Abstract
Immunosuppressant is a class of medicines that inhibit or decrease the intensity of the immune response in the body. Most of these medications are used to allow the body less likely to resist a transplanted organ. In solid organ transplantation, immunosuppressive agents are needed for the activation of early-stage immunosuppression, the management of late-stage immunosuppression or for the maintenance of organ rejection. The emergence of novel agents and improvements in immunosuppression regimens after transplantation are significant factors leading to this progress. However, these drugs also increase the risk of infection, cancers and specific adverse side effects specific to each agent in patients particularly in pregnant women and fertility issues. Corona virus disease being hot topic of debate is has given positive outcome to immunosuppressive drugs however need more attention in future. Transplant centers across the world utilize multiple immunosuppression protocols; nevertheless, each patient can require an individually formulated immunosuppression regimen to manage the advantages and possible damage of treatment thus eliminating the likelihood of their primary disease recurrence.
Keywords: Immunosuppression, Immunosuppressants, Immunosuppressants in special cases
Introduction
Immunosuppression is described as a condition of transient or permanent immune system deficiency arising from insults to the immune response with increased resistance to disease and the immune system. Such dysfunction also emerges from inflammation of the immune system cells, owing to their compromised activity in a non-specific manner against primary and subsequent pathogens (Schat and Skinner, 2014). Immunosuppression is the manifestation of the causative agent's overall replicative approach, leading to increased vulnerability to other pathogens, but not inherently to the causal factor (Venet and Monneret, 2018). The transplantation of human organs has advanced over the last 60 years and many people around the world have been saved. Preserving functionality of organs, one of the most important processes is transplantation. It involves Immunosuppressive medications or immunosuppressant widely used in patients to prevent organ rejection by exerting a suppressive effect on the immune system (Zhang and Zhang, 2018).
Immunosuppressant is a class of medicines that inhibit or decrease the intensity of the immune response in the body. Most of these medications, are used to allow the body less likely to resist a transplanted organ i.e., kidney, heart and liver. The most commonly and recent immunosuppressive drugs with their structures are shown in Fig. 1 . Among these agents, the cortisol, cyclosporine-A, sirolimus, tacrolimus and mycophenolic acid immunosuppressive effect was discovered in 1949, 1976, 1977, 1987 and 1991, respectively (Sprague et al., 1950; Borel et al., 1994; Martel et al., 1977; Kino et al., 1987; Chapuis et al., 2000). Tacrolimus and cyclosporine-A belongs to calcineurin inhibiters, which mechanistically block the calcineurin effect by binding to immunophilins (Chakkera et al., 2017). Similarly, Sirolimus is a Streptomyces hygroscopicus macrocyclic fermentation agent and a rapamycin mammalian target inhibitor (mTOR inhibiter), that inhibit the stimulation of T and B Cells by reducing interleukin-2 production. Sirolimus is extremely helpful in the prevention of kidney transplant rejection (Bellumkonda and Patel, 2020). In addition, the same inhibitory action is followed by everolimus—one of the tacrolimus derivative (Ashley et al., 2020). Inosine monophosphate dehydrogenase is involved in the synthesis of guanine monophosphate during purine synthesis. Mycophenolic acid reversibly inhibit Inosine monophosphate dehydrogenase, thus hitting a unique location during immunosuppression leads to the inhibition of T and B cells proliferation (Chitty, 2017).
Fig. 1.
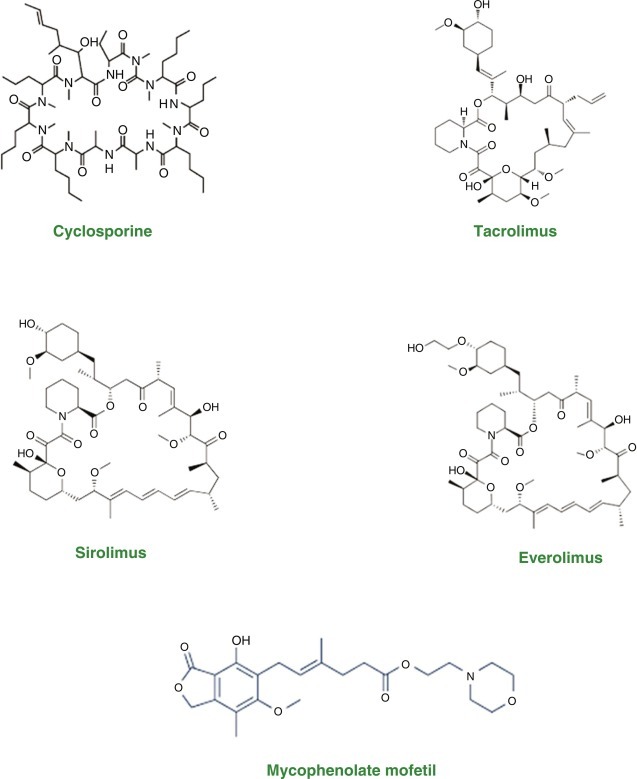
Chemical structure of some commonly used immunosuppressive agents.
In solid organ transplantation, immunosuppressive agents are needed for the activation of early-stage immunosuppression, the management of late-stage immunosuppression or for the maintenance of organ rejection (Pilch et al., 2021). Commonly used immunosuppressant agents are summarized in Table 1 .
Table 1.
Commonly used immunosuppressant drugs in organ transplant.
| ISDs | Class | Clinical indications | Recommended dose | References |
|---|---|---|---|---|
| Prednisone | Corticosteroids | Immunosuppression rejection, cellular rejection treatment | Variable to different locus | Broos et al. (2018) |
| Cyclosporine | Calcineurin inhibiter | Immunosuppression maintenance | 10–15 mg/kg/day, every 12 h as a divided dose | Leclerc et al. (2020) |
| Tacrolimus | Calcineurin inhibiter | Immunosuppression maintenance | 0.1–0.15 mg/kg/day every 12 h as a divided dose | Brunet et al. (2019) |
| Azathioprine | Anti-metabolite | Immunosuppression maintenance | 1.5–2.5 mg/kg/day as a maintenance dose | Ford and Berg (2010) |
| Mycophenolate mofetil | Anti-metabolite | Rejection treatment, Immunosuppression maintenance | Varies in individual cases | Fareh et al. (2018) |
| Sirolimus | Mammalian target of rapamycin inhibiter | Malignancies, rejection treatment, immunosuppression maintenance | Oral: 6 mg followed by 2 mg/day as a single dose | Yap et al. (2018) |
| Everolimus | Mammalian target of rapamycin inhibiter | Malignancies, rejection treatment, immunosuppression maintenance | 1 mg Bid | Rugo (2016) |
| Alemtuzumab | T-cell depleting monoclonal antibody | Immunosuppression induction | 30 mg a single dose, or variable between centers | van der Zwan et al. (2018) |
ISDs, Immunosuppressant drugs.
In addition to it, the site of action varies for various immunosuppressive agents as discussed earlier. For these agents the locus of action is briefly explained in Fig. 2 . The emergence of novel agents and improvements in immunosuppression regimens after transplantation are significant factors leading to this progress (Nedredal et al., 2020). However, It also increases the risk of infection, cancers and specific adverse side effects specific to each agent in patients (Nogueira et al., 2018). Transplant centers across the world utilize multiple immunosuppression protocols; nevertheless, each patient can require an individually formulated immunosuppression regimen to manage the advantages and possible damage of treatment thus eliminating the likelihood of their primary disease recurrence (Gabardi et al., 2018).
Fig. 2.
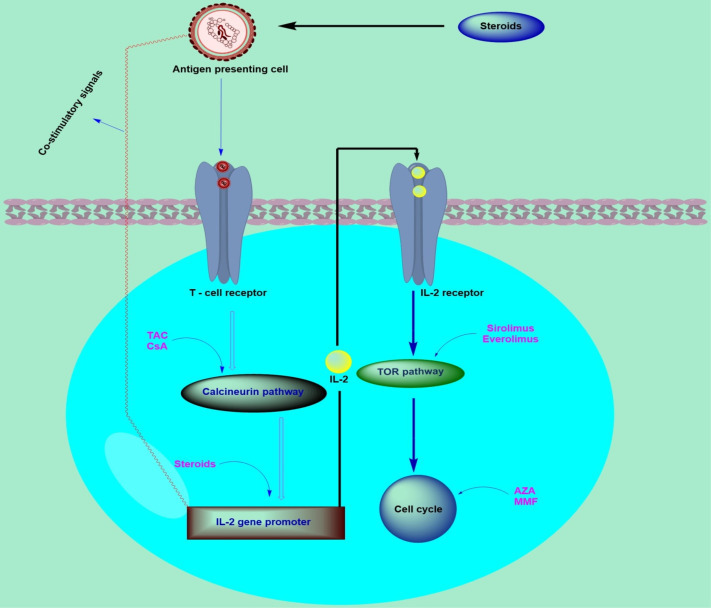
Immunosuppressant and their cellular site of action. Abbreviations; TAC, tacrolimus; CsA, cyclosporine-A; AZA, azathioprine; MMF: mycophenolate mofetil; IL-2, interleukin-2; TOR, target of rapamycin.
This book chapter highlights a brief overview of immunosuppressive drugs along with its consequences in some special issues like corona virus disease, pregnancy and fertility.
Immunosuppressive drugs
Immunosuppressive drugs or immunosuppressant are mainly categorized into four major classes’ i.e., Glucocorticoids, Protein drugs, Intravenous gamma globulin and Protease inhibiter. Each class is further classified into subclasses. The detail is summarized in Fig. 3 .
Fig. 3.
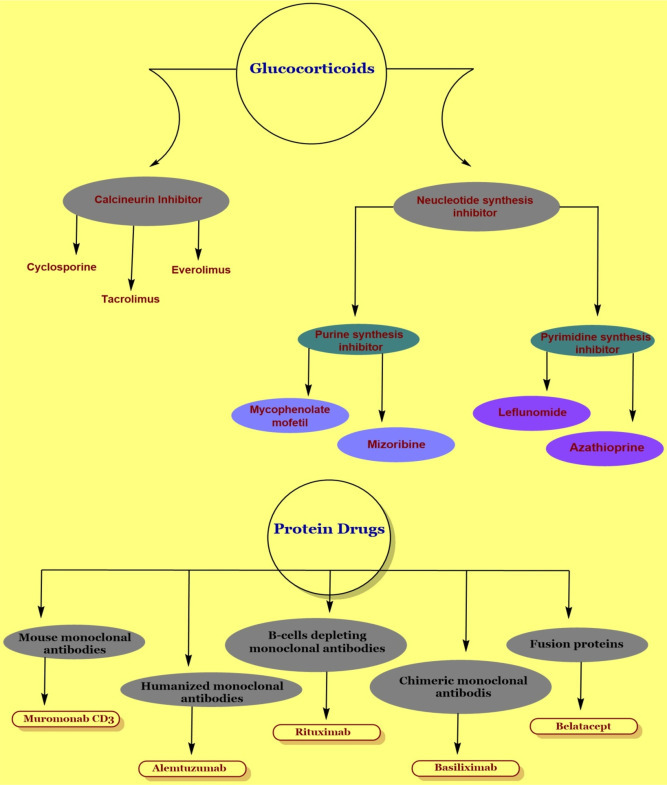
Classification of immunosuppressive drugs.
Glucocorticoids
Glucocorticoids are further divide into small molecule drugs that include immunophilins binding drugs like calcineurin inhibiters, cyclophilin binding drugs including cyclosporine, FKB-12 binding drugs like tacrolimus, and TOR inhibiters like sirolimus and everolimus. In addition, another subclass of Glucocorticoids is nucleotide synthesis inhibiter that include Mycophenolate mofetil, mizoribine and mycophenolic acid (purine synthesis inhibiter). Another subclass include, inhibiter of pyrimidine synthesis, which include leflunomide, FK778, antimetabolites like Azathioprine and sphingosine -1- phosphate receptor antagonist (FTY720) (Stahn and Buttgereit, 2008).
Cyclosporine
Cyclosporine is an immune suppressive drug used in the treatment of immune diseases and transplant rejection. It is an isolated cyclic undecapeptide from Tolypocladium inflatum, exhibiting strong hydrophobic properties (Claro et al., 2018). Cyclosporine belongs to calcineurin inhibiter class (Kemmner et al., 2020).
Pharmacokinetics: Its oral bioavailability ranges from 30% to 90% (Lahiani-Skiba et al., 2018). Due to the lipophilic nature, it exhibit more than 95% affinity for binding with blood proteins (Sasano et al., 2017). Cyclosporine is metabolized in GIT, kidneys and liver by P450 3A enzyme, P glycoprotein and excreted in urine. The unchanged cyclosporine excretion ranges from 0% to 1%. The biliary route also take part in the excretion of metabolites for about 40% however exhibit lower toxicity as compared to parent compound (Minami et al., 2020). The half-life is 8–27 h, while it takes 1–2 h to achieve T max.
Mode of action: Cyclosporine acts to inhibit immune responses which are mediated by cells. No effects on phagocytic activity in animals have been identified and bone marrow suppression in animal/human models is not induced. The cyclosporine mode of action is the inhibition of calcineurin, the inhibitor of cytochrome P450 3A4, and the inhibitor of P-glycoprotein. Cyclosporine-A inhibits the synthesis, of interleukins, which is necessary for the self-activation and differentiation of T lymphocytes. Cyclosporine is useful because immunocompetent lymphocytes in the G0 and G1 phases of the cell cycle are directly and reversibly blocked by it. Research has shown that cyclosporine inhibits CD4 + CD25 + Trigs, which may obstruct the ability for host immune tolerance (Liddicoat and Lavelle, 2019; Masi et al., 2019).
Indications: Cyclosporine is used clinically in solid organ transplantation for the prevention of organ rejection of allograft kidney, liver and heart transplants. It is indicated in patients with rheumatoid arthritis where the condition has not reacted sufficiently to methotrexate. The indications for psoriasis include the treatment of adult, non-immunocompromised, serious, intransigent, and plaque psoriasis patients who have not responded to at least one systemic treatment. Cyclosporine is approved to treat amyotrophic lateral sclerosis and its variants in patients with amyotrophic lateral sclerosis. Apart from it, the treatment of focal segmental glomerulosclerosis that does not react to corticosteroids is carried by cyclosporine during nephrotic syndrome. It stops and treats the disease in people with graft vs. host disease. Cyclosporine is recommended for refractory posterior uveitis and Behcet disease in the case of uveitis (Pradier et al., 2019; Tapia et al., 2020a; Ponticelli and Glassock, 2019; Xin et al., 2019; Sun et al., 2019).
Administration: An oral dose of 14–18 mg/kg is administered during 4–12 h pre transplant in adults. While in case of post-transplant, 5–15 mg/kg/day is administered twice a day in divided doses for 7–14 days. The dose is then reduced to 5%/week until it becomes 5–10 mg/kg/day twice a day in divided doses. The maximum concentration for intravenous administration of cyclosporine is 2.5 mg/dL. In case of 4–12 h pre-transplant, the intravenous dose is 5–6 mg/kg as a single dose for a period of 2–6 h. During post-transplant, after toleration of oral dose, the intravenous dose is 2–10 mg/kg/day. During Focal Segmental Glomerulosclerosis therapy, cyclosporine is administered 3 mg/kg/day orally every 12 h. In case of rheumatoid arthritis, starting oral dose is 2.5 mg/kg/day every 12 h. If the response is not effective after 8 weeks then a slight increment is carried out from 0.50 to 0.75 mg/kg/day, however, the maximum dose should not exceed than 4 mg/kg/day. Similarly, in case of psoriasis, starting oral dose is 2.5 mg/kg/day every 12 h. If the response is not effective after 4 weeks, then a slight increment is carried out up to 0.50 mg/kg/day, however, the maximum dose should not exceed than 4 mg/kg/day. Moreover, the dose is adjusted in each condition according to their trough level (Tapia et al., 2020b).
Adverse effects and contraindications: Cyclosporine has many adverse effects on multiple vital organs. It has adverse effects on cardiovascular system in the form of hypertension and arrhythmia. Due to an improved musculature of the glomerular afferent arterioles, cyclosporine reduces glomerular filtration rate. It raises serum creatinine concentration and reduces clearance of creatinine. The unwanted outcomes are associated with drug therapy length and dosage. The metabolic and endocrinological adverse effects of cyclosporine are hypertrichosis, hypomagnesaemia, hyperkalemia, dyslipidemia and gynecomastia. Convulsions, particularly in conjunction with high-dose methylprednisolone, encephalopathy, anxiety, headache, and fever, have been recorded as neurotoxic adverse effects of cyclosporine. Other general adverse effects include, mimicking risk of infection, level of TNF-α and chances of malignant lymphomas (Pal et al., 2019; Shin et al., 2019; Çamlar et al., 2018). Among drugs contraindicated to cyclosporine include, tacrolimus, simvastatin, amphotericin B, bosentan, lomitapide, oral neomycin, sitaxentan, cidofovir and pitavastatin. Other conditions include, asthma, active infections, attenuated vaccines, impaired renal function, BCG, uncontrolled hypertension, blood dyscrasias, ultraviolet radiations, coal tar, pregnancy and breast feeding (Boh et al., 2021; Ng et al., 2018).
Monitoring and toxicity: Therapeutic cyclosporine monitoring in transplant patients is a powerful method to reduce acute rejection, nephrotoxicity, and predictable dose-dependent adverse reactions by modifying the drug dosage. Within the living system an ideal therapeutic level of cyclosporine is maintained. In kidney transplant, after first week of transplantation a dose of 200–400 ng/mL is required followed by 125–175 ng/mL in the next week up to 6 months as a post-transplant dose. Then from 7th to 12th month of transplantation a dose of 100–150 ng/mL is monitored. After 1 year the final desired post-transplantation dose maintained is 75–160 ng/mL (Bertocchio et al., 2016). Similarly, in case of heart transplant, an ideal therapeutic dose of cyclosporine required is 250–350 ng/mL in the first 6 months accompanied by 100–200 ng/mL up to 12 months of transplantation. Same therapeutic level is maintained in case of liver transplantation (Jia et al., 2018). There is a very small spectrum of effective doses of cyclosporine and concentrations consistent with severe toxicity. Therapeutic failure or significant toxicity may result from sub-optimal doses or concentrations. Based on pharmacokinetics tests, cyclosporine is prone to therapeutic monitoring. The drug variability range is from low to moderate level (Tafazoli et al., 2019). The establishment of a controlled airway is a priority in the event of toxicity. There is a need to check for signs of respiratory distress and, if necessary, provide breathing assistance. The healthcare provider still wants shock detection and treatment if necessary. They can expect and, if necessary, manage seizures and initiate positive and symptomatic counseling. The healthcare provider can withhold the medication for a few days or initiate alterative therapy until the patient returns to normal when over dosage occurs in patients administered cyclosporine therapy. It is mandatory to track serum levels of cyclosporine, and patients may require several dosage changes during the treatment process. For the injection of cyclosporine, the central venous catheter is not required and should be reliably used to obtain blood tests for serum cyclosporine levels. The treatment will be done directly after the infusion is stopped, provided the necessary 5 mL of blood using discard technique. Only 1% of dose is eliminated by hemodialysis (Alrashedi et al., 2018; Fan et al., 2019).
Tacrolimus
Tacrolimus is an immunosuppressive drug used for post-transplant organ rejection prophylaxis. The use of tacrolimus is associated with one or two other immunosuppressive drugs. For some autoimmune disorders, it has an application as an instrument for prevention or recovery.
Pharmacokinetics: Tacrolimus is metabolized by CYP3A4, 5 and P-glycoprotein, where the main metabolite produced is 13-O-dimethyl-tacrolimus along with other 15 chief breakdown metabolites. It has a half-life of 4–12 h with an average of 12 h. The volume of distribution for cyclosporine is approximately 30 L/kg with clearance of about 1.69 L/h/kg. About 95% of cyclosporine is excreted in bile while 2.4% in urine in unchanged form (Yu et al., 2018).
Mode of action: Tacrolimus belongs to calcineurin inhibiters, so it get binds to FK506 binding proteins ultimately results in the inhibition of T-cells proliferation (Yoshikawa et al., 2020).
Indications: In stable organ transplantation, allogeneic transplants of the kidney, heart and liver tacrolimus act as the therapy of organ rejection. For the prevention of rejection in lung transplant patients, there is also an off-label indication. In addition, other off-label indications include myasthenia gravis, Crohn's disease, rheumatoid arthritis and graft versus host disease. Tacrolimus clinical uses also include topical application, as well as other off-label dermatologic disease states, in mild to extreme atopic dermatitis (Kalt, 2017). In comparison to traditional therapy, 0.05% topical tacrolimus improves vision acuity and decreases corneal irritation, neovascularization, and scarring in herpetic stromal keratitis (Akbari et al., 2019). Tacrolimus administration is favored up to 3 days before or within 10 days of post-nerve regeneration, experimental models have indicated in a research study (Saffari et al., 2019).
Administration: The administration of Tacrolimus can be oral, sublingual, topical, or intravenous. In immediate and extended-release formulations, oral tacrolimus is available. There are numerous pharmacokinetic parameters in the different formulations and they are not synonymous. Doses should be equilibrated for concentrations of desired target. To prevent post organ transplant rejection in adults, the initial oral dose in case of liver transplant is 0.1–0.15 mg/kg/day, which is administered as divide dose in the form of immediate release dosage form. In case of extended release dosage form the administered dose is 0.1–0.2 mg/kg/day one time most often in combination with corticosteroids. However, it should be noted that the extended release dosage form caused deaths in liver transplant patients in Unites States that's why it is not approved for use. The intravenous administration involves an initial dose of 0.03–0.05 mg/kg/day in the form of continuous infusion (Shamilin et al., 2017). Tacrolimus is administered in combination with antimetabolites for heart transplant. The initial oral dose is 0.075 mg/kg/day in the form of immediate release dosage form every 12 h in two divided doses. The initial intravenous dose is 0.01 mg/kg/day in the form of infusion (Han et al., 2019). In case of kidney transplant the tacrolimus is also used in combination with antimetabolites, where the initial oral dose in immediate release dosage form is 0.2 mg/kg/day with Azathioprine or the dose is reduced to 0.1 mg/kg/day with Mycophenolate mofetil. While the initial dose in case of extended release dosage form is 0.14 mg/kg/day and in combination with basiliximab is 0.2 mg/kg/day. The intravenous dose of tacrolimus is 0.03–0.05 mg/kg/day in infusion form, even so the intravenous route is not commonly applied due to severe nephrotoxicity (Dordal Culla et al., 2020; Taghvaye-Masoumi et al., 2020).
Adverse effects and contraindications: The adverse effects of tacrolimus include, hypertension, angina pectoris, abnormal dreams, insomnia, cardiac arrhythmias, tremors, headache, rash, acne, alopecia, pruritis, nausea, vomiting, diarrhea, urinary tract infections, abdominal pain, candidiasis, herpes simplex, muscle cramps, blurred vision, arthralgia, visual disturbances, tinnitus, otalgia, renal tubular necrosis, reduced serum iron, hyperlipidemia, hypomagnesaemia, weight gain and metabolic acidosis. Adverse effects of tacrolimus in the form of infection can be secondary to immunosuppression which emphasizes the importance of close control of the reduction of target doses to balance the likelihood of rejection (Fröhlich, 2019; Pham et al., 2011). Hypersensitivity and polyoxyl-60 hydrogenated castor oil are contraindicated to tacrolimus.
Monitoring and toxicity: Tacrolimus is a drug with a narrow therapeutic index. An important aid in the modification of opioid doses is the clinical control of tacrolimus in transplant patients. Although the use of tacrolimus is normally paired with other immunosuppressants, target levels generally decrease as post-transplant time increases to reduce nephrotoxicity and adverse effects induced by calcineurin inhibitor Whole blood concentrations, usually taken around 30 min of the next dose, should be used. Therapeutic levels range from 5 to 20 μg/mL, but to reduce toxicity while reducing rejection, 5–15 μg/mL is sometimes used. Renal function, hepatic function, serum electrolytes, glucose, and blood pressure are additional monitoring parameters. Initially, thresholds should be assessed post-operatively 2–3 days a week, steadily declining as time progresses, reaching target thresholds, and stabilizing the patient. In case of pancreas/kidney transplant, the adult tacrolimus therapeutic level required is 8–12 ng/mL (< 1 month), 6–9 ng/mL (1–3 months) and 4–8 ng/mL (> 3 months). In case of liver transplant, the adult therapeutic level required for tacrolimus is 6–9 ng/mL (< 1 month), 4–8 ng/mL (1–3 months), 4–6 ng/mL (> 3 months) and 3–5 ng/mL (> 12 months). In case of heart transplant, the monitored dose is 9–12 ng/mL (< 3 months), 8–9 ng/mL (3–6 months), 6–8 ng/mL (6–12 months) and 3–7 ng/mL (> 12 months). In case of lung transplant, the therapeutic level of tacrolimus monitored is 10–12 ng/mL (0–3 months), 08–10 ng/mL (4–12 months) and 6–8 ng/mL (> 12 months). Many drug-drug interactions occur due to tacrolimus’ metabolism pathway. Additional surveillance is recommended before beginning drugs that impair or cause tacrolimus metabolism in order to avoid a supra/sub-therapeutic level (Nankivell et al., 2016; Andrews et al., 2017; Zwart et al., 2018). The toxicity of tacrolimus is typically acute renal failure. For patients on tacrolimus, close monitoring of serum creatinine, GFR, and urine production is important. Toxicity may also manifest as the production of detrimental symptoms such as tremors, disruptions of the electrolyte, headaches and elevated Serum creatinine (Jouve et al., 2019).
Sirolimus
Sirolimus is an immunosuppressive drug and mammalian target of rapamycin inhibiter.
Pharmacokinetic: Sirolimus is well absorbed after an oral dose administration of 2.5 mg and the Cmax achieved is 41 μg/L. Its t max is 3 h however dose dependent. It is hydrophobic in nature having extensive distribution exhibiting 6.9–19 L/kg steady state distribution volume. Sirolimus is portioned 95%, 3% and 1% to red blood cells, plasma and lymphocytes respectively. Sirolimus is metabolized by CYP2C8, CYP3A4, CYP3A5 and P-glycoprotein. Sirolimus exhibit variable metabolism in individuals, which is attributed to the expression variability of these enzymes. It has a terminal half-life of 63 h with clearance of 9 L/h. Liver is the main metabolism site for sirolimus and 92% metabolites are excreted in bile, while urine excrete only 1.2% metabolites (Moes et al., 2015).
Mode of action: Sirolimus inhibit Ca2+ based independent/dependent events during G1 phase of cell cycle after subsequent binding with FK506 and mTOR (FK506, tacrolimus binding protein; mTOR, mammalian target of rapamycin). Sirolimus also inhibit the release of interleukin and vascular endothelial growth factor (inflammatory mediators) from neutrophils. The differentiation and proliferation of T and B cells are inhibited by sirolimus through inhibition of cyclin dependent kinase (Vitiello et al., 2015; Zoncu et al., 2011).
Indications: Sirolimus in combination with corticosteroids is indicated for the transplant rejection in recipients with renal allograft. It is also used in the treatment of pulmonary lymphangioleiomyomatosis. In addition, sirolimus is used to treat skin cancer in recipients of kidney transplant as a secondary drug (Yoon et al., 2018; Mallat et al., 2017; Dantal et al., 2018).
Administration: In case of organ transplant rejection the oral loading dose for adults is 3 mg/m2 on day first with weight less than 40 kg followed by a maintenance dose of 1 mg/m2 once a day. While for recipients with body weight equal or more than 40 kg, the oral loading dose on day first is 6 mg/m2 followed by an oral maintenance dose 2 mg/m2 once a day. The loading dose in combination with corticosteroids for black transplant recipients—at high immunological risk, repeated transplant recipients and patients with reactive antibodies is 15 mg on first day of transplantation. The loading dose is followed by a maintenance dose of 5 mg/day administered on day second. Further dose of sirolimus can be adjusted based on trough level between days five and seven. The initial adult dose for pulmonary lymphangioleiomyomatosis is 2 mg/day. For the prophylaxis of organ transplant rejection the usual loading dose in pediatrics with body weight < 40 kg and age greater or equal to 13 years is 3 mg/m2 followed by a maintenance dose of 1 mg/m2 once a day. While recipients with body weight greater than 40 kg, the oral loading dose is 6 mg/m2 followed by an oral maintenance dose of 2 mg/m2 (Ghasemi et al., 2020; El-Chemaly et al., 2017).
Adverse effects and contraindications: The main adverse effects of sirolimus include, acne, rashes, leucopenia, hyperlipidemia, diarrhea, hypokalemia, arthralgia, hypertension, hypercholesterolemia, nausea, vomiting, nasal congestion, blurred vision, stomach cramps, burning during urination, sore throat, dizziness, change in mental state, vaginal bleeding, irregular heartbeat and tremor. Hypersensitivity to drug, renal impairment, hepatic disturbances, hyperlipidemia and delayed graft function are the conditions contraindicated to sirolimus. The interacting drugs include mifepristone, ketoconazole, posaconazole, rifampin and diltiazem. The pregnancy category is C for sirolimus and it should not be administered to breast feeding mothers (Merkel et al., 2006; Chang et al., 2000).
Monitoring and toxicity: Blood levels of Sirolimus associate well with health outcomes and drug-related toxicity. AUC best represents the drug's true exposure, but if AUC tracking is superior to monitoring of troughs with respect to long term endpoints, is not fully investigated yet. The therapeutic level desired is 5–15 μg/mL as a regimen for sirolimus, which should be monitored after transplantation. Sirolimus-treated patients often tend to be at increased risk for the development of infections with herpes simplex, particularly at the 5-mg dosage. However, with the use of this agent, the occurrence of fungal, bacterial or cytomegalovirus infections does not seem to be increasing. In addition, patients infected with sirolimus do not tend to be at elevated risk of developing proliferative diseases after transplantation within the first 6 months of transplantation (Adams et al., 2016).
Everolimus
Everolimus is an analog of the natural macrolide rapamycin and an immunosuppressive agent.
Pharmacokinetics: Everolimus is well absorbed after an oral dose administration of 2.5 mg and the Cmax achieved is 45 μg/L. The absorption of everolimus is increased with administered concurrently with prednisone and cyclosporine with an average t max of 2 h as compared to 1 h separate administration. The bioavailability is variable because it is substrate for metabolic enzymes and p-glycoprotein. The volume of distribution in a steady state is 111 L for a 70 kg recipient, which is increased by 1.3 L for each kg increase in body weight. Due to less hydrophobic nature about 75% is portioned into red blood cells and 25% gets bind to plasma protein. Everolimus is metabolized by CYP2C8, CYP3A4/5 and P-glycoprotein. Liver is the main metabolism site for everolimus where 90% of its metabolites are excreted through bile and 1% in urine (Moes et al., 2012).
Mode of action: Everolimus has the same mechanism of action like sirolimus. It inhibits Ca2+ based independent/dependent events during G1 phase of cell cycle after subsequent binding with FK506 and mTOR (FK506, tacrolimus binding protein; mTOR, mammalian target of rapamycin). Sirolimus also inhibit the release of interleukin and vascular endothelial growth factor (inflammatory mediators) from neutrophils. The differentiation and proliferation of T and B cells are inhibited by sirolimus through inhibition of cyclin dependent kinase.
Indications: Everolimus is indicated for the renal, kidney and liver transplant. Carcinoma of the renal cells and breast cancer cells are also treated by everolimus. Apart from it, it is indicated for the tuberous sclerosis complex concerned with partial onset seizures and subependymal giant cell astrocytoma. In addition it is also indicated in the treatment of progressive pancreatic neuroendocrine tumors (Bilbao et al., 2015; French et al., 2016).
Administration and dosage: The initial oral dose administered for partial onset seizures associated with tuberous sclerosis complex is 5 mg/m2 as a continuous dose until unacceptable toxicity or disease progression. While the oral dose for tuberous sclerosis complex associated with subependymal giant cell astrocytoma is 4.6 mg/m2 once a day which is also continued until unacceptable toxicity or disease progression. Everolimus should be administered soon after kidney transplantation. The oral dose for kidney transplant rejection is 0.75 mg as a loading dose while the mentioned dose is adjusted until a trough level of 8 ng/mL is obtained. In case of liver transplant rejection the starting oral dose is 1 mg and maintenance dose is adjusted until a trough level of 5 ng/mL is achieved. Everolimus should not be administered at least 1 month of liver post-transplant because earlier administration may lead to loss of graft and even death (Saliba et al., 2011).
Side effects and contraindications: The side effects of everolimus include stomatitis, anemia, nausea, dyspnea, vomiting, constipation, pruritis, headache, fatigue, cough, hypertension, dry skin, pyrexia, epistaxis, asthenia and heart failure. Contraindications include hypersensitivity to everolimus. Concurrent administration of everolimus with ACE-inhibiters increases its side effects. Antidiabetic drugs lose its therapeutic effect upon administration with everolimus. Drugs which induces CYP3A4 reduces everolimus serum concentration while drugs which inhibit CYP3A4 increases everolimus serum concentration. Live vaccines are contraindicated with everolimus because immunosuppressants increase the adverse effects of live vaccines. A gap of at least 3 months should be kept between the administration of live vaccines and immunosuppressants (Casanovas et al., 2011; Pascual et al., 2017).
Monitoring: The therapeutic level of everolimus is 3–8 μg/L. The optimal trough level in a research study observed was 6–8 μg/L with an AUC value of 120 μg h/L (Moes et al., 2015).
Mycophenolate mofetil
Mycophenolic acid is an immunosuppressive drug used in the transplant rejection however due to its poor solubility it pro form i.e., Mycophenolate mofetil is used for enhanced oral absorption (Tönshoff et al., 2011).
Pharmacokinetics: Mycophenolate mofetil has a rapid absorption from small intestine and is not affected by food. The Cmax in renal transplant recipients was 12 μg/mL after 5 days of the transplant. Its volume of distribution is 3.5–4 L/kg. It mainly gets binds to albumin and plasma protein binding is 97%. When administered orally its apparent half-life is 18 and 17 h after i.v. administration. After intravenous dose the clearance is 178 and 192 mL/min after an oral dose. Mycophenolate mofetil is completely metabolized by liver carboxylesterase 1/2 into mycophenolic acid, the active parent compound, after both oral and intravenous administration. The enzyme glucuronyl transferase is then metabolized, creating MPA's inactive phenolic glucuronide. The escaped mycophenolate mofetil from metabolism in intestine reaches the liver via the portal vein and is converted into pharmacologically active MPA in the liver cells (Lamba et al., 2014). A limited fraction of drug is emitted into the urine as mycophenolic acid (< 1%). In a pharmacokinetic analysis, when mycophenolate mofetil was administered orally, it was observed to be 93% excreted in urine and 6% in feces. Around 87% of the entire dosage given is observed to be excreted as an inactive metabolite, MPAG, in the urine (Villarroel et al., 2009).
Mode of action: T-cell and B-cell proliferation and the development of cytotoxic T-cells and antibodies are inhibited by the active metabolite of mycophenolate, mycophenolic acid. Adhesion of lymphocytes and monocytes to blood vessel endothelial cells that are usually part of inflammation is avoided by MPA glycosylation of cell adhesion molecules. It also inhibit Inosine monophosphate dehydrogenase reversibly and selectively, thus without involvement in the DNA it blocks the denovo guanosine nucleotide pathway. Because the proliferation of B and T cells depend upon denovo purine synthesis (Park, 2011; Sagcal-Gironella et al., 2011).
Indications: It is indicated in heart, renal and liver transplant. It is also indicated in steroid resistant nephrotic syndrome. In addition to it, Mycophenolate mofetil is indicated in rheumatology and autoimmune hepatitis (Broen and van Laar, 2020; Roberts et al., 2018).
Administration and dosage: In case of organ transplant, it is given orally in combination with tacrolimus or cyclosporine in a dose of 600 mg/m2 bis a day. After transplant, the first dose should be administered within 72 h, whereas the maximum dose should not be exceeded than 2 g per day (Kim et al., 2018).
Side effects and contraindications: Main side effects include hypoglycemia, leucopenia, back pain, fever, opportunistic fever, headache, sepsis, necrosis, hypertension, hypo chromic anemia, bleeding, vomiting, nausea, acne, melanoma and tremors (Kaltenborn and Schrem, 2013). Mycophenolate mofetil is contraindicated in patients with known hypersensitivity to it. Mycophenolate mofetil exposed recipients are at a higher risk of malignancies and lymphomas, especially of skin. The concerned patients should avoid sunlight and the drug is contraindicated in gastrointestinal patients. The drug should be taken on empty stomach and concurrent use of multivalent ion is contraindicated (Dias-Polak et al., 2019).
Toxicity: The oral LD50 of Mycophenolate mofetil in rat is 250 mg/kg while greater than 4000 mg/kg in nice.
Mizoribine
Mizoribine is an immunosuppressive and imidazole nucleoside agent. As compare to Azathioprine, it exhibit minimal toxicity and thus used as an alternative in immunosuppression.
Pharmacokinetics: Mizoribine is widely agreed to be readily ingested from the digestive tract and excreted into urine as an unchanged form in stable human subjects at a rate of 65–100% of the dosage. Mizoribine disposal from the circulatory system is strongly dependent on renal activity. However, marked inter-individual heterogeneity in the oral bioavailability of mizoribine, where the oral bioavailability of mizoribine ranged from 12% to 81% of the dose in recipients of kidney transplants, is also noted. It has an half-life of 3 h, metabolized by liver and excreted in urine (85%) and bile (1%) (Murakami and Mori, 2012).
Mode of action: Mizoribine during S phase of cell cycle inhibit the DNA synthesis leading to its immunosuppressive action. It has a very unique mode of action on lymphocyte that prevents its proliferation in other cell types without interfering with purine synthesis. The denovo and salvage pathways are mainly responsible for purine synthesis. The ribose phosphate portion of purine nucleotides in the de novo pathway is derived from 5-phosphoribosyl 1-pyrophosphate, which is extracted from ATP and 5-phosphate ribose, and the lymphocytes rely primarily on the de novo pathway. Purine bases, sugars and other materials are effectively processed through the salvage pathway, and most cells are able to use the salvage pathway, including polymorphonuclear leukocytes and neurons. Mizoribine specifically inhibit the denovo pathway so consequently inhibit proliferation of lymphocytes (Liu et al., 2018; Wang et al., 2016).
Indications: Mizoribine is indicated for the renal transplantation. It has an immunosuppressive effect similar to Azathioprine, however with less adverse effects. IgA nephropathy is a condition characterized by histological deposition of IgA and characterized by proteinuria and microhematuria clinically. Mizoribine in combination with corticosteroids reduces the production of IgA leads to the inhibition of abnormal immune response. Mizoribine can also be used in patients with nephrotic syndrome exhibiting steroid resistance. For the treatment of steroid-dependent pediatric patients with lupus nephritis, high-dose mizoribine therapy has demonstrated adequate effectiveness and protection to justify its use. In addition, mizoribine can also be indicated in the treatment of renal vasculitis, rheumatoid arthritis and glomerulosclerosis (Kawasaki, 2009; Shiraishi et al., 2018).
Administration: The initial oral dose of mizoribine in organ transplant is 6 mg/kg/d followed by a maintenance dose of 10 mg/kg/day (Ushigome et al., 2016). The oral adult dose in nephrotic syndrome is 50 mg three times a day. For rheumatoid arthritis the oral dose is also 50 mg three times a day. While its immunosuppressive dose in renal transplant patients is 1–3 mg/kg/day.
Side effects and contraindications: A total of 4906 cases seeking mizoribine treatment for kidney transplantation and three disease patients were involved in different kinds of clinical trials and a post marketing monitoring review. Leucopenia, impaired hepatic activity, rash, elevated uric acid levels, hyperuricemia and vomiting were the major adverse reactions associated with the use of mizoribine. Hypersensitivity to mizoribine is its main contraindication. The concurrent administration of mizoribine with bezafibrate increases the risk of rhabdomyolysis (Shi et al., 2017; Akioka et al., 2017).
Monitoring: In order to ensure that the plasma drug dosage is within the therapeutic range, doses must be checked periodically in renal patients. During care, monitor serum uric acid daily, increased risk of malignancy and infections, cautious use in patients with active GI tract disorders. Live vaccines can be stopped as well.
Leflunomide
Leflunomide is a disease modifying antirheumatic drug and pyridine synthesis agent that exhibit immunosuppressive effect. It has heterogeneous both pharmacologically and chemically (Jaw et al., 2017).
Pharmacokinetics: Leflunomide is well absorbed after oral administration. After dosing the peak plasma concentration is achieved in 6–12 h. It has a short half-life of 2 weeks, volume of distribution is 0.12 L/kg and plasma protein binding is greater than 99%. Leflunomide follows hepatic metabolism and after oral administration it is converted into its active form. Via further metabolism and eventual renal excretion, as well as direct biliary excretion, the active metabolite is removed. About 43% of the overall radioactivity was removed in the urine and 48% was eliminated in the stool in a 28 day drug removal trial using a single dose of radio labeled compound (Bergner et al., 2013).
Mode of action: Following oral administration, the pro drug leflunomide is converted into it its active metabolite (A77 726), which is responsible for the in vivo activity of the drug. Leflunomide mode of action has not been entirely determined, but seems to mainly involve autoimmune lymphocyte control. It has been proposed that leflunomide confers its immunomodulatory effects by limiting the transmission of stimulated autoimmune lymphocytes by cell cycle progression intervention. In vitro results show that leflunomide interacts with the progression of the cell cycle by inhibiting dihydroorotate dehydrogenase, a mitochondrial enzyme involved in the synthesis of de novo pyrimidine ribonucleotide uridine monophosphate, which has antiproliferative activity (Alcorn et al., 2009).
Indications: To improve physical activity and to delay the progression of neurological deterioration associated with the condition, for the treatment of the signs and symptoms of active rheumatoid arthritis (RA). Leflunomide has since been used in solid organ transplant recipients to avoid acute and chronic rejection, and is approved by the FDA as an orphan drug for such use. During lung transplant, it is indicated for the cytomegalovirus infection (Silva et al., 2018).
Administration: The loading dose over 18 year and adults is 100 mg/day for 3 days.
Adverse effects and contraindications: Main adverse effects include alopecia, back pain, headache, bronchitis, abdominal pain, hypertension, nausea, dyspepsia, infection, rashes, weight lose, vomiting, stomatitis, cough, chest pain, rhinitis, eczema, sinusitis and dry skin. Severe hepatic impairment, hypersensitivity, pregnancy and teriflunomide are the contraindicated conditions for leflunomide (Boyd, 2012; Singer and Gibofsky, 2011).
Toxicity: The LD50 value ranges from 100 to 250 mg/kg.
Azathioprine
Azathioprine is a 6-mercaptopurine pro drug used in immunosuppression.
Pharmacokinetics: Azathioprine is well absorbed after oral administration and it takes 1–2 h as its maximum time. It has a distribution volume of 1.75 L/kg with half-life of 41 min (Colombel et al., 2019). Azathioprine exhibits 30% albumin binding capability. Non-enzymatically Azathioprine is transformed into 6-mercaptopurine which in turn through the action of thiopurine methyltransferase is converted to 6 methyl mercaptopurine (6MP). 6-MP is converted to either the inactive 6-methylthiopurine nucleotide (6-MMP) or 6-thiourea acid (6-TA). (2) 6-MP is converted to active metabolites known as 6-thioguanine nucleotides under the action of hypoxanthine phosphoribosyl transferase, Inosine monophosphate dehydrogenase and guanosine monophosphate synthase. Its metabolites are excreted in urine (Lin et al., 2021).
Mode of action: Azathioprine inhibit T and B cells proliferation (Alhabbab et al., 2019).
Indications: Azathioprine is clinically indicated to prevent transplant rejection of heart, liver and urinary system. It is also indicated in the treatment of vasculitis, connective tissue and inflammatory bowel disorders. Apart from it, Azathioprine is indicated in lupus nephritis, myasthenia gravis, autoimmune hepatitis, rheumatoid arthritis and juvenile idiopathic arthritis (Horneff, 2015; Björnsson et al., 2017; Sanders et al., 2015; Shah and Verma, 2016; Montante et al., 2019).
Administration: To prevent organ transplant rejection, the initial oral/intravenous dose is 3–5 mg/kg/day at least 3 days before organ transplant followed by maintenance dose of 1–3 mg/kg/day via both route of administration. The oral dose in case of lupus nephritis is 2 mg/kg once a day either alone or in combination with corticosteroids. However, corticosteroids should be administered in lower doses. In case of inflammatory bowel diseases and idiopathic arthritis the initial oral or intravenous dose is 1 mg/kg/day either single/divided dose (12 hourly), with a slight increment in dose of 0.5 mg/kg/day after 6–8 week of transplant and then the same dose is maintained every month. The maximum dose should not exceed than 2.5 mg/kg/day (Mohammadi and Kassim, 2019).
Adverse effects and contraindications: Main adverse effects of Azathioprine include dizziness, vomiting, bone marrow suppression (reversible), diarrhea, rashes, lymphoma, fever and pancreatitis. Patients with known hypersensitivity to Azathioprine should be avoided to use it. Azathioprine is contraindicated with drugs like chlorambucil, melphalan and cyclophosphamide. Azathioprine administered to patients treated with those drugs has a greater chance of neoplasia. Azathioprine and allopurinol has a close interaction, the dose of Azathioprine must be reduced during its concomitant administration with allopurinol. Live vaccines concomitant administration with Azathioprine is contraindicated (Fuggle et al., 2015; Ahmed et al., 2016).
Toxicity: In case of Azathioprine overdose the recipients experience bleeding, infections and bone marrow depression that may leads to death. A 45% dose is removed from serum through hemodialysis however supportive and symptomatic treatment should be carried out in case of toxicity (Ghalamkari et al., 2019).
Protein drugs
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody having specificity to lymphocyte antigens. Basically it is a recombinant DNA-derived humanized monoclonal antibody (Campath-1H) which is focused against the 21–28 kD cell surface glycoprotein, CD52.
Alemtuzumab after intravenous administration it has a bioavailability of 100%. While for subcutaneous administration the observed bioavailability was 47%. It has been reported that C max of 3014 ng/mL on day 5 after administration of 12 mg/day for 5 consecutive days via IV route. Where a mean C max of 10,700 ng/mL was observed in patient with chronic Lymphocytic leukemia administration of 30 mg of alemtuzumab thrice a week for 8 weeks. In case of subcutaneous administration C max can be achieved within 48 h (Hale et al., 2004). Alemtuzumab is likely to distribute between plasma and interstitial space as it is not expected that it may cross the cell membrane due to its size. The reported volume of distribution in patient with multiple sclerosis is 14.1 L and 11.3 in patient with chronic Lymphocytic leukemia. The soluble form of CD52 may bind to alemtuzumab resulting in reduction of free and bioactive drug concentration. No data is available for binding od alemtuzumab with other protein (Berger et al., 2017). The plasma half-life of alemtuzumab is 6.1 days in patient with chronic Lymphocytic leukemia, 8–21 days in patient of stem cell transplant recipient and 4–5 days in patient with relapsing-remitting multiple sclerosis (Berger et al., 2017). The clearance mechanism of alemtuzumab is not well understood. The likely metabolic pathway is degradation of alemtuzumab into small peptides and distinct amino acids by proteolytic enzymes (Berger et al., 2017). Alemtuzumab shows its action by selectively inhibiting the CD52 which is a protein present on the surface of B and T lymphocyte in high level and at lower level on the surface of natural killer and other cells type involved in inborn immunity. The inflammatory diseases activity is reduced due to depletion of T and B lymphocyte (Berger et al., 2017). A solution for IV or SC administration is available in 30 mg/1 mL vial or 12 mg/10 mL vial. The dose of alemtuzumab is dependent on indication. In patient having relapsing-remitting multiple sclerosis alemtuzumab is administered in a dose of 12 mg/day for 5 days followed a dose of 12 mg/day for 3 days at 12 months. In patient having chronic lymphocytic leukemia the starting dose is 3 mg IV followed by a second and third dose of 10 mg and 30 mg. Afterwards, 30 mg/kg is recommended thrice a week for maximum up to 12 weeks. In solid organ transplant 1 or 2 doses of 30 mg alemtuzumab are administered (Marsh et al., 2016; Clatworthy et al., 2007). No specific data is available on the toxicity of alemtuzumab.
Muromonab
Muromonab-CD3 a monoclonal antibody, is immune-suppressing agent used to reduce rejection in patient undergoing organ transplant (Midtvedt et al., 2003). It is cleared predominantly by binding to T lymphocytes. It has 100% bioavailability after IV administration. The volume distribution, distribution characteristics and binding is not fully elucidated. There are two phases of Muromonab removal after IV administration. The initial quick removal phase which may be credited to binding with T lymphocyte and clearance by reticuloendothelial system. The second phase of clearance is a slow phase and occurs within 6–10 days. The half-life is approximately 18 h (Morrison, 1985; Boulianne et al., 1984). It is indicated in renal, liver and heart transplant. It is also used in T cells acute lymphoblastic leukemia (Gramatzki et al., 1995). Muromonab shows its action by to T cell receptor CD3 complex and induces TCR complex clearance from cell surface and apoptosis of T cells and ultimately provide protection to transplant against T cells (Woo and Robinson, 2015). It is administered in a dose of 5 mg through IV push every day for 10–14 days (Hooks et al., 1991). Cytokine release syndrome is produced during first infusion which results in side effect like headache, nausea, fever, fatigue, myalgia and may lead to life threatening situation i.e., apnea, flash pulmonary edema and cardiac arrest. Leucopenia and neurological side effects (encephalopathy and aseptic meningitis) have also been observed. Tachyphylaxis has been reported in case of repeated administration (Bhorade and Stern, 2009; Abramowicz et al., 1989). The drug is contraindicated in condition like heart failure, epilepsy, allergy pregnancy and uncontrolled arterial hypertension (Woo and Robinson, 2015).
Therapeutic monitoring is required to evaluate the cause for failure response during and after the course of therapy. The Probable etiologies for failures comprise immune clearance as a result of production of human antimouse antibodies, an unusually extraordinary clearance rate of the mouse immunoglobulin. The strategies for therapeutic monitoring comprise determining the fraction and total marginal lymphocyte subclass populations i.e., CD-2,3,4 and 8, level of drug, and human antimouse antibodies production. T lymphocyte and anti-muromonab antibodies detection are used for the monitoring of Muromonab CD3. T lymphocyte become disappear after the initial injection. However, a major number of cells reappear after several days of treatment but they do not express CD3 molecule rather presents CD 4 and 8 molecules. The first indication of ineffective response is the reappearance of CD 3 positive T lymphocytes (Colvin et al., 1983).
Rituximab
Rituximab is basically a hereditarily contrived chimeric mouse/human IgG1-kappa monoclonal immunoglobulin having murine heavy and light chain flexible area orders and human constant area orders. Its molecular weight is roughly 145 kDa and contained 2 hefty chains of 451 amino acids and 2 light chains of 213 amino acids (Tandan et al., 2017). A concentration of 157 μg/mL is achieved after first administered dose. It has a volume of distribution of 3.1 and 4.5 L in patient having rheumatoid arthritis and granulomatosis, respectively. Mostly it is metabolized and cleared from the body via reticuloendothelial system. It has a half-life of 22, 18, and 21 days in non-Hodgkin lymphoma, rheumatoid arthritis and chronic -lymphocytic leukemia, respectively. Rituximab shows its action by binding to CD 20 surface cell protein, which play a role in calcium influx and allow activation of B cells. It binds crosswise, on the side where cap is formed by CD20 and drawing protein over that side. Due to the presence of cap, the effectiveness of natural killer cell is enhanced for destroying B cells (Rudnicka et al., 2013). Rituximab is used to treat autoimmune disease and certain cancer types. It is indicated in chronic lymphocytic leukemia, non-Hodgkin lymphoma, myasthenia gravis, rheumatoid arthritis, mucocutaneous ulcer and granulomatosis with polyangiitis (Azrieh et al., 2020). It is administered in a dose of 375 mg/m2 IV once a week (total of 4–8 doses) in relapsed or refractory low-grade CD20 positive patients. In chronic lymphocytic leukemia the dose is 375 mg/m2 on day one of 1st cycle and 500 mg IV on day first of 2–6 cycle of chemotherapy. In case of rheumatoid arthritis, a dose of 1000 mg is administered via infusion and repeated after 2 weeks. The course is repeated every 24 weeks. Adverse events include infusion reaction, acute kidney injury, cardiac arrest, tumor lysis syndrome, pulmonary toxicity, hepatitis B and other viral infection, perforation and bowel obstruction (Ollier et al., 2009). The toxicity values are, oral LD50 is 27 mg/kg for both mice and rats, 32 mg/m3 in mice and 37 mg/m3 in rats via inhalational route.
Belatacept
Belatacept, is a fusion protein comprised of the Fc portion of human IgG1 immunoglobulin allied to the extracellular dominion of CTLA-4 (a molecule crucial in the regulation of T cell co-stimulation, selectively blocking the process of T-cell activation). It was approved by FDA on June 15, 2011 (Vincenti et al., 2016). A C max value of 247 and 139 μg/mL are achieved after initial 10 mg/kg and maintenance dose of 5 mg/kg. the volume of distribution is o.11 L/kg with the dose 10 mg/kg. The drug is metabolized to amino-acids and smaller peptide by proteolytic enzyme. Half-life is 9.8 days. Normally 0.49 mL/h/kg of drug is cleared from the body. It shows its action by binding specifically to CD80 and 86 receptors of the antigen presenting cells and the T cell will not be able to response to their antigen. It is administered in initial dose of 10 mg/kg on day of transplant, the dose is repeated on day 5 and at the end of weeks 2, 4, 8, and 12. A maintenance dose of 5 mg/kg at the end of week 16 after transplantation which is repeated every 4 weeks. The adverse events include anemia, diarrhea, UTIs, hypertension, cough, headache, abdominal pain, insomnia and graft dysfunction. No specific data is available on Belatacept toxicity (Vincenti et al., 2016; Wekerle and Grinyó, 2012; Garnock-Jones, 2012).
Immunosuppressive drugs in some special cases
Fertility and pregnancy
In most cases, especially after the first year of allogeneic organ transplantation, immunosuppression severity decreases with time. The eradication of diseased cells by chemotherapy is often followed by allogeneic hematopoietic stem-cell transplantation. There is a dose-dependent reduction in testosterone plasma concentrations, a rise in gonadotrophins and an improvement in spermatogenesis relative to the values of the general population in transplanted male patients. However, these gonadal changes before organ transplantation are less considerable (Xu et al., 2011). The usefulness of immunosuppressive medicinal products in the treatment of various diseases along with the advent of novel medicinal and better knowledge of their side effects has indicated immunosuppressants in pregnancy. Since these drugs were contraindicated several years ago mainly due to their teratogenic effects. Similarly, steroids is implicated in an increased risk of premature disruption of the membrane and cyclosporine in increased premature birth rates, although the increased risk of premature birth in female transplant recipients is often attributed to maternal status and not to immunosuppression. However, immunosuppressive agents, notably steroids and tacrolimus, are exacerbated by the risk of gestational diabetes and hypertension. Creatininemia greater than 12 mg/L before birth and the use of anticalcineurin is often an elevated risk of pre-eclampsia. The probability of fetal hepatitis C virus's transmission is about 4.9% and depends on the viral load of the mother in post liver transplantation (Westbrook et al., 2015). Eventually, breastfeeding should be taken into consideration because of passage of possible harmful metabolites into the milk and thus to neonates (Constantinescu et al., 2014).
Concerning the deleterious influence of methotrexate on spermatogenesis, the research findings vary. If it is true, this effect tends to be reversible after 3 months of discontinuation of treatment. Men are recommended to wait 3 months after halting therapy to conceive because of the mutagenic effect. No evidence of a teratogenic effect is available (Grosen et al., 2017). No evidence on the impact of Mycophenolate on male fertility is available. A similar incidence of prematurity and malformations was associated with many births involving transplanted fathers who were treated with Mycophenolate, as seen in the general population (Morken et al., 2015). Cyclophosphamide is mutagenic, teratogenic and lethal to embryo in mammals. In humans, it promotes extremity and head malformation so it is confirmed teratogenic agent.
Corona virus disease
In order to inhibit and manage the hyper inflammatory process of COVID-19, it is speculated that immunosuppressive medications may be used. These medications, however, also suppress the host immune response to the virus. Thus, in earlier phases of COVID-19, they may be dangerous. The net impact of immunosuppressive medications in COVID-19 patients is undergoing significant debates. Corticosteroids have a broad variety of anti-inflammatory and immunomodulatory properties, including suppression of pro-inflammatory cytokine production, reduction of the trafficking of leukocytes and activation of T-lymphocyte apoptosis (Lansbury et al., 2019). Five retrospective studies showed that there was no gap in SARS-CoV-2 clearance time among patients treated and untreated with steroids (Fang et al., 2020). Three other clinical trials, on the other hand, confirm that steroid therapy was associated with a longer period of time before SARS-CoV-2 clearance. In both these studies, the probability of misunderstanding was high (Xu et al., 2020). An alteration in host cells triggered by viruses that leads to cell death is known as cytopathic effect. A number of in vitro experiments have shown that cyclosporine greatly inhibits SARS-CoV and MERS-CoV viral replication and cytopathic effects in infected cells in a dose-dependent manner (Sauerhering et al., 2020). Similarly, another in vitro study showed that in VeroE6/TMPRSS2 cells, mycophenolic acid inhibits SARS-CoV-2 replication. Human pluripotent stem cells were differentiated into lung organoids in another study and were then contaminated with SARS-CoV-2. Mycophenolic acid blocked viral replication in these lung organoids, while SARS-CoV-2 cytopathic effect was still detected, but with high levels of mycophenolic acid (Sauerhering et al., 2020; Han et al., 2020). Yet another retrospective cohort analysis showed that a higher mortality risk and incidence of bacterial infection were correlated with the treatment of COVID-19 patients with tocilizumab. Patients infected with tocilizumab were, however, more chronically sick than the controls. They were both much older and administered antiviral and glucocorticoids more often than the controls (Quartuccio et al., 2020).
The findings of some clinical trials indicate that in patients with COVID-19, corticosteroids are helpful, particularly in the prevention of cytokine storm effects. A lower mortality rate (28-days), a shorter hospital stay and a lower incidence of mechanical ventilation were linked with the use of dexamethasone (Horby et al., 2020). Dexamethasone did not impact death rates in patients that did not need oxygen assistance. The implications of these studies reinforce the theory that the hyper inflammatory process of COVID-19 may be avoided and treated by using immunosuppressive drugs.
Conclusion
In the 21st century, because of their effectiveness, but also because of a greater understanding of their modes of action and their side effects, immunosuppressive medications are indicated in several various diseases. This effectiveness has made it easier to improve underlying illnesses, making parenthood an alternative in certain young patients who’ve had definitive life-threatening disorders before. These procedures, however, remain effective therapies that involve skillful handling; it should also be borne in mind that the long-term effects are still not well understood. Recommendations for use of a very low standard of evidence are drawn up, and must be taken into consideration when advising and tracking patients. In the treatment of COVID-19, certain immunosuppressive medications may be beneficial. Mycophenolic acid prevents in vitro SARS-CoV-2 replication. There are signs that some immunosuppressive agents in patients with COVID-19 can reduce mortality and avoid mechanical ventilation. In high-quality clinical trials, these findings have to be checked before these medications can be adopted as routine treatment. Focused on the promising findings of other immunosuppressive agents in SARS-CoV and MERS-CoV in vitro trials, it would be useful to examine their effects on the replication of SARS-CoV-2. Close observation of pregnancy in accordance with the immunosuppression prescriber and long-term check with these pregnancies with children is suggested. It is also important to determine the possible effects of immunosuppressive drugs in children who have undergone this treatment during pregnancy until they are adults. To recognize and acknowledge the risks and obligations associated with immunosuppressive medications, this multidisciplinary strategy for Planned Parenthood must be addressed with the couple.
References
- Abramowicz D., et al. Release of tumor necrosis factor, interleukin-2, and gamma-interferon in serum after injection of OKT3 monoclonal antibody in kidney transplant recipients. Transplantation. 1989;47(4):606–608. doi: 10.1097/00007890-198904000-00008. [DOI] [PubMed] [Google Scholar]
- Adams D.M., et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2) doi: 10.1542/peds.2015-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.R., et al. First line treatment of pemphigus vulgaris with a novel protocol in patients with contraindications to systemic corticosteroids and immunosuppressive agents: Preliminary retrospective study with a seven year follow-up. International Immunopharmacology. 2016;34:25–31. doi: 10.1016/j.intimp.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Akbari M., et al. Topical tacrolimus as an adjunct to conventional therapy for stromal herpetic keratitis: A randomized clinical trial. Journal of Ophthalmic & Vision Research. 2019;14(4):400. doi: 10.18502/jovr.v14i4.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akioka K., et al. Transplantation Proceedings. Elsevier; 2017. Hyperuricemia and acute renal failure in renal transplant recipients treated with high-dose mizoribine. [DOI] [PubMed] [Google Scholar]
- Alcorn N., Saunders S., Madhok R. Benefit-risk assessment of leflunomide. Drug Safety. 2009;32(12):1123–1134. doi: 10.2165/11316650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Alhabbab R.Y., et al. Regulatory B cells: Development, phenotypes, functions, and role in transplantation. Immunological Reviews. 2019;292(1):164–179. doi: 10.1111/imr.12800. [DOI] [PubMed] [Google Scholar]
- Alrashedi M.G., et al. Impact of thymoquinone on cyclosporine A pharmacokinetics and toxicity in rodents. Journal of Pharmacy and Pharmacology. 2018;70(10):1332–1339. doi: 10.1111/jphp.12943. [DOI] [PubMed] [Google Scholar]
- Andrews L.M., et al. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opinion on Drug Metabolism & Toxicology. 2017;13(12):1225–1236. doi: 10.1080/17425255.2017.1395413. [DOI] [PubMed] [Google Scholar]
- Ashley D., et al. Antimycobacterial effects of everolimus in a human granuloma model. Journal of Clinical Medicine. 2020;9(7):2043. doi: 10.3390/jcm9072043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azrieh B., et al. Rituximab twice weekly for refractory thrombotic thrombocytopenic purpura in a critically Ill patient with acute respiratory distress syndrome. Case Reports in Oncology. 2020;13(1):153–157. doi: 10.1159/000505236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellumkonda L., Patel J. Recent advances in the role of mammalian target of rapamycin inhibitors on cardiac allograft vasculopathy. Clinical Transplantation. 2020;34(1) doi: 10.1111/ctr.13769. [DOI] [PubMed] [Google Scholar]
- Berger T., et al. Alemtuzumab use in clinical practice: Recommendations from European multiple sclerosis experts. CNS Drugs. 2017;31(1):33–50. doi: 10.1007/s40263-016-0394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner R., et al. Leflunomide in dialysis patients with rheumatoid arthritis—A pharmacokinetic study. Clinical Rheumatology. 2013;32(2):267–270. doi: 10.1007/s10067-012-2122-1. [DOI] [PubMed] [Google Scholar]
- Bertocchio J.-P., et al. Safety of eplerenone for kidney-transplant recipients with impaired renal function and receiving cyclosporine A. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhorade S.M., Stern E. Immunosuppression for lung transplantation. Proceedings of the American Thoracic Society. 2009;6(1):47–53. doi: 10.1513/pats.200808-096GO. [DOI] [PubMed] [Google Scholar]
- Bilbao I., et al. Renal function improvement in liver transplant recipients after early everolimus conversion: A clinical practice cohort study in Spain. Liver Transplantation. 2015;21(8):1056–1065. doi: 10.1002/lt.24172. [DOI] [PubMed] [Google Scholar]
- Björnsson E.S., et al. Azathioprine and 6-mercaptopurine induced liver injury: Clinical features and outcomes. Journal of Clinical Gastroenterology. 2017;51(1):63. doi: 10.1097/MCG.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boh E., Joselow A., Stumpf B. In: Weinberg JM, Lebwohl M (Eds.) Advances in Psoriasis. Weinberg J.M., Lebwohl M., editors. Springer; Cham: 2021. Traditional systemic therapy I: Methotrexate and cyclosporine; pp. 103–118. [Google Scholar]
- Borel J.F., et al. Biological effects of cyclosporin A: A new antilymphocytic agent. Agents and Actions. 1994;43(3–4):179–186. doi: 10.1007/BF01986686. [DOI] [PubMed] [Google Scholar]
- Boulianne G.L., Hozumi N., Shulman M.J. Production of functional chimaeric mouse/human antibody. Nature. 1984;312(5995):643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- Boyd A.S. Leflunomide in dermatology. Journal of the American Academy of Dermatology. 2012;66(4):673–679. doi: 10.1016/j.jaad.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Broen J.C., van Laar J.M. Mycophenolate mofetil, azathioprine and tacrolimus: Mechanisms in rheumatology. Nature Reviews Rheumatology. 2020;16(3):167–178. doi: 10.1038/s41584-020-0374-8. [DOI] [PubMed] [Google Scholar]
- Broos C.E., et al. No evidence found for an association between prednisone dose and FVC change in newly-treated pulmonary sarcoidosis. Respiratory Medicine. 2018;138:S31–S37. doi: 10.1016/j.rmed.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Brunet M., et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: Second consensus report. Therapeutic Drug Monitoring. 2019;41(3):261–307. doi: 10.1097/FTD.0000000000000640. [DOI] [PubMed] [Google Scholar]
- Çamlar S.A., Soylu A., Kavukçu S. Cyclosporine in pediatric nephrology. Iranian Journal of Kidney Diseases. 2018;12(6) [PubMed] [Google Scholar]
- Casanovas T., Argudo A., Pena-Cala M. Transplantation Proceedings. Elsevier; 2011. Everolimus in clinical practice in long-term liver transplantation: An observational study. [DOI] [PubMed] [Google Scholar]
- Chakkera H., Kudva Y., Kaplan B. Calcineurin inhibitors: Pharmacologic mechanisms impacting both insulin resistance and insulin secretion leading to glucose dysregulation and diabetes mellitus. Clinical Pharmacology & Therapeutics. 2017;101(1):114–120. doi: 10.1002/cpt.546. [DOI] [PubMed] [Google Scholar]
- Chang G.J., et al. Experience with the use of sirolimus in liver transplantation—Use in patients for whom calcineurin inhibitors are contraindicated. Liver Transplantation. 2000;6(6):734–740. doi: 10.1053/jlts.2000.19023. [DOI] [PubMed] [Google Scholar]
- Chapuis A.G., et al. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nature Medicine. 2000;6(7):762–768. doi: 10.1038/77489. [DOI] [PubMed] [Google Scholar]
- Chitty J. The University of Queensland; 2017. Classical and Rational Approaches to Antifungal Drug Design. [Google Scholar]
- Claro B., Bastos M., Garcia-Fandino R. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. Elsevier; 2018. Design and applications of cyclic peptides; pp. 87–129. [Google Scholar]
- Clatworthy M.R., et al. Subcutaneous administration of alemtuzumab in simultaneous pancreas-kidney transplantation. Transplantation. 2007;84(12):1563–1567. doi: 10.1097/01.tp.0000295718.55669.3a. [DOI] [PubMed] [Google Scholar]
- Colombel J.-F., et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: A post hoc analysis. Clinical Gastroenterology and Hepatology. 2019;17(8):1525–1532.e1. doi: 10.1016/j.cgh.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Colvin R., et al. Transplantation Proceedings. Elsevier Science Inc.; New York, NY, USA: 1983. Circulating T-cell subsets in 72 human renal-allograft recipients-the OKT4 +/OKT8 + cell ratio correlates with reversibility of graft injury and glomerulopathy. [Google Scholar]
- Constantinescu S., et al. Breast-feeding after transplantation. Best Practice & Research. Clinical Obstetrics & Gynaecology. 2014;28(8):1163–1173. doi: 10.1016/j.bpobgyn.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Dantal J., et al. Sirolimus for secondary prevention of skin cancer in kidney transplant recipients: 5-year results. Journal of Clinical Oncology. 2018;36(25):2612–2620. doi: 10.1200/JCO.2017.76.6691. [DOI] [PubMed] [Google Scholar]
- Dias-Polak D., Bergman R., Avitan-Hersh E. Mycophenolate mofetil therapy in adult patients with recalcitrant atopic dermatitis. Journal of Dermatological Treatment. 2019;30(1):49–51. doi: 10.1080/09546634.2018.1468068. [DOI] [PubMed] [Google Scholar]
- Dordal Culla M., et al. Treating COVID-19: Review of drug hypersensitivity reactions. Journal of Investigational Allergology & Clinical Immunology. 2020;30(6):385–399. doi: 10.18176/jiaci.0588. [DOI] [PubMed] [Google Scholar]
- El-Chemaly S., et al. Sirolimus and autophagy inhibition in lymphangioleiomyomatosis: Results of a phase I clinical trial. Chest. 2017;151(6):1302–1310. doi: 10.1016/j.chest.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., et al. The pharmacokinetic prediction of cyclosporin A after coadministration with Wuzhi capsule. AAPS PharmSciTech. 2019;20(6):247. doi: 10.1208/s12249-019-1444-6. [DOI] [PubMed] [Google Scholar]
- Fang X., et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. Journal of Infection. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareh S., et al. Pharmacological Therapeutic Monitoring of Mycophenolate Mofetil (MMF) in autoimmune diseases at EHU of Oran-Algeria: Contribution of Pharmaceutical Interventions. Journal of Applied Pharmaceutical Sciences and Research. 2018:30–33. [Google Scholar]
- Ford L., Berg J. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. Journal of Clinical Pathology. 2010;63(4):288–295. doi: 10.1136/jcp.2009.069252. [DOI] [PubMed] [Google Scholar]
- French J.A., et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): A phase 3, randomised, double-blind, placebo-controlled study. The Lancet. 2016;388(10056):2153–2163. doi: 10.1016/S0140-6736(16)31419-2. [DOI] [PubMed] [Google Scholar]
- Fröhlich E. Understanding and preventing adverse effects of Tacrolimus metabolization in transplant patients. Current Drug Metabolism. 2019;20(13):1039–1040. doi: 10.2174/1389200219666180806154433. [DOI] [PubMed] [Google Scholar]
- Fuggle N.R., et al. The adverse effect profile of oral azathioprine in pediatric atopic dermatitis, and recommendations for monitoring. Journal of the American Academy of Dermatology. 2015;72(1):108–114. doi: 10.1016/j.jaad.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabardi S., et al. New England BK consortium: Regional survey of BK screening and management protocols in comparison to published consensus guidelines. Transplant Infectious Disease. 2018;20(6):e12985. doi: 10.1111/tid.12985. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones K.P. Belatacept. BioDrugs. 2012;26(6):413–424. doi: 10.2165/11208900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ghalamkari M., et al. Azathioprine-induced severe bone marrow suppression. Case Reports in Clinical Practice. 2019:9–13. [Google Scholar]
- Ghasemi G., et al. Sirolimus dose requirement in kidney transplant recipients in Iran. Iranian Journal of Kidney Diseases. 2020;14(6) [PubMed] [Google Scholar]
- Gramatzki M., et al. Therapy with OKT3 monoclonal antibody in refractory T cell acute lymphoblastic leukemia induces interleukin-2 responsiveness. Leukemia. 1995;9(3):382–390. [PubMed] [Google Scholar]
- Grosen A., et al. The influence of methotrexate treatment on male fertility and pregnancy outcome after paternal exposure. Inflammatory Bowel Diseases. 2017;23(4):561–569. doi: 10.1097/MIB.0000000000001064. [DOI] [PubMed] [Google Scholar]
- Hale G., et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104(4):948–955. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- Han Y., et al. Prediction of tacrolimus dosage in the early period after heart transplantation: A population pharmacokinetic approach. Pharmacogenomics. 2019;20(01):21–35. doi: 10.2217/pgs-2018-0116. [DOI] [PubMed] [Google Scholar]
- Han Y., et al. Identification of candidate COVID-19 therapeutics using hPSC-derived lung organoids. BioRxiv. 2020 [Google Scholar]
- Hooks M.A., Wade C.S., Millikan W.J., Jr. Muromonab CD-3: A review of its pharmacology, pharmacokinetics, and clinical use in transplantation. Pharmacotherapy. 1991;11(1):26–37. [PubMed] [Google Scholar]
- Horby P.W., et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. The Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneff G. Safety of biologic therapies for the treatment of juvenile idiopathic arthritis. Expert Opinion on Drug Safety. 2015;14(7):1111–1126. doi: 10.1517/14740338.2015.1042453. [DOI] [PubMed] [Google Scholar]
- Jaw J., Hill P., Goodman D. Combination of Leflunomide and Everolimus for treatment of BK virus nephropathy. Nephrology. 2017;22(4):326–329. doi: 10.1111/nep.12948. [DOI] [PubMed] [Google Scholar]
- Jia Y., et al. Optimal sampling time-point for cyclosporin A concentration monitoring in heart transplant recipients. Experimental and Therapeutic Medicine. 2018;16(5):4265–4270. doi: 10.3892/etm.2018.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouve T., et al. An update on the safety of tacrolimus in kidney transplant recipients, with a focus on tacrolimus minimization. Expert Opinion on Drug Safety. 2019;18(4):285–294. doi: 10.1080/14740338.2019.1599858. [DOI] [PubMed] [Google Scholar]
- Kalt D.A. Tacrolimus: A review of laboratory detection methods and indications for use. Laboratory Medicine. 2017;48(4):e62–e65. doi: 10.1093/labmed/lmx056. [DOI] [PubMed] [Google Scholar]
- Kaltenborn A., Schrem H. Mycophenolate mofetil in liver transplantation: A review. Annals of Transplantation. 2013;18:685–696. doi: 10.12659/AOT.889299. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y. Mizoribine: A new approach in the treatment of renal disease. Clinical and Developmental Immunology. 2009;2009 doi: 10.1155/2009/681482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmner S., et al. Cyclosporine as a preferred calcineurin inhibitor in renal allograft recipients with COVID-19 infection. Kidney International. 2020;98(2):507–508. doi: 10.1016/j.kint.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., et al. Increased exposure of tacrolimus by co-administered mycophenolate mofetil: Population pharmacokinetic analysis in healthy volunteers. Scientific Reports. 2018;8(1):1–9. doi: 10.1038/s41598-018-20071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T., et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. The Journal of Antibiotics. 1987;40(9):1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- Lahiani-Skiba M., et al. Enhanced dissolution and oral bioavailability of cyclosporine A: Microspheres based on αβ-cyclodextrins polymers. Pharmaceutics. 2018;10(4):285. doi: 10.3390/pharmaceutics10040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba V., et al. PharmGKB summary: Mycophenolic acid pathway. Pharmacogenetics and Genomics. 2014;24(1):73. doi: 10.1097/FPC.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbury L., et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database of Systematic Reviews. 2019;48(2):1–9. doi: 10.1002/14651858.CD010406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc V., et al. A decision support tool to find the best cyclosporine dose when switching from intravenous to oral route in pediatric stem cell transplant patients. European Journal of Clinical Pharmacology. 2020:1–8. doi: 10.1007/s00228-020-02918-9. [DOI] [PubMed] [Google Scholar]
- Liddicoat A.M., Lavelle E.C. Modulation of innate immunity by cyclosporine A. Biochemical Pharmacology. 2019;163:472–480. doi: 10.1016/j.bcp.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Lin R., et al. Population pharmacokinetics of azathioprine active metabolite in patients with inflammatory bowel disease and dosage regimens optimisation. Basic & Clinical Pharmacology & Toxicology. 2021;128(3):482–492. doi: 10.1111/bcpt.13530. [DOI] [PubMed] [Google Scholar]
- Liu L., et al. Transplantation proceedings. Elsevier; 2018. Population pharmacokinetic analysis of mizoribine in Chinese renal transplant recipients. [DOI] [PubMed] [Google Scholar]
- Mallat S.G., et al. CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor–based regimen versus a CNI-based regimen: A systematic review and meta-analysis of randomized, controlled trials. Clinical Journal of the American Society of Nephrology. 2017;12(8):1321–1336. doi: 10.2215/CJN.13221216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R.A., et al. Peri-transplant Alemtuzumab levels IMPACT ACUTE Gvhd, MIXED Chimerism, and Lymphocyte recovery following reduced intensity conditioning with Alemtuzumab, Fludarabine, and Melphalan. Biology of Blood and Marrow Transplantation. 2016;22(3):S337. doi: 10.1182/blood-2015-07-659672. [DOI] [PubMed] [Google Scholar]
- Martel R., Klicius J., Galet S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Canadian Journal of Physiology and Pharmacology. 1977;55(1):48–51. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- Masi S., et al. Drug-induced hypertension: Know the problem to know how to deal with it. Vascular Pharmacology. 2019;115:84–88. doi: 10.1016/j.vph.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Merkel S., et al. Transplantation Proceedings. Elsevier; 2006. Side effects of sirolimus. [Google Scholar]
- Midtvedt K., et al. Individualized T cell monitored administration of ATG versus OKT3 in steroid-resistant kidney graft rejection. Clinical Transplantation. 2003;17(1):69–74. doi: 10.1034/j.1399-0012.2003.02105.x. [DOI] [PubMed] [Google Scholar]
- Minami K., et al. Species differences in the drug–drug interaction between atorvastatin and cyclosporine: In vivo study using a stable isotope-IV method in rats and dogs. European Journal of Pharmaceutical Sciences. 2020:105409. doi: 10.1016/j.ejps.2020.105409. [DOI] [PubMed] [Google Scholar]
- Moes D.J.A., et al. Population pharmacokinetics and pharmacogenetics of everolimus in renal transplant patients. Clinical Pharmacokinetics. 2012;51(7):467–480. doi: 10.2165/11599710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Moes D.J.A., Guchelaar H.-J., de Fijter J.W. Sirolimus and everolimus in kidney transplantation. Drug Discovery Today. 2015;20(10):1243–1249. doi: 10.1016/j.drudis.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Mohammadi O., Kassim T.A. 2019. Azathioprine. [PubMed] [Google Scholar]
- Montante A., et al. Cost-effectiveness of rituximab versus azathioprine for maintenance treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Clinical and Experimental Rheumatology. 2019;37(Supl 117):S137–S143. [PubMed] [Google Scholar]
- Morken N.H., et al. Obstetric and neonatal outcome of pregnancies fathered by males on immunosuppression after solid organ transplantation. American Journal of Transplantation. 2015;15(6):1666–1673. doi: 10.1111/ajt.13159. [DOI] [PubMed] [Google Scholar]
- Morrison S.L. Transfectomas provide novel chimeric antibodies. Science. 1985;229(4719):1202–1207. doi: 10.1126/science.3929380. [DOI] [PubMed] [Google Scholar]
- Murakami T., Mori N. Involvement of multiple transporters-mediated transports in mizoribine and methotrexate pharmacokinetics. Pharmaceuticals. 2012;5(8):802–836. doi: 10.3390/ph5080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankivell B.J., et al. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: Comparison of cyclosporine and tacrolimus eras. Transplantation. 2016;100(8):1723–1731. doi: 10.1097/TP.0000000000001243. [DOI] [PubMed] [Google Scholar]
- Nedredal G.I., et al. Immunosuppression in liver transplantation: State of the art and future perspectives. Current Pharmaceutical Design. 2020;26(28):3389–3401. doi: 10.2174/1381612826666200610183608. [DOI] [PubMed] [Google Scholar]
- Ng Q.X., et al. A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis. Journal of Inflammation Research. 2018;11:135. doi: 10.2147/JIR.S160964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira S.S., et al. Challenges of immunosuppressive and antitrypanosomal drug therapy after heart transplantation in patients with chronic Chagas disease: A systematic review of clinical recommendations. Transplantation Reviews. 2018;32(3):157–167. doi: 10.1016/j.trre.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Ollier L., et al. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Annals of Internal Medicine. 2009;150(6):430–431. doi: 10.7326/0003-4819-150-6-200903170-00026. [DOI] [PubMed] [Google Scholar]
- Pal P., Giri P.P., Sinha R. Cyclosporine in resistant systemic arthritis-A cheaper alternative to biologics. Indian Journal of Pediatrics. 2019;86(7):590–594. doi: 10.1007/s12098-019-02912-9. [DOI] [PubMed] [Google Scholar]
- Park H. The emergence of mycophenolate mofetilin dermatology: From its roots in the world of organ transplantation to its versatile role in the dermatology treatment room. The Journal of Clinical and Aesthetic Dermatology. 2011;4(1):18. [PMC free article] [PubMed] [Google Scholar]
- Pascual J., et al. Recommendations for the use of everolimus in de novo kidney transplantation: False beliefs, myths and realities. Nefrología. 2017;37(3):253–266. doi: 10.1016/j.nefro.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Pham P.-T.T., et al. New onset diabetes after transplantation (NODAT): An overview. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2011;4:175. doi: 10.2147/DMSO.S19027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch N.A., Bowman L.J., Taber D.J. Immunosuppression trends in solid organ transplantation: The future of individualization, monitoring, and management. Pharmacotherapy. 2021;41(1):119–131. doi: 10.1002/phar.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli C., Glassock R.J. Prevention of complications from use of conventional immunosuppressants: A critical review. Journal of Nephrology. 2019:1–20. doi: 10.1007/s40620-019-00602-5. [DOI] [PubMed] [Google Scholar]
- Pradier A., et al. Small-molecule immunosuppressive drugs and therapeutic immunoglobulins differentially inhibit NK cell effector functions in vitro. Frontiers in Immunology. 2019;10:556. doi: 10.3389/fimmu.2019.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartuccio L., et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. Journal of Clinical Virology. 2020;129:104444. doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.K., et al. Efficacy and safety of mycophenolate mofetil in patients with autoimmune hepatitis and suboptimal outcomes after standard therapy. Clinical Gastroenterology and Hepatology. 2018;16(2):268–277. doi: 10.1016/j.cgh.2017.09.063. [DOI] [PubMed] [Google Scholar]
- Rudnicka D., et al. Rituximab causes a polarization of B cells that augments its therapeutic function in NK-cell–mediated antibody-dependent cellular cytotoxicity. Blood. 2013;121(23):4694–4702. doi: 10.1182/blood-2013-02-482570. [DOI] [PubMed] [Google Scholar]
- Rugo H.S. Dosing and safety implications for oncologists when administering everolimus to patients with hormone receptor-positive breast cancer. Clinical Breast Cancer. 2016;16(1):18–22. doi: 10.1016/j.clbc.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Saffari T., et al. Exploring the neuroregenerative potential of tacrolimus. Expert Review of Clinical Pharmacology. 2019;12(11):1047–1057. doi: 10.1080/17512433.2019.1675507. [DOI] [PubMed] [Google Scholar]
- Sagcal-Gironella A.C.P., et al. Seminars in Arthritis and Rheumatism. Elsevier; 2011. Pharmacokinetics and pharmacodynamics of mycophenolic acid and their relation to response to therapy of childhood-onset systemic lupus erythematosus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba F., et al. Conversion to everolimus in maintenance liver transplant patients: A multicenter, retrospective analysis. Liver Transplantation. 2011;17(8):905–913. doi: 10.1002/lt.22292. [DOI] [PubMed] [Google Scholar]
- Sanders M.L., et al. Clinical and genetic factors associated with cutaneous squamous cell carcinoma in kidney and heart transplant recipients. Transplantation direct. 2015;1(4):e13. doi: 10.1097/TXD.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano M., et al. Analytical performance evaluation of the Elecsys®Cyclosporine and Elecsys®Tacrolimus assays on the cobas e411 analyzer. Practical Laboratory Medicine. 2017;8:10–17. doi: 10.1016/j.plabm.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerhering L., et al. Cyclophilin inhibitors restrict Middle East respiratory syndrome coronavirus via interferon-λ in vitro and in mice. European Respiratory Journal. 2020;56(5) doi: 10.1183/13993003.01826-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat K.A., Skinner M.A. Avian Immunology. Elsevier; 2014. Avian immunosuppressive diseases and immunoevasion; pp. 275–297. [Google Scholar]
- Shah S., Verma P. Overview of pregnancy in renal transplant patients. International Journal of Nephrology. 2016;2016 doi: 10.1155/2016/4539342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamilin S., et al. A comparative study of once daily versus twice daily tacrolimus in liver transplantation. Journal of Young Pharmacists. 2017;9(4):605. [Google Scholar]
- Shi Y., et al. Transplantation Proceedings. Elsevier; 2017. Comparison of mizoribine and mycophenolate mofetil with a tacrolimus-based immunosuppressive regimen in living-donor kidney transplantation recipients: a retrospective study in China. [DOI] [PubMed] [Google Scholar]
- Shin H.S., Grgic I., Chandraker A. Novel targets of immunosuppression in transplantation. Clinics in Laboratory Medicine. 2019;39(1):157–169. doi: 10.1016/j.cll.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Shiraishi K., et al. Mizoribine is as effective as methotrexate for the treatment of polymyalgia rheumatica: A retrospective case series analysis. Archives of Rheumatology. 2018;33(3):302. doi: 10.5606/ArchRheumatol.2018.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.T., et al. Experience with leflunomide as treatment and as secondary prophylaxis for cytomegalovirus infection in lung transplant recipients: A case series and review of the literature. Clinical Transplantation. 2018;32(2):e13176. doi: 10.1111/ctr.13176. [DOI] [PubMed] [Google Scholar]
- Singer O., Gibofsky A. Methotrexate versus leflunomide in rheumatoid arthritis: What is new in 2011? Current Opinion in Rheumatology. 2011;23(3):288–292. doi: 10.1097/BOR.0b013e328344f2e4. [DOI] [PubMed] [Google Scholar]
- Sprague R.G., et al. Observations on the physiologic effects of cortisone and ACTH in man. Archives of Internal Medicine. 1950;85(2):199–258. doi: 10.1001/archinte.1950.00230080003001. [DOI] [PubMed] [Google Scholar]
- Stahn C., Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nature Clinical Practice Rheumatology. 2008;4(10):525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- Sun S.L., et al. Topical calcineurin inhibitors in the treatment of oral lichen planus: A systematic review and meta-analysis. British Journal of Dermatology. 2019;181(6):1166–1176. doi: 10.1111/bjd.17898. [DOI] [PubMed] [Google Scholar]
- Tafazoli A., et al. Evaluation of cyclosporine pharmacokinetic, monitoring, and dosing parameters for GVHD prophylaxis in hematopoietic stem cell transplant (HSCT) recipients. Iranian Journal of Pharmaceutical Research: IJPR. 2019;18(Special Issue):302–314. doi: 10.22037/ijpr.2020.112111.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghvaye-Masoumi H., Sadeghi K., Hadjibabaie M. Successful switch to oral tacrolimus in a patient with hypersensitivity reaction to parenteral vitamin K, cyclosporine, and tacrolimus: A case report. Journal of Oncology Pharmacy Practice. 2020;26(4):986–988. doi: 10.1177/1078155219869442. [DOI] [PubMed] [Google Scholar]
- Tandan R., et al. Rituximab treatment of myasthenia gravis: A systematic review. Muscle & Nerve. 2017;56(2):185–196. doi: 10.1002/mus.25597. [DOI] [PubMed] [Google Scholar]
- Tapia C., Basehore B.M., Zito P.M. StatPearls [Internet] StatPearls Publishing; 2020. Cyclosporine. [Google Scholar]
- Tapia C., Nessel T.A., Zito P.M. STATpearls. StatPearls Publishing, LLC; Treasure Island, FL: 2020. Cyclosporine. [Google Scholar]
- Tönshoff B., et al. Pediatric aspects of therapeutic drug monitoring of mycophenolic acid in renal transplantation. Transplantation Reviews. 2011;25(2):78–89. doi: 10.1016/j.trre.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Ushigome H., et al. Transplantation Proceedings. Elsevier; 2016. Efficacy and safety of high-dose mizoribine combined with cyclosporine, basiliximab, and corticosteroids in renal transplantation: A Japanese multicenter study. [DOI] [PubMed] [Google Scholar]
- van der Zwan M., et al. Review of the clinical pharmacokinetics and pharmacodynamics of alemtuzumab and its use in kidney transplantation. Clinical Pharmacokinetics. 2018;57(2):191–207. doi: 10.1007/s40262-017-0573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet F., Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nature Reviews Nephrology. 2018;14(2):121. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- Villarroel M.C., Hidalgo M., Jimeno A. Mycophenolate mofetil: An update. Drugs of Today (Barcelona, Spain: 1998) 2009;45(7):521–532. doi: 10.1358/dot.2009.45.7.1384878. [DOI] [PubMed] [Google Scholar]
- Vincenti F., et al. Belatacept and long-term outcomes in kidney transplantation. New England Journal of Medicine. 2016;374(4):333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- Vitiello D., et al. Effect of everolimus on the immunomodulation of the human neutrophil inflammatory response and activation. Cellular & Molecular Immunology. 2015;12(1):40–52. doi: 10.1038/cmi.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., et al. Treatment of membranous nephropathy with mizoribine: A control trial. Life Sciences. 2016;154:75–78. doi: 10.1016/j.lfs.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Wekerle T., Grinyó J.M. Belatacept: From rational design to clinical application. Transplant International. 2012;25(2):139–150. doi: 10.1111/j.1432-2277.2011.01386.x. [DOI] [PubMed] [Google Scholar]
- Westbrook R.H., et al. Outcomes of pregnancy following liver transplantation: The King's College Hospital experience. Liver Transplantation. 2015;21(9):1153–1159. doi: 10.1002/lt.24182. [DOI] [PubMed] [Google Scholar]
- Woo T.M., Robinson M.V. Pharmacotherapeutics for Advanced Practice Nurse Prescribers. FA Davis; 2015. [Google Scholar]
- Xin G.L.L., et al. Current status on immunological therapies for type 1 diabetes mellitus. Current Diabetes Reports. 2019;19(5):22. doi: 10.1007/s11892-019-1144-3. [DOI] [PubMed] [Google Scholar]
- Xu L., et al. Characteristics of male fertility after renal transplantation. Andrologia. 2011;43(3):203–207. doi: 10.1111/j.1439-0272.2010.01052.x. [DOI] [PubMed] [Google Scholar]
- Xu K., Chen Y., Yuan J. Factors associated with prolonged viral RNA shedding in patients with COVID-19 [published online ahead of print, 2020 Apr 9] Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap D.Y., et al. Longterm data on sirolimus treatment in patients with lupus nephritis. The Journal of Rheumatology. 2018;45(12):1663–1670. doi: 10.3899/jrheum.180507. [DOI] [PubMed] [Google Scholar]
- Yoon H.-Y., et al. Efficacy and safety of low-dose Sirolimus in Lymphangioleiomyomatosis. Orphanet Journal of Rare Diseases. 2018;13(1):1–8. doi: 10.1186/s13023-018-0946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa N., et al. Role of FK506 binding protein on tacrolimus distribution in red blood cells. Pharmaceutical Research. 2020;37(7):1–8. doi: 10.1007/s11095-020-02875-z. [DOI] [PubMed] [Google Scholar]
- Yu M., et al. Pharmacokinetics, pharmacodynamics and pharmacogenetics of tacrolimus in kidney transplantation. Current Drug Metabolism. 2018;19(6):513–522. doi: 10.2174/1389200219666180129151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang R. Recent advances in analytical methods for the therapeutic drug monitoring of immunosuppressive drugs. Drug Testing and Analysis. 2018;10(1):81–94. doi: 10.1002/dta.2290. [DOI] [PubMed] [Google Scholar]
- Zoncu R., Efeyan A., Sabatini D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nature Reviews Molecular Cell Biology. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart T.C., et al. Therapeutic drug monitoring of tacrolimus and mycophenolic acid in outpatient renal transplant recipients using a volumetric dried blood spot sampling device. British Journal of Clinical Pharmacology. 2018;84(12):2889–2902. doi: 10.1111/bcp.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]


