Abstract
Background
The kinetics of neutralizing antibodies against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) play an important role in evaluating vaccine efficacy and durability, herd immunity, additional vaccination, and prediction models of immune protection against coronavirus disease 2019.
Materials and Methods
Serum collection times were 4 and 8 weeks after 1st inoculation of AZD1222 (AstraZeneca, Cambridge, UK), and 2 and 16 weeks after 2nd inoculation with 12-week dosing intervals. Neutralizing antibody (Nab) titers were measured indirectly using commercially available R-FIND SARS-CoV-2 Neutralizing Antibody ELISA Kit (SG Medical Inc., Seoul, Korea). Possible influences of gender, age, and adverse events on neutralizing antibody titer were also investigated.
Results
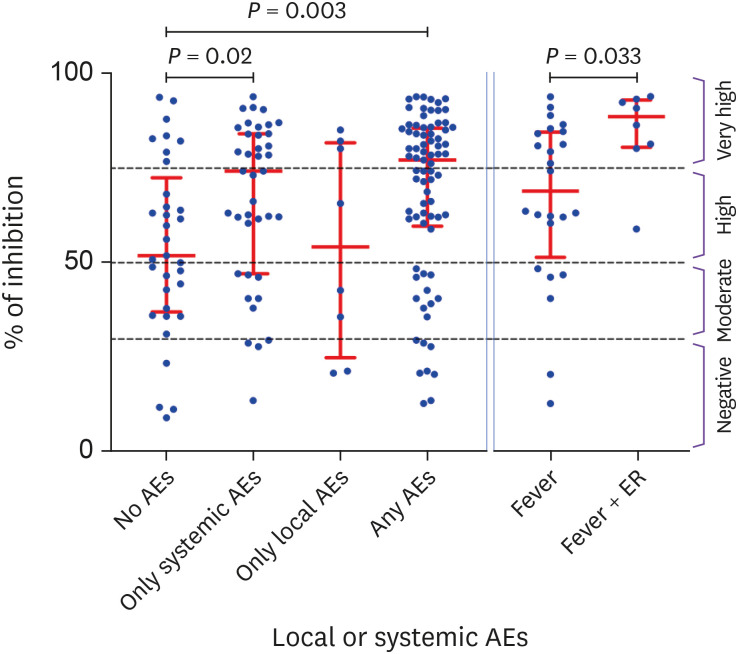
Nab titers (median inhibition %) started to decrease shortly after reaching peaks. This decrease was more pronounced in the elderly group (≥56 years) than in the young group (≤39 years) at 8 weeks (49.5% vs. 55.4%, P = 0.021) and 16 weeks (40.6% vs. 53.9%, P = 0.006) after the 1st and 2nd inoculation. And Nab titers were inversely correlated with age in the 8-week (r = -0.2091, P = 0.0284) and the 28-week group (r = -0.2811, P = 0.0029). Seropositive conversion of Nab reached 89.1% and 100% following 1st and 2nd inoculation. This 100% seropositivity was dropped sharply to 74.5% after 16 weeks. Compared to subjects without adverse events (51.8%), median inhibition was higher in subjects with one or more systemic adverse events (74.2%, P = 0.0203) or those with one or more local and systemic adverse events (77.1%, P = 0.0003).
Conclusion
Nab induced by AZD1222 (AstraZeneca, UK) vaccination started to degrade shortly after the production period. Nab titers were lower in the elderly than in younger group during the degradation period. This seems to be because the degradation process of Nab is more pronounced in the elderly. This may explain why the frequency of breakthrough infections, disease severity, and mortality were higher in the elderly and may require revaccination to ensure robust immunity.
Keywords: COVID-19, Vaccination, Neutralizing antibody, Adverse events, AZD1222
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in 446 million confirmed cases and over 6 million deaths after two years after onset (http://covid19.who.int) [1]. Many scientists have believed that the only way out of this catastrophe is to gain herd immunity through natural infection or vaccination. And this has sparked the most extensive and rapid global vaccine development program in history (http://www.gavi.org) [2]. So far, 23 vaccines have been approved for emergency use by regulatory agencies in many countries. Four have been approved in Korea, including two mRNA vaccines coated with a lipid nanoparticle delivery system such as BNT162b2 (Pfizer/BioNTech, Philadelphia, PA, USA) [3] and mRNA-1273 (Moderna, Cambridge, MA, USA) [4] and two DNA vaccines using an adenoviral vector system such as ChAdOx1 nCoV-19 (AZD1222, AstraZeneca, Cambridge, UK) [5] and Ad26.COV2 (Janssen, Titusville, NJ, USA) [6] vaccines. All of these introduce genetic information in the form of RNA or DNA encoding SARS-CoV-2 spike protein into host cells. The generated spike protein can then induce binding antibodies to spike protein and neutralizing antibodies (Nabs).
During natural infection, SARS-CoV-2 can bind to human angiotensin-converting enzyme 2 receptor (hACE2) via the receptor binding domain (RBD) of the viral spike glycoprotein. This RBD-hACE2 complex is responsible for viral entry and replication in host cell. RBD-targeted Nab generated from a natural infection or vaccination can competitively bind to RBD and inhibit the RBD-hACE2 complex, thereby preventing infection or reinfection and reducing the disease severity [7]. This assumption is supported by several reports. Up to 89.0% of Nab seropositive subjects who have recovered from COVID-19 are reported to be protected from reinfection [8]. There are also some reports showing protection against reinfection in non-human primate data [9,10]. Therapeutic effects of passive delivery of Nabs have also been reported [11]. There is also an inverse correlation between Nab concentration and breakthrough infection [12]. Unfortunately, Nab titers in convalescent sera are known to continue to decline over time [13]. Nab immune response against SARS-CoV-2 vaccines have been extensively described in the literature. However, kinetics about the long-term duration of Nabs after vaccination are still lacking, which is a key issue determining the immune protection against COVID-19 breakthrough infection or its variant, and is also important for designing vaccination strategies during the current pandemic. Therefore, quantification and kinetics of long term duration of Nab after vaccination are very important and urgently needed.
The current standard methods for measuring Nab rely on using wild-type SARS-CoV-2 or pseudo-virus and the degree of infection using cell culture-based system as a read-out [14]. However, these assays are labor-intensive. They also require adequate biosafety facilities. In addition, they are difficult to standardize. Neutralization tests using recombinant RBD acting as a surrogate of real virus based on antibody-mediated blocking of the RBD-hACE2 protein complex have recently been reported [15,16]. And they are already commercially available (ELISA, cPassTM SARS-CoV-2 NAbs detection kit; GenScript, Piscataway, NJ, USA). A similar kit has recently been developed and is also available in Korea (R-Find SARSCoV-2 Neutralizing Antibody ELISA; SG Medical Inc., Seoul, Korea).
To determine the kinetics of Nab response, we measured Nab levels at four time points for 110 healthcare workers at Pyeongtaek St. Mary’s Hospital who received two doses of AZD1222 (AstraZeneca, UK) with 12-week dosing interval. Possible influence of gender, age, and adverse events on Nab level were also investigated.
Materials and methods
1. Enrolled subjects and procedure
We enrolled 110 healthcare workers (HCWs) who received two doses of AZD1222 (AstraZeneca, UK) (0.5 ml in each dose containing 5 × 1010 viral particles) 12 weeks apart in accordance with the Korean government policy. These 110 subjects were divided into three age groups: 19 - 39 years, 40 - 55 years, and 56 - 77 years. All subjects were asked to report adverse events (AEs) during two days after vaccination (1st and 2nd inoculations, respectively) via a mobile self-report questionnaire. Questionnaire consisted of a total of nine AEs, including local reactions (pain & tenderness, swelling, itching, and redness at the injection site) and systemic reactions (fever, headache, muscle pain, chillness, and nausea) [17].
Blood samples were collected into serum-gel tubes (BD SST II Advance®, Becton Dickinson, Franklin lakes, NJ, USA). Sera were separated within 4 hours after blood collection and stored at -20oC until the day of measurement. Blood collection times were 4 and 8 weeks after 1st inoculation of AZD1222 (AstraZeneca, UK), and 2 and 16 weeks after 2nd inoculation with 12-week dosing interval. Nab against SARS-CoV-2 was measured indirectly using commercially available ELISA-based surrogate SARS-CoV-2 neutralization test (R-Find SARS-CoV-2 Neutralizing Antibody ELISA; SG Medical, Korea). This Kit contains two key components: horseradish peroxidase (HRP) conjugated recombinant SARS-CoV-2 RBD fragment (HRP-RBD) and hACE2. The test was performed according to the manufacturer’s instructions. Briefly, 25 μl of test serum was added to the ELISA plate well coated with hACE2 pre-filled with 25 μl sample diluent. 50 μl of provided positive and negative controls were also added to appropriated wells. And 50 μl of HRP-RBD conjugated were mixed to each well and incubated for 45 minutes at room temperature. The supernatant including unbound HRP-RBD was removed and washed 3 times using provided washing buffer. 100 μl of tetramethylbenzidine substrate was added for 15 minutes at room temperature before the reaction was stopped by addition of 50 μl stop solution. The absorbance of the samples was measure at 450 nm using microplate reader. Manufacturer provided performance evaluation data between two commercial kits (SG Medical, Korea and GenScript, USA). After the 1st vaccination of BNT162b2, the neutralizing antibody positive rates were 92.4% (SG Medical) and 87.6% (GenScript). Positive rate after 2nd vaccination was 100.0% in both companies. In addition, the test evaluation results for the same sample for the kit of GenScript and SG Medical showed very high agreement (r = 0.9424, P <0.0001).
Results were interpreted by percent inhibition and calculated as follows:
According to the manufacturer’s instructions, a percent inhibition of ≥30.0% was considered positive and percent inhibition of <30.0% was considered negative. For convenience, the inhibition rate of 30.0 – 50.0% was set as moderate, 51.0 - 75.0% as high, and 76.0% or more as very high.
2. Ethics statement
This study was approved by Institutional Review Board (IRB) of the College of Medicine, The Catholic University of Korea (IRB approval number: MC22RADI0006), and written informed consent was obtained from all participants.
3. Statistical analysis
Nab titer (inhibition %) were expressed as median with interquartile range (IQR). Paired t-test or one-way ANOVA were used to compare Nab titer after vaccination. In addition, we used an independent, two tailed, unpaired t-test for comparing Nab titers between different age groups, and between different adverse events. To determine the correlation between age and the Nab titer was used Pearson’s correlation test. Categorical variables were compared using Pearson’s chi-squared test. Trend analysis using the Cochran-Armitage test was performed to determine a significant increase or decrease in the frequency of AEs across age groups. All statistical analyses were performed using GraphPad Prism® software (version 9.1.0, San Diego, CA, USA).
Results
1. Demographic data
We obtained blood samples for measuring Nab from a total of 110 HCWs each at 4 and 8 weeks after the 1st inoculation and at 2 and 16 weeks after the 2nd inoculation. Of the 110 vaccinated HCWs, 65 were females and 45 were males. Their median age was 48.5 years (range, 19 - 77 years).
2. Kinetics of Nab titers after 1st and 2nd inoculation of AZD1222 vaccine
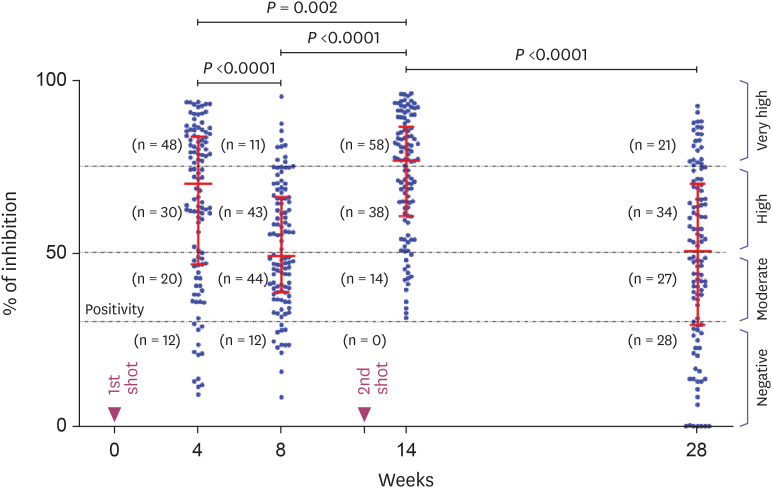
As shown in Figure 1, In the 4-week group (4 weeks after 1st inoculation), inhibition% increased sharply to 70.1% (range: 9.2 - 93.7%, IQR: 46.7 - 83.7%) and seropositive rate was 89.1% (98/110). Of the 98 seropositive subjects, 78 (79.6%) had Nab titers greater than 50.0% of median inhibition, 48 were in very high (>75.0%) and 30 were in high titer range (50.0 – 75.0%). At 8 weeks after the 1st inoculation (8-week group), the median inhibition was rapidly decreased (P <0.0001) from 70.1% to 49.2% (range: 8.4 - 95.3%, IQR: 38.7% - 66.3%). Interestingly, the number of seronegative subject (12 of 110) was the same as in the 4-week group. However, the number of subjects in the very high titers range (>75.0%) decreased from 48 (50.0%, 48/98) to 11 (11.2%, 11/98). And these reduced 37 subjects were dispersed into the lower titer range group. In 14-week group (2 weeks after the 2nd inoculation), all subjects were seropositive, and the median inhibition was increased (P = 0.0001) to 76.7% of inhibition (range: 31.3 - 96.2%, IQR: 60.8 - 86.5%). Most (96/110, 87.3%) subjects were above 50.0% inhibition line in the very high (n = 58) or high titers range (n = 38). In the 28-week group (16 weeks after the 2nd inoculation), the median inhibition was decreased (P <0.0001) from 76.7% in 14-week group to 50.5% of inhibition (range: 0 - 92.6%, IQR: 29.3 - 70.0%). The number of subjects in very high titers was drastically decreased from 58 (52.7%) to 21 (19.1%). Mostly are located in high (n = 34, 30.9%) and moderate titer ranges (n = 27, 24.5%). The number of seronegative subjects was also increased 0 to 28 (25.5%). In addition, only 14 (12.7%) subjects were less than 50.0% of inhibition at 2 weeks after 2nd inoculation, but increased to 55 (50.0%) subjects after 16 weeks.
Figure 1. Kinetics of Nab at four different time points after two doses of AZD1222 vaccine. The vertical axis represents the % inhibition (Nab titer) for SARS-CoV-2 Nab tested using R-Find SARS-CoV-2 Neutralizing Antibody ELISA. Horizontal red lines indicate median values ± (IQR). The black dotted lines correspond to the seropositivity threshold at 30.0% inhibition and 50.0 and 75.0% lines indicates the borderline between moderate, high and very high protection areas.
Nab, neutralizing antibody; ELISA, enzyme linked immunosorbent assay; IQR, interquartile range; SARS-Cov-2, severe acute respiratory syndrome coronavirus-2.
3. Differences in Nab response according to age
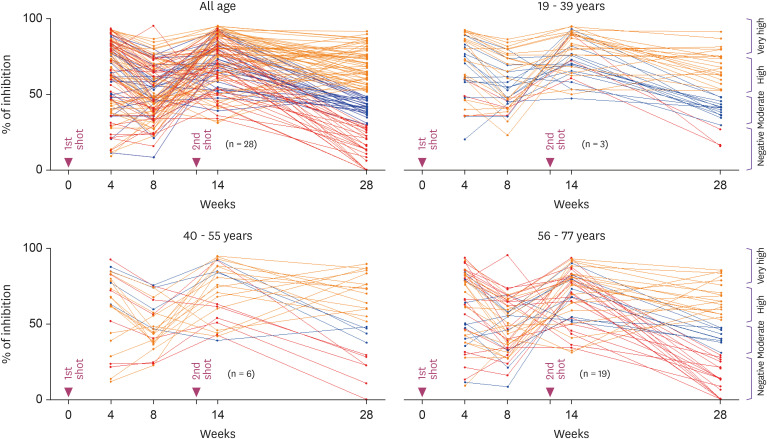
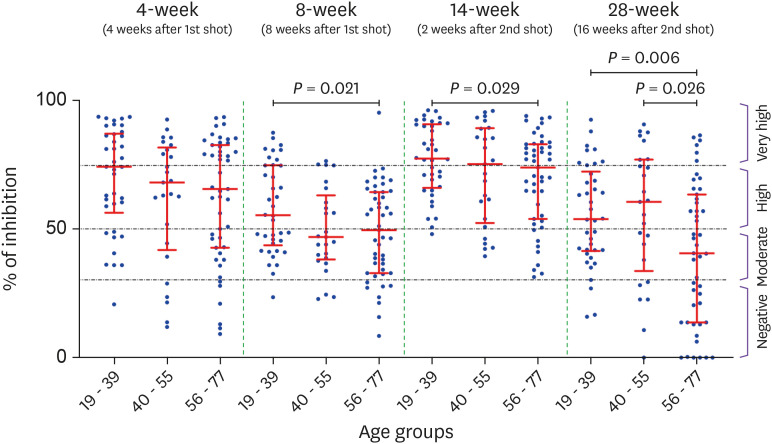
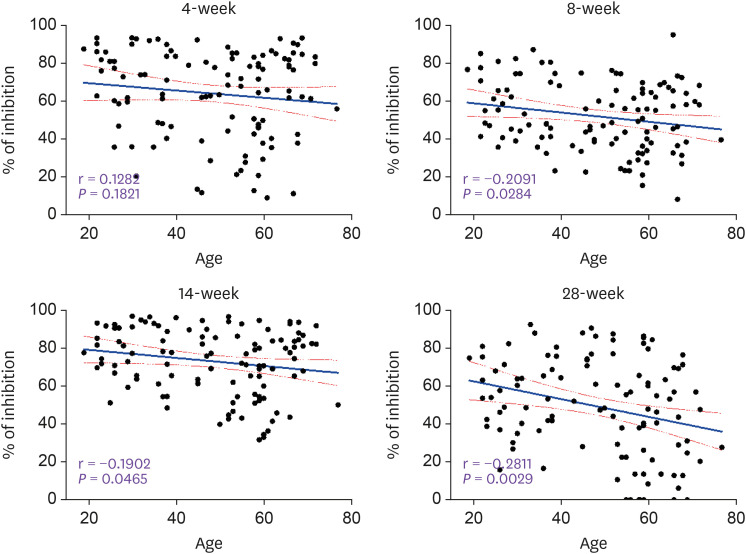
All 110 subjects were seropositive and the inhibition% ranged from 31.3 to 96.2% in 14-week group (Fig. 2). However, the 28-week group with relatively a long degradation period (16 weeks after the 2nd inoculation) showed broad distribution pattern (0 to 92.6% of inhibition). Also, the number of seronegative subjects increased rapidly from 0 in the 14-week group to 28 in the 28-week group. This seronegative conversion rate was 40.4% (19/47) in the ≥56 years group, which was significantly greater than 14.3% (9/63) in ≤55 years group (P = 0.0015) or 7.9% (3/38) in ≤39 years group (P = 0.0005). During Nabs production period, the median inhibition% was not significantly different between the three age groups in the 4-week group. However at 2 weeks after 2nd inoculation, it was slightly higher in younger age group (≤39 years, 77.7%) than in the elderly (≥56 years, 73.9%, P = 0.029) (Fig. 3). However, even at 2 weeks after 2nd inoculation, one of the production period, Nab titer was slightly lower in the elderly group (≥56 years) than in the young group (≤39 years) (73.9% vs. 77.4%, P = 0.0213). It also showed an inverse correlation with age (r = -0.1902, P = 0.0465). During the degradation period of Nab, the median inhibition% of the elderly (≥56 years) compared to young group (≤39 years) was greatly reduced at 8-week (55.4% vs. 49.5%, P = 0.021) and at 28-week (53.9% vs. 40.6%, P = 0.006). And Nab titers were conversely correlated with age in the 8-week group (r = -0.2091, P = 0.0284) and in the 28-week group (r = -0.2811, P = 0.0029) (Fig. 4).
Figure 2. The time course of the measured values of Nab titers (inhibition %) for all vaccinated individuals. For ease of viewing, a merged line graph with all age groups was separated into 3 ages groups of 19 - 39, 40 - 55 and 56 - 77 years. In the 28-week group, subjects with inhibition% of less than 30.0% (red), 31.0 – 50.0% (blue) and >51.0% (orange) are indicated by different color lines.
Nab, neutralizing antibody.
Figure 3. Comparison of Nab titers between three different age groups during the production period (4-week, and 14-week) and during the degradation period (8-week and 28-week).
Nab, neutralizing antibody.
Figure 4. Linear correlations between subject’s age and Nab titer (inhibition %) after AZD1222 vaccination. Correlation were analyzed using simple linear regression and bounded by 95.0% CI.
CI, confidence interval; Nab, neutralizing antibody.
4. Difference in Nab response according to the adverse events
Table 1. shows the frequency of local and systemic AEs following 1st and 2nd inoculations of AZD1222 (AstraZeneca, UK). In general, AEs developed on the night or the next day following vaccination and continued for 1 or 2 days. Fever (28.2%, 31/110) and pain & tenderness (30.0%. 33/110) were the most common AEs in systemic and local AEs respectively. And these two AEs (P for trend = 0.0001 and P = 0.0025) tended to be more common in younger age group. Subjects with one or more systemic AEs (59.1%, 65/110) also tended to be common in younger subjects (P for trend = 0.0032). There were no gender differences in the frequency of AEs. And the frequency of AEs after 2nd inoculation was too low to obtain meaningful statistical data.
Table 1. Frequency of local and systemic adverse events after AZD1222 vaccination according to gender and age difference.
| Variables | Gender | P value | Age group | P for trend | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | ≤39 | 40 - 55 | ≥56 | |||||
| Vaccinated HCWs | 110 | 45 (40.9%) | 65 (59.1%) | 38 (34.6%) | 25 (22.7%) | 47 (42.7%) | ||||
| Adverse event after 1st inoculation | ||||||||||
| Any systemic adverse event | 65 (59.1%) | 26 | 39 | 0.8157 | 28 (73.7%) | 17 (68.0%) | 20 (42.6%) | 0.0032 | ||
| Fever | 31 (28.2%) | 14 | 17 | 0.5699 | 20 (52.6%) | 5 (20.0%) | 6 (12.8%) | <0.0001 | ||
| Headache | 21 (19.1%) | 8 | 13 | 0.7706 | 7 | 7 | 7 | 0.6339 | ||
| Nausea | 8 (7.3%) | 2 | 6 | 0.3419 | 5 | 2 | 1 | 0.0509 | ||
| Muscle pain | 11 (10.0%) | 2 | 9 | 0.1061 | 3 | 4 | 4 | 0.9710 | ||
| Chillness | 16 (14.5%) | 7 | 9 | 0.8026 | 7 | 4 | 5 | 0.3066 | ||
| Any local adverse event | 38 (34.5%) | 13 | 25 | 0.2992 | 17 | 9 | 12 | 0.0632 | ||
| Pain & tenderness | 33 (30.0%) | 10 | 23 | 0.1386 | 17 (44.7%) | 9 (36.0%) | 7 (14.9%) | 0.0025 | ||
| Swelling | 26 (23.6%) | 11 | 15 | 0.8682 | 12 | 4 | 10 | 0.2899 | ||
| Erythema | 14 (12.7%) | 3 | 11 | 0.1125 | 8 | 2 | 4 | 0.0926 | ||
| Itching | 17 (15.5%) | 7 | 10 | 0.9805 | 8 | 5 | 4 | 0.1042 | ||
| Adverse event after 2nd inoculation | ||||||||||
| Any systemic adverse event | 14 (12.7%) | 4 | 10 | 0.3149 | 6 | 4 | 4 | 0.3039 | ||
| Fever | 4 (3.6%) | 2 | 2 | 0.7064 | 2 | 1 | 1 | 0.4399 | ||
| Headache | 8 (7.3%) | 2 | 6 | 0.3419 | 2 | 4 | 2 | 0.7836 | ||
| Nausea | 5 (4.5%) | 2 | 3 | 0.9662 | 3 | 1 | 1 | 0.2077 | ||
| Muscle pain | 1 (0.9%) | 1 | 0 | 0.2273 | 0 | 1 | 0 | 0.9252 | ||
| Chillness | 3 (2.7%) | 2 | 1 | 0.3576 | 1 | 1 | 1 | 0.8696 | ||
| Any local adverse event | 12 (10.9%) | 7 | 5 | 0.1934 | 4 | 6 | 2 | 0.2974 | ||
| Pain & tenderness | 11 (10.0%) | 7 | 4 | 0.1061 | 4 | 5 | 2 | 0.2923 | ||
| Swelling | 9 (8.2%) | 6 | 3 | 0.1010 | 4 | 4 | 1 | 0.1375 | ||
| Erythema | 8 (7.3%) | 5 | 3 | 0.1971 | 3 | 4 | 1 | 0.2655 | ||
| Itching | 4 (3.6%) | 1 | 3 | 0.5097 | 1 | 2 | 1 | 0.8490 | ||
HCW=Healthcare worker.
As shown in Figure 5, subjects without AEs after the 1st inoculation showed a median inhibition of 51.8% (range: 9.2 - 93.6%, IQR: 37.1 - 72.4%). Compared to subjects without AEs (51.8%), median inhibition was higher in subjects with one or more systemic AEs (74.2%, P = 0.0203) or those with one or more local and systemic AEs (77.1%, P = 0.0003). Of 32 subjects with fever, 8 visited emergency room (ER) or had hospitalization for antipyretic treatment (Fever + ER). In comparison between these two groups, the Nab titer was 88.4% (IQR: 80.4 - 92.8%) in the severe fever group (Fever + ER), distinct from 68.9% (IQR: 51.4 - 84.4%) of inhibition in simple fever group (P = 0.0331).
Figure 5. Comparison of Nab titers between the subjects with or without AEs after the 1st inoculation of AZD1222.
AEs, adverse events; ER, emergency room; Nab, neutralizing antibody.
Discussion
Several COVID-19 vaccines including AZD1222 (AstraZeneca, UK) have received emergency approval early last year in Korea. The AZD1222 (AstraZeneca, UK) vaccine was initially one of avoiding vaccines due to some mistakes in clinical trials in the early stages of its development, lower efficacy compared to mRNA vaccines, and exaggerated rumors about adverse events such as thrombosis with thrombocytopenia syndrome [18]. However, all healthcare workers in Pyeongtaek St. Mary’s Hospital received the AZD1222 (AstraZeneca, UK) vaccine according to the government policy without any option. Measurement of Nab titer using recombinant RBD, a surrogate of real virus, based on antibody-mediated blocking of the RBD-hACE2 protein complex has been recently reported. It is already commercially available [15,16]. Therefore, we had a chance to evaluate kinetics of Nab following AZD1222 (AstraZeneca, UK) vaccination using sera obtained from at four different time points from 110 healthcare workers who voluntarily participated in this study.
Studies have shown that the Nab response peaks at 3 - 5 weeks after infection, and the magnitude of the peak correlates with clinical disease severity [19,20]. However, Nab responses can degrade rapidly in early convalescence, having an overall half-life of 90 days over the first 8 months after infection [21]. Although there are reports of Nab degradation after vaccination, the literature on the long-term responses of Nab titers, especially after AZD1222 (AstraZeneca, UK) vaccination, is still lacking. Kang et al. [22] also reported Nab responses at 3 different time points identical to ours, except for 28-week group. They claim that there was no significant difference in Nab titer between 4 and 8 weeks after 1st dose of AZD1222 (AstraZeneca, UK). In our data, despite the same number of seronegative, Nab titer (median inhibition) decreased sharply (P <0.0001) from 70.1% (4-week group) to 49.2% (8-week group). More specifically, the number of subjects in the very high titers range (>75.0%) decreased from 48 (50.0%, 48/98) to 11 (11.2%, 11/98). And these reduced 37 subjects were dispersed into the lower titer ranges. After 2 weeks of the 2nd inoculation, the median inhibition increased to 76.1% again, and decreased to 50.3% after 14 weeks (28-week group) (Fig. 1). Therefore, it is believed that the Nab produced by the vaccine starts to decrease immediately after reaching the peak. Moreover, the decrease in Nab titers was more pronounced in the elderly (≥56 years) than in the young (≤39 Years) at 8-week (49.5% vs. 55.4%) and 28-week (40.6% vs. 53.9%) (Fig. 3). This was strongly supported by the fact that Nab titers were inversely correlated with age in the 8-week (r = -0.2091, P = 0.0284) and 28-week groups (r = -0.2811, P = 0.0029) obtained by linear regression analysis (Fig. 4). Müller et al [20] also reported that antibody titers after 1st and 2nd dose of BNT162b2 vaccine were significantly lower in elderly (>80 years) than in younger group (<60 years). On the other hand, the number of seronegative subjects increased rapidly from 0 in the 14-week group to 28 in the 28-week group (Fig. 1). In the 28-week group, the seronegative conversion rate was also higher in the ≥56 years group (40.4%) than the ≤55 years (14.3%) or the ≤39 years group (7.9%) (Fig. 2). Although currently unexplained, our data suggested that the degradation process of Nab was more pronounced in elderly rather than the generation process of Nab. This may be related to why the frequency of breakthrough infections, disease severity, and mortality are higher in the elderly. According to the latest data from the Center for Disease Control and Prevention, people 65 years and older account for 63.5% (1,286/2,025) of hospitalizations and 86.0% (363/428) of deaths from breakthrough cases [23].
Kang et al. reported that seropositive rates after 1st and 2nd inoculation were 66.8% and 98.0% for AZD1222 (AstraZeneca, UK), and 87.5% and 100% for BNT162b2 vaccine [22]. Nam et al. [24]. also reported that seropositive rates of AZD1222 (AstraZeneca, Cambridge, UK) vaccine were 68.8 and 97.3% after 1st and 2nd inoculation, similar to those reported by Kang et al. However, in the case of BNT162b2, the positivity rate after the 1st inoculation was 96.6%, which was significantly higher than that of Kang et al [22] (87.5%). Our seropositive rates were 89.1% and 100% after the 1st and 2nd AZD1222 (AstraZeneca, Cambridge, UK) vaccination (Fig. 1). Among them, the positive rate after 1st inoculation was significantly higher (89.1%) than in the above two papers. We used the SG Medical kit, which has the same principle as the GenScript kit, but with some difference in the testing process. In the case of the GenScript kit, serum (Nab) and RBD are sufficiently reacted first, and then transferred to the well-coated hACE2. Thus, the only remaining RBDs that fail to bind Nab bind hACE2. However, the SG Medical kit differs in that serum (Nab) and RBD can be administered simultaneously to hACE2-coated well, allowing RBD to competitively bind to Nab or hACE2. The high seropositivity of this first paper using the SG Medical kit compared to GenSript kit means lower discrimination power, especially at low Nab titers. Papers using GenScript kit are divided into cases using 20.0% or 30.0% of inhibition as a cutoff [25]. Although neither of these methods is quantitative analysis, the determination of cutoff is critical and should be standardized with greater precision in the future. Nevertheless, we had no problems observing the longitudinal kinetics of Nab titers using the SG Medical kit.
Many papers have reported that AZD1222 (AstraZeneca, UK) has lower vaccine efficacy and/or Nab production rate compared to RNA vaccines [22,24,26,27]. The most important determinant of producing a high level of Nab is SARS-CoV-2 infection itself. One subject was infected with SARS-CoV-2 at our hospital 4 months prior to the start of the AZD1222 (AstraZeneca, UK) vaccine schedule. And his Nab titers showed absolutely high levels of Nab titers without any decrease during for a total of 44 weeks, including 16 weeks after infection and 28 weeks of study period. He was, of course, excluded from this analysis.
According to two articles [17,28], vaccine-related AEs in Korean healthcare workers were more common in AZD1222 (AstraZeneca, UK) than in the BNT162b2 group. In contrast to BNT162b2, most types of AEs in AZD1222 (AstraZeneca, UK) tend to decrease with old age group and female experienced AEs more frequent. Lee et al also reported that pain around injection site was the most common AEs, and Nab response was not related to age, sex, and obesity [28]. However, our data have no gender difference. Fever and pain around injection site were most common AEs in systemic and local AEs respectively. And these two AEs tend to be more in younger age group (Table 1). We showed that the Nab titers were higher in the group with one or more systemic AEs (P = 0.02) and in the group with at least one or more local or systemic AEs (P = 0.003) than in the group without AEs (Fig. 5). When we compared subjects who had simple fever and subjects with severe fever requiring emergency room visit or hospitalization, Nab titer was higher in the severe fever group (88.4% vs. 68.9% of inhibition, P = 0.0331) (Fig. 5). This is consistent with the fact that the stronger the inflammatory response following vaccination can achieve better the immunity [29].
This study has some limitations. First, the Nab degradation process was not sufficiently observed by tracking the Nab titer only for 16 weeks after 2nd inoculation. Second, in this study, it was rather difficult to evaluate the adverse events after vaccination because there were not enough subjects. Third, the Nab measurement method in this study is a semi-quantitative and an indirect method using surrogate marker instead of authentic SARS-CoV-2 or pseudo-virus, and no clear cut-off criterion. Finally, humoral immunity, such as Nab production, is also important for protective immunity obtained after vaccination, but other immune factor such as T cell immunity also has a great impact on the protection from disease severity.
In conclusion, Nab induced by AZD1222 (AstraZeneca, UK) vaccination started to degrade shortly after the production period. Nab titers were lower in the elderly than in younger group during the degradation period. This seems to be because the degradation process of Nab is more pronounced in the elderly. This may explain why the frequency of breakthrough infections, disease severity, and mortality were higher in the elderly and may require revaccination to ensure robust immunity.
ACKNOWLEDGMENTS
We would like thank to healthcare workers in Pyeongtaek St. Mary’s Hospital for their commitment to patients care and in screening clinic, respiratory safety clinic and drive-thru test during the COVID-19 pandemic. We also thank their participation in this study.
Footnotes
Funding: This study was supported by a grant from the private foundation of Pyeongtaek St. Mary’s Hospital. The funder had no role in study design, data collection, decision to publish, or preparation of the manuscript.
Conflict of Interest: No conflict of interest.
- Conceptualization: JYL, JHY, WSP.
- Data Curation: SL, YL, DWK.
- Formal analysis: JHY, SL, YL, DWK.
- Funding acquisition: JYL.
- Investigation: JYL, JHY.
- Methodology: JHY, DWK.
- Resources: SL, YL, DWK.
- Supervision: JYL, JHY.
- Validation: JYL, JHY, WSP.
- Visualization: JHY, SL, YL, DWK.
- Writing – original draft: JYL.
- Writing – review & editing: JYL, WSP, JHY.
References
- 1.World Health Organization (WHO) WHO coronavirus (COVID-19) dashboard. [Accessed 5 March 2022]. Available at: https://covid19.who.int.
- 2.Gavi. The Gavi COVAX facility. [Accessed 5 March 2022]. Available at: https://www.gavi.org/vaccineswork.
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP, Dooling K. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O’Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ Oxford COVID vaccine trial group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, Berghmans PJ, Kimmel M, Van Damme P, de Hoon J, Smith W, Stephenson KE, De Rosa SC, Cohen KW, McElrath MJ, Cormier E, Scheper G, Barouch DH, Hendriks J, Struyf F, Douoguih M, Van Hoof J, Schuitemaker H. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 8.Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, Warren F, Peck LJ, Ritter TG, de Toledo Z, Warren L, Axten D, Cornall RJ, Jones EY, Stuart DI, Screaton G, Ebner D, Hoosdally S, Chand M, Crook DW, O’Donnell AM, Conlon CP, Pouwels KB, Walker AS, Peto TEA, Hopkins S, Walker TM, Jeffery K, Eyre DW Oxford university hospitals staff testing group. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng W, Bao L, Liu J, Xiao C, Liu J, Xue J, Lv Q, Qi F, Gao H, Yu P, Xu Y, Qu Y, Li F, Xiang Z, Yu H, Gong S, Liu M, Wang G, Wang S, Song Z, Liu Y, Zhao W, Han Y, Zhao L, Liu X, Wei Q, Qin C. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, Nkolola JP, Liu J, Li Z, Chandrashekar A, Martinez DR, Loos C, Atyeo C, Fischinger S, Burke JS, Slein MD, Chen Y, Zuiani A, Lelis FJN, Travers M, Habibi S, Pessaint L, Van Ry A, Blade K, Brown R, Cook A, Finneyfrock B, Dodson A, Teow E, Velasco J, Zahn R, Wegmann F, Bondzie EA, Dagotto G, Gebre MS, He X, Jacob-Dolan C, Kirilova M, Kordana N, Lin Z, Maxfield LF, Nampanya F, Nityanandam R, Ventura JD, Wan H, Cai Y, Chen B, Schmidt AG, Wesemann DR, Baric RS, Alter G, Andersen H, Lewis MG, Barouch DH. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Teran C, Tiruthani K, McSweeney M, Ma A, Pickles R, Lai SK. Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy. Adv Drug Deliv Rev. 2021;169:100–117. doi: 10.1016/j.addr.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Levin EG, Rubin C, Indenbaum V, Tal I, Zavitan M, Zuckerman N, Bar-Chaim A, Kreiss Y, Regev-Yochay G. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayart JL, Douxfils J, Gillot C, David C, Mullier F, Elsen M, Eucher C, Van Eeckhoudt S, Roy T, Gerin V, Wieers G, Laurent C, Closset M, Dogné JM, Favresse J. Waning of IgG, total and neutralizing antibodies 6 months post-vaccination with BNT162b2 in healthcare workers. Vaccines (Basel) 2021;9:1092. doi: 10.3390/vaccines9101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong HL, Wu YT, Cao JL, Yang R, Liu YX, Ma J, Qiao XY, Yao XY, Zhang BH, Zhang YL, Hou WH, Shi Y, Xu JJ, Zhang L, Wang SJ, Fu BR, Yang T, Ge SX, Zhang J, Yuan Q, Huang BY, Li ZY, Zhang TY, Xia NS. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect. 2020;9:2105–2113. doi: 10.1080/22221751.2020.1815589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, Hu Z, Chen VC, Young BE, Sia WR, Tan YJ, Foo R, Yi Y, Lye DC, Anderson DE, Wang LF. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 16.Meyer B, Reimerink J, Torriani G, Brouwer F, Godeke GJ, Yerly S, Hoogerwerf M, Vuilleumier N, Kaiser L, Eckerle I, Reusken C. Validation and clinical evaluation of a SARS-CoV-2|surrogate virus neutralisation test (sVNT) Emerg Microbes Infect. 2020;9:2394–2403. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, Park S, Kim SK, Lim YJ, Kim EO, Jung J, Kwon HS, Kim TB, Kim SH. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36:e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Röltgen K, Powell AE, Wirz OF, Stevens BA, Hogan CA, Najeeb J, Hunter M, Wang H, Sahoo MK, Huang C, Yamamoto F, Manohar M, Manalac J, Otrelo-Cardoso AR, Pham TD, Rustagi A, Rogers AJ, Shah NH, Blish CA, Cochran JR, Jardetzky TS, Zehnder JL, Wang TT, Narasimhan B, Gombar S, Tibshirani R, Nadeau KC, Kim PS, Pinsky BA, Boyd SD. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5:eabe0240. doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, Ostermann PN, Robitzsch R, Hauka S, Walker A, Menne C, Grutza R, Timm J, Adams O, Schaal H. Age-dependent immune response to the biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang YM, Minn D, Lim J, Lee KD, Jo DH, Choe KW, Kim MJ, Kim JM, Kim KN. Comparison of antibody response elicited by ChAdOx1 and BNT162b2 COVID-19 vaccine. J Korean Med Sci. 2021;36:e311. doi: 10.3346/jkms.2021.36.e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scobie HM, Johnson AG, Suthar AB, Severson R, Alden NB, Balter S, Bertolino D, Blythe D, Brady S, Cadwell B, Cheng I, Davidson S, Delgadillo J, Devinney K, Duchin J, Duwell M, Fisher R, Fleischauer A, Grant A, Griffin J, Haddix M, Hand J, Hanson M, Hawkins E, Herlihy RK, Hicks L, Holtzman C, Hoskins M, Hyun J, Kaur R, Kay M, Kidrowski H, Kim C, Komatsu K, Kugeler K, Lewis M, Lyons BC, Lyons S, Lynfield R, McCaffrey K, McMullen C, Milroy L, Meyer S, Nolen L, Patel MR, Pogosjans S, Reese HE, Saupe A, Sell J, Sokol T, Sosin D, Stanislawski E, Stevens K, Vest H, White K, Wilson E, MacNeil A, Ritchey MD, Silk BJ. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status - 13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1284–1290. doi: 10.15585/mmwr.mm7037e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam M, Seo JD, Moon HW, Kim H, Hur M, Yun YM. Evaluation of humoral immune response after SARS-CoV-2 vaccination using two binding antibody assays and a neutralizing antibody assay. Microbiol Spectr. 2021;9:e0120221. doi: 10.1128/Spectrum.01202-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun S, Ryu JH, Jang JH, Bae H, Yoo SH, Choi AR, Jo SJ, Lim J, Lee J, Ryu H, Cho SY, Lee DG, Lee J, Kim SC, Park YJ, Lee H, Oh EJ. Comparison of SARS-CoV-2 antibody responses and seroconversion in COVID-19 patients using twelve commercial immunoassays. Ann Lab Med. 2021;41:577–587. doi: 10.3343/alm.2021.41.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terpos E, Trougakos IP, Karalis V, Ntanasis-Stathopoulos I, Sklirou AD, Bagratuni T, Papanagnou ED, Patseas D, Gumeni S, Malandrakis P, Korompoki E, Dimopoulos MA. Comparison of neutralizing antibody responses against SARS-CoV-2 in healthy volunteers who received the BNT162b2 mRNA or the AZD1222 vaccine: Should the second AZD1222 vaccine dose be given earlier? Am J Hematol. 2021;96:E321–E324. doi: 10.1002/ajh.26248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, Seo KH, Lee SH, Peck KR. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021;36:e107. doi: 10.3346/jkms.2021.36.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SW, Moon JY, Lee SK, Lee H, Moon S, Chung SJ, Yeo Y, Park TS, Park DW, Kim TH, Sohn JW, Yoon HJ, Kim SH. Anti-SARS-CoV-2 spike protein RBD antibody levels after receiving a second dose of ChAdOx1 nCov-19 (AZD1222) vaccine in healthcare workers: Lack of association with age, sex, obesity, and adverse reactions. Front Immunol. 2021;12:779212. doi: 10.3389/fimmu.2021.779212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang CL, Lin ZJ, Bi ZF, Qiu LX, Hu FF, Liu XH, Lin BZ, Su YY, Pan HR, Zhang TY, Huang SJ, Hu YM, Qiao YL, Zhu FC, Wu T, Zhang J, Xia NS. Inflammation-related adverse reactions following vaccination potentially indicate a stronger immune response. Emerg Microbes Infect. 2021;10:365–375. doi: 10.1080/22221751.2021.1891002. [DOI] [PMC free article] [PubMed] [Google Scholar]