Background:
Although it was initially described for improved myoelectric control, targeted muscle reinnervation (TMR) has quickly gained popularity as a technique for neuroma control. With this rapid increase in utilization has come broadening indications and variability in the described technique. As a result, it becomes difficult to interpret published outcomes. Furthermore, there is no literature discussing the management of failed cases which are undoubtedly occurring.
Methods:
This is a retrospective case series of two patients who underwent revision surgery for failed TMR. The authors also review the current literature on TMR and outline technical and conceptual pitfalls and pearls based on our local experience.
Results:
Excessive donor nerve redundancy, kinking, donor–recipient nerve size mismatch, superficial placement of the nerve coaptation, inappropriate target selection, and incomplete target muscle denervation were identified as technical pitfalls of TMR surgery. Techniques to avoid these pitfalls were described.
Conclusions:
Although TMR has been a major development in amputee care for both pain management and improved myoelectric control, it is important to acknowledge that it is not a foolproof surgery and does not provide a guaranteed result. Failed cases of TMR represent opportunities to learn about factors contributing to unfavorable outcomes and refine our techniques empirically.
Takeaways
Question: This article aims to outline cases in which preemptive targeted muscle reinnervation (pTMR) failed to prevent painful neuromas and suggest technical pearls for revision surgery.
Findings: Using intraoperative findings from two failed cases of pTMR, this article highlights certain technical pitfalls: excessive nerve redundancy, kinking, size mismatch and incomplete recipient muscle denervation. Suggested techniques to address these pitfalls are included.
Meaning: pTMR is not a foolproof surgery and its outcomes and techniques vary widely. By highlighting the major technical pitfalls, we identify opportunities to refine the surgical technique both at preemptive and revision TMR procedures.
INTRODUCTION
Targeted muscle reinnervation (TMR) was first described by Dumanian et al as a technique to improve myoeletric prosthetic control.1–5 Interestingly, upper extremity amputees noticed an improvement in their phantom limb and residual limb pain postoperatively, which led to TMR surgery becoming adopted as a treatment for pain management as well.6–8 Over time, the indications have broadened from delayed reconstructions to performing TMR at the time of the amputation as a preemptive measure against neuroma formation and the subsequent phantom limb and residual limb pain.9–14 Lately, the technique has been increasingly applied even to treatment of painful neuromas outside of the amputee population.15–18
However, the reported success rates of TMR for prevention of phantom and residual limb pain vary in the literature.6,7,9–11 As the technique continues to be more widely adopted in both the prophylactic and reconstructive setting, it is being used for a wider variety of amputation types,19,20 and more variation in surgical technique is being applied.4,21–25
Little attention has been paid in the literature to failed or revision cases of TMR, although these clinical cases are bound to be accruing. Neither does there appear to have been consideration for how variations in technique may affect outcomes. Therefore, this paper examines our experience with failed cases of preemptive TMR (pTMR) undergoing revision surgery. The goal is to identify technical and conceptual factors contributing to failure of TMR, based on our experience in revision cases and a review of the current literature.
METHODS
In this article, the authors retrospectively present two consecutive cases of failed TMR, review the current literature on primary TMR, and describe technical and conceptual pitfalls that contribute to failed TMR and how to avoid them. The principles outlined in the Declaration of Helsinki have been followed.
Case Reports
In case 1, a 35-year-old, right-hand dominant man underwent left transradial forearm amputation for a rare malignant tumor of the common digital nerve to the second webspace. pTMR was performed transferring the median nerve to flexor carpi radialis, the ulnar nerve to palmaris longus, and the radial sensory nerve to extensor carpi radialis brevis (Video 1; Fig. 1). (See Video 1 [online], which displays the details and summary of case 1.) At 1 year postoperative, the patient developed MRI-proven symptomatic neuromas of the median and ulnar nerves along with bony exostoses at the distal ends of the radius and ulna. Due to a lack of available motor targets, a free chimeric serratus muscle and thoracodorsal artery perforator flap was elevated using the two most distal slips of the muscle for a revision TMR surgery. Intraoperatively, the median and ulnar nerves were found to be redundant and coiled in a circular pattern and terminating in large neuromas (Fig. 2). Each nerve was neurolysed carefully to preserve functioning branches, transected distally, and transferred to the long thoracic nerve branch entering each slip of serratus where the nerve entered the muscle (Fig. 3). The patient experienced immediate improvement of his neuroma pain from a level of 10/10 preoperatively to 2–3/10 after revision TMR. However, he had a recurrence of median and ulnar nerve neuromas at three months postoperative. This was managed with excision of neuromas, nerve transposition to upper arm, and nerve allograft “to nowhere” (i.e., no distal neurorrhaphy). Pain consistent with recurrent symptomatic neuroma once again recurred shortly thereafter.
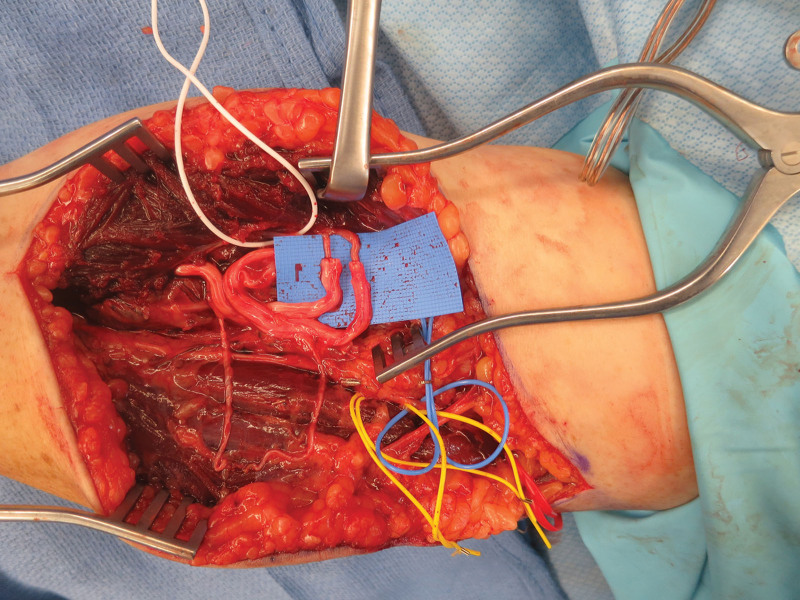
Fig. 1.
Preemptive targeted muscle reinnervation at the time of transradial amputation in case 1. The median nerve was coapted to flexor carpi radialis and the ulnar nerve to palmaris longus. Note the relatively long distance of the nerve coaptation from the target muscle and the excess redundancy and coiling of the proximal median and ulnar nerves.
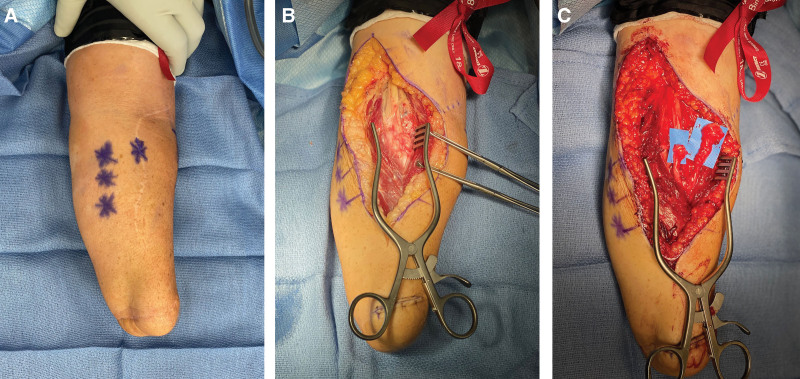
Fig. 2.
Intraoperative findings at the time of revision surgery for failed transradial pTMR. A, Preoperative marking of Tinel’s signs at revision targeted muscle reinnervation for case 1. B, Intraoperative findings included dense scarring around and coiling of proximal median and ulnar nerves. C, The distal median and ulnar nerves terminated in large neuromas at the site of the previous nerve coaptation and corresponding to the location of Tinel’s signs.
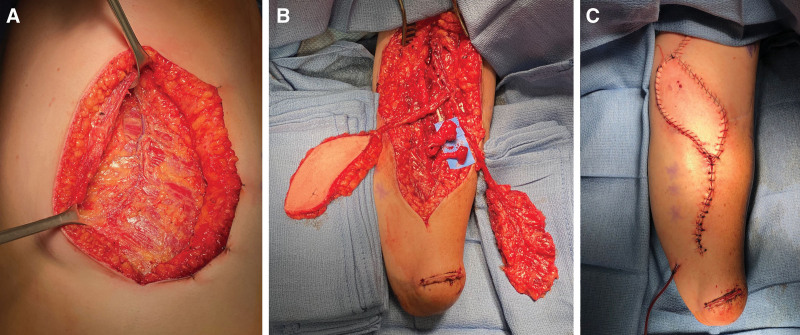
Fig. 3.
Management of failed transradial TMR using a free serratus muscle flap to provide additional muscle targets for revision TMR without sacrificing remaining critical muscles. A, Intraoperative photograph demonstrating the neurovascular pedicle to the serratus muscle, two distal slips of which were used as recipients at revision targeted muscle reinnervation in case 1. B, A fasciocutaneous thoracodorsal artery perforator flap was harvested along with the serratus muscle as a chimeric flap for soft-tissue reconstruction at the antecubital fossa. The median and ulnar nerve neuromas are shown in relation to the serratus muscle. C, Final flap inset and closure.
Video 1. Targeted muscle reinnervation: case 1.
In case 2, a 70-year-old, right-hand dominant man was transferred to the emergency room for an acute left upper extremity ischemia from a thromboembolus to the distal subclavian artery (Video 2). (See Video 2 [online], which displays the surgical findings in case 2, including a video demonstrating electrical stimulation of a prior TMR coaptation site.) On arrival, his extremity was found to be nonsalvageable and an emergent elbow disarticulation amputation was performed. After multiple trips to the operating room for debridement of nonviable biceps and brachialis muscles, a transhumeral amputation with TMR was performed, transferring the musculocutaneous, median, and ulnar nerves to branches to the medial triceps, the radial nerve to the brachialis, and the posterior cutaneous nerve to the lateral triceps. The medial antebrachial cutaneous nerve was crushed, cauterized, and buried proximally in residual brachialis muscle. The patient developed significant phantom limb and neuroma pain, with a soft-tissue mass 5 cm proximal to the tip of the stump on the medial aspect of the residual limb. At the time of revision TMR, large neuromas of the median, ulnar, and medial brachial cutaneous nerves were noted, along with two 90-degree kinks of the median nerve within and around the scar tissue and imbricated triceps muscle (Video 2; Fig. 4). Stimulation of the median and ulnar nerves did produce weak contraction of the medial triceps, indicating the muscle reinnervation had been successful (Video 2). Revision TMR was performed transferring the median nerve to the long head of biceps (preserving the strongly contracting short head for elbow flexion signal), the ulnar nerve to the medial head of the triceps, and the radial nerve to the lateral head of triceps. The medial brachial and antebrachial cutaneous nerves were transferred to intramuscular branches to the most distal slips of the serratus on the chest wall. At 1 year postoperatively, the patient experienced complete resolution of his neuroma pain from a level of 8–9/10 preoperatively to 0/10 after revision TMR, but continued to have phantom limb pain. His frequency of prosthetic use improved.
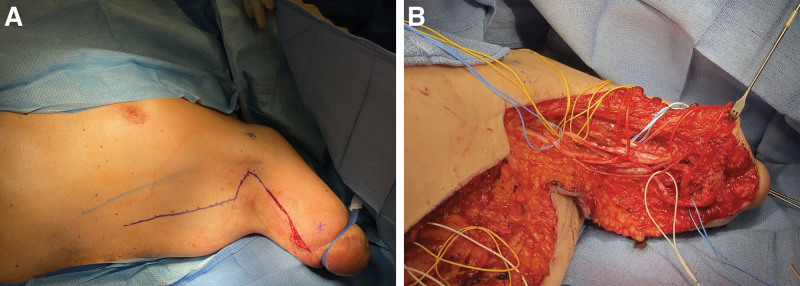
Fig. 4.
Intraoperative findings at the time of revision surgery for failed transhumeral pTMR. A, Marking of the incision and Tinel’s signs for case 2 at revision targeted muscle reinnervation. Preemptive targeted muscle reinnervation had been performed at the time of the transhumeral amputation. B, Both median (red vessel loops) and ulnar (blue vessel loops) nerves terminated in neuromas despite having successfully reinnervated their target (the medial head of the triceps). Note also that the median nerve takes a sharp 90-degree turn just proximal to the site of the muscle imbrication at the preemptive targeted muscle reinnervation (distal red vessel loop).
Video 2. Targeted muscle reinnervation: case 2.
Literature Review and Comparison to Traditional Neuroma Management Techniques
Traditional neuroma management techniques have emphasized proximal transposition and burial of the transected nerve end away from surface contact points. A study by Ducic et al26 utilizing this traditional technique in amputees showed a pain reduction from 8.04 ± 1.18 preoperative to 1.07 ± 1.59 postoperative (P < 0.0001). They also found that intermittent painful spasms, reported by 12 of 15 amputees (80%), were eliminated in 10 patients (83%) (P < 0.0001).26 A meta-analysis of all surgical interventions for neuroma pain by Poppler et al27 found that in 54 included studies, the overall meaningful reduction in pain for all techniques was 77% and that the excision and transposition group had the highest proportion of patients with a meaningful pain reduction (81%). Although post amputation pain is more complex than pure neuroma pain, there is no doubt that neuroma formation contributes to at least residual limb pain and likely to phantom limb pain to some degree as well.12–14,28–33 Thus, these traditional technique outcomes serve as the baseline to compare the novel TMR related techniques.
TMR was first suggested to be an effective tool for neuroma pain by Souza et al7 when they reported complete resolution of pain in 14 of 15 patients who had neuroma pain prior to TMR and partial resolution in the other patient. Additionally, they found that none of the other 11 patients in the study without symptomatic neuromas before the TMR surgery, developed them afterward.7 A subsequent publication by Dumanian et al6 is the only randomized controlled trial in this field. Unfortunately, despite the intention to enroll 200 patients, the study was terminated after 28 were enrolled. As a result, their primary outcome of NRS pain score reduction failed to reach statistical significance. For their secondary outcomes, they report a 72% and 67% complete resolution of phantom limb and residual limb pain respectively in the TMR group compared to 40% and 27% in the non-TMR group.6 Another limitation of this paper is that a large number of patients were either ineligible for randomization due to a need for myoelectric prosthetic control or refused to be randomized because they read on the internet that TMR was effective.6 This second subset of non randomized patients was then also followed and results were published by Mioton et al.8 They showed that in 33 patients with a minimum of 1-year follow-up, NRS scores for residual limb pain decreased from 6.4 ± 2.6 to 3.6 ± 2.2 (P < 0.001) and phantom limb pain decreased from 6.0 ± 3.1 to 3.6 ± 2.9 (P < 0.001). They also reported that the percentage of individuals experiencing severe residual limb pain (defined as an NRS score of 7–10) decreased from 58% (19/33) preoperatively to 6% (2/33) postoperatively. The percentage of individuals experiencing severe phantom limb pain decreased from 52% (17/33) preoperatively to 15% (5/33) postoperatively.8 Valerio et al11 showed that when TMR is performed preemptively at the time of amputation, the percentage of patients who were completely pain free from phantom limb and residual limb pain were 45.3% and 49.2%, respectively, compared to only 21.5% and 19.5% in the non-TMR group. Another study by Frantz et al9 investigating the outcome of preemptive TMR at the time of amputation found it to be effective at preventing phantom limb and residual limb pain in 92% of patients.
DISCUSSION
Despite promising initial published results, subsequent TMR outcomes studies have demonstrated substantial variability. Exact surgical techniques for TMR are variable, and in some reports minimal technical detail is provided.21 Furthermore, multiinstitutional studies have been authored by surgeons who individually have described different techniques, indicating that the treatments rendered may have been variable to some degree. As a result, it is difficult to generalize the published outcomes across the variability of technical procedures being performed, and impossible using these reports to infer which technical variants are superior. It stands to reason therefore, that the absence of standardized techniques for a novel surgical intervention could lead to surgeons running into failures and poor outcomes as seen in the revision TMR case reports described here. The following sections will explore some of the apparent pitfalls that we have encountered while managing patients with failed TMR.
Technical Pitfalls and Pearls
Nerve Redundancy
Technical education for nerve transfers has always emphasized adequate redundancy both to avoid the contribution of tension to neural ischemia, and to prevent rupture of the coaptation with motion related to excursion of nearby joints.34–37 However, in the setting of TMR for amputees, some of these factors are less applicable. For instance, in amputees, the coaptation is typically being done immediately above the level of the amputation and as a result is not compromised by joint movement since there is no joint distal to the neurorrhaphy. Second, unlike a motor-to-motor nerve transfer, TMR involves transferring a mixed or sensory nerve that will be sensitive to kinking which creates mechanical or ischemic injury to pain fibers located around the nerve periphery. For instance, sensitivity to kinking of the ulnar nerve has been documented as a cause of failed ulnar nerve transposition.38 Last, unlike in a nerve transfer where it is possible to distribute redundancy proximal and distal to the coaptation, TMR coaptations are performed as close to the target muscle as possible which creates a fixed point at the coaptation that is prone to kinking from more proximal redundancy. This situation is exacerbated when the coaptation is imbricated within or fixed to the muscle at the site of the coaptation. Thus, considering the above, although a minimal degree of redundancy is still important to avoid ischemia, excess redundancy in TMR may be problematic.
This pitfall was evident in patient 2 where the median and ulnar nerves had sharp 90-degree turns as they reached the location of the TMR coaptation (Video 2; Fig. 4) and in patient 1 where the median and ulnar nerves were coiled in circles (Fig. 2).
Size Mismatch and Neuroma Formation
When performing TMR from a large mixed major nerve into a smaller motor branch, size mismatch is inevitable. We find it implausible that all donor nerve axons will all funnel into the small target nerve. Rather, it is likely that many regenerating axons will escape the target nerve and aberrantly sprout into the adjacent area (Video 2, demonstrating presence of symptomatic neuroma despite reinnervation of the target muscle). Axon escape must be accounted for by placing the coaptation as close as possible to the denervated muscle, allowing escaped axons to grow into the muscle and find targets. This pitfall was evident in patient 1 where TMR coaptations were performed too far from the target muscle (Fig. 1) resulting in axons growing into “empty space” that by default becomes scar tissue and leads to the classic mechanism for a painful neuroma.
One suggested technique to guide escaped axons into the muscle involves gathering surrounding muscle near the target nerve and imbricating it around the coaptation with a few loose sutures. With this muscle wrap, the aberrant sprouting axons that escape the target nerve will in theory have a greater likelihood of finding denervated nerve pathways or guiding epimysial membranes to conduct them towards motor end plates. However, as mentioned previously, when performing the muscle imbrication, it is important to avoid creating a kink point with any excess redundancy leading up to the imbrication (Video 2).
Placement of Coaptation Site
Another technique to avoid symptomatic neuroma formation uses the same principles as standard neuroma transposition and burial techniques—keep the site of potential neuroma formation proximal, away from the end of the stump. This pitfall was evident in patient 2, in whom stimulation demonstrated that TMR was clearly successful in motor reinnervation of the target muscle but still developed symptomatic painful neuromas at the tip of the stump due to the superficial and distal placement of the TMR coaptations (Video 2).
Target Muscle Denervation
For the mixed major nerve that is being coapted into the small target motor nerve to properly reinnervate the target muscle segment, the latter must first be truly and completely denervated. Surgeons cannot simply explore the stump for the first available target motor nerve and perform the TMR without any further thought. Doing so does not guarantee that the target muscle is not still innervated by another nearby nerve branch or that the target muscle will not quickly become reinnervated by nearby collateral sprouting. If the target muscle becomes locally reinnervated before the coapted major nerve has a chance to regenerate into it, the coapted major nerve regeneration will be blocked and will result in a neuroma at that site. Additionally, it will not provide the intended myoelectric prosthetic signal if it does not successfully reinnervate the target muscle.
This issue can be managed in one of two ways. If the target muscle is nonfunctional or expendable, then the main nerve trunk into this target muscle should be transected proximally to denervate the entire muscle. TMR can then be done to a small motor branch near the neuromuscular junction as usual. If the muscle is not expendable, then TMR can be performed to an individual belly of the target muscle and then separated from the other muscle bellies with tissue such as an adipofascial flap, as described by Gart et al.4
Target Selection for Prosthetic Functional Optimization
Surgeons performing amputations may have the urge to include “any available” nerve coaptation acutely to prevent neuroma formation. Although this is a valid consideration, attention should still be paid to the target motor nerve selection. Surgeons performing TMR without adequate consideration of the myoelectric implications may be doing a disservice when the myoelectric signals are antagonistic or they have difficulty separating the signals from surrounding background muscle activity. Although it is unknown whether this contributes to pain relief, this failure was evident in patient 2 who underwent pTMR at the time of his amputation and had both the median and ulnar nerve coapted into two branches of the same triceps head. Not only was this antagonistic, but it also made it impossible to separate the signals from each other and from the other nearby triceps heads.
Surgeons who are uncertain about their confidence to properly select and prepare the target nerves and muscles for TMR should instead perform simple traction neurectomies with minimal dissection proximal to the amputation level. This preserves the appropriate nerve length and tissue planes for a secondary TMR.
Conceptual Pitfalls
Is There Evidence the Sensory Nerve Can Reinnervate Motor Endplates?
Although pTMR has been quickly adopted clinically, there has been little discussion regarding the microanatomic and biologic underpinnings of the procedure. Many early basic science and animal models of nerve regeneration have shown the effect of neurotropism on the preferential regeneration of nerves toward other nerve targets compared to nonnerve targets, and the preferential regeneration of motor axons to motor targets.39–41 However, there is less literature examining the effect of muscle reinnervation with sensory axons. In 1970, Zalewski42 demonstrated that although sensory axons will not reinnervate denervated muscle to reverse the effect of atrophy, evidence of sensory nerve fibers in the denervated muscle can be found. This would suggest that it is possible for the sensory component of the mixed donor nerve to actually grow into the target muscle during TMR surgery and achieve the effect of reduced neuroma formation. However, more modern specific research into this question using a mixed donor nerve would still be beneficial.
Unregulated “Conceptual Expansion” of TMR
Although innovation is a hallmark of our specialty and is likely to lead to continued improvements in TMR outcomes, allowing unchecked variations of technique to all be considered under the TMR moniker dilutes and confuses outcomes. For instance, the practice of implanting a mixed nerve at the site of a transected smaller intramuscular nerve branch, while leaving the dominant innervation to the muscle intact, is conceptually more akin to what has been previously been described as “targeted nerve implantation” than it is to “targeted muscle reinnervation.”43 Although these two concepts certainly exist along the same spectrum, they are unlikely to produce the same result given the underlying difference in physiology of the target.
Is Revision TMR Effective?
Future studies need to be performed to determine whether revision surgery is even indicated, or if another modality should be employed.
CONCLUSION
The advent of TMR has been a major development in amputee care for both pain management and improved myoelectric control. However, it is important to acknowledge that it is not a foolproof surgery and does not provide a guaranteed result. By recognizing and discussing failed cases of TMR, we have the opportunity to learn about factors which may contribute to unfavorable outcomes, and refine our techniques empirically. Furthermore, the use of precise language surrounding operative techniques will allow appropriate comparison of reported outcomes and accelerate advancement of this field.
Footnotes
Published online 6 April 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com
REFERENCES
- 1.Kuiken TA, Dumanian GA, Lipschutz RD, et al. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–253. [DOI] [PubMed] [Google Scholar]
- 2.Hijjawi JB, Kuiken TA, Lipschutz RD, et al. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118:1573–1578. [DOI] [PubMed] [Google Scholar]
- 3.Farina D, Castronovo AM, Vujaklija I, et al. Common synaptic input to motor neurons and neural drive to targeted reinnervated muscles. J Neurosci. 2017;37:11285–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gart MS, Souza JM, Dumanian GA. Targeted muscle reinnervation in the upper extremity amputee: a technical roadmap. J Hand Surg Am. 2015;40:1877–1888. [DOI] [PubMed] [Google Scholar]
- 5.Bowen JB, Wee CE, Kalik J, et al. Targeted muscle reinnervation to improve pain, prosthetic tolerance, and bioprosthetic outcomes in the amputee. Adv Wound Care (New Rochelle). 2017;6:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270:238–246. [DOI] [PubMed] [Google Scholar]
- 7.Souza JM, Cheesborough JE, Ko JH, et al. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;472:2984–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mioton LM, Dumanian GA, Shah N, et al. Targeted muscle reinnervation improves residual limb pain, phantom limb pain, and limb function: a prospective study of 33 major limb amputees. Clin Orthop Relat Res. 2020;478:2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frantz TL, Everhart JS, West JM, et al. Targeted muscle reinnervation at the time of major limb amputation in traumatic amputees. Jbjs Open Access. 2020;5:e0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien AL, Jordan SW, West JM, et al. Targeted muscle reinnervation at the time of upper-extremity amputation for the treatment of pain severity and symptoms. J Hand Surg Am. 2021;46:72.e1–72.e10. [DOI] [PubMed] [Google Scholar]
- 11.Valerio IL, Dumanian GA, Jordan SW, et al. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019;228:217–226. [DOI] [PubMed] [Google Scholar]
- 12.Bolognini N, Olgiati E, Maravita A, et al. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. 2013;154:1274–1280. [DOI] [PubMed] [Google Scholar]
- 13.Jensen TS, Krebs B, Nielsen J, et al. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983;17:243–256. [DOI] [PubMed] [Google Scholar]
- 14.Ephraim PL, Wegener ST, MacKenzie EJ, et al. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–1919. [DOI] [PubMed] [Google Scholar]
- 15.Eberlin KR, Ducic I. Surgical algorithm for neuroma management: a changing treatment paradigm. Plast Reconstr Surg Glob Open. 2018;6:e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chappell AG, Jordan SW, Dumanian GA. Targeted muscle reinnervation for treatment of neuropathic pain. Clin Plast Surg. 2020;47:285–293. [DOI] [PubMed] [Google Scholar]
- 17.Fracol ME, Dumanian GA, Janes LE, et al. Management of sural nerve neuromas with targeted muscle reinnervation. Plast Reconstr Surg Glob Open. 2020;8:e2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier ST, Jordan SW, Ko JH, et al. Targeted muscle reinnervation as a solution for nerve pain. Plast Reconstr Surg. 2020;146:651e–663e. [DOI] [PubMed] [Google Scholar]
- 19.Elmaraghi S, Albano NJ, Israel JS, et al. Targeted muscle reinnervation in the hand: treatment and prevention of pain after ray amputation. J Hand Surg Am. 2020;45:884.e1–884.e6. [DOI] [PubMed] [Google Scholar]
- 20.Daugherty THF, Mailey BA, Bueno RA, Jr, et al. Targeted muscle reinnervation in the hand: an anatomical feasibility study for neuroma treatment and prevention. J Hand Surg Am. 2020;45:802–812. [DOI] [PubMed] [Google Scholar]
- 21.Bowen JB, Ruter D, Wee C, et al. Targeted muscle reinnervation technique in below-knee amputation. Plast Reconstr Surg. 2019;143:309–312. [DOI] [PubMed] [Google Scholar]
- 22.Daugherty THF, Parikh R, Mailey BA, et al. Surgical technique for below-knee amputation with concurrent targeted muscle reinnervation. Plast Reconstr Surg Glob Open. 2020;8:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agnew SP, Schultz AE, Dumanian GA, et al. Targeted reinnervation in the transfemoral amputee: a preliminary study of surgical technique. Plast Reconstr Surg. 2012;129:187–194. [DOI] [PubMed] [Google Scholar]
- 24.Morgan EN, Kyle Potter B, Souza JM, et al. Targeted muscle reinnervation for transradial amputation: description of operative technique. Tech Hand Up Extrem Surg. 2016;20:166–171. [DOI] [PubMed] [Google Scholar]
- 25.Fracol ME, Janes LE, Ko JH, et al. Targeted muscle reinnervation in the lower leg: an anatomical study. Plast Reconstr Surg. 2018;142:541e–550e. [DOI] [PubMed] [Google Scholar]
- 26.Ducic I, Mesbahi AN, Attinger CE, et al. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg. 2008;121:908–914. [DOI] [PubMed] [Google Scholar]
- 27.Poppler LH, Parikh RP, Bichanich MJ, et al. Surgical interventions for the treatment of painful neuroma: a comparative meta-analysis. Pain. 2018;159:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badalamente MA, Hurst LC, Ellstein J, et al. The pathobiology of human neuromas: an electron microscopic and biochemical study. J Hand Surg Br. 1985;10:49–53. [DOI] [PubMed] [Google Scholar]
- 29.Harris AJ. Cortical origin of pathological pain. Lancet. 1999;354:1464–1466. [DOI] [PubMed] [Google Scholar]
- 30.Preissler S, Feiler J, Dietrich C, et al. Gray matter changes following limb amputation with high and low intensities of phantom limb pain. Cereb Cortex. 2013;23:1038–1048. [DOI] [PubMed] [Google Scholar]
- 31.Kooijman CM, Dijkstra PU, Geertzen JHB, et al. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87:33–41. [DOI] [PubMed] [Google Scholar]
- 32.Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. 2002;1:182–189. [DOI] [PubMed] [Google Scholar]
- 33.Subedi B, Grossberg GT. Phantom limb pain: mechanisms and treatment approaches. Pain Res Treat. 2011;2011:864605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. [DOI] [PubMed] [Google Scholar]
- 35.Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JM, Mackinnon SE. Nerve transfers in the forearm and hand. Hand Clin. 2008;24:319–40, v. [DOI] [PubMed] [Google Scholar]
- 37.Tung TH, Mackinnon SE. Nerve transfers: indications, techniques, and outcomes. J Hand Surg Am. 2010;35:332–341. [DOI] [PubMed] [Google Scholar]
- 38.Davidge KM, Ebersole GC, Mackinnon SE. Pain and function following revision cubital tunnel surgery. Hand (N Y). 2019;14:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundborg G, Dahlin LB, Danielsen N, et al. Tissue specificity in nerve regeneration. Scand J Plast Reconstr Surg. 1986;20:279–283. [DOI] [PubMed] [Google Scholar]
- 40.Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brushart TM, Seiler WA, 4th. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987;97:289–300. [DOI] [PubMed] [Google Scholar]
- 42.Zalewski AA. Effects of reinnervation on denervated skeletal muscle by axons of motor, sensory, and sympathetic neurons. Am J Physiol. 1970;219:1675–1679. [DOI] [PubMed] [Google Scholar]
- 43.Pet MA, Ko JH, Friedly JL, et al. Does targeted nerve implantation reduce neuroma pain in amputees? Clin Orthop Relat Res. 2014;472:2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]