer incidence for 1997–2017 and projections by the APC model for 2018 to 2025 are presented in Fig. 1. The age-standardized incidence rate increased considerably from 1997, with the incidence rate doubled in 2009 compared with 1997, but then the increasing trend slowed. The projection indicated that the trend would start to decline in 2017 and further decline until 2025.
Figure 1.
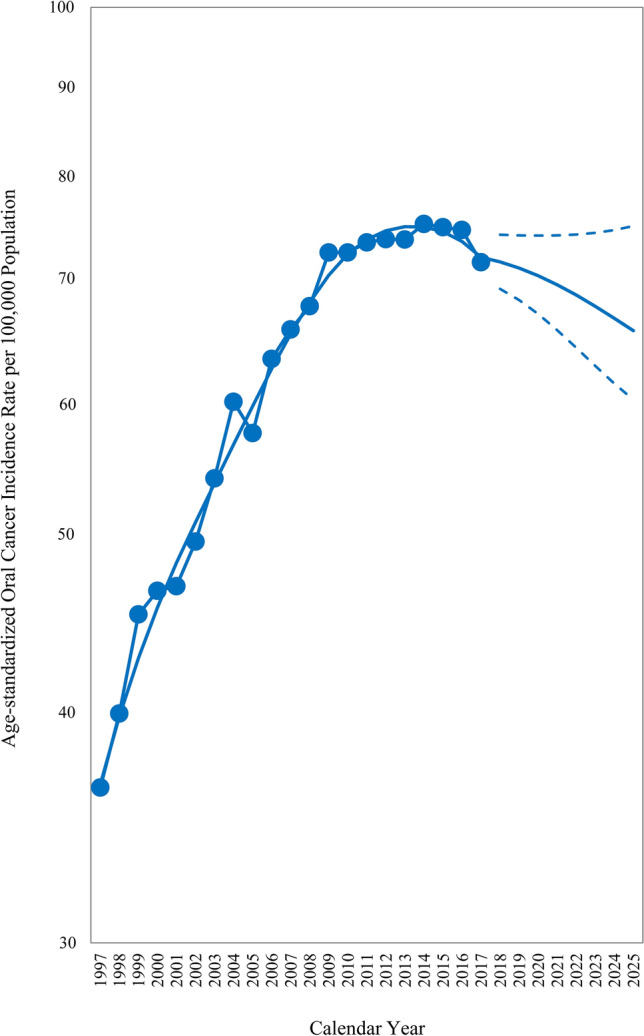
Age-standardized oral cancer incidence rates from 1997 to 2017 and the age–period–cohort model projections from 2018 to 2025. The World Health Organization 2000 Standard Population was used to compute the truncated age-standardized incidence rates (age range: 25–84 years), see Table S6.
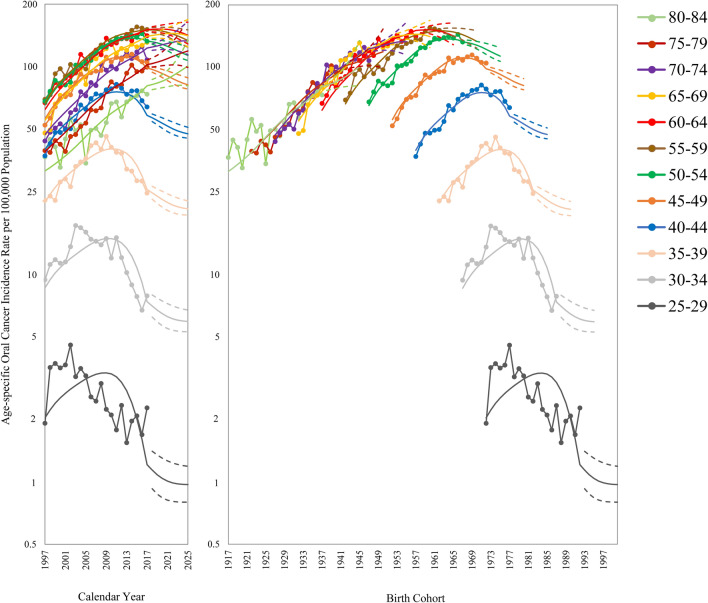
Data on age-specific oral cancer incidence rate among men in Taiwan by calendar year and birth cohort are displayed in Fig. 2. Period trends diverged across age groups. Period trends for older age groups (70–74, 75–79, and 80–84) were rapidly increasing, and the projections also show an increasing trend. Period trends for the middle-age groups (40–44, 45–49, 50–54, 55–59, 60–64, and 65–69) were increasing at first but then leveled off, with projections indicating a stable trend (60–64 and 65–69) or decrease (40–44, 45–49, 50–54, and 55–59). Period trends for the younger age groups (25–29, 30–34, and 35–39) initially increased rapidly, peaked—the younger, the earlier, and then decreased. Period trends for these younger age groups are projected to further decline until 2025. Birth cohort trends were consistent for all age groups: trends increased before 1973 and decreased after that.
Figure 2.
Age-specific oral cancer incidence rates among men in Taiwan by calendar year and birth cohort (age range: 25–84 years).
The age-standardized and age-specific oral cancer incidence per 100,000 population (observed rates in 2017 and projected rates in 2025) and projected percentage changes from 2018 to 2025 are shown in Table 1. The age-standardized incidence rate is poised to decrease by 8.4% from 2017 to 2025. The age-specific incidence rates of age groups 80–84, 75–79, and 70–74 are predicted to increase substantially by 20% from 2017 to 2025. The age-specific incidence rates for 65–69 and 60–64 are expected to increase slightly by < 10% from 2017 to 2025. The age-specific incidence rate of 55–59 will decrease by approximately 10% from 2017 to 2025. The age-specific incidence rates of 50–54 and 35–39 will reduce by about 15% from 2017 to 2025. The age-specific incidence rates of the remaining age groups (45–49, 40–44, 30–34, and 25–29) are expected to decrease substantially by > 20% from 2017 to 2025.
Table 1.
Age-standardized and age-specific oral cancer incidence per 100,000 population (observed rates in 2017 and projected rates for 2025) and projected percentage changes from 2018 to 2025.
| Incidence in 2017 per 100,000 population | Projected incidence in 2025 per 100,000 population [95% Uncertainty Interval] | Projected percentage change from 2018 [95% Uncertainty Interval] | |
|---|---|---|---|
| Age-standardized rates# | 72.05 | 65.98 [60.36, 75.49] | − 8.42 [− 16.21, 4.78] |
| Age-specific rates | |||
| Age 25–29 | 2.27 | 0.97 [0.80, 1.19] | − 57.32 [− 64.89, − 47.71] |
| Age 30–34 | 7.85 | 5.92 [5.28, 6.72] | − 24.66 [− 32.79, − 14.51] |
| Age 35–39 | 24.58 | 20.63 [19.24, 22.53] | − 16.05 [− 21.73, − 8.34] |
| Age 40–44 | 63.86 | 47.39 [45.17, 50.93] | − 25.78 [− 29.26, − 20.25] |
| Age 45–49 | 104.74 | 81.60 [77.83, 88.18] | − 22.09 [− 25.69, − 15.81] |
| Age 50–54 | 132.40 | 113.10 [106.74, 124.25] | − 14.57 [− 19.38, − 6.15] |
| Age 55–59 | 150.77 | 133.43 [123.84, 150.01] | − 11.50 [− 17.86, − 0.50] |
| Age 60–64 | 140.67 | 141.42 [128.47, 163.60] | 0.53 [− 8.68, 16.30] |
| Age 65–69 | 130.79 | 140.66 [124.42, 168.38] | 7.55 [− 4.87, 28.74] |
| Age 70–74 | 107.56 | 132.79 [113.90, 165.02] | 23.45 [5.89, 53.41] |
| Age 75–79 | 99.43 | 118.81 [99.05, 152.85] | 19.49 [− 0.39, 53.73] |
| Age 80–84 | 73.54 | 100.59 [80.77, 134.50] | 36.79 [9.83, 82.90] |
#The World Health Organization 2000 World Standard Population was used to compute the truncated age-standardized incidence rates (age 25–84 years), see Table S6.
The results of the sensitivity analysis were presented in the supplementary materials. The optimal model based on 0% attenuation in 2011 and 80% attenuation from 2012 to 2017 was the type 26 model with and . The results for men based on the SMAPE-T (Fig. S3) or a 5-fold validation (Figs.S6-S7) showed similar projected trends. In addition, the projection for women (Figs. S4-S5) showed a similar age-standardized incidence trend to men. However, the age-specific incidence rate among women increased slower than among men for 65–84.
Discussion
The age-standardized incidence rate of oral cancer among men in Taiwan presented a rapidly increasing trend at first, but the increasing trend slowed after 2009. The historical trend of age-standardized rate alone makes it challenging to visualize peak attainment and imminent decline after 2017. By contrast, the APC analysis showed that period trends for young adults aged 25–44 years had already peaked before 2017—the younger, the earlier, and then the trends declined. Moreover, a significant cohort effect was revealed, decreasing incidence rates at roughly the 1972 birth cohort for all ages. The combined results indicate a peak has been attained, and the trend is now for oral cancer incidence to decrease among men in Taiwan. Previously, Hsu et al.30 predicted a future decline but not before 2025. However, they only created one APC model and did not use techniques to prevent overfitting. By contrast, we used a data-splitting method and constructed an ensemble APC model to allow for flexible model specification. Furthermore, Hsu et al.30 only studied the trend in age-standardized incidence rate but not the period trends and birth cohort trends by age.
We did not estimate the linear cohort effect for making parameters identifiable in the study. The use of additional constraints can successfully decompose the APC effect. However, different constraints may cause drastically different or even contradictory results. There is no consensus in the APC literature on which constraints are the best and used. Besides, the incidence projections in this study were impervious to the non-identifiability problem because the fitted values in the nonidentifiable APC model were the same for all possible sets of parameter estimates. According to Figs. 1 and 2, a discontinuity could be observed when the prediction started, caused by imposing the 80% attenuation on the predictive value. The idea of attenuating the projected trend comes from the belief that historical trends will not continue indefinitely and was shown to be valuable empirically for making future predictions by Møller et al.11–13,42 When alleviating the attenuation level from 80 to 0%, the result showed a rapidly decreasing trend after 2017 (Fig. S8). Still, the SMAPE would increase from 8.59 to 11.52, and the LOGS would drop from − 469.75 to − 490.82. With the experience by Møller et al.13,42, we also found that it worked better to gradually reduce the projected trend (SMAPE = 8.59 and LOGS = − 469.75) than attenuate the trend a period after the last observation (SMAPE = 9.04 and LOGS = − 486.84). Therefore, it seems favorable to gradually attenuate the projected trend in the study. In addition, the selected type 26 model with 80% attenuation had the lowest SMAPE (8.59) but the second-highest LOGS (− 469.75). Notably, the same model with a bit higher 85% attenuation had the second-lowest SMAPE (8.78) and the highest LOGS (− 460.61). Therefore, it is suitable to predict the oral cancer incidence among men in Taiwan with the type 26 model with 80% or 85% attenuation.
The data splitting method in the study was referred to in the previous studies20,25 in Taiwan. However, unlike a 2-fold validation in those studies, we applied a 3-fold validation, two-thirds of the calendar years (1997–2010) used for training and the remaining (2011–2017) for validating, for the following motivation and reasons: (1) given the calendar year ended in 2010, we could include enough data cells for the 1972 birth cohort and later for training (Table S3) to capture the cohort effect for oral cancer among men in Taiwan36, (2) a sensitivity analysis with a 5-fold validation was considered, and the similar projected trends were obtained (Figs. S6-S7), and (3) Taiwan have implemented the National Health Insurance Program and passed the legislation of Tobacco Hazards Prevention Act since 1997, which might impact the oral cancer incidence trend in Taiwan.
Betel quid chewing and smoking are risk factors for oral cancer among men in Taiwan46,47. In 2005 in Taiwan, the betel quid chewing rate was approximately 10%48, and the smoking rate among men was about 50%49. Notably, the betel quid chewing rate for the indigenous peoples of Taiwan was four times the overall rate in Taiwan50. The World Health Organization Global Oral Health Programme presented the common risk factor approach for preventing and controlling non-communicable diseases51,52. Public health interventions, including tax policies and health education49,53–56, for preventing excessive risk exposure of these lifestyle factors57 have been implemented since 1997 in Taiwan. These measures have continuously declined Taiwan's betel quid chewing and smoking rates49,54. Su et al.36 conducted an APC analysis to examine the incidence rate of oral cancer among men in Taiwan from 1997 to 2016. They found a strong association between the cohort effect on oral cancer incidence rate among men and average betel nut consumption with a lag time of 30 years. A decrease in smoking rates may also contribute to the decreasing oral cancer incidence rate after the 1972 birth cohort. Per our projections, by 2025, the age-specific incidence rate will have decreased for those between 25 and 59 years old but increased for those between 60 and 84 years old. Health care professionals have actively implemented long-term care services since 2007 to alleviate the excessive burden of an aging population in Taiwan to ensure older adults can live healthier lives58,59.
Early detection of oral cancer can prolong life expectancy60. Taiwan started a national oral cancer screening program in 200461,62 that provides a free oral mucosal examination every two years for Taiwanese residents with habits of smoking or betel quid chewing. Between 2004 and 2009, the overall oral cancer screening rate was 55.1% in Taiwan62. A shift has been observed, indicating that patients with malignant oral cancer can be detected earlier because of the screening program62. In addition, we obtained contrasting projected oral incidence trends in men and women for 65–84. Because men might have a higher prevalence of risk factors and more opportunities to be screened for oral cancer, the early detection due to the screening program might have caused the increase in oral cancer incidence among men aged between 65 and 84 since 2004.
In conclusion, despite the increasing trend in the age-standardized incidence rate of oral cancer among men in Taiwan from 1997 to 2017, we determined that a peak was reached in 2017, and the incidence has subsequently been in decline, with a decrease of 8.4% in age-standardized incidence from 2017 to 2025. Therefore, the findings of this study contribute to developing efficient and comprehensive strategies for oral cancer prevention and control.
Supplementary Information
Abbreviations
- APC
Age-period-cohort
- HPV
Human papillomavirus virus
- SMAPE
Symmetric mean absolute percentage error
Author contributions
J.-R.J. took responsibility for conceptualization, original draft writing, review and editing, formal analysis, and the final version of the manuscript. S.-Y.S. contributed to the methodology and approval of the final version of the manuscript. C.-J.C., Y.-W.Y., L.-J.L., and T.-H.H. contributed to managing data from the Taiwan Cancer Registry and the final version of the manuscript. Wen-Chung Lee helped with the conceptualization and approval of the final version of the manuscript.
Funding
This work was supported by grants from the Health Promotion Administration, the Ministry of Health and Welfare in Taiwan (A1091115; the tobacco control and health care funds), the Ministry of Science and Technology in Taiwan (MOST 108-3017-F-002–001, MOST 108-2314-B-002-127-MY3), and the Innovation and Policy Center for Population Health and Sustainable Environment (Population Health Research Center, PHRC) from Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan (NTU‐109L900308). The funder had no role in study design, data collection, analysis, or manuscript preparation.
Data availability
The authors confirm that all data underlying the findings are fully available without restriction. The data underlying the results of this study are either available in the manuscript or upon request from the corresponding author, Wen-Chung Lee. The raw data cannot be made available in supplemental files or a public repository because of privacy or ethical reasons. However, the dataset used for analysis can be obtained in an anonymized form upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-09736-2.
References
- 1.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward ME. The global burden of cancer: Priorities for prevention. Carcinogenesis. 2010;31:100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, et al. Global cancer statistics, 2012. C.A. Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Calver L, Tickle A, Moghaddam N, Biswas S. The effect of psychological interventions on quality of life in patients with head and neck cancer: A systematic review and meta-analysis. Eur. J. Cancer Care. 2018 doi: 10.1111/ecc.12789. [DOI] [PubMed] [Google Scholar]
- 5.Osazuwa-Peters N, Arnold LD, Loux TM, Varvares MA. Factors associated with increased risk of suicide among survivors of head and neck cancer: A population-based analysis. Oral. Oncol. 2018;81:29–34. doi: 10.1016/j.oraloncology.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Osazuwa-Peters N, et al. Suicide risk among cancer survivors: Head and neck versus other cancers. Cancer. 2018;124:4072–4079. doi: 10.1002/cncr.31675. [DOI] [PubMed] [Google Scholar]
- 7.Zaorsky NG, et al. Suicide among cancer patients. Nat. Commun. 2019;10:207. doi: 10.1038/s41467-018-08170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du L, et al. Incidence of suicide death in patients with cancer: A systematic review and meta-analysis. J. Affect. Disord. 2020;276:711–719. doi: 10.1016/j.jad.2020.07.082. [DOI] [PubMed] [Google Scholar]
- 9.Du M, Nair R, Jamieson L, Liu Z, Bi P. Incidence trends of lip, oral cavity, and pharyngeal cancers: Global burden of disease 1990–2017. J. Dent. Res. 2020;99:143–151. doi: 10.1177/0022034519894963. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health and Welfare, Taiwan, ROC. Cancer Registry Annual Report 2018. https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/13498/File_15611.pdf (2021).
- 11.Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: Projections to the year 2030. Br. J. Cancer. 2011;105(11):1795–1803. doi: 10.1038/bjc.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the U.K. until 2035. Br. J. Cancer. 2016;115(9):1147–1155. doi: 10.1038/bjc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Møller B, et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur. J. Cancer Prev. 2002;11(Suppl 1):S1–96. [PubMed] [Google Scholar]
- 14.Virani S, et al. Breast cancer incidence trends and projections in Northeastern Thailand. J. Epidemiol. 2018;28(7):323–330. doi: 10.2188/jea.JE20170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng R, et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res. 2018;30(6):571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: A modelling study. Lancet Public Health. 2018;3(1):e34–e43. doi: 10.1016/S2468-2667(17)30222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JE, Lee SA, Kim TH, Park S, Choy YS, Ju YJ, Park EC. Projection of breast cancer burden due to reproductive/lifestyle changes in Korean Women (2013–2030) using an age-period-cohort model. Cancer Res. Treat. 2018;50(4):1388–1395. doi: 10.4143/crt.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018;8(1):1129. doi: 10.1038/s41598-018-19199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Offman J, Pesola F, Sasieni P. Trends and projections in adenocarcinoma and squamous cell carcinoma of the oesophagus in England from 1971 to 2037. Br. J. Cancer. 2018;118(10):1391–1398. doi: 10.1038/s41416-018-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su SY, Chiang CJ, Yang YW, Lee WC. Secular trends in liver cancer incidence from 1997 to 2014 in Taiwan and projection to 2035: An age-period-cohort analysis. J. Formos. Med. Assoc. 2019;118:444–449. doi: 10.1016/j.jfma.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen SM, Deppen S, Nguyen GH, Pham DX, Bui TD, Tran TV. Projecting cancer incidence for 2025 in the 2 largest populated cities in Vietnam. Cancer Control. 2019 doi: 10.1177/1073274819865274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu XQ, et al. Statistical projection methods for lung cancer incidence and mortality: A systematic review. BMJ Open. 2019;9(8):e028497. doi: 10.1136/bmjopen-2018-028497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirier AE, et al. The future burden of cancer in Canada: Long-term cancer incidence projections 2013–2042. Cancer Epidemiol. 2019;59:199–207. doi: 10.1016/j.canep.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Lei S, et al. Breast cancer incidence and mortality in women in China: Temporal trends and projections to 2030. Cancer Biol. Med. 2021;18(3):900–909. doi: 10.20892/j.issn.2095-3941.2020.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsiao BY, et al. Ensemble forecasting of a continuously decreasing trend in bladder cancer incidence in Taiwan. Sci. Rep. 2021;11:8373. doi: 10.1038/s41598-021-87770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Dahlstrom KR, Lairson DR, Sturgis EM. Projected oropharyngeal carcinoma incidence among middle-aged U.S. men. Head Neck. 2019;41(9):3226–3234. doi: 10.1002/hed.25810. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Fakhry C, D'Souza G. Projected association of human papillomavirus vaccination with oropharynx cancer incidence in the U.S., 2020–2045. JAMA Oncol. 2021;7(10):e212907. doi: 10.1001/jamaoncol.2021.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat. Med. 1987;6(4):469–481. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 30.Hsu WL, Yu KJ, Chiang CJ, Chen TC, Wang CP. Head and neck cancer incidence trends in Taiwan, 1980–2014. Int. J. Head Neck Sci. 2017;1:180–189. [Google Scholar]
- 31.Ko YC, et al. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J. Oral. Pathol. Med. 1995;24:450–453. doi: 10.1111/j.1600-0714.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee CH, Ko YC, Huang HL, et al. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br. J. Cancer. 2003;88(3):366–372. doi: 10.1038/sj.bjc.6600727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YS, et al. Epidemiology of oral cavity cancer in Taiwan with emphasis on the role of betel nut chewing. ORL. J. Otorhinolaryngol Relat. Spec. 2005;67:230–236. doi: 10.1159/000089214. [DOI] [PubMed] [Google Scholar]
- 34.Chung CH, Yang YH, Wang TY, Shieh TY, Warnakulasuriya S. Oral precancerous disorders associated with areca quid chewing, smoking, and alcohol drinking in southern Taiwan. J. Oral. Pathol. Med. 2005;34:460–466. doi: 10.1111/j.1600-0714.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 35.Wen, C.P. et al. Uncovering the relation between betel quid chewing and cigarette smoking in Taiwan. Tob. Control 14 Suppl 1(Suppl 1), i16–22 (2005). [DOI] [PMC free article] [PubMed]
- 36.Su SY, Chen WT, Chiang CJ, Yang YW, Lee WC. Oral cancer incidence rates from 1997 to 2016 among men in Taiwan: Association between birth cohort trends and betel nut consumption. Oral Oncol. 2020;107:104798. doi: 10.1016/j.oraloncology.2020.104798. [DOI] [PubMed] [Google Scholar]
- 37.Chiang CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015;45:291–296. doi: 10.1093/jjco/hyu211. [DOI] [PubMed] [Google Scholar]
- 38.Chiang CJ, Yang YW, Lee WC. Taiwan's nationwide cancer registry system of 40 years: Past, present, and future. J. Formos. Med. Assoc. 2019;118:856–858. doi: 10.1016/j.jfma.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Kao CW, et al. Accuracy of long-form data in the Taiwan cancer registry. J. Formos. Med. Assoc. 2021;120(11):2037–2041. doi: 10.1016/j.jfma.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Fritz A, et al. International Classification of Diseases for Oncology (ICD-O) 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 41.Holford TR. Approaches to fitting age-period-cohort models with unequal intervals. Stat. Med. 2006;25(6):977–993. doi: 10.1002/sim.2253. [DOI] [PubMed] [Google Scholar]
- 42.Møller B, et al. Prediction of cancer incidence in the Nordic countries: Empirical comparison of different approaches. Stat. Med. 2003;22:2751–2766. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 43.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39(2):311–324. [PubMed] [Google Scholar]
- 44.Tzeng IS, Lee WC. Forecasting hepatocellular carcinoma mortality in Taiwan using an age-period-cohort model. Asia Pac. J. Public Health. 2015;27:NP65–NP73. doi: 10.1177/1010539511422941. [DOI] [PubMed] [Google Scholar]
- 45.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat. Med. 2007;26:3018–3045. doi: 10.1002/sim.2764. [DOI] [PubMed] [Google Scholar]
- 46.Lin WJ, Jiang RS, Wu SH, Chen FJ, Liu SA. Smoking, alcohol, and betel quid and oral cancer: A prospective cohort study. J. Oncol. 2011;2011:525976. doi: 10.1155/2011/525976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guha N, Warnakulasuriya S, Vlaanderen J, Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int. J. Cancer. 2014;135:1433–1443. doi: 10.1002/ijc.28643. [DOI] [PubMed] [Google Scholar]
- 48.Yang MJ, Chung TC, Yang MJ, Hsu TY, Ko YC. Betel quid chewing and risk of adverse birth outcomes among aborigines in eastern Taiwan. J. Toxicol Environ. Health A. 2001;64:465–472. doi: 10.1080/152873901753215920. [DOI] [PubMed] [Google Scholar]
- 49.Chiang CY, Chang HY. A population study on the time trend of cigarette smoking, cessation, and exposure to secondhand smoking from 2001 to 2013 in Taiwan. Popul. Health Metr. 2016;14:38. doi: 10.1186/s12963-016-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin CF, et al. Predictors of betel quid chewing behavior and cessation patterns in Taiwan aborigines. BMC Public Health. 2006;6:271. doi: 10.1186/1471-2458-6-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petersen PE. The world oral health report 2003: Continuous improvement of oral health in the 21st century—the approach of the WHO global oral health programme. Commun. Dent. Oral. Epidemiol. 2006;31(Suppl 1):3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 52.Petersen PE. Oral cancer prevention and control—the approach of the World Health Organization. Oral. Oncol. 2009;45:454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 53.Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: A brief review. Jpn. J. Clin. Oncol. 2002;32(Suppl):S66–81. doi: 10.1093/jjco/hye138. [DOI] [PubMed] [Google Scholar]
- 54.Yang YH, Warnakulasuriya S, Yang HF, Lin LJ, Wang YW. Public health measures to reduce areca nut and betel quid use for control of oral cancer in Taiwan. Oral. Oncol. 2020;108:104915. doi: 10.1016/j.oraloncology.2020.104915. [DOI] [PubMed] [Google Scholar]
- 55.Lee JM, Chen MG, Hwang TC, Yeh CY. Effect of cigarette taxes on the consumption of cigarettes, alcohol, tea and coffee in Taiwan. Public Health. 2010;124:429–436. doi: 10.1016/j.puhe.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Chen SH, Lee JM, Liu HH, Wang HC, Ye CY. The cross-effects of cigarette and betel nut consumption in Taiwan: Have tax increases made a difference? Health Policy Plan. 2011;26:266–273. doi: 10.1093/heapol/czq041. [DOI] [PubMed] [Google Scholar]
- 57.Petti S. Lifestyle risk factors for oral cancer. Oral. Oncol. 2009;45:340–350. doi: 10.1016/j.oraloncology.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Wang HH, Tsay SF. Elderly and long-term care trends and policy in Taiwan: Challenges and opportunities for health care professionals. Kaohsiung J. Med. Sci. 2012;28:465–469. doi: 10.1016/j.kjms.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hollenbeak CS, et al. Determinants of medicare costs for elderly patients with oral cavity and pharyngeal cancers. JAMA Otolaryngol. Head Neck Surg. 2015;141:628–635. doi: 10.1001/jamaoto.2015.0940. [DOI] [PubMed] [Google Scholar]
- 60.Huang CC, et al. Life expectancy and expected years of life lost to oral cancer in Taiwan: A nation-wide analysis of 22,024 cases followed for 10 years. Oral Oncol. 2015;51:349–354. doi: 10.1016/j.oraloncology.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Huang CC, et al. Cost-effectiveness analysis of the oral cancer screening program in Taiwan. Oral Oncol. 2019;89:59–65. doi: 10.1016/j.oraloncology.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Chuang SL, et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer. 2017;123:1597–1609. doi: 10.1002/cncr.30517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The data underlying the results of this study are either available in the manuscript or upon request from the corresponding author, Wen-Chung Lee. The raw data cannot be made available in supplemental files or a public repository because of privacy or ethical reasons. However, the dataset used for analysis can be obtained in an anonymized form upon request from the corresponding author.