Abstract
Racial differences of prostate cancer incidence and mortality among Asian, Black, and Caucasian men have been known, however, comprehensive update of this topic is not yet reported. In the present review, an overview of the racial differences in prostate cancer characteristics and cancer-specific mortality is collected and reviewed. Regarding racial differences of incidence and mortality, surprising differences in the incidence of prostate cancer are seen among different populations around the world, with some countries having rates that are 60 to 100 times higher than others. African-American men have a higher incidence of prostate cancer, higher prostate cancer mortality, and are diagnosed with prostate cancer at a younger age than Caucasian American men. Furthermore, race is gaining attention as an important factor to consider for planning active surveillance for localized prostate cancer, especially among African-Americans. In addition, the causes of these differences are being elucidated by genomic profiling. Determinants of racial disparities are multifactorial, including socioeconomic and biologic factors. Although race-specific differences in prostate cancer survival estimates appear to be narrowing over time, there is an ongoing need to continue to understand and mitigate racial factors associated with disparities in health care outcomes.
Keywords: DNA mismatch repair, Genetic background, Prostate cancer, Race factors, Race relations
INTRODUCTION
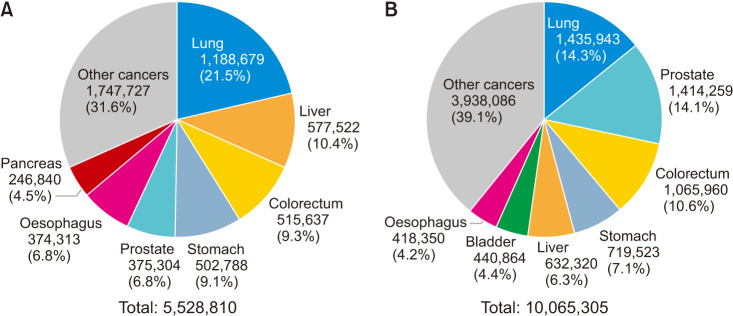
A number of new cases of prostate cancer is reported to be 1,414,259 in 2020. Other than lung cancer, prostate cancer is the most common cancer among men in the world. Estimated number of deaths from prostate cancer is also reported to be 375,304 according to the Global Cancer Observatory (Fig. 1) [1]. Incidence of prostate cancer is increasing in Asia according to increased prostate-specific antigen (PSA) screening, westernized food, as well as the life extension. Racial differences of prostate cancer incidence and mortality have been known, however, comprehensive update of this topic is not yet reported. In the present review, an overview of the racial differences in prostate cancer characteristics and cancer-specific mortality is collected and reviewed.
Fig. 1. Estimated numbers of (A) deaths and (B) new cases in 2020 worldwide. Data from International Ageacy for Research on Cancer (IARC, 2020) [1].
EVIDENCE ACQUISITION
PubMed, MEDLINE, Embase, Web of Science, Scopus, the Cochrane database, and recent abstracts were searched to identify all available studies addressing acial differences of prostate cancer published before April 30, 2021. The following keywords were used in the search strategy to identify potential reports: (prostate cancer OR prostate carcinoma OR prostatic cancer OR prostatic cancer disease) AND (racial difference OR human race specificity OR ethnic group). Two independent reviewers (N.H., M.F.) performed initial screening of the existing literature. Reasons for study exclusions were noted. We then performed a critical evaluation of the studies selected and relevant reports were subjected to a full-text review. The relevance of each study was confirmed on a case-by-case basis. Disagreements were resolved via consensus with all co-authors.
RACIAL DIFFERENCES OF INCIDENCE AND MORTALITY
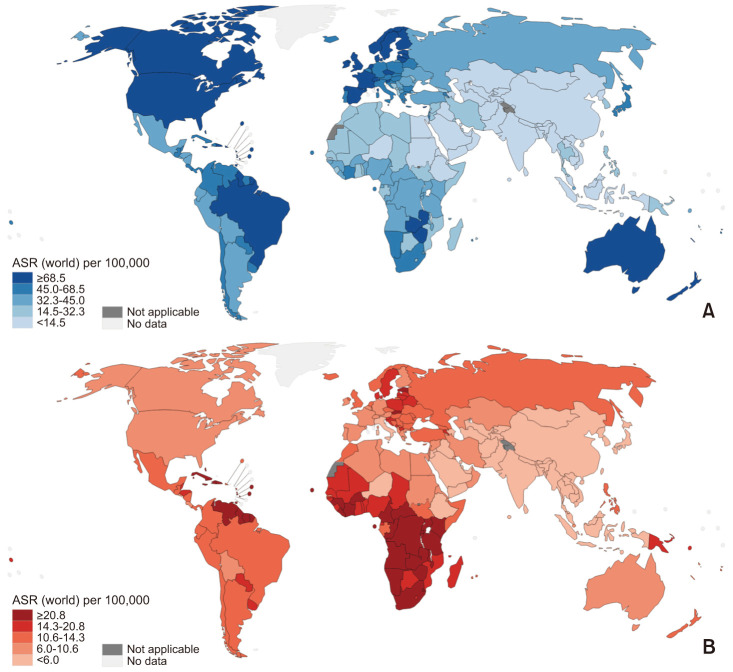
Surprising differences in the incidence of prostate cancer are seen among different populations around the world (Fig. 2A), with some countries having rates that are 60 to 100 times higher than others [2]. The incidence of prostate cancer is typically low in Asian men, with age-adjusted incidences ranging from 2 to 10 per 100,000 men. The incidence in northern Europe is generally high. African-American men have the highest incidence of prostate cancer in the world. Incidence and mortality rates of prostate cancer in each country are shown in Fig. 2. Differences in the mortality of prostate cancer are also observed among different populations around the world (Fig. 2B). In the United States, the incidence among African-American men is 60% higher than among Caucasian men [3]. The prostate cancer-specific mortality rate of African-American men is reported to be more than twice that of non-Hispanic Caucasian men [4]. For example, in the USA, African Americans have the highest incidence and mortality rates, which are approximately twice as those of European American men and 3 to 4 times higher than those of Asian Americans (Fig. 1) [5]. However, race-specific differences in survival estimates of prostate cancer appear to be narrowing over time [6].
Fig. 2. Estimated age-standardized (A) incidence rates and (B) mortality rates in each countries, 2020 (prostate, male, all ages). ASR: age-standardized race. Data from International Ageacy for Research on Cancer (IARC, 2020) [1].
RACIAL DIFFERENCE AND PROSTATE-SPECIFIC ANTIGEN SCREENING
The incidence of prostate cancer among African-American men is 64% higher than among Caucasian men, and the mortality rate of prostate cancer is 2.3 times higher [7,8]. Further, autopsy data shows that prostate cancer transforms into forms that are more active among African-American men at a younger age compared to Caucasian men [8]. Data also suggests that prostate cancer develops at a younger age in African-American men. Analysis of the 2010 SEER data revealed that non-Hispanic Caucasian men were diagnosed with prostate cancer 1.2 years later than non-Hispanic African-American men [9], while an earlier SEER analysis found that African-American men were diagnosed with prostate cancer at age which was mean of 3 years younger than Caucasian men [10]. A population-based retrospective cohort study in Great Britain found that Caucasian men were diagnosed with prostate cancer a mean of 5.1 years later than men of African descent [11]. Another study estimated that men of African descent were approximately twice as likely to be diagnosed with prostate cancer before the age of 45 as Caucasian men [12]. Based on these results, race-based PSA reference ranges have been proposed for PSA cutoff values for performing a biopsy [13]. Finally, modeling studies have suggested that African-American men have a higher incidence of preclinical disease and are at higher risk of metastasis than Caucasian American men [14]. Hence, baseline PSA levels are seen as a stronger predictor than race [15].
Further, it was found in a recent study including 41,250 men in the Veterans Affairs (VA) Health Care System database that the optimal PSA threshold for predicting prostate cancer diagnosis within 4 years was higher in Caucasians than in African-American men (2.5 ng/mL vs. 1.9 ng/mL) [16]. A prospective study found that Black men aged 40 to 64 with PSA levels above the 90th percentile were at a much higher risk of active prostate cancer than people whose PSA levels were below the median [17]. Factors contributing to this racial difference include genetic risk factors, differences in environmental exposure, differences in patient and doctor behavior, and poorer access to quality medical care such as early cancer screenings and follow-up care, delayed diagnosis, and inadequate treatment [18,19,20,21,22]. In addition, recently Hur and Giovannucci reported that the earlier onset of puberty in males could be a risk factor for prostate carcinogenesis and potentially account for the observed racial disparities in the disease burden [23].
African-American men have a higher incidence of prostate cancer, higher prostate cancer mortality, and are diagnosed with prostate cancer at a younger age than Caucasian American men. This is due to a high rate of prostate cancer onset before the appearance of symptoms and the high likelihood of tumor progression before the onset of symptoms. Therefore, for African-Americans, it may be sensible to consider initiating shared decision-making about PSA screening at age 40, and to consider screening yearly rather than every two years [14].
Regarding Asian populations, Kimura [24] reported that the incidence and mortality was much lower in Asian countries compared to the countries in North America, Europe, and Oceania. Also, the incidence in Asian immigrants in Western countries had higher incidence of prostate cancer compared to those in their countries of birth. She concluded that the lower PSA screening rate in Asian countries and some of the Asian populations in Western countries may partially explain the low incidence of prostate cancer in Asian populations [24].
RACIAL DIFFERENCE AND TREATMENT
Race is gaining attention as an important factor to consider for planning active surveillance for localized prostate cancer, especially among African-Americans. From 2010 to 2012, Black men had a higher lifetime risk of developing prostate cancer and a higher prostate cancer mortality rate than Caucasian American men (18.2% vs. 13.3% and 4.4% vs. 2.4%, respectively) [25]. One study found that the increased prostate cancer-specific mortality seen in African-American men was limited to grade group 1 patients [26]. Multiple studies have shown that Black men with very low-risk of prostate cancer may have high grade (grade group ≥2) cancers that are not detected by pre-treatment biopsies. It has been reported that pathological upgrading is 1.7 to 2.3 times more common in African-Americans than in Caucasian Americans with matching clinical parameters [27,28]. However, some studies have not observed differences in stage assessments or upgrading rates [29,30]. For example, a retrospective study of 895 men showed no significant differences in the frequency of pathological upgrading, up-staging, or biochemical recurrence between African-American and Caucasian-American men [26]. Several studies have reported that among men with low prostate cancer risk who enrolled in active surveillance programs, Caucasian Americans have a lower risk of progressing to a higher Gleason grade cancer or high-volume status than African-Americans [31,32,33]. African-Americans in low to moderate-risk groups also appear to be at a higher risk for biochemical recurrence after treatment [34]. In addition, African-American patients with low risk or moderate-risk prostate cancer with a good prognosis have a higher overall post-treatment mortality rate, primarily due to post-ADT cardiovascular complications [35]. The reasons for these clinical discrepancies are being studied. One explanation may be differences in the areas tumors occupy in the prostate, which could reflect differences in prostate cancer sub-types associated with variations in gene expression [21,36,37,38]. Disparities in treatment and access to medical care may also play important roles [39,40]. A multi-parametric magnetic resonance imaging (mpMRI) is a strategy that improves risk stratification in African-American patients that considers active surveillance. mpMRI uses image-guided targeted biopsies and has been found to improve the detection of clinically significant tumors in some men [41]. Vengaloor Thomas et al [42] retrospectively reviewed prostate cancer patient data (n=1,319,225) from the National Cancer Database (NCDB) to determine whether racial differences exist among the target population. As a result, no statistically supported racial disparities were observed for African American and Caucasian men with bone metastasis (p=0.885). Similarly, there were no racial disparities in survival for men suffering from other metastases (liver, lung, or brain). However, racial disparities in survival were observed among African American and Caucasian men with non-metastatic prostate cancer (p<0.001) or when metastasis status was not taken into account (p<0.001) [42].
Recently, Wen et al [43] evaluated the impact of clinical characteristics and factors related to access to care on survival by race using data from the NCDB for 526,690 patients with prostate cancer who underwent radical prostatectomy. In their study, when adjustments were made for age and year of diagnosis only, it was observed that the Black, AAPIs (Asian Americans and Pacific Islanders), and Hispanic population had 51% higher mortality, 22% lower mortality, and 6% lower mortality, respectively compared to the Caucasian population. However, after adjustments for all clinical factors and nonclinical factors, the Black-White survival disparity narrowed to 20%, whereas the AAPI-White disparity increased to 35%. Among the controlled-for factors, education, median household income, and insurance status primarily contributed to racial disparity [43]. Regarding radiation therapy, Kodiyan et al [44] utilized the NCDB and compared overall survival (OS) in 27,150 Black and Caucasian men receiving radiotherapy. The study reported that OS was equivalent between Black and Caucasian race in favorable risk (hazard ratio [HR], 0.928; 95% confidence interval [CI], 0.583–1.477; p=0.753) and unfavorable-risk subgroups (HR, 1.078; 95% CI, 0.843–1.379; p=0.550). No significant interaction existed between treatment and race for either cohort but there was a significant interaction between race and age in those with unfavorable risk (HR, 1.046; 95% CI, 1.009–1.084; p=0.015), with greater OS in those of Caucasian race ≤60 years (HR, 0.320, 95% CI, 0.137–0.752; p=0.009) [44].
Regarding Asian populations, Hassanipour et al [45] performed a systematic review and meta-analysis to provide a comprehensive estimate of the survival of prostate cancer patients in Asian countries. According to the results of this study, the prostate cancer survival rate in Asian countries is relatively lower than in Europe and North America, which may be due to less access to diagnostic facilities and higher age at recognition of disease than to advanced countries. Another result of our study was the higher survival rate of prostate cancer in countries with very high human development index (such as South Korea and Japan), with similar survival rates as those of advanced countries such as Europe and North America.
RACIAL DIFFERENCES IN GENOMIC PROFILING OF PROSTATE CANCER
Mahal et al [46] analyzed tumor genomic profiles across race in a diverse cohort of patients. This study included 2,393 men (80 Asian, 2,109 White, and 204 Black), 1,484 patients had primary disease (43 Asian, 1,308 White, and 133 Black) and 909 patients had metastatic disease (37 Asian, 801 White, and 71 Black). 11.5% of Asian men had more than 20 mutations among the patients with primary prostate cancers. The occurrence of FOXA1 mutations was greater in Black men than in White men (18.6% vs. 11.9%; 95% CI, 0.8–13.5; p=0.03), in addition, such mutations occurred more frequently in Asian men as well (37.8% vs. 11.9%; 95% CI, 10.2–41.7; p<0.001). The occurrence of TP53 mutations was observed to be lesser in Black men than in White men (14.2% vs. 20.6%; 95% CI, −12.7 to 0; p=0.045). Mutations in the gene encoding androgen receptor (AR) were rare, regardless of race. The frequencies of genes with actionable mutations, those are alterations that are the intended targets of precision-oncology drugs, and mutations in DNA-repair genes did not differ substantially among the racial groups.
Among the patients with metastatic prostate cancer, 6.8% of Black men had more than 20 mutations. The occurrence of AR mutations was greater in Black men than in White men (18.3% vs. 8.1%; 95% CI, 1.0–19.4; p=0.004). The occurrence of TP53 mutations was greater in Asian men than Black men (62.0% vs. 22.5%; 95% CI, 21.2–58.0; p=0.008) and White men (62.0% vs. 36.4%; 95% CI, 9.7–41.7; p=0.004). The occurrence of genes with actionable mutations was greater in Black men than in White men (26.7% vs. 18.0%; 95% CI, 0–19.5; p=0.05), which was similar to the occurrence observed for DNA-repair genes (22.5% vs. 15.6%; 95% CI, 0.5–18.1; p=0.05). In addition, BRAF mutations, which are rare in prostate cancer and are considered to be actionable in many tumor sites, were frequently observed in Black men than in White men (7.0% vs. 1.5%; 95% CI, 0.4–11.5; p=0.002).
Clinically significant alterations may occur at different frequencies across races. Notably, Black men with metastatic prostate cancer were more likely have tumor mutations in AR, along with mutations in DNA-repair genes and actionable genetic mutations than White and Asian men [46].
Germline mutations in BRCA1 and BRCA2 (related to hereditary breast and ovarian cancer syndrome) are found in 0.2% to 0.3 % of the general population and are more common in certain racial and ethnic groups [47]. Many studies have observed associations between germline mutations in BRCA1 and BRCA2 and increased risk of prostate cancer [48,49,50,51,52,53,54,55,56,57]. In particular, BRCA2 mutations have been found to be associated with a risk of prostate cancer that is 2 to 6 times higher, though findings detailing the association between BRCA1 mutations and prostate cancer risk have not been very consistent [49,51,52,57,58,59]. In addition, prostate cancer in men with BRCA germline mutations are more active, occur earlier, and have significantly shorter survival periods than prostate cancer in non-carriers [60,61,62,63,64,65]. In cases of fatal prostate cancer, 60% of BRCA1/2 and ATM mutation carriers are reported to have no family history [62].
A number of genes and genetic mutations associated with prostate cancer risk are also known. A linkage analysis of high-risk family lines identified RNASEL (1q25), ELAC2 (17q12), MSR1 (8p22), HOXB13 (17q21), and other responsible genes [66]. The HOXB13 G84E mutation (rs138213197) is a relatively new genetic mutation reported by researchers at Johns Hopkins University in 2012. This missense mutation involves the replacement of glutamic acid for glycine at codon 84. This mutation was found in 1.4% (72/5,083 cases) of prostate cancer patients and 0.1% (1/1,401 cases) of a control group, which constitutes a 20.1 times higher risk of disease (95% CI, 3.5–803.3) [67]. A summary of various studies stated that the risk of developing prostate cancer among carriers of this mutation is 3.3 to 20.1 times higher [66]. The proportion of carriers of the HOXB13 G84E mutation in families with familial prostate cancer is high. The mutation was found in 31% (42/137 cases) of men who do not have prostate cancer and 51% (194/382 cases) of men who were diagnosed with prostate cancer. Regional differences are observed in the frequency of the HOXB13 G84E mutation in families with familial prostate cancer, with the frequency in Finland, Sweden, North America, and Australia at 22.4%, 8.2%, 0%–6.1%, 2.6%, respectively [68]. When classified by race, this mutation was observed in 4.8% of European families with familial prostate cancer, however, it was not found in African, Jewish, or Chinese families [68,69].
GWAS have been carried out since the mid-2000s [70,71]. There are nearly 100 single-nucleotide polymorphisms (SNPs) associated with prostate cancer [72]. Of the genetic loci associated with a risk of prostate cancer, about 60 start with the 8q24 region. However, GWAS have found racial differences in the frequency of SNP alleles, with some SNP being associated with increased risk in some races [72,73]. A brief synopsis of racial differences in genomic profiling of prostate cancer is summarized in Table 1.
Table 1. A brief synopsis of racial differences in genomic profiling of prostate cancer.
| 1. Black patients are more likely to harbor DNA repair mutations of any kind. |
| 2. Black patients with metastatic disease were more likely to have actionable mutations. |
| 3. Androgen receptor mutations are rare but more prevalent in Black patients than the others. |
| 4. Seven percent of Black patients had BRAF mutations, which are prevalent in other cancers and are targetable. |
| 5. TP53 mutations are much more common in Asian patients than in the other groups. |
| 6. Asian patients are far more likely to harbor FOXA1 mutations in the primary tumor. |
BRCA MUTATION CARRIERS AND TREATMENT
The occurrence of prostate cancer is greater in men who have BRCA mutations than the general population. It should be noted that there are large racial differences in BRCA mutations, which makes it difficult to generalize morbidity and mortality [74,75]. Prostate cancer has a high degree of familial aggregation. If one of a person's first-degree relatives is a prostate cancer patient, the person’s risk of being diagnosed with prostate cancer doubles. Further, if there are 2 or more prostate cancer patients among the first-degree relatives of individuals, the individuals risk of being diagnosed with prostate cancer is 5 to 11 times higher [76]. If the presence of BRCA1 mutation and BRCA2 mutation is reported in individuals, their of developing prostate cancer is 2 times and 5 to 7 times higher, respectively [77].
As discussed below, the therapeutic approach for BRCA mutation carriers who have not developed prostate cancer is different from the one for general prostate cancer patients. First of all, there is a higher possibility of cancer that is highly malignant and has a poor prognosis; thus, early detection and radical treatment are recommended. For advanced cancers, antineoplastic chemotherapy mainly involving cisplatin or novel PARP inhibitors are recommended.
According to a German study that searched for BRCA2 mutations in 382 patients with familial prostate cancer and 92 patients who developed prostate cancer at age 60 or younger but had no family history of prostate cancer, mutations were found in 5 patients with familial prostate cancer, however none were found in the 92 patients with no family history [78]. In an Australian family cohort of 1,423 people that included multiple breast cancer patients, 148 prostate cancers had poor prognoses and the mortality risk of BRCA2 mutation carriers was reported to be 4.5 times higher than non-carriers. In contrast, a U.S. study that searched 266 patients with familial prostate cancer did not detect any BRCA2 mutations. Prostate cancer in BRCA1 mutation carriers is thought to have a worse prognosis than general prostate cancer, but not as poor as in BRCA2 mutation carriers [63].
The NCCN guidelines recommends PSA screening at age 40 for mutation carriers [10,79]. Prostate cancer was reported in 47% of people with high PSA levels [10]. The IMPACT trial used a cohort with known BRCA mutation data. Subjects with levels >3.0 ng/mL underwent biopsies. Of the 791 BRCA1 mutation carriers and 531 non-carriers, and 731 BRCA2 mutation carriers and 428 non-carriers, 162 people with PSA levels over this threshold underwent biopsies. Of them, 59 were diagnosed with prostate cancer. Among these 59 patients, 18 were BRCA1 mutation carriers, 10 were BRCA1 non-carriers, 24 were BRCA2 mutation carriers, and 7 were BRCA2 mutation non-carriers. The positive predictive value in BRCA2 mutation carriers was the highest at 48%.
TREATMENT OF BRCA MUTATION CARRIERS
Prostate cancer in BRCA mutation carriers should be seen differently than regular prostate cancer, and cases of early detection should be treated aggressively instead of undergoing active surveillance. Particularly, patients with BRCA2 mutations have a poor prognosis. In cases of metastasis, cisplatin-based chemotherapy or PARP inhibitors can be expected to be effective.
Men with BRCA mutations not only have a higher risk of developing prostate cancer, but their cancers also progress more quickly than prostate cancer in patients without mutations. Histopathologically, they have high Gleason scores (>8) [63], a high frequency of lymph node metastasis, and poor prognoses [65]. The search for BRCA mutations can thus be interpreted as looking for a biomarker for highly malignant prostate cancer with a poor prognosis. In BRCA2 mutation carriers in particular, cancer onset tends to be relatively young, diagnosed at an advanced stage, highly malignant, and has a median survival period of only 2.1 years [61]. In a U.S. cohort of 182 patients with prostate cancer from families with BRCA2 mutations and 119 patients with prostate cancer from families with BRCA1 mutations, the median OS periods were 4.0 years and 8.0 years, respectively. The prognosis of prostate cancer in BRCA2 mutation carriers is extremely poor. The prognosis remains poor even after adjusting for Gleason score and stage, and cannot be explained by pathological background factors [80]. Recently, a histological type called intraductal carcinoma of the prostate (IDC-P) has been attracting attention. IDC-P has a poor prognosis, and prostate cancer with a BRCA2 mutation tends to be of this histological type [81]. A study of 1,090 high-risk prostate cancer patients who underwent total prostatectomy that examined genetic expression profiles related to the DNA damage response (DDR) pathway observed significantly worse prognoses in cases with dysregulation of the DDR pathway that includes BRCA [82]. Therefore, BRCA mutation carriers should be seen differently than regular prostate cancer, and cases that are discovered early should undergo aggressive treatment instead of active surveillance. In cases of metastasis, neoplastic chemotherapy with cisplatin [83] or PARP inhibitor therapy should be considered. In a phase II trial of the PARP inhibitor (Olaparib) in patients with metastatic castration-resistant prostate cancer who had been treated with docetaxel and had abnormal DNA repair genes, DNA repair mutations were found in 16 of the 49 patients who underwent genetic analysis, and among those 16 patients, 14 patients responded to olaparib. Among the 14 patients, seven were confirmed to have somatic BRCA2 mutations, and 3 had germline mutations [9]. The trial is transitioning to phase III and PARP inhibitor was associated with longer progression-free survival and better measures of response and patient-reported end points than either enzalutamide or abiraterone In men with metastatic castration-resistant prostate cancer who had disease progression while receiving enzalutamide or abiraterone and who had alterations in genes with a role in homologous recombination repair [84].
CONCLUSIONS
Population-based studies demonstrate that differences in the incidence of prostate cancer are seen among different populations around the world. Determinants of racial disparities are multifactorial, including socioeconomic and biologic factors. Although race-specific differences in prostate cancer survival estimates appear to be narrowing over time, there is an ongoing need to continue to understand and mitigate racial factors associated with disparities in health care outcomes.
Footnotes
CONFLICT OF INTEREST: The authors have nothing to disclose.
- Conceptualization: NH, MF.
- Data curation: NH.
- Formal analysis: NH.
- Investigation: NH, MF.
- Methodology: NH, MF.
- Resources: NH, MF.
- Supervision: MF.
- Validation: MF.
- Writing – original draft: NH.
- Writing – review & editing: MF.
References
- 1.International Agency for Research on Cancer (IARC) Global Cancer Observatory: Cancer Today [Internet] Lyon: IARC; [cited 2021 Jun 8]. Available from: https://gco.iarc.fr/today. [Google Scholar]
- 2.Stanford JL, Stephenson RA, Coyle LM, Cerhan J, Correa R, Eley JW, et al. Prostate cancer trends 1973–1995, SEER program. Bethesda (MD): National Cancer Institute (US); 1999. Report No.: 99-4543. [Google Scholar]
- 3.Miller BA, Kolonel LN, Bernstein L, Young JL, Jr, Swanson GM, West D, et al. Racial/ethnic patterns of cancer in the United States 1988–1992. Bethesda (MD): National Cancer Institute (US); 1996. Report No.: 96-4104. [Google Scholar]
- 4.American Cancer Society. Cancer facts & figures 2021. Atlanta (GA): American Cancer Society; 2021. [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 6.Kaur D, Ulloa-Pérez E, Gulati R, Etzioni R. Racial disparities in prostate cancer survival in a screened population: reality versus artifact. Cancer. 2018;124:1752–1759. doi: 10.1002/cncr.31253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Cancer stat facts: prostate cancer [Internet] Bethesda (MD): National Cancer Institute; [cited 2021 Jun 8]. Available from: http://seer.cancer.gov/statfacts/html/prost.html. [Google Scholar]
- 8.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792–1796. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins HA, Engels EA, Pfeiffer RM, Shiels MS. Age at cancer diagnosis for blacks compared with whites in the United States. J Natl Cancer Inst. 2015;107:dju489. doi: 10.1093/jnci/dju489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karami S, Young HA, Henson DE. Earlier age at diagnosis: another dimension in cancer disparity? Cancer Detect Prev. 2007;31:29–34. doi: 10.1016/j.cdp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Fairey A, Chetner M, Metcalfe J, Moore R, Todd G, Rourke K, et al. Associations among age, comorbidity and clinical outcomes after radical cystectomy: results from the Alberta Urology Institute radical cystectomy database. J Urol. 2008;180:128–134. doi: 10.1016/j.juro.2008.03.057. discussion 134. [DOI] [PubMed] [Google Scholar]
- 12.Metcalfe C, Evans S, Ibrahim F, Patel B, Anson K, Chinegwundoh F, et al. PROCESS Study Group. Pathways to diagnosis for Black men and White men found to have prostate cancer: the PROCESS cohort study. Br J Cancer. 2008;99:1040–1045. doi: 10.1038/sj.bjc.6604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moul JW. Targeted screening for prostate cancer in African-American men. Prostate Cancer Prostatic Dis. 2000;3:248–255. doi: 10.1038/sj.pcan.4500472. [DOI] [PubMed] [Google Scholar]
- 14.Tsodikov A, Gulati R, de Carvalho TM, Heijnsdijk EAM, Hunter-Merrill RA, Mariotto AB, et al. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer. 2017;123:2312–2319. doi: 10.1002/cncr.30687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vertosick EA, Poon BY, Vickers AJ. Relative value of race, family history and prostate specific antigen as indications for early initiation of prostate cancer screening. J Urol. 2014;192:724–728. doi: 10.1016/j.juro.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton SS, Crawford ED, Moul JW, Hardin JW, Kruep E. Determining optimal prostate-specific antigen thresholds to identify an increased 4-year risk of prostate cancer development: an analysis within the Veterans Affairs Health Care System. World J Urol. 2016;34:1107–1113. doi: 10.1007/s00345-015-1754-6. [DOI] [PubMed] [Google Scholar]
- 17.Preston MA, Gerke T, Carlsson SV, Signorello L, Sjoberg DD, Markt SC, et al. Baseline prostate-specific antigen level in midlife and aggressive prostate cancer in Black men. Eur Urol. 2019;75:399–407. doi: 10.1016/j.eururo.2018.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barocas DA, Grubb R, 3rd, Black A, Penson DF, Fowke JH, Andriole G, et al. Association between race and follow-up diagnostic care after a positive prostate cancer screening test in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer. 2013;119:2223–2229. doi: 10.1002/cncr.28042. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Rand KA, Hazelett DJ, Ingles SA, Kittles RA, Strom SS, et al. Prostate cancer susceptibility in men of African ancestry at 8q24. J Natl Cancer Inst. 2016;108:djv431. doi: 10.1093/jnci/djv431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahal BA, Aizer AA, Ziehr DR, Hyatt AS, Sammon JD, Schmid M, et al. Trends in disparate treatment of African American men with localized prostate cancer across National Comprehensive Cancer Network risk groups. Urology. 2014;84:386–392. doi: 10.1016/j.urology.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Yamoah K, Johnson MH, Choeurng V, Faisal FA, Yousefi K, Haddad Z, et al. Novel biomarker signature that may predict aggressive disease in African American men with prostate cancer. J Clin Oncol. 2015;33:2789–2796. doi: 10.1200/JCO.2014.59.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 23.Hur J, Giovannucci E. Racial differences in prostate cancer: does timing of puberty play a role? Br J Cancer. 2020;123:349–354. doi: 10.1038/s41416-020-0897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012;31:421–429. doi: 10.5732/cjc.011.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 26.Mahal BA, Berman RA, Taplin ME, Huang FW. Prostate cancer-specific mortality across Gleason scores in Black vs Nonblack men. JAMA. 2018;320:2479–2481. doi: 10.1001/jama.2018.11716. [DOI] [PubMed] [Google Scholar]
- 27.Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991–2997. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vora A, Large T, Aronica J, Haynes S, Harbin A, Marchalik D, et al. Predictors of Gleason score upgrading in a large African-American population. Int Urol Nephrol. 2013;45:1257–1262. doi: 10.1007/s11255-013-0495-y. [DOI] [PubMed] [Google Scholar]
- 29.Leapman MS, Freedland SJ, Aronson WJ, Kane CJ, Terris MK, Walker K, et al. Pathological and biochemical outcomes among African-American and Caucasian men with low risk prostate cancer in the SEARCH database: implications for active surveillance candidacy. J Urol. 2016;196:1408–1414. doi: 10.1016/j.juro.2016.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi R, Moul J. African American men with low-risk prostate cancer are candidates for active surveillance: the Will-Rogers effect? Am J Mens Health. 2017;11:1765–1771. doi: 10.1177/1557988317721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abern MR, Bassett MR, Tsivian M, Bañez LL, Polascik TJ, Ferrandino MN, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis. 2013;16:85–90. doi: 10.1038/pcan.2012.38. [DOI] [PubMed] [Google Scholar]
- 32.Iremashvili V, Soloway MS, Rosenberg DL, Manoharan M. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187:1594–1599. doi: 10.1016/j.juro.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 33.Sundi D, Faisal FA, Trock BJ, Landis PK, Feng Z, Ross AE, et al. Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology. 2015;85:155–160. doi: 10.1016/j.urology.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faisal FA, Sundi D, Cooper JL, Humphreys EB, Partin AW, Han M, et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology. 2014;84:1434–1441. doi: 10.1016/j.urology.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovtun KA, Chen MH, Braccioforte MH, Moran BJ, D'Amico AV. Race and mortality risk after radiation therapy in men treated with or without androgen-suppression therapy for favorable-risk prostate cancer. Cancer. 2016;122:3608–3614. doi: 10.1002/cncr.30224. [DOI] [PubMed] [Google Scholar]
- 36.Pettaway CA, Troncoso P, Ramirez EI, Johnston DA, Steelhammer L, Babaian RJ. Prostate specific antigen and pathological features of prostate cancer in Black and White patients: a comparative study based on radical prostatectomy specimens. J Urol. 1998;160:437–442. [PubMed] [Google Scholar]
- 37.Powell IJ, Dyson G, Land S, Ruterbusch J, Bock CH, Lenk S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prev. 2013;22:891–897. doi: 10.1158/1055-9965.EPI-12-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in Black American men. J Urol. 2014;191:60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bickell NA, Lin JJ, Abramson SR, Hoke GP, Oh W, Hall SJ, et al. Racial disparities in clinically significant prostate cancer treatment: the potential health information technology offers. J Oncol Pract. 2018;14:e23–e33. doi: 10.1200/JOP.2017.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedlander DF, Trinh QD, Krasnova A, Lipsitz SR, Sun M, Nguyen PL, et al. Racial disparity in delivering definitive therapy for intermediate/high-risk localized prostate cancer: the impact of facility features and socioeconomic characteristics. Eur Urol. 2018;73:445–451. doi: 10.1016/j.eururo.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vengaloor Thomas T, Gordy XZ, Lirette ST, Albert AA, Gordy DP, Vijayakumar S, et al. Lack of racial survival differences in metastatic prostate cancer in National Cancer Data Base (NCDB): a different finding compared to non-metastatic disease. Front Oncol. 2020;10:533070. doi: 10.3389/fonc.2020.533070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen W, Luckenbaugh AN, Bayley CE, Penson DF, Shu XO. Racial disparities in mortality for patients with prostate cancer after radical prostatectomy. Cancer. 2021;127:1517–1528. doi: 10.1002/cncr.33152. [DOI] [PubMed] [Google Scholar]
- 44.Kodiyan J, Ashamalla M, Guirguis A, Ashamalla H. Race does not affect survival in patients with prostate cancer treated with radiation therapy. Anticancer Res. 2020;40:3307–3314. doi: 10.21873/anticanres.14313. [DOI] [PubMed] [Google Scholar]
- 45.Hassanipour S, Delam H, Arab-Zozani M, Abdzadeh E, Hosseini SA, Nikbakht HA, et al. Survival rate of prostate cancer in Asian countries: a systematic review and meta-analysis. Ann Glob Health. 2020;86:2. doi: 10.5334/aogh.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahal BA, Alshalalfa M, Kensler KH, Chowdhury-Paulino I, Kantoff P, Mucci LA, et al. Racial differences in genomic profiling of prostate cancer. N Engl J Med. 2020;383:1083–1085. doi: 10.1056/NEJMc2000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298:2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 48.Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 49.Agalliu I, Gern R, Leanza S, Burk RD. Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations. Clin Cancer Res. 2009;15:1112–1120. doi: 10.1158/1078-0432.CCR-08-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE Breast Cancer Linkage Consortium. Risks of cancer in BRCA1-mutation carriers. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 51.Gallagher DJ, Gaudet MM, Pal P, Kirchhoff T, Balistreri L, Vora K, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115–2121. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirchhoff T, Kauff ND, Mitra N, Nafa K, Huang H, Palmer C, et al. BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clin Cancer Res. 2004;10:2918–2921. doi: 10.1158/1078-0432.ccr-03-0604. [DOI] [PubMed] [Google Scholar]
- 53.Leongamornlert D, Mahmud N, Tymrakiewicz M, Saunders E, Dadaev T, Castro E, et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106:1697–1701. doi: 10.1038/bjc.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22:735–742. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 55.Thompson D, Easton DF Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 56.Tulinius H, Olafsdottir GH, Sigvaldason H, Arason A, Barkardottir RB, Egilsson V, et al. The effect of a single BRCA2 mutation on cancer in Iceland. J Med Genet. 2002;39:457–462. doi: 10.1136/jmg.39.7.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, et al. Netherlands Collaborative Group on Hereditary Breast Cancer (HEBON) Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42:711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121:269–275. doi: 10.1002/cncr.29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moran A, O'Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012;11:235–242. doi: 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 60.Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitra A, Fisher C, Foster CS, Jameson C, Barbachanno Y, Bartlett J, et al. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98:502–507. doi: 10.1038/sj.bjc.6604132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Na R, Zheng SL, Han M, Yu H, Jiang D, Shah S, et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur Urol. 2017;71:740–747. doi: 10.1016/j.eururo.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narod SA, Neuhausen S, Vichodez G, Armel S, Lynch HT, Ghadirian P, et al. Rapid progression of prostate cancer in men with a BRCA2 mutation. Br J Cancer. 2008;99:371–374. doi: 10.1038/sj.bjc.6604453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thorne H, Willems AJ, Niedermayr E, Hoh IM, Li J, Clouston D, et al. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res (Phila) 2011;4:1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 65.Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, Jonasson JG, Olafsdóttir EJ, Olafsdóttir GH, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 66.Xu J, Sun J, Zheng SL. Prostate cancer risk-associated genetic markers and their potential clinical utility. Asian J Androl. 2013;15:314–322. doi: 10.1038/aja.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Lange EM, Lu L, Zheng SL, Wang Z, Thibodeau SN, et al. International Consortium for Prostate Cancer Genetics. HOXB13 is a susceptibility gene for prostate cancer: results from the International Consortium for Prostate Cancer Genetics (ICPCG) Hum Genet. 2013;132:5–14. doi: 10.1007/s00439-012-1229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin X, Qu L, Chen Z, Xu C, Ye D, Shao Q, et al. A novel germline mutation in HOXB13 is associated with prostate cancer risk in Chinese men. Prostate. 2013;73:169–175. doi: 10.1002/pros.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amundadottir LT, Sulem P, Gudmundsson J, Helgason A, Baker A, Agnarsson BA, et al. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38:652–658. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 71.Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet. 2007;39:631–637. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- 72.Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46:1103–1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Na R, Liu F, Zhang P, Ye D, Xu C, Shao Q, et al. Evaluation of reported prostate cancer risk-associated SNPs from genome-wide association studies of various racial populations in Chinese men. Prostate. 2013;73:1623–1635. doi: 10.1002/pros.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Page EC, Bancroft EK, Brook MN, Assel M, Hassan Al Battat M, Thomas S, et al. Interim results from the IMPACT study: evidence for prostate-specific antigen screening in BRCA2 mutation carriers. Eur Urol. 2019;76:831–842. doi: 10.1016/j.eururo.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bancroft EK, Page EC, Castro E, Lilja H, Vickers A, Sjoberg D, et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66:489–499. doi: 10.1016/j.eururo.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC. Family history and the risk of prostate cancer. Prostate. 1990;17:337–347. doi: 10.1002/pros.2990170409. [DOI] [PubMed] [Google Scholar]
- 77.Mitra AV, Bancroft EK, Barbachano Y, Page EC, Foster CS, Jameson C, et al. Targeted prostate cancer screening in men with mutations in BRCA1 and BRCA2 detects aggressive prostate cancer: preliminary analysis of the results of the IMPACT study. BJU Int. 2011;107:28–39. doi: 10.1111/j.1464-410X.2010.09648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maier C, Herkommer K, Luedeke M, Rinckleb A, Schrader M, Vogel W. Subgroups of familial and aggressive prostate cancer with considerable frequencies of BRCA2 mutations. Prostate. 2014;74:1444–1451. doi: 10.1002/pros.22860. [DOI] [PubMed] [Google Scholar]
- 79.National Comprehensive Cancer Network (NCCN) Prostate cancer (version 2.2021) [Internet] Plymouth Meeting (PA): NCCN; c2021. [cited 2021 Jun 8]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [Google Scholar]
- 80.Euhus DM, Robinson L. Genetic predisposition syndromes and their management. Surg Clin North Am. 2013;93:341–362. doi: 10.1016/j.suc.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 81.Risbridger GP, Taylor RA, Clouston D, Sliwinski A, Thorne H, Hunter S, et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol. 2015;67:496–503. doi: 10.1016/j.eururo.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Evans JR, Zhao SG, Chang SL, Tomlins SA, Erho N, Sboner A, et al. Patient-level DNA damage and repair pathway profiles and prognosis after prostatectomy for high-risk prostate cancer. JAMA Oncol. 2016;2:471–480. doi: 10.1001/jamaoncol.2015.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freedland S, Aronson W. Commentary on “Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer” Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Eur Urol. Jun 2016;69(6):992-5. Urol Oncol. 2017;35:536. doi: 10.1016/j.urolonc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 84.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]