Abstract
Background
Real-world clinical data concerning regdanvimab, a monoclonal antibody treatment for patients with mild-to-moderate coronavirus disease 2019 (COVID-19), are urgently needed. Here, we describe our experience with regdanvimab.
Materials and Methods
This retrospective cohort study enrolled high-risk adults with mild-to-moderate COVID-19 who were admitted to a dedicated COVID-19 hospital in Korea from March to September 2021. We used multiple logistic regression and propensity score-matching to compare the outcomes of patients who did or did not receive regdanvimab. The primary outcome was in-hospital progression to severe or critical status, or death.
Results
Of 586 patients eligible for regdanvimab, 256 patients who received regdanvimab and 251 untreated patients were included. The median age was 66 years and 47.5% were men. The most common underlying illnesses were hypertension (53.8%) and diabetes (36.9%). Patients were admitted to the hospital at a median of 2 days after symptom onset; regdanvimab was administered at a median of 3 days after symptom onset. Multivariate analysis indicated that regdanvimab significantly reduced the risk of disease progression during hospitalization [odds ratio (OR): 0.285; 95% confidence interval (CI): 0.144 - 0.564]. In a 1:1 propensity score-matched cohort (172 patients in either group), regdanvimab also decreased the risk of progression (OR: 0.162; 95% CI: 0.068 - 0.386).
Conclusion
In high-risk patients with mild-to-moderate COVID-19, regdanvimab decreased the risk of progression to severe COVID-19.
Keywords: Regdanvimab, Monoclonal antibody, COVID-19
Introduction
Although more than 1 year has passed since the World Health Organization declared coronavirus disease 2019 (COVID-19) to be a global pandemic, hundreds of thousands of daily cases and thousands of daily deaths continue to be reported worldwide [1]. Most patients with mild-to-moderate COVID-19 recover without specific treatment, but approximately 8.0% to 30.0% eventually progress to severe or critical disease [2]. Risk factors for disease progression and poor outcomes include older age; cardiovascular, chronic lung, and chronic kidney disease; diabetes; obesity; cancer; and immunosuppression [3]. Neutralizing monoclonal antibodies have been developed for high-risk patients [4,5,6,7,8]. Regdanvimab (CT-P59, Celltrion, Incheon, Korea) is a monoclonal antibody that targets the receptor-binding domain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein. A preclinical study in animals demonstrated a 100-fold reduction in viral load and the alleviation of clinical symptoms [9]. A Phase II/III clinical trial reported reduced progression to severe COVID-19 in 54.0% of patients with mild-to-moderate symptoms and 68.0% of patients with moderate symptoms aged >50 years, as well as significantly more rapid clinical recovery (3.4 to 6.4 days) than in placebo-treated patients [10]. Thus, regdanvimab received conditional approval for emergency use in February 2021, and full approval in September 2021, from the Korea Ministry of Food and Drug Safety (MFDS) [11]. The European Medicines Agency Committee has encouraged regdanvimab treatment for COVID-19 patients [12]. However, more real-world data concerning treatment effects and adverse events are required. Here, we describe our experience with regdanvimab.
Materials and Methods
1. Study setting
This observational cohort study was conducted at Masan Medical Center (MMC), a 300-bed public hospital in Changwon, Gyeongsangnam-do Province (population 3.5 million), Korea. MMC has operated as a dedicated hospital for patients with mild-to-moderate COVID-19 since February 2020. During the study period, all adult COVID-19 cases were hospitalized immediately or transported to residential treatment centers for treatment and isolation. MMC provided treatment for patients who required low-flow oxygen therapy; severe or critical patients who required high-flow therapy or mechanical ventilation were transferred to university hospitals. Patients were discharged if they met the clinical or testing criteria of the Korean Government. In the clinical criteria, any symptomatic case must not have fever in the absence of fever reducers and other clinical symptoms must improve for at least 72 h after 10 days from symptom onset. Asymptomatic patients were discharged if they lacked any clinical symptoms for 10 days. The testing criteria required cases to be polymerase chain reaction test-negative twice, with at least 24 h between the tests [13].
2. Study design and participants
The indications for regdanvimab (Celltrion, as conditionally approved by the MFDS) were adults (age >60 years) with mild COVID-19 and at least one of diabetes mellitus, hypertension, cardiovascular disease, or chronic lung disease; or radiologically confirmed pneumonia (regardless of concomitant risk factors). To allow comparisons with other studies, we modified the clinical criteria based on the recommendations of the United States Food and Drug Administration (US FDA). Our clinical criteria were mild-to-moderate COVID-19 developing within 10 days of symptom onset, and at least one of age ≥65 years or diabetes mellitus; or age >55 years with hypertension, cardiovascular disease, or chronic lung disease. All consenting patients received a single intravenous infusion of 40 mg/kg regdanvimab over 90 min. Patients with severe COVID-19 received steroids and remdesivir. Regdanvimab became available at MMC on March 15, 2021. We included adults hospitalized from March 1, 2021, to September 30, 2021, with laboratory-confirmed (reverse transcriptase polymerase chain reaction) COVID-19 who met the above clinical criteria. We compared patients who received and did not receive regdanvimab. We reviewed medical records (demographics, chronic health conditions, COVID-19 vaccination status, symptoms, laboratory findings, and disease severity at admission). The disease severity at admission was classified based on the National Institute of Health guidelines: mild, symptoms and signs without evidence of lower respiratory tract disease; moderate, symptoms with radiologically confirmed pneumonia but no hypoxemia; severe, respiratory rate >30 breaths/min, oxygen saturation <94.0% on room air at sea level, PaO2/FiO2 <300 mmHg, or lung infiltrates >50.0%; Critical cases were patients who had respiratory failure, septic shock, and/or multiorgan failure [14]. Patients who had clinical manifestations of severe COVID-19 within 2 days of hospitalization and those who received both regdanvimab and oxygen (within 6 h after monoclonal antibody treatment) were excluded because regdanvimab was unlikely to exert any effects in such patients [15]. Patients with incomplete medical records were also excluded. The primary outcome was progression of asymptomatic-to-moderately ill COVID-19 patients to severe or critical status during hospitalization. The duration of hospitalization, transfers to university hospitals, and deaths during hospitalization were also evaluated.
3. Ethics statement
The study protocol was approved by the Gyeongsang National University Changwon Hospital Institutional Review Board (GNUCH no. 2021-07-039). Informed consent was waived because of the retrospective nature of the study.
4. Statistical analysis
Baseline characteristics are summarized using standard descriptive statistics. Continuous variables were compared using the Mann–Whitney U-test; categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate. Univariate analysis was performed to identify variables significantly associated with COVID-19 progression. All variables with P-values <0.1 on univariate analysis, and other significant parameters (vaccination status and regdanvimab treatment), were included in multivariate logistic regression to identify risk factors for disease progression. Physicians tended to prescribe the monoclonal antibody for patients with more severe symptoms (Table 1). Thus, we used propensity score-matching to adjust for differences in baseline characteristics; we sought to reduce selection bias and confounding. Nearest-propensity score-matching (without replacement; ratio 1: 1; and caliper = 0.25) between the treatment and control groups was based on age (<65 and ≥65 years); sex; body mass index (BMI) (BMI <30 and ≥30 kg/m2); comorbidities (diabetes, hypertension, chronic lung disease, cerebrovascular disease, cardiovascular disease, or malignancy); COVID-19 severity; vaccination status; and body temperature and symptoms on admission. Comorbidities with <5 cases were not included. Covariate balances in the matched cohort were evaluated using standardized mean differences [16]. Additional multivariate logistic analysis was performed to identify risk factors for COVID-19 disease progression in the propensity score-matched cohort. Kaplan–Meier curves were drawn to compare the outcomes of the two groups using the log-rank test. To evaluate the effect of regdanvimab on SARS-CoV-2 B.1.617.2 (Delta) variants indirectly, subgroup analyses were performed with similar approaches by dividing before and after July 2021, when the Delta variant became dominant (the “fourth wave”). All tests were two-tailed, and a P-value <0.05 was considered statistically significant. All analyses were performed using SPSS software (version 24.0, IBM Corporation, Armonk, NY, USA) and R software (version 4.1.1 with the R packages; The R Project for Statistical Computing, Vienna, Austria).
Table 1. Clinical characteristics and outcomes of COVID-19 patients before and after propensity score matching.
| Variables | Pre-matched cohort | Propensity score-matched cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 507) | Regdanvimab (n = 256) | Control (n = 251) | P | Total (n = 344) | Regdanvimab (n = 172) | Control (n = 172) | P | ||
| Age (years) | 66 (60 – 72) | 66 (60 – 72) | 67 (60 – 72) | 0.646 | 67 (62 – 72) | 66 (61 – 72) | 67.5 (63 – 72) | 0.479 | |
| Age ≥65 years | 316 (62.3) | 158 (61.7) | 158 (62.9) | 0.775 | 232 (67.4) | 112 (65.1) | 120 (69.8) | 0.357 | |
| Male | 241 (47.5) | 122 (47.7) | 119 (47.4) | 0.956 | 159 (46.2) | 80 (46.5) | 79 (45.9) | 0.914 | |
| BMI, kg/m2 | 24.1 (22.1 – 26.2) | 24.5 (22.6 – 26.4) | 23.5 (21.5 – 25.6) | 0.017 | 24.0 (22.2 – 26.2) | 24.5 (22.6 – 26.6) | 23.7 (21.9 – 25.9) | 0.053 | |
| BMI ≥30 kg/m2 | 22 (4.3) | 12 (4.7) | 10 (4.0) | 0.698 | 11 (3.2) | 6 (3.5) | 5 (2.9) | 0.999 | |
| Days from symptom onset | |||||||||
| To admissiona, median (range) | 2 (0 – 9) | 2 (0 – 8) | 3 (0 – 9) | 0.012 | 2 (0 – 9) | 2 (0 – 8) | 3 (0 – 9) | 0.056 | |
| To Regdanvimab treatment, median (range) | 3 (0 – 9) | 3 (0 – 9) | NA | NA | 3 (0 – 9) | 3 (0 – 9) | NA | NA | |
| Days from diagnosis to admission, median (range) | 0 (0 – 4) | 0 (0 – 3) | 0 (0 – 4) | 0.674 | 0 (0 – 4) | 0 (0 – 3) | 0 (0 – 4) | 0.873 | |
| Underlying illness | |||||||||
| Diabetes | 187 (36.9) | 102 (39.8) | 85 (33.9) | 0.163 | 132 (38.4) | 68 (39.5) | 64 (37.2) | 0.657 | |
| Hypertension | 273 (53.8) | 131 (51.2) | 142 (56.6) | 0.223 | 178 (51.7) | 88 (51.2) | 90 (52.3) | 0.829 | |
| Coronary artery disease | 46 (9.1) | 27 (10.5) | 19 (7.6) | 0.243 | 33 (9.6) | 17 (9.9) | 16 (9.3) | 0.855 | |
| Chronic lung disease | 19 (3.7) | 16 (6.3) | 3 (1.2) | 0.004 | 8 (2.3) | 5 (2.9) | 3 (1.7) | 0.723 | |
| Chronic kidney disease | 5 (1.0) | 1 (0.4) | 4 (1.6) | 0.212 | 4 (1.2) | 0 (0.0) | 4 (2.3) | 0.123 | |
| Malignancy | 11 (2.2) | 5 (2.0) | 6 (2.4) | 0.770 | 7 (2.0) | 3 (1.7) | 4 (2.3) | 0.999 | |
| Immunocompromised | 2 (0.4) | 0 (0.0) | 2 (0.8) | 0.245 | 2 (0.6) | 0 (0.0) | 2 (1.2) | 0.499 | |
| Liver cirrhosis | 2 (0.4) | 2 (0.8) | 0 (0.0) | 0.499 | 2 (0.6) | 2 (1.2) | 0 (0.0) | 0.499 | |
| Cerebrovascular disease | 35 (6.9) | 18 (7.0) | 17 (6.8) | 0.909 | 27 (7.8) | 13 (7.6) | 14 (8.1) | 0.999 | |
| COVID-19 vaccination | 128 (25.2) | 63 (24.6) | 65 (25.9) | 0.739 | 92 (26.7) | 46 (26.7) | 46 (26.7) | 0.999 | |
| One dose | 99 (19.5) | 51 (19.9) | 48 (19.1) | 0.821 | 75 (21.8) | 37 (21.5) | 38 (22.1) | 0.896 | |
| Two doses | 29 (5.7) | 12 (4.7) | 17 (6.8) | 0.312 | 17 (4.9) | 9 (5.2) | 8 (4.7) | 0.804 | |
| Initial symptoms and sign | |||||||||
| Fever or chill | 205 (40.4) | 124 (48.4) | 81 (32.3) | <0.001 | 144 (41.9) | 72 (41.9) | 72 (41.9) | 0.999 | |
| Myalgia | 110 (21.7) | 70 (27.3) | 40 (15.9) | 0.002 | 76 (22.1) | 40 (23.3) | 36 (20.9) | 0.603 | |
| Cough | 270 (53.3) | 151 (59.0) | 119 (47.4) | 0.009 | 180 (52.3) | 91 (52.9) | 89 (51.7) | 0.829 | |
| Sputum | 135 (26.6) | 78 (30.5) | 57 (22.7) | 0.048 | 99 (28.8) | 49 (28.5) | 50 (29.1) | 0.905 | |
| Dyspnea | 19 (3.7) | 15 (5.9) | 4 (1.6) | 0.017 | 6 (1.7) | 3 (1.7) | 3 (1.7) | 0.999 | |
| Congestion or runny nose | 53 (10.5) | 26 (10.2) | 27 (10.8) | 0.825 | 35 (10.2) | 20 (11.6) | 15 (8.7) | 0.373 | |
| Sore throat | 144 (28.4) | 76 (29.7) | 68 (27.1) | 0.517 | 90 (26.2) | 45 (26.2) | 45 (26.2) | 0.999 | |
| Chest discomfort | 18 (3.6) | 8 (3.1) | 10 (4.0) | 0.601 | 14 (4.1) | 7 (4.1) | 7 (4.1) | 0.999 | |
| Abdominal pain or diarrhea | 21 (4.1) | 10 (3.9) | 11 (4.4) | 0.788 | 12 (3.5) | 7 (4.1) | 5 (2.9) | 0.770 | |
| Headache | 114 (22.5) | 70 (27.3) | 44 (17.5) | 0.008 | 79 (23.0) | 40 (23.3) | 39 (22.7) | 0.898 | |
| Loss of taste or smell | 33 (6.5) | 14 (5.5) | 19 (7.6) | 0.338 | 25 (7.3) | 11 (6.4) | 14 (8.1) | 0.533 | |
| Fever ≥38°C on admission | 83 (16.4) | 65 (25.4) | 18 (7.2) | <0.001 | 43 (12.5) | 25 (14.5) | 18 (10.5) | 0.254 | |
| Severityb on admission | |||||||||
| No symptom | 34 (6.7) | 9 (3.5) | 25 (10.0) | 0.004 | 21 (6.1) | 9 (5.2) | 12 (7.0) | 0.499 | |
| Mild | 236 (46.5) | 88 (34.4) | 148 (59.0) | <0.001 | 159 (46.2) | 76 (44.2) | 83 (48.3) | 0.449 | |
| Moderate | 237 (46.7) | 159 (62.1) | 78 (31.1) | <0.001 | 164 (47.7) | 87 (50.6) | 77 (44.8) | 0.280 | |
| Laboratory findings on admissionc | |||||||||
| WBCs (× 103/mm3) | 4.94 (4.08 – 6.04) | 4.83 (4.05 – 5.87) | 5.14 (4.11 – 6.23) | 0.110 | 4.92 (4.11 – 5.90) | 4.90 (4.10 – 5.80) | 4.95 (4.11 – 5.93) | 0.507 | |
| Platelet (× 103/mm3) | 199 (160 – 238) | 197 (158 – 234) | 202 (165 – 243) | 0.195 | 200 (164 – 241) | 200 (163 – 237) | 198 (165 – 243) | 0.673 | |
| AST (IU/L) | 24 (19 – 31) | 25 (20 – 32) | 23 (19 – 31) | <0.001 | 24 (19 – 31) | 24 (19 – 30) | 24 (19 – 33) | 0.909 | |
| ALT (IU/L) | 19 (14 – 29) | 19 (14 – 28) | 19 (13 – 31) | 0.185 | 18 (14 – 29) | 18 (14 – 27) | 19 (13 – 31) | 0.758 | |
| Creatinine (mg/dL) | 0.79 (0.65 – 0.94) | 0.79 (0.65 – 0.94) | 0.79 (0.66 – 0.94) | 0.863 | 0.79 (0.65 – 0.95) | 0.79 (0.65 – 0.95) | 0.78 (0.65 – 0.95) | 0.948 | |
| C-reactive protein (mg/dL) | 0.47 (0.14 – 1.28) | 0.63 (0.23 – 1.70) | 0.29 (0.10 – 0.68) | <0.001 | 0.44 (0.14 – 1.28) | 0.57 (0.19 – 1.48) | 0.33 (0.12 – 0.93) | 0.002 | |
| Outcomes | |||||||||
| Progress to severe disease | 51 (10.1) | 20 (7.8) | 31 (12.4) | 0.089 | 38 (11.0) | 8 (4.7) | 30 (17.4) | <0.001 | |
| Transfer to tertiary hospitals | 6 (1.2) | 3 (1.2)d | 3 (1.2)e | 0.999 | 4 (1.2) | 1 (0.6) | 3 (1.7) | 0.311 | |
| Duration of hospitalization | 11 (5 – 33) | 11 (6 – 22) | 11 (5 – 33) | 0.010 | 11 (5 – 33) | 11 (6 – 20) | 11 (5 – 33) | 0.329 | |
Data are no. (%) of patients or median (interquartile range), unless otherwise indicated.
aOf the total cohort, 374 had COVID-19 symptoms at the time of admission.
bSeverity was classified based on NIH-guidelines.
cOf the total cohort, 37 patients did not get laboratory tests.
dOne patient was treated with high flow oxygen therapy and two were treated with low flow oxygen therapy in tertiary hospitals.
eTwo patients were treated with high flow oxygen therapy and one was treated with mechanical ventilation.
COVID-19, coronavirus disease 2019; BMI, body mass index; NA, not applicable; WBC, white blood cell; AST, aspartate transaminase; ALT, alanine transaminase.
Results
1. Patient demographics
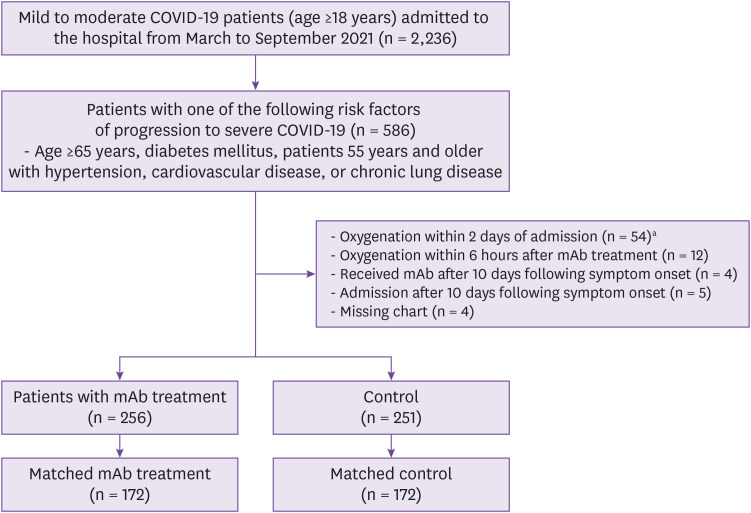
Of 2,236 adults admitted to MMC, 586 had at least one risk factor rendering monoclonal antibody treatment appropriate. In total, 507 patients were included in the analysis: a monoclonal antibody group (n = 256) and a control group (n = 251). Furthermore, 79 patients met the exclusion criteria (Fig. 1). The baseline characteristics of both groups are listed in Table 1. The median age was 66 years (interquartile range, 60 - 72 years) and 47.5% of the patients were men. The most common underlying illnesses were hypertension (53.8%), diabetes (36.9%), coronary artery disease (9.1%), cerebrovascular disease (6.9%), and chronic lung disease (3.7%). Patients were admitted to the hospital at a median of 2 days (range, 0 - 9 days) from symptom onset, indicating admission on the day of diagnosis (median, 0 days; range, 0 - 4 days). Regdanvimab was administered at a median of 3 days (range, 0 - 9 days) from symptom onset and at a median of 1 day (range, 0 - 7 days) from admission. Before matching (compared with controls), the treatment group exhibited more COVID-19 symptoms, more chronic lung disease, more temperatures >38°C on admission, and more cases of moderate COVID-19 on admission. Propensity score-matching yielded 172 patients in each group. The baseline characteristics were adequately balanced, as revealed by the standardized mean differences (Supplementary Table 1 and Fig. 1).
Figure 1. The study population.
aOf the 54 patients, 49 (90.7%) required oxygenation on the day of admission.
COVID-19, coronavirus disease 2019; mAb, monoclonal antibody.
2. Clinical outcomes
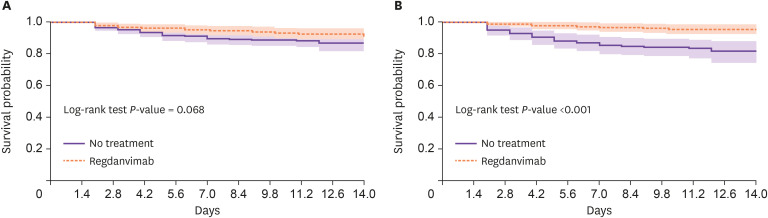
Progression to severe COVID-19 occurred in 20 (7.8%) monoclonal antibody-treated patients and 31 (12.4%) untreated patients (P = 0.089), including 3 of each pre-matched cohort who were transferred to university hospitals. There were no deaths. Although the duration of hospitalization slightly differed between the pre-matched groups, this difference disappeared after propensity score-matching (P = 0.329) (Table 1). After adjustments for age, hypertension status, body temperature on admission, C-reactive protein (CRP) level, platelet count, COVID-19 vaccination status, and disease severity, regdanvimab was significantly associated with a reduced risk of disease progression during hospitalization [odds ratio (OR), 0.285; 95% confidence interval (CI), 0.144 - 0.564]. Hypertension, CRP level ≥1 mg/dL, platelet count <150 × 103/mm3 and moderate disease severity on admission were significantly associated with disease progression (Table 2). In the propensity score-matched cohort, progression to severe COVID-19 occurred in 8 (4.7%) monoclonal antibody-treated patients and 30 (17.4%) untreated patients (P <0.001), including 1 and 3 patients (respectively) who were transferred to university hospitals (Table 1). After adjustments for hypertension, body temperature on admission, CRP level, platelet count, COVID-19 vaccination status, and disease severity, regdanvimab significantly reduced the risk of disease progression during hospitalization (OR: 0.162; 95% CI: 0.068 - 0.386) in the propensity score-matched cohort (Table 2). Kaplan–Meier survival analysis also revealed a significant difference in disease progression within 2 weeks after hospitalization between the two groups (log-rank test P <0.001; Fig. 2B).
Table 2. Association of baseline characteristics and regdanvimab treatment on disease progression in patients with COVID-19.
| Variables | Pre-matched cohort (n = 507) | Propensity score-matched cohort (n = 344) | |||
|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | ||
| Age, ≥65 years | 1.872 (0.970 – 3.611) | 1.722 (0.840 – 3.532) | 1.635 (0.746 – 3.583) | ||
| Male | 0.686 (0.380 – 1.239) | 0.734 (0.369 – 1.460) | |||
| Diabetes | 1.116 (0.617 – 2.021) | 1.708 (0.868 – 3.361) | |||
| Hypertension | 2.464 (1.297 – 4.681) | 2.766 (1.388 – 5.509) | 3.395 (1.555 – 7.412) | 3.170 (1.379 – 7.286) | |
| Coronary artery disease | 0.839 (0.288 – 2.443) | 0.789 (0.229 – 2.719) | |||
| Chronic lung disease | 0.487 (0.064 – 3.723) | ||||
| Cerebrovascular disease | 1.167 (0.395 – 3.450) | 1.007 (0.288 – 3.517) | |||
| BMI ≥30 kg/m2 | 0.890 (0.202 – 3.922) | 1.833 (0.381 – 8.819) | |||
| Fever ≥38°C on admission | 2.654 (1.391 – 5.063) | 1.929 (0.874 – 4.257) | 2.955 (1.318 – 6.624) | 2.144 (0.824 – 5.578) | |
| C- reactive protein ≥1 mg/dL | 3.977 (2.186 – 7.234) | 3.572 (1.836 – 6.951) | 3.655 (1.829 – 7.304) | 3.950 (1.838 – 8.489) | |
| WBCs >10 × 103/mm3 | 1.030 (0.126 – 8.407) | 1.926 (0.210 – 17.693) | |||
| Platelet <150 × 103/mm3 | 2.781 (1.466 – 5.275) | 2.322 (1.141 – 4.727) | 2.269 (1.049 – 4.905) | 1.702 (0.688 – 4.214) | |
| COVID-19 vaccination | 1.014 (0.522 – 1.971) | 0.673 (0.314 – 1.443) | 1.132 (0.537 – 2.385) | 0.753 (0.314 – 1.806) | |
| Two doses of vaccination | 0.649 (0.150 – 2.810) | 1.078 (0.237 – 4.906) | |||
| Moderate severity vs. No symptom to mild | 3.045 (1.622 – 5.717) | 3.049 (1.489 – 6.244) | 2.310 (1.139 – 4.685) | 2.167 (0.996 – 4.717) | |
| Regdanvimab treatment | 0.601 (0.333 – 1.086) | 0.285 (0.144 – 0.564) | 0.231 (0.103 – 0.520) | 0.162 (0.068 – 0.386) | |
COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; BMI, body mass index; WBC, white blood cell.
Figure 2. Kaplan–Meier curves of disease progression within 2 weeks of hospitalization.
The shaded area indicates the 95% confidence interval. (A) pre-matched cohort; (B) propensity score-matched cohort.
We performed subgroup analyses before and after July 2021 (i.e., divided by the Delta variant-dominant wave in Korea). Multivariate analyses of the pre-matched cohorts showed that the preventive effect of regdanvimab appeared to decrease after commencement of the Delta variant-dominant wave [OR: 0.100 (95% CI: 0.031 - 0.320) before the wave vs. OR, 0. 464 (95% CI: 0.183 - 1.175) after the wave]. The data from the propensity score-matched cohort showed a similar trend [OR: 0.032 (95% CI: 0.004 - 0.260) before the wave vs. OR: 0.414 (95% CI: 0.143 - 1.195) after the wave]. However, vaccination appeared to prevent disease progression after commencement of the Delta variant-dominant wave (OR: 0.414; 95% CI: 0.173 - 0.990) in the pre-matched cohort (Supplementary Table 2, 3). We noted no serious adverse infusion-related events among regdanvimab-treated patients during the study period.
Discussion
In this retrospective study, we found that regdanvimab treatment of mild-to-moderate COVID-19 was significantly associated with a decreased risk of progression to severe disease during hospitalization. It is difficult to compare the effect of regdanvimab to the effects of other neutralizing antibody therapies approved by the US FDA (i.e., bamlanivimab–etesevimab, casirivimab–imdevimab, and sotrovimab) because of differences in the clinical settings and outcome measures. We included only patients who met the US FDA criteria for monoclonal antibody use. Our results are consistent with previous reports that monoclonal antibody treatment prevented mild-to-moderate COVID-19 from progressing to severe disease (i.e., hospitalization) by 70.0 - 80.0% [17].
Unlike other monoclonal antibodies, limited data are available concerning regdanvimab. One Phase II trial reported that the proportions of patients requiring hospitalization or oxygen to day 28 were 4.0% and 4.9% in regdanvimab 40 and 80 mg/kg groups, but 8.7% in a placebo group. A preliminary Phase III trial found that regdanvimab reduced the risk of hospitalization or death with 28 days by 72% (compared to placebo) in high-risk patients with mild-to-moderate COVID-19 (P <0.01) [18]. We found that regdanvimab was useful for treating mild-to-moderate COVID-19 in a real-world setting.
In one randomized controlled trial, bamlanivimab was no better than standard therapy in hospitalized COVID-19 patients; the primary outcome was sustained recovery over a 90-day period [19]. Of 314 patients, 77.0% exhibited a disease status exceeding “severe” and monoclonal antibodies were administered at a median of 7 days from symptom onset. Based on this work and other clinical trials, the US guideline recommends monoclonal antibody treatment for ambulatory at-risk patients with mild-to-moderate COVID-19 [20]. Although our primary outcome was progression to severe COVID-19 in hospitalized patients with mild-to-moderate COVID-19, we acknowledge that it is difficult to directly compare our results with the results of previous studies that employed hospitalization or emergency room visits as primary outcomes [17].
As SARS-CoV-2 variants such as Delta and Omicron, there has been increasing concern that monoclonal antibody effects will decrease. An in vitro study showed that bamlanivimab was not active against the Delta variant, while etesevimab, casirivimab, and imdevimab remained active [21]. In vitro and in a mouse model, regdanvimab was active against Delta, but showed less activity than it did against Delta precursors [22]. However, we found that the preventive effect of regdanvimab appeared to decrease during the Delta variant-dominant wave. Importantly, it is difficult to draw firm conclusions because more patients became vaccinated and there were a relatively small number of cases during the Delta variant-dominant wave. Furthermore, we do not know the proportions of Delta-infected cases; genomic sequencing data were lacking. According to the Korea Disease Control and Prevention Agency, >50.0% of random samples collected in mid-July and >90.0% collected in early August were Delta. It may thus be possible to compare the effect of regdanvimab before and after the Delta variant-dominant wave in this study. Omicron is rapidly outpacing Delta globally and is driving an upsurge of infections in most regions [23]. In vitro data suggest that several monoclonal antibodies, including regdanvimab, lost neutralizing activity against Omicron [24]. Although some monoclonal antibodies in clinical use may retain benefit, the use of monoclonal antibody therapeutics will require caution depending on whether Omicron is dominant in a region [25].
Our work had several limitations. First, although propensity score-matching was used to reduce selection bias and confounding, many cases were discarded during this process, possibly creating (rather than eliminating) selection bias. However, multiple logistic regression analysis yielded similar results. Second, we excluded patients who progressed to severe or critical status within 2 days of hospitalization or within 6 h of regdanvimab administration. Because progression to severe or critical COVID-19 disease usually occurred soon after hospitalization, such patients were less likely to receive regdanvimab; this may have exaggerated the effect of the drug. We excluded such patients to render our analysis conservative. Third, neither the duration of hospitalization nor the time to symptom improvement served as a principal outcome, because patients were discharged only when they met the government guideline regardless of whether they had recovered earlier. Finally, minor symptoms might not have been recorded by attending physicians or nurses.
In conclusion, our real-world study suggests that regdanvimab treatment of mild-to-moderate COVID-19 significantly reduced disease progression; the effect appeared to wane during the Delta variant-dominant wave. A large-scale prospective study or randomized clinical trial is needed to explore the effects of regdanvimab in the era of Omicron domination and high numbers of vaccinated individuals.
ACKNOWLEDGMENTS
The authors thank Dr. Su Jin Lim, Department of Internal Medicine, Gyeongsangnam-do Masan Medical Center, Dr. Seungjin Lim, Department of Internal Medicine, Pusan National University School of Medicine, and Dr. Moonsuk Bae, Department of Internal Medicine, Pusan National University School of Medicine, for data curation.
Footnotes
Funding: None.
Conflict of Interest: No conflict of interest.
- Conceptualization: SIH, OHC.
- Data curation: BHR, KWH, IGB, OHC.
- Formal analysis: SIH, BHR, KWH, IGB.
- Methodology: SIH, OHC.
- Supervision: OHC.
- Validation: SIH, OHC.
- Visualization: SIH.
- Writing - original draft: SIH.
- Writing - review & editing: SIH, OHC.
SUPPLEMENTARY MATERIALS
Standardized mean differences before and after propensity score matching
Association of clinical characteristics and disease progression in the pre-matched cohort with COVID-19 before and after the Delta variant-dominant wave in Korea
Association of clinical characteristics and disease progression in the propensity score-matched cohort with COVID-19 before and after the Delta variant-dominant wave in Korea
Visualization of the distribution of propensity score by the jitter plot.
References
- 1.World Health Organization (WHO) WHO coronavirus (COVID-19) dashboard. [Updated Nevember 28, 2021]. [Accessed 28 November 2021]. Available at: https://covid19.who.int/
- 2.Cen Y, Chen X, Shen Y, Zhang XH, Lei Y, Xu C, Jiang WR, Xu HT, Chen Y, Zhu J, Zhang LL, Liu YH. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-centre observational study. Clin Microbiol Infect. 2020;26:1242–1247. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, Sarkis E, Solis J, Zheng H, Scott N, Cathcart AL, Hebner CM, Sager J, Mogalian E, Tipple C, Peppercorn A, Alexander E, Pang PS, Free A, Brinson C, Aldinger M, Shapiro AE COMET-ICE Investigators. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 5.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B, Morris J, Crystal C, Igbinadolor A, Huhn G, Cardona J, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Dabora MC, Klekotka P, Shen L, Skovronsky DM BLAZE-1 Investigators. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marovich M, Mascola JR, Cohen MS. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020;324:131–132. doi: 10.1001/jama.2020.10245. [DOI] [PubMed] [Google Scholar]
- 9.Kim C, Ryu DK, Lee J, Kim YI, Seo JM, Kim YG, Jeong JH, Kim M, Kim JI, Kim P, Bae JS, Shim EY, Lee MS, Kim MS, Noh H, Park GS, Park JS, Son D, An Y, Lee JN, Kwon KS, Lee JY, Lee H, Yang JS, Kim KC, Kim SS, Woo HM, Kim JW, Park MS, Yu KM, Kim SM, Kim EH, Park SJ, Jeong ST, Yu CH, Song Y, Gu SH, Oh H, Koo BS, Hong JJ, Ryu CM, Park WB, Oh MD, Choi YK, Lee SY. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021;12:288. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celltrion Heathcare. Celltrion Group announces positive top-line efficacy and safety data from global Phase II/III clinical trial of COVID-19 treatment candidate CT-P59. [Accessed 28 November 2021]. Available at: https://www.celltrionhealthcare.com/en-us/board/newsdetail?modify_key=433&pagenumber=1&keyword=&keyword_type=
- 11.Ministry of Food and Drug Safety (MFDS) Press release: Celltrion’s regdanvimab (CT-P59) becomes the first authorized COVID-19 treatment approved from the Korean MFDS. [Accessed 1 December 2021]. Available at: https://www.mfds.go.kr/brd/m_99/view.do?seq=45778.
- 12.European Meicines Agency. EMA issues advice on use of regdanvimab for treating COVID-19. [Updated March 26, 2021]. [Accessed 29 November 2021]. Available at: https://www.ema.europa.eu/en/news/ema-issues-advice-use-regdanvimab-treating-covid-19.
- 13.Ministry of Health and Welfare. Coronavirus (COVID-19), Republic of Korea. Patient treatment & management. [Updated June 25, 2020]. [Accessed 29 November 2021]. Available at: http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=112&dataGubun=&ncvContSeq=&contSeq=&board_id=
- 14.National Institutes of Health (NIH) COVID-19 treatment guidelines. Clinical spectrum of SARS-CoV-2 infection. [Updated October 19, 2021]. [Accessed 7 February 2022]. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum.
- 15.Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8:ofab315. doi: 10.1093/ofid/ofab315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84–S90.e1. doi: 10.1016/j.jclinepi.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruzzesi E, Ranzenigo M, Castagna A, Spagnuolo V. Neutralizing monoclonal antibodies for the treatment and prophylaxis of SARS-CoV-2 infection. New Microbiol. 2021;44:135–144. [PubMed] [Google Scholar]
- 18.Syed YY. Regdanvimab: First approval. Drugs. 2021;81:2133–2137. doi: 10.1007/s40265-021-01626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ACTIV-3/TICO LY-CoV555 Study Group. Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, Brown SM, Knowlton KU, Self WH, Files DC, Jain MK, Benfield T, Bowdish ME, Leshnower BG, Baker JV, Jensen JU, Gardner EM, Ginde AA, Harris ES, Johansen IS, Markowitz N, Matthay MA, Østergaard L, Chang CC, Davey VJ, Goodman A, Higgs ES, Murray DD, Murray TA, Paredes R, Parmar MKB, Phillips AN, Reilly C, Sharma S, Dewar RL, Teitelbaum M, Wentworth D, Cao H, Klekotka P, Babiker AG, Gelijns AC, Kan VL, Polizzotto MN, Thompson BT, Lane HC, Neaton JD. A Neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Infectious Diseases Society of America (IDSA) IDSA guidelines on the treatment and management of patients with COVID-19. [Updated November 18, 2021]. [Accessed 29 November 2021]. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
- 21.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Péré H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Lorière E, Rey FA, Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 22.Ryu DK, Kang B, Noh H, Woo SJ, Lee MH, Nuijten PM, Kim JI, Seo JM, Kim C, Kim M, Yang E, Lim G, Kim SG, Eo SK, Choi JA, Song M, Oh SS, Chung HY, Tijsma AS, van Baalen CA, Kwon KS, Lee SY. The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021;578:91–96. doi: 10.1016/j.bbrc.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO) Enhancing response to Omicron SARS-CoV-2 variant. [Updated January 21 2022]. [Accessed 7 February 2022]. Available at: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states.
- 24.VanBlargan LA, Errico JM, Halfmann PJ, Zost SJ, Crowe JE, Jr, Purcell LA, Kawaoka Y, Corti D, Fremont DH, Diamond MS. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022:1–6. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S Food & Drug Administration. FDA statement. Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant. [Updated January 24, 2022]. [Accessed 8 February 2022]. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standardized mean differences before and after propensity score matching
Association of clinical characteristics and disease progression in the pre-matched cohort with COVID-19 before and after the Delta variant-dominant wave in Korea
Association of clinical characteristics and disease progression in the propensity score-matched cohort with COVID-19 before and after the Delta variant-dominant wave in Korea
Visualization of the distribution of propensity score by the jitter plot.