Abstract
Retrograde ejaculation (RE) is a condition defined as the backward flow of the semen during ejaculation, and when present can result in male infertility. RE may be partial or complete, resulting in either low seminal volume or complete absence of the ejaculate (dry ejaculate). RE can result from anatomic, neurological or pharmacological conditions. The treatment approaches outlined are determined by the cause. Alkalinizing urinary pH with oral medications or by adding sperm wash media into the bladder prior to ejaculation may preserve the viability of the sperm. This article provides a step-by-step guide to diagnose RE and the optimal techniques to retrieve sperm.
Keywords: Ejaculation; Infertility, male; Sperm count
INTRODUCTION
Retrograde ejaculation (RE) is an ejaculatory disorder that results in substantial redirection of semen from the posterior urethra into the bladder. It occurs secondary to suboptimal bladder neck closure, due to sympathetic neuropathic dysfunction or to organic lesions [1]. Hence, minimal to no seminal fluid is expelled antegrade through the urethral meatus, and instead, passes backward into the bladder. This results in hypospermia or aspermia, conditions where the ejaculated fluid is less than 1.5 mL in volume or is completely absent [2,3]. RE is responsible in 0.3% to 2% of male infertility cases [1]. Diagnosing RE and investigating the underlying cause is a crucial step of male infertility investigation that enables the clinicians to offer the best available treatment option. A list of medical and surgical treatment modalities such as alpha-adrenergic agonists or anticholinergic and antihistaminic drugs as well as surgical retrieval of sperm are available with variable degrees of success [4,5,6].
This article aims to provide: 1) an overview of the common etiological factors of RE and the mechanisms underlying RE-mediated male infertility; 2) a step-by-step laboratory protocol to assess retrograde semen samples; and 3) a rational approach to the clinical management of RE from a laboratory perspective, along with the clinical interpretation of the laboratory results.
CAUSES OF RETROGRADE EJACULATION AND MECHANISM OF RETROGRADE EJACULATION-MEDIATED MALE INFERTILITY
Normal ejaculation includes an emission phase that is regulated by sympathetic nerves (T10-L2) and involves the participation of distal epididymis, vas deferens, ampulla of vas, seminal vesicles, prostate, and the bulbourethral glands [7]. Sperm are released in an antegrade manner from the vas deferens through the ejaculatory ducts into the prostatic urethra. This is followed by the bladder neck contraction (involuntary internal urethral sphincter) to prevent the backflow of semen in the bladder [2,8]. The expulsion phase is regulated by the somatic nerves from S2-4 spinal segments, controlling the rhythmic contraction of the voluntary external urethral sphincter, and the pelvic floor muscles (levator ani, ischiocavernosus, and bulbospongiosus) [2,8]. Any disruption of this centrally mediated reflex arc inhibits bladder neck contraction, causing backflow of semen into the bladder, although, the exact role of parasympathetic system on ejaculation remains unclear [9].
In RE, the bladder neck does not contract adequately during ejaculation, due to either an anatomical or neurogenic functional defect of the internal sphincter. This permits the expelled semen to follow the passage with lower resistance and therefore pass into the urinary bladder, in a retrograde fashion [10].
Various disorders can result in bladder neck dysfunction and RE. Anatomical disruption following prostatectomy, cystectomy, traumatic bladder injury or radiation therapy to treat cancer in the pelvic area, are common causes of RE [2]. Neuropathy resulting from nerve damage secondary to uncontrolled diabetes, multiple sclerosis, non-nerve sparing retroperitoneal lymph node dissection or spinal cord injury could lead to a functional bladder neck closure incompetency [2]. Pharmacologically mediated bladder neck dysfunction is another common cause for RE and may include; effects of medications used to treat benign prostatic hyperplasia (BPH) such as such as alpha blockers tamsulosin, alfuzosin, or terazosin; mood disorders such as selective serotonin reuptake inhibitors including fluoxetine and sertraline among several others; antipsychotics - such as chlorpromazine, thioridazine, and risperidone [2,11]. There has been more evidence that many alpha blockers may not be causing RE but rather lack of emission or anejaculation [7,12].
Unlike other ejaculatory disorders, which exhibit a mixture of both psychological and organic origins, causes of RE are mainly organic and include pharmacological, neurogenic, and anatomical abnormalities [3].
The pH and osmolality of the urine specimen have effects on sperm motility and velocity parameters. The fresh semen sample has an average pH of 7.2 to 8.2 and osmolality of 300 to 380 mOsm/kg. If the pH and osmolality are lower or higher than the normal reference values, it leads to progressive decline in sperm motility [13]. In a study where spermatozoa were exposed to fresh urine specimens there was an immediate loss of motility [13]. The pH and osmolality of the urine samples have to be both in the optimal range to enhance sperm survival in post-ejaculatory urine.
STEP-BY-STEP PROTOCOL FOR HANDLING POST-EJACULATORY URINE SAMPLES IN PATIENTS WITH RE
Retrieval of retrograde ejaculated sperm from urine remains as a valid approach. Once the clinician establishes the impossibility of obtaining an antegrade ejaculation, Andrology laboratory can initiate the procedure of sperm retrieval for therapeutic purposes. Finding sperm in post-ejaculatory urine is the main method for diagnosis of RE, as well as a treatment option for retrieved sperm for use in assisted reproductive technology (ART) [8,14,15]. In this context, an accurate laboratory analysis and clinical interpretation of the results are essential.
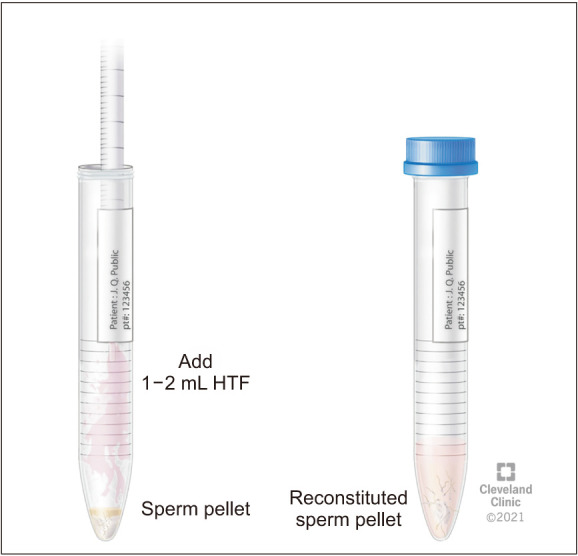
For the assessment of RE, it is important for the patient to deliver both the semen and post-ejaculatory urine samples for RE assessment at the Andrology laboratory [16]. The laboratory prepares for a patient scheduled for RE assessment by warming the sperm wash media to 37℃. Subsequently, 9 mL of sperm wash media is placed in a sterile container marked as “Urine” in the incubator for 20 minutes. Patient is instructed to urinate and partially empty his bladder. Next, the patient is instructed to attempt ejaculation into a sterile collection container, marked “Semen” or to reach orgasm in cases of aspermia. This is immediately followed by having the patient provide a urine sample into a separate sterile container containing ~9 mL of warm (37℃) sperm wash media (Fig. 1, 2). The specimen is transferred from the container into several sterile conical tubes and centrifuged at 300 g for 10 minutes (Fig. 3, 4). After centrifugation, the supernatant is discarded from the tube without disturbing the pellet (Fig. 5, 6). Sterile technique is used throughout the reconstitution process. The first tube sample pellet is reconstituted with 1–2 mL of sperm wash media (Fig. 6). Transfer 2 mL of the mixture from the first tube to the second tube and reconstitute the pellet. The process of reconstitution is continued until all the tubes are reconstituted. The final reconstituted pellet obtained after removal of the supernatant is re-suspended in 2.0 mL of sperm wash media and the re-suspended sample is examined for the presence of sperm. The initial urine specimen volume and final volume after reconstitution are recorded. A 6 µL droplet of the well-mixed re-suspended sample is plated on a fixed cell chamber. Manual analysis for sperm parameters is performed. Both concentration and motility are then assessed by utilizing fixed cell counting chamber and recorded [16].
Fig. 1. Semen and retrograde urine sample collection.

Fig. 2. Semen collection and urine collection containers with sperm wash media.
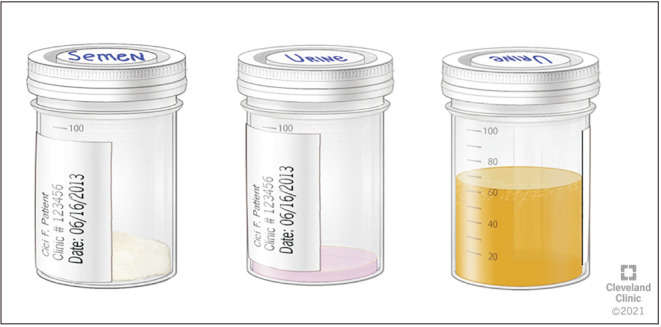
Fig. 3. Transfer of urine sample aliquots into polystyrene centrifuge tubes.
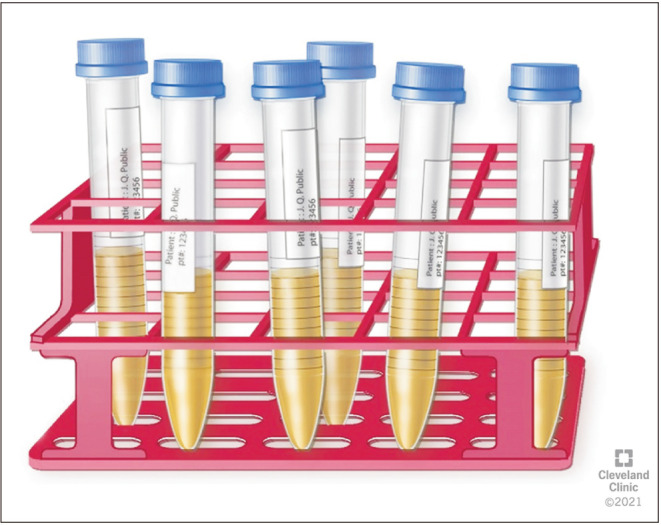
Fig. 4. Centrifuge for the processing of retrograde sample.

Fig. 5. Aspiration of supernatant from centrifuged retrograde urine.
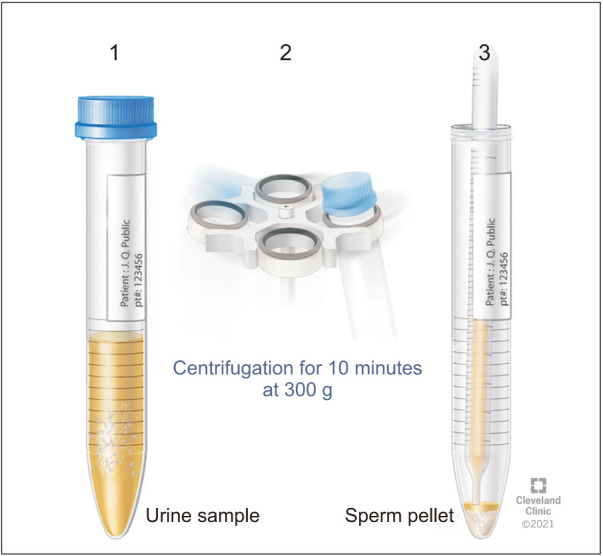
Fig. 6. Reconstitution of pellet with sperm wash media.
RETROGRADE URINE SPERM PREPARATION FOR ART BY DOUBLE DENSITY GRADIENT
In the ART setting, the patient is advised by his clinician to consume fluids with alkaline pH such as sodium bicarbonate to neutralize the highly acidic and detrimental pH that spermatozoa are exposed to in urine sample. Sperm processing for intrauterine insemination (IUI), is performed by layering the reconstituted urine suspension as explained in our publication on a double density gradient and subsequently following the gradient sperm preparation protocol as described below [16].
The specimen is urine, and this should be recorded on the report form with documentation of the actual urine volume and indicating that the specimen was centrifuged and reconstituted using sperm wash media. The reconstituted volume is used for the calculation of total sperm count (in millions). The sperm density gradients are prepared according to the instructions for the sperm density gradient procedure. The retrograde urine sample is processed by a double density gradient technique in a sterile manner. The reconstituted urine specimen is carefully layered on the upper phase of the gradient. Continue the procedure following the steps of gradient sperm wash technique [16]. A post-wash semen analysis (SA) is performed with a 6 µL drop of the post-wash specimen according to the protocol [16].
SPERM CONCENTRATION AND MOTILITY ASSESSMENT IN THE CONTEXT OF RETROGRADE EJACULATION: QUALITY CONTROL, QUALITY ASSURANCE AND COMPETENCY
Weekly quality control (QC) for sperm concentration and motility must be performed with a sample from a normal subject. A manual count and motility reading are performed simultaneously while the specimen is run on the computer-assisted semen analyzer (CASA) [16]. The passing criterion for the QC is that all manual results should have less than 20% difference compared to CASA value. The data for QC is monitored monthly and a quality assurance report is generated on a monthly basis. Competency assessment for laboratory personnel performing the tests is assessed. The assessment is done for both SA and density gradient techniques on an annual basis using competency checklists [16].
LAB SCENARIOS
A number of laboratory situations can be encountered while performing a post-ejaculatory urine analysis, and being aware of such scenarios allows for better preparedness.
1. Case A
1) Scenario
A patient has been referred by his physician to provide a semen sample for suspected RE. The patient is provided with two sterile collection cups, one labelled for semen and other containing sperm wash medium for urine sample.
The patient after 45 minutes says he is unable to collect a semen sample and only provides a urine sample. On examination of the urine sample, no sperm were seen.
2) Solution
Ask the patient if he has been able to achieve an orgasm or not? If not, assure the patient that the inability to orgasm and collect semen sample is not uncommon. The physician is informed regarding the patient's inability to provide a sample.
2. Case B
1) Scenario
The patient is referred to the laboratory for evaluation of RE. However, the patient does not follow instructions and attempts to collect the sample at home, furthermore, he reports inability to collect any semen sample. Instead, he collects the urine sample at home and brings it to the laboratory.
2) Solution
The sample should be rejected. Provide the patient with correct instructions for semen and retrograde urine collection at the laboratory according to the WHO 5th edition guidelines [17]. The patient is instructed to collect the sample at the laboratory in a warm media for correct assessment.
3. Case C
1) Scenario
Patient is referred to the laboratory for assessment of RE. Patient follows the instructions and collects the sample at the laboratory. He collects the semen sample in the first sterile container labelled as “semen” and the urine sample is collected in the second container with warm sperm wash media. However, upon examination the semen sample was found to be highly viscous.
2) Solution
The semen sample is treated with viscosity treatment system as per the protocol [18]. A vial of 5 mg Chymotrypsin enzyme is added to the semen sample. Semen sample is incubated for additional 10 minutes at 37℃ in the incubator. Once the viscosity treatment has been completed, 6 µL of the well mixed semen sample is plated on the fixed cell chamber and examined for sperm concentration and motility.
INTERPRETATION OF POST-EJACULATORY URINE ANALYSIS
In RE, the post-ejaculatory urine is examined for presence of spermatozoa, which would not be present in the post-ejaculatory urine of men with antegrade ejaculation. There is a reported overlap in the presence of sperm on post-ejaculatory urine analysis (PEUA) in infertile men versus normal men [2,19]. This may be due to traces of semen remaining inside the urethra, and these will be washed by micturition resulting in the presence of sperm in the urine specimen [20,21]. The presence of sperm in PEUA is more prevalent in infertile men and is a higher proportion of the total sperm count [22]. In infertile men presenting with either hypospermia or aspermia, the presence of spermatozoa in the PEUA usually confirms RE. Aspermia or hypospermia cannot differentiate between emission disorders and true RE. This can be confirmed by the presence of spermatozoa in the post-ejaculatory urine sample [23]. Fructose can also be found in the urine analysis in patients with RE, indicating the presence of semen [24]. The presence of 10–15 sperm per high power field in the post-ejaculatory urine after centrifugation [19,25] or more than a million sperm in the PEUA is defined as the limit for RE diagnosis. [26].
A study was conducted to compare the PEUA results between infertile and fertile men [22]. The study reported that PEUA showed higher number of infertile men harboring spermatozoa in the urine compared to fertile controls. The study also reported that low semen volume was associated with higher percentage of spermatozoa in urine; and severe oligozoospermia with low semen volume was directly correlated with high sperm counts in post-ejaculatory urine. In addition, the number of recovered spermatozoa from the urine were inversely related with the ejaculated seminal volume.
OPTIMIZATION OF URINE pH AND OSMOLALITY FOR IMPROVED SPERM VIABILITY
Both, high urine osmolality and low pH are considered toxic to spermatozoa, and hence, both parameters need optimization to enhance sperm survival [5,27,28,29]. Urine preparation in the patients with RE can be performed by ingestion of oral alkalinizing agents [29]. In the latter study, sperm motility was reduced in the unprepared urine samples (mean, 42.4%; range, 7.7%–79.5%) compared to higher recorded motility in the prepared urinary samples (mean, 99.1%; range, 86.7%–110.7%). The sperm retrieved from the retrograde urine can be used in either IUI or for in vitro fertilization (IVF) [28,30,31,32,33,34].
Three different methods of sperm retrieval from urine have been described for the management of infertility in patients suffering from RE [3]. These include: 1) centrifugation and re-suspension of post-ejaculatory urine specimens; 2) the Hotchkiss (or modified Hotchkiss) technique; and 3) ejaculation on a full bladder.
The centrifugation and re-suspension technique is performed in order to improve ambient sperm conditions. In this technique the patient is asked to increase his fluid intake and take sodium bicarbonate to dilute and alkalinize the urine, respectively. Taking oral acetazolamide, baking soda, or potassium citrate or instilling sperm wash medium into the empty bladder with catheterization after the patient voids, alkalinize the environment of the sperm to be retrieved [2]. A post-ejaculatory urine sample is collected by either introducing a catheter or spontaneous voiding. The urine sample is then centrifuged and suspended in a medium containing human serum albumin in Earle's/Hank's, phosphate buffered medium. The resultant modified sperm mixture can then be used in ART. The aforementioned method was reported to result in a 15% pregnancy rate per cycle based on 15 studies using either IUI or IVF/intracytoplasmic sperm injection (ICSI) [35].
The Hotchkiss method involves emptying the bladder prior to ejaculation using a catheter and then washing out and instilling a small quantity of Ringer's lactate to improve the ambient conditions of the bladder [36]. This is followed by the patient ejaculating and semen is retrieved by catheterization or voiding. A meta-analysis reviewed the ART outcome of 8 retrospective studies where the patients with RE underwent centrifugation and re-suspension of post-ejaculatory urine samples using the Hotchkiss technique [35]. In the latter review, the pregnancy rate (per cycle) of 25% and live birth rate of 28% was reported in IVF or ICSI cycles. For the technique of ejaculation with a full bladder, the patient is encouraged to ejaculate with a full bladder and the semen is suspended in Baker's buffer [37,38]. The meta-analysis by Jeffreys et al [35] reports that there were only 2 studies that used the technique of ejaculation on a full bladder for sperm retrieval. The pregnancy outcomes with use of the full bladder technique for IUI were inconclusive due to the poor methodology of the studies.
In patients with RE refractory to medical treatment, modified Hotchkiss procedure was utilized to retrieve sperm, and the retrieved sperm were frozen [14]. The authors of the latter study indicated successful outcomes and healthy live births with the use of frozen sperm retrieved with modified Hotchkiss procedure for ICSI. An average live birth rate per transfer of 28% was reported in the latter study.
CLINICAL INTERPRETATION OF RE IN MALE INFERTILITY
There are several clinical and diagnostic conditions or findings which indicate the need to perform PEUA test, including azoospermia with low semen volume, and aspermia, oligozoospermia associated with low semen volume and history of previous urological surgery for BPH or distal urethra stenosis. Some of the clinical scenarios associated with RE and male infertility are presented here as management options.
CLINICAL SCENARIOS
1. Case A
1) Scenario
A 36-year-old male with history of type 1 Diabetes presents with primary infertility. No female factor is noted. He reports lack of fluid expulsion during ejaculation, so he is unable to produce a semen sample for SA.
2) Management
If the patient has a typical orgasm and no semen upon ejaculation or low volume ejaculate (<1.5 mL), a PEUA should be performed. This follows the European Association of Urology (EAU) guidelines for RE evaluation and management, which recommends PEUA for patients with total or partial absence of an antegrade ejaculation and for patients experiencing a normal or decreased orgasmic sensation, except in paraplegia [39]. The PEUA is positive if: there is presence of any sperm in patients. It is reported that similar or more spermatozoa are present in patients with low volume ejaculate and oligozoospermia [22].
2. Case B
1) Scenario
A couple presents with primary infertility for 2 years and is discussing pursuing IUI cycles. The male partner presents his latest SA and PEUA results. The SA shows low volume (0.30 mL) with a sperm concentration of 2.0×106/mL and motility of 25% and normal morphology. Retrograde sperm concentration and motility were reported to be 24.0×106/mL and 20%, respectively.
2) Management
As per the EAU guidelines, sperm collection from the post-ejaculatory urine is recommended for use in ART if pharmacologic therapy is ineffective or not tolerable due to side-effects or in cases of patient with a spinal cord injury [39]. In specific cases, medications that causes RE should be withheld unless there is a risk of exacerbating the underlying medical condition by stopping the medication.
CONCLUSIONS
This article aims to provide the practitioner with a comprehensive guide/recommendations on optimal laboratory and clinical management of RE. Various scenarios that the practitioner may be faced with are analyzed highlighting the fact that when RE is involved, preparedness for the procedure is of added value. Critical analysis of the literature coupled by our extensive clinical experience indicates the merits of certain practices presented herein. Fertility problems associated with RE can be managed by addressing the cause whether it is anatomic, neurological or pharmacologic. Screening of post-ejaculatory urine is necessary in infertile patients with hypospermia or aspermia. Special conditions including collection at the laboratory and collection of the urine sample directly into sperm wash media are crucial in RE assessment. Oral intake of urinary alkalinizing agent such as sodium bicarbonate should be considered to regulate urinary pH and osmolality, and hence the sample should be optimized for use in ART. A retrograde urine sample with proper collection can be processed for potential use in ART depending on the sperm parameters and female factor with acceptable pregnancy outcomes.
ACKNOWLEDGEMENTS
Authors are thankful to the artists from the Cleveland Clinic's Center for Medical Art & Photography for their help with the illustrations. The study was supported by the American Center for Reproductive Medicine.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: AA, Rakesh S, SG.
- Writing—original draft: all the authors.
- Writing—review & editing: all the authors
References
- 1.Yavetz H, Yogev L, Hauser R, Lessing JB, Paz G, Homonnai ZT. Retrograde ejaculation. Hum Reprod. 1994;9:381–386. doi: 10.1093/oxfordjournals.humrep.a138513. [DOI] [PubMed] [Google Scholar]
- 2.Mehta A, Sigman M. Management of the dry ejaculate: a systematic review of aspermia and retrograde ejaculation. Fertil Steril. 2015;104:1074–1081. doi: 10.1016/j.fertnstert.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Parnham A, Serefoglu EC. Retrograde ejaculation, painful ejaculation and hematospermia. Transl Androl Urol. 2016;5:592–601. doi: 10.21037/tau.2016.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds JC, McCall A, Kim ED, Lipshultz LI. Bladder neck collagen injection restores antegrade ejaculation after bladder neck surgery. J Urol. 1998;159:1303. [PubMed] [Google Scholar]
- 5.Okada H, Fujioka H, Tatsumi N, Kanzaki M, Inaba Y, Fujisawa M, et al. Treatment of patients with retrograde ejaculation in the era of modern assisted reproduction technology. J Urol. 1998;159:848–850. [PubMed] [Google Scholar]
- 6.Kondoh N. Ejaculatory dysfunction as a cause of infertility. Reprod Med Biol. 2011;11:59–64. doi: 10.1007/s12522-011-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med. 2011;8 Suppl 4:310–315. doi: 10.1111/j.1743-6109.2011.02450.x. [DOI] [PubMed] [Google Scholar]
- 8.Mieusset R, Walschaerts M, Isus F, Almont T, Daudin M, Hamdi SM. Diagnosis of partial retrograde ejaculation in non-azoospermic infertile men with low semen volume. PLoS One. 2017;12:e0168742. doi: 10.1371/journal.pone.0168742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement P, Giuliano F. Physiology and pharmacology of ejaculation. Basic Clin Pharmacol Toxicol. 2016;119 Suppl 3:18–25. doi: 10.1111/bcpt.12546. [DOI] [PubMed] [Google Scholar]
- 10.Yakass MB, Woodward B, Otoo MA, Hiadzi EK. Case report: a healthy live birth following icsi with retrograde ejaculated sperm. Afr J Reprod Health. 2014;18:123–125. [PubMed] [Google Scholar]
- 11.Roughley M, Lyall M. Retrograde ejaculation associated with quetiapine and treatment with low-dose imipramine. BMJ Case Rep. 2019;12:e228539. doi: 10.1136/bcr-2018-228539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hisasue S, Furuya R, Itoh N, Kobayashi K, Furuya S, Tsukamoto T. Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol. 2006;13:1311–1316. doi: 10.1111/j.1442-2042.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 13.Makler A, David R, Blumenfeld Z, Better OS. Factors affecting sperm motility. VII. Sperm viability as affected by change of pH and osmolarity of semen and urine specimens. Fertil Steril. 1981;36:507–511. doi: 10.1016/s0015-0282(16)45802-4. [DOI] [PubMed] [Google Scholar]
- 14.Philippon M, Karsenty G, Bernuz B, Courbiere B, Brue T, Saïas-Magnan J, et al. Successful pregnancies and healthy live births using frozen-thawed sperm retrieved by a new modified Hotchkiss procedure in males with retrograde ejaculation: first case series. Basic Clin Androl. 2015;25:5. doi: 10.1186/s12610-015-0021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Garcia J, Jarow JP, Wallach EE. Successful management of infertility due to retrograde ejaculation using assisted reproductive technologies: a report of two cases. Arch Androl. 2004;50:391–394. doi: 10.1080/01485010490484110. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Gupta S, Sharma R. In: Andrological evaluation of male infertility. Agarwal A, Gupta S, Sharma R, editors. Cham: Springer; 2016. Procedure for retrograde ejaculate; pp. 97–100. [Google Scholar]
- 17.World Health Organization (WHO) WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO; 2010. [Google Scholar]
- 18.Baskaran S, Finelli R, Agarwal A, Henkel R. Diagnostic value of routine semen analysis in clinical andrology. Andrologia. 2021;53:e13614. doi: 10.1111/and.13614. [DOI] [PubMed] [Google Scholar]
- 19.Sigman M, Jarow J. In: Campbell's urology. 8th ed. Walsh PC, Retik AB, Vaughan ED, Wein AJ, Kavoussi LR, Novick AC, editors. Philadelphia: Elsevier; 2002. Male infertility; pp. 1475–1531. [Google Scholar]
- 20.Colpi GM, Negri L, Mariani M, Balerna M. Semen anomalies due to voiding defects of the ampullo-vesicular tract. Infertility due to ampullo-vesicular voiding defects. Andrologia. 1990;22 Suppl 1:206–218. doi: 10.1111/j.1439-0272.1990.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 21.Ariagno JI, Mendeluk GR, Pugliese MN, Sardi SL, Acuña C, Repetto HE, et al. The only presence of sperm in urine does not imply retrograde ejaculation. Arch Androl. 2005;51:431–436. doi: 10.1080/014850190953294. [DOI] [PubMed] [Google Scholar]
- 22.Mehta A, Jarow JP, Maples P, Sigman M. Defining the “normal” postejaculate urinalysis. J Androl. 2012;33:917–920. doi: 10.2164/jandrol.111.015974. [DOI] [PubMed] [Google Scholar]
- 23.Vroege JA, Gijs L, Hengeveld MW. Classification of sexual dysfunctions: towards DSM-V and ICD-11. Compr Psychiatry. 1998;39:333–337. doi: 10.1016/s0010-440x(98)90044-x. [DOI] [PubMed] [Google Scholar]
- 24.Cruz N, Porst H. In: The ESSM syllabus of sexual medicine. Porst H, Reisman Y, editors. Amsterdam: Medix Publishers BV; 2012. Ejaculatory and orgasmic disorders other than premature ejaculation; pp. 737–790. [Google Scholar]
- 25.McMahon C. In: Campbell-Walsh urology. 11th ed. Wein AJ, Kavoussi LR, Partin AW, Peters CA, editors. Philadelphia: Elsevier; 2016. Disorders of male orgasm and ejaculation; pp. 692–708. [Google Scholar]
- 26.Fedder J, Kaspersen MD, Brandslund I, Højgaard A. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology. 2013;1:602–606. doi: 10.1111/j.2047-2927.2013.00083.x. [DOI] [PubMed] [Google Scholar]
- 27.Braude PR, Ross LD, Bolton VN, Ockenden K. Retrograde ejaculation: a systematic approach to non-invasive recovery of spermatozoa from post-ejaculatory urine for artificial insemination. Br J Obstet Gynaecol. 1987;94:76–83. doi: 10.1111/j.1471-0528.1987.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsai TC, Lin MC, Cheng CJ. A new sperm collection method for treatment of retrograde ejaculation. J Formos Med Assoc. 1990;89:484–486. [PubMed] [Google Scholar]
- 29.Aust TR, Brookes S, Troup SA, Fraser WD, Lewis-Jones DI. Development and in vitro testing of a new method of urine preparation for retrograde ejaculation; the Liverpool solution. Fertil Steril. 2008;89:885–891. doi: 10.1016/j.fertnstert.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Ranieri DM, Simonetti S, Vicino M, Cormio L, Selvaggi L. Successful establishment of pregnancy by superovulation and intrauterine insemination with sperm recovered by a modified Hotchkiss procedure from a patient with retrograde ejaculation. Fertil Steril. 1995;64:1039–1042. doi: 10.1016/s0015-0282(16)57927-8. [DOI] [PubMed] [Google Scholar]
- 31.Elliot S, Szasz G, Zouves C. The combined use of vibrostimulation and in vitro fertilization: successful pregnancy outcome from a retrograde specimen obtained from a spinal cord-injured male. J In Vitro Fert Embryo Transf. 1991;8:348–352. doi: 10.1007/BF01133027. [DOI] [PubMed] [Google Scholar]
- 32.van der Linden PJ, Nan PM, te Velde ER, van Kooy RJ. Retrograde ejaculation: successful treatment with artificial insemination. Obstet Gynecol. 1992;79:126–128. [PubMed] [Google Scholar]
- 33.Tay JI, Whitehead A, Joyce AD, Rutherford AJ. Twin pregnancy following IVF treatment using frozen-thawed retrograde ejaculated sperm. J Assist Reprod Genet. 1996;13:731–732. doi: 10.1007/BF02066428. [DOI] [PubMed] [Google Scholar]
- 34.Trofimenko V, Hotaling JM. Fertility treatment in spinal cord injury and other neurologic disease. Transl Androl Urol. 2016;5:102–116. doi: 10.3978/j.issn.2223-4683.2015.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefferys A, Siassakos D, Wardle P. The management of retrograde ejaculation: a systematic review and update. Fertil Steril. 2012;97:306–312. doi: 10.1016/j.fertnstert.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Hotchkiss RS, Pinto AB, Kleegman S. Artificial insemination with semen recovered from the bladder. Fertil Steril. 1954;6:37–42. doi: 10.1016/s0015-0282(16)31863-5. [DOI] [PubMed] [Google Scholar]
- 37.Crich JP, Jequier AM. Infertility in men with retrograde ejaculation: the action of urine on sperm motility, and a simple method for achieving antegrade ejaculation. Fertil Steril. 1978;30:572–576. doi: 10.1016/s0015-0282(16)43640-x. [DOI] [PubMed] [Google Scholar]
- 38.Templeton A, Mortimer D. Successful circumvention of retrograde ejaculation in an infertile diabetic man. Case report. Br J Obstet Gynaecol. 1982;89:1064–1065. doi: 10.1111/j.1471-0528.1982.tb04668.x. [DOI] [PubMed] [Google Scholar]
- 39.Salonia A, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioğlu A, et al. Sexual and reproductive health [Internet] Arnhem: European Association of Urology; c2020. [cited 2021 Mar 27]. Available from: https://uroweb.org/guideline/sexual-and-reproductive-health/ [Google Scholar]


